Chapter 24
FUNGI
Craig S. Glazer and Cecile S. Rose
A variety of fungi are associated with occupational illnesses (see Tables 24.1, 24.2, and 24.3 on page 426.)
TABLE 24.1 Common fungi associated with hypersensitivity diseases.
| Fungus | Exposure | Syndrome |
| Alternaria spp. | Wood pulp | Wood pulp worker’s lung, mold allergy, asthma |
| Aspergillus spp. | Moldy hay | |
| Water | Farmer’s lung | |
| Aspergillus fumigatus | General environment | Ventilation pneumonitis |
| Aspergillus clavatus | Barley | Allergic bronchopulmonary aspergillosis |
| Aureobasidium pullulans | Water | |
| Cladosporium spp. | Hot tub mists | Malt worker’s lung |
| General environment | Humidifier lung | |
| Cryptostroma corticale | Wood bark | Hot tub HP |
| Graphium, Aureobasidium pullulans | Wood dust | Mold allergy, asthma |
| Merulius lacrymans | Rotten wood | Maple bark stripper’s lung |
| Penicillium spp. | Fuel chips, sawmills, tree cutting | Sequoiosis |
| Dry rot lung | ||
| Shiitake mushroom manufacturing, humidifier water | Hypersensitivity pneumonitis, asthma | |
| Penicillium frequentans | Cork dust | Suberosis |
| Penicillium casei, Penicillium roqueforti | Cheese | Cheese washer’s lung |
| Rhizopus, Mucor | Damp basements | Asthma, mold allergy |
| Trichosporon cutaneum | Damp wood and mats | Japanese summer-type HP |
TABLE 24.2 Common toxigenic fungi.
| Toxin | Fungal Source | Occupational Exposure |
| Aflatoxin | Aspergillus flavus | Farmers |
| Aspergillus parasiticus | Peanut handlers | |
| Fumitoxin | Aspergillus fumigatus | Compost workers |
| Satratoxin | Stachybotrys chartarum | Maintenance workers (from insulated pipes) |
| Sterigmatocystin | Avicularia versicolor | Housekeepers |
| T-2 toxin | Fusarium | Machinists |
| Farmers |
TABLE 24.3 Occupational fungal infections.
| Fungus | Source | Exposure |
| Blastomyces dermatitidis | Acid soil near rivers, streams, and swamps | Hunters |
| Campers | ||
| Coccidioides immitis | Semiarid or desert soils | Construction workers |
| Farmers | ||
| Archeologists | ||
| Laboratory workers | ||
| Textile workers | ||
| Cryptococcus neoformans | Pigeon/avian droppings | Pigeon breeders |
| Histoplasma capsulatum | Bat-infested caves, staring/chicken roosts | Spelunkers |
| Construction workers | ||
| Laboratory workers | ||
| Sporothrix schenckii | Contaminated soil and vegetation in warm or tropical areas | Gardeners |
| Farmers | ||
| Florists | ||
| Hunters | ||
| Gold miners | ||
| Laboratory workers |
ALTERNARIA SPECIES
Common name for disease: Wood pulp workers’ disease
Occupational setting
Woodworkers exposed via inhalation to wood dusts contaminated with the mold Alternaria may develop hypersensitivity pneumonitis, asthma, and allergic rhinitis.
Exposure (route)
Inhalation of the pyriform-shaped spores with an average length of 18 µm and a diameter of 5 µm (Figure 24.1) can cause hypersensitivity lung disease. Infection of the nails and cornea in an immunocompromised wood pulp worker has been reported,1 as have corneal infections in farmers working in humid environments.2
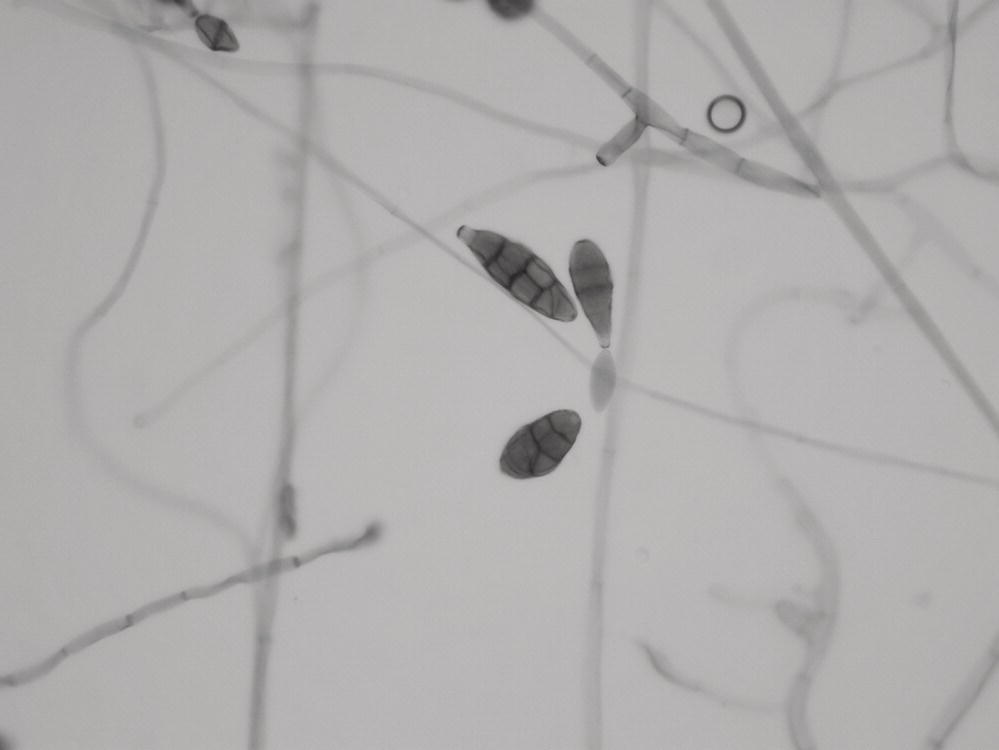
FIGURE 24.1 Photomicrograph showing Alternaria spores.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Pathobiology
In 1930, Hopkins described a 37-year-old man whose asthma was associated with exposure to damp musty areas contaminated with Alternaria spp. and confirmed by bronchial challenge.3 Bronchial challenge with both fungal extracts and whole spores of Alternaria can trigger both immediate and late asthmatic reactions in sensitized asthmatics.4 Immunologic sensitization to Alternaria has been linked to increased bronchial hyperreactivity and to life-threatening asthma.5–7 Alternaria is also a rare cause of allergic bronchopulmonary mycosis.8 Progressive hypersensitivity pneumonitis leading to chronic interstitial fibrosis has been documented in two workers with prolonged exposure to Alternaria during the manufacture of wood pulp.9 Eosinophilic pneumonia related to Alternaria exposure in a water-damaged home has been reported.10
Diagnosis
Diagnosis of sensitizing occupational asthma relies on symptom and exposure histories, findings on pulmonary function testing (typically airflow limitation with a significant bronchodilator response or positive methacholine challenge), and the results of peak flow monitoring at and away from work. Allergy skin prick testing to Alternaria is often but not always positive.7 Diagnosis of hypersensitivity pneumonitis relies on a constellation of clinical findings, including a careful symptom and occupational history.11 Physical examination may be normal or show basilar crackles; the chest radiography may be normal or show diffuse alveolar or interstitial opacities; pulmonary function tests show restriction, obstruction, or a mixed picture. Fiber-optic bronchoscopy with bronchoalveolar lavage and transbronchial biopsies may be helpful when the clinical suspicion is strong but routine tests are nondiagnostic. Exercise physiology testing may be helpful in patients who have dyspnea but normal resting pulmonary function. Serum precipitating antibodies to Alternaria are often found in asymptomatic exposed workers and may be negative if the wrong antigen preparation is used. Infections are diagnosed by culture.
Treatment
The treatment of hypersensitivity lung diseases begins with removal from antigen exposure. Inhaled corticosteroids are useful as first-line pharmacotherapy for asthma, often in combination with inhaled bronchodilators. Oral corticosteroids may be indicated in patients with hypersensitivity pneumonitis or eosinophilic pneumonia manifested by severe symptoms and radiographic or functional abnormalities. Amphotericin B and itraconazole are the most frequently used agents to treat infections, but recent data suggest posaconazole may have better in vitro activity.12
Prevention
Adequate ventilation and process enclosure, appropriate respiratory protection, and work practices that reduce airborne dust levels are recommended. Workers in at-risk industries should be educated regarding exposure risks and encouraged to seek early medical attention for persistent respiratory and/or systemic symptoms.11
References
- 1. Arrese JE, Pierard-Franchimont C, Pierard GE. Onychomycosis and keratomycosis caused by Alternaria spp. A bipolar opportunistic infection in a wood-pulp worker on chronic steroid therapy. Am J Dermatopathol 1996; 18:611–3.
- 2. Jiang K, Brownstein S, Baig K, et al. Clinicopathologic case reports of Alternaria and Fusarium keratitis in Canada. Can J Ophthalmol 2013; 48:e151–4.
- 3. Hopkins J, Benham R, Kesten B. Asthma due to a fungus – Alternaria. JAMA 1930; 94:6–11.
- 4. Licorish K, Novey H, Kozak P. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol 1985; 76:819–25.
- 5. Nelson HS, Szefler SJ, Jacobs J, et al. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol 1999; 104:775–85.
- 6. Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000; 55:501–4.
- 7. Fernandez C, Bevilacqua E, Fernandez N, et al. Asthma related to Alternaria sensitization: an analysis of skin-test and serum-specific IgE efficiency based on the bronchial provocation test. Clin Exp Allergy 2011; 41:649–56.
- 8. Chowdhary A, Agarwal K, Randhawa HS, et al. A rare case of allergic bronchopulmonary mycosis caused by Alternaria alternata. Med Mycol 2012; 50:890–6.
- 9. Schlueter D, Fionk J, Hensley G. Wood pulp workers’ disease: a hypersensitivity pneumonitis caused by Alternaria. Ann Intern Med 1972; 77:907–14.
- 10. Ogawa H, Fujimura M, Amaike S, et al. Eosinophilic pneumonia caused by Alternaria alternata. Allergy 1997; 52:1005–8.
- 11. Rose C, Lara A. Hypersensitivity pneumonitis. In: Murray JF, Nadel JF (eds.), Textbook of Respiratory Medicine, 5th edn. Philadelphia: Saunders, 2010:1587–600.
- 12. Alastruey-Iazquierdo A, Cuesta I, Ros L, et al. Antifungal susceptibility profile of clinical Alternaria spp. identified by molecular methods. J Antimicrob Chemother 2011; 66:2585–7.
ASPERGILLUS SPECIES
Common names for diseases: Hypersensitivity diseases: Extrinsic allergic alveolitis, farmer’s lung, malt worker’s lung (Aspergillus clavatus), allergic bronchopulmonary aspergillosis (ABPA), allergic Aspergillus sinusitis, allergic fungal sinusitis (AFS), baker’s asthma, mushroom grower’s asthma; Infections: Invasive aspergillosis, aspergilloma. Mycotoxins: Mycotoxicosis, aflatoxin-induced liver cancer.
Occupational setting
There are over 600 species in the genus Aspergillus. Most Aspergillus species are found in soil. Many species are found on a wide variety of substrates, including forage products, food products, cotton, and other organic debris. Aspergillus fumigatus, the most common species, accounts for most disease, both allergic and infectious. Farmers, sawmill workers, mushroom workers, greenhouse workers, tobacco workers, bakers, garbage workers, and bird hobbyists are among the many groups at risk from this fungal exposure.1–12 Workers who deal with compost piles, decomposing haystacks, or moldy grains may develop hypersensitivity responses and are at increased risk of developing liver cancer.13–16 Power plant workers are an emerging at-risk group in plants that are transitioning to biomass fuels as an alternative to traditional carbon-based fuels.17 Allergy to Aspergillus-derived enzymes has been associated with baker’s asthma.18
Exposure (route)
Aspergillus species produce conidia in chains measuring 2–5 µm in diameter that are readily airborne and easily respirable (Figure 24.2). Aspergillus aflatoxin may become airborne as well.19 Most Aspergillus diseases are acquired from inhalation of spores, but airborne spores probably also infect tissues exposed during surgery. Hospital renovations may increase the risk of aspergillosis in immunocompromised hosts due to release of spore-bearing dust. This risk can be attenuated by the addition of either high-efficiency particulate air filtration or laminar airflow.20 Contaminated ventilation systems and compost sites have been associated with case clusters.21 Other portals of entry (including the eye, paranasal sinuses, skin, GI tract, and via intravenous drug use) have been associated with invasive aspergillosis.
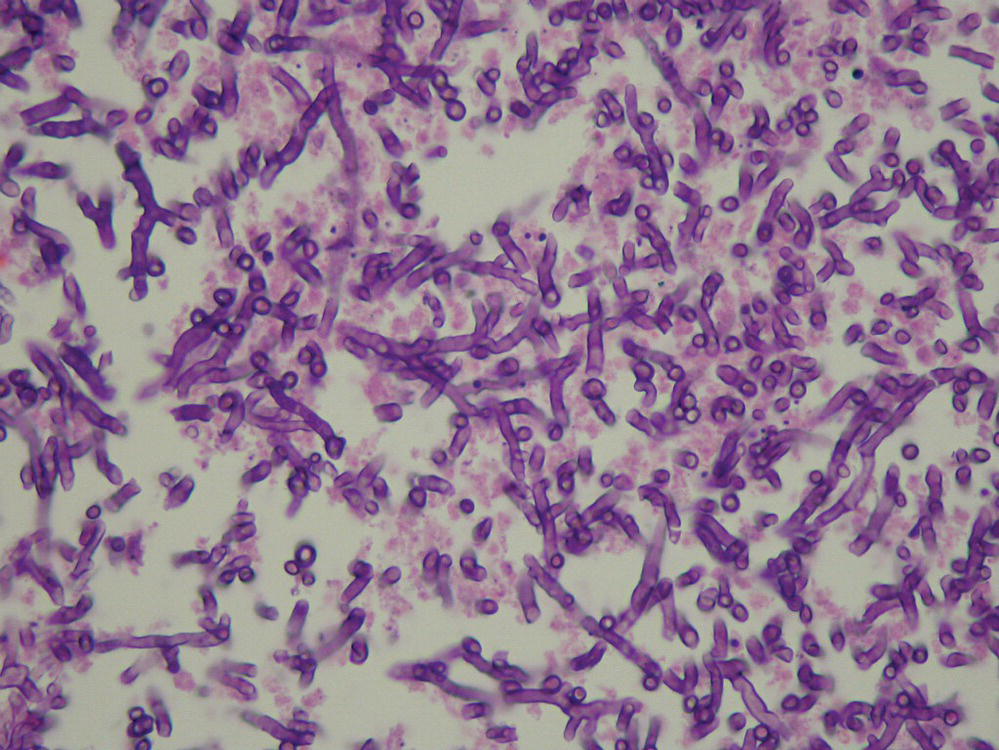
FIGURE 24.2 Tissue sample hematoxylin and eosin stain revealing Aspergillus hyphae in tissue.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Pathobiology
There are four main categories of disease involving Aspergillus spp.—allergic aspergillosis, colonizing aspergillosis, invasive infections (both pulmonary and nonpulmonary), and aflatoxin-induced malignancy.
ALLERGIC ASPERGILLOSIS: The four major allergic manifestations of Aspergillus sensitization are asthma, hypersensitivity pneumonitis, allergic bronchopulmonary aspergillosis, and allergic sinusitis.
The clinical manifestations of Aspergillus-related asthma are no different from other forms of extrinsic asthma: symptoms of cough, wheezing, chest tightness, and dyspnea; wheezing on examination; and obstructive changes on pulmonary function testing during acute exacerbations. Peripheral eosinophilia is common, and Aspergillus-specific IgE antibodies are detectable with serum RAST or ELISA.
Hypersensitivity pneumonitis (extrinsic allergic alveolitis) can occur in individuals with repeated exposure to organic dusts containing Aspergillus species. Symptoms may resemble an acute, self-limited, flu-like illness occurring 6–12 hours after exposure. Signs include crackles on lung exam, peripheral leukocytosis, infiltrates on chest radiographs, and normal, restrictive, or obstructive changes on lung function testing. Symptoms of hypersensitivity pneumonitis may also be subacute or chronic. They include myalgias, weight loss, fatigue, chest tightness, cough, and subtle, progressive dyspnea on exertion. The chest radiograph and pulmonary function tests are often normal in early disease. More sensitive diagnostic testing, such as fiber-optic bronchoscopy, may be needed to detect the characteristic lymphocytic alveolitis and granulomatous lung disease. Continued antigen exposure typically leads to worsening symptoms, restrictive physiologic changes, and permanent interstitial fibrosis.
Allergic bronchopulmonary aspergillosis (ABPA) is an inflammatory disease caused by an immunologic response to Aspergillus fumigatus (Af) and other Aspergillus species growing in the bronchi of patients with asthma and cystic fibrosis.22 Classically, patients present with chest radiographic infiltrates and peripheral blood eosinophilia. ABPA can range from mild asthma to end-stage fibrotic lung disease. The latest ABPA diagnostic criteria include (i) the presence of a predisposing condition such as asthma or cystic fibrosis; (ii) obligatory criteria including both elevated total IgE (>1000) and immunologic sensitization as shown by immediate cutaneous reactivity to Aspergillus or elevated serum IgE antibodies to Aspergillus; and (iii) two out of the following three other criteria: (a) precipitating (IgG) antibodies to Aspergillus, (b) radiographic abnormalities consistent with ABPA, or (c) total eosinophil count greater than 500. 22
Allergic fungal sinusitis (AFS) due to Aspergillus species typically occurs in immunocompetent atopic patients. Most patients have asthma; 85% have nasal polyposis.23 Patients present with chronic recurrent sinusitis are unresponsive to conventional therapy. Many describe blowing dark, rubbery plugs out of their nose. Fungal hyphae are identified with Gomori methenamine silver stain of the allergic mucin. Diagnostic criteria are similar to those for ABPA and include peripheral eosinophilia, increased serum IgE levels, and precipitating antibodies to fungal extracts.
COLONIZING ASPERGILLOSIS: Saprophytic colonization of air spaces by Aspergillus species can result in a number of outcomes, including otomycosis, fungus ball of the paranasal sinuses,24 endobronchial colonization, and fungus ball of the lung (aspergilloma). Fungus balls of the lung typically occur in patients with preexisting cavitary lung diseases or chronic allergic aspergillosis, with an aspergilloma forming in an ectatic bronchus. The condition is associated with eosinophilic pneumonia and bronchiectasis. Primary aspergillomas may also occur in patients without allergic disease; they present as fuzzy infiltrates that progress to rounded cavities on the chest radiograph. The major symptom of pulmonary aspergilloma is recurrent hemoptysis. Masses of fungal elements are found on surgical resection. Resolution with residual fibrosis is uncommon. Aspergillomas may occur in association with other cavitary diseases, including tuberculosis and sarcoidosis. Fungus balls occur rarely in the urinary bladder, gallbladder, and bile ducts.
INVASIVE ASPERGILLOSIS: This is an uncommon form of Aspergillus-related disease. It is also the most serious. It typically occurs in immunocompromised patients, most notably those with leukemia and lymphoma. Fatal invasive microgranulomatous aspergillosis occurred after shoveling moldy cedar wood chips in a patient with chronic granulomatous disease.25 An unusual case of fatal invasive pulmonary aspergillosis occurred in a nonimmunocompromised gardener exposed to heavy environmental concentrations of A. fumigatus.3 The disease presents as pneumonia with fever, cough, leukocytosis, and respiratory distress. Radiographically, there may be diffuse, patchy, alveolar infiltrates, or consolidation with mass effect (lung abscess). The necrotizing pneumonia that develops is usually fulminant, and death typically occurs in 1–2 weeks. Diagnosis is difficult because Aspergillus antibodies are often not detectable in the immunocompromised patient. Depending on the patient’s clinical status, invasive diagnostic procedures, such as bronchoscopy or surgical lung biopsy, early in the course of disease may enable early diagnosis and treatment with intravenous amphotericin B. Recent advances in the diagnosis of invasive disease include serum and respiratory sample testing for aspergillus galactomannan and aspergillus PCR.26 The combination of both has the best positive predictive values.27
Extrapulmonary forms of invasive aspergillosis may involve the eye (keratitis), ear, paranasal sinuses, skin, CNS, heart, and GI tract. Intravenous drug injection and iatrogenic procedures (i.e., dialysis, cardiac surgery, and intrathecal medication) can create portals of entry for fungal dissemination.28
MYCOTOXIN DISEASE: Aflatoxin, a mycotoxin produced by many aspergillus species, is an IARC Class I carcinogen.29 Specifically, aflatoxin increases the risk for developing liver cancer in exposed subjects. Increased standardized mortality ratios (SMRs) for liver cancer have been described in Swedish grain millers, animal feed production plant workers, and other agricultural occupations in both Denmark and the United States where aflatoxin exposures likely occur.14–16
Treatment
Management of Aspergillus-related asthma includes treatment with inhaled steroids and bronchodilators and avoidance of exposure to high airborne concentrations of conidia. Similarly, the mainstay of management of hypersensitivity pneumonitis is the removal from further antigen exposure. Treatment with oral corticosteroids may be indicated in persistently or severely symptomatic individuals with abnormal physiology. For patients with ABPA, treatment with systemic corticosteroids is indicated to prevent or minimize bronchiectasis that occurs with each episode of pneumonia. Antifungal therapy with itraconazole may allow a reduction in corticosteroid dose.22,30 Therapy for AFS usually includes surgical debridement followed by systemic corticosteroids; however, evidence of efficacy is largely anecdotal. Early diagnosis and treatment are important in the management of invasive aspergillosis. Voriconazole is now the preferred agent for treatment of invasive aspergillosis based on head to head trials showing superiority to amphotericin B.30 The voriconazole dose is 6 mg/kg IV every 12 hours on the first day, followed by 4 mg/kg IV every 12 hours. Alternative agents include caspofungin, posaconazole, and liposomal amphotericin.30 Combination therapy is only recommended for salvage after initial treatment failure.30
Prevention
Although Aspergillus spp. are ubiquitous, immunocompromised patients should be protected from exposure to high concentrations of fungal conidia and should avoid occupational settings where high spore concentrations are likely. High-efficiency filters remove Aspergillus from the air of operating rooms and laminar flow rooms. Isolating immunosuppressed patients from dusty hospital renovation and construction appears useful, as does keeping potted plants out of their rooms. High-efficiency particulate air filtration has been used during renovations to minimize exposure risk.20 Occupational groups such as agricultural workers, bird breeders, sawmill workers, and brewery workers should be provided with adequate ventilation or respiratory protection in circumstances where exposure concentrations are likely to be high. These occupational groups should be educated regarding the exposure risks and instructed to seek early medical attention for recurrent or persistent respiratory and systemic symptoms. Aggressive diagnostic evaluation of suspect hypersensitivity pneumonitis (including early bronchoscopy) is essential, as is the removal from exposure if disease is confirmed.
References
- 1. Yoshida K, Ueda A, Yamasaki H, et al. Hypersensitivity pneumonitis resulting from Aspergillus fumigatus in a greenhouse. Arch Environ Health 1993; 48:260–262.
- 2. Yoshida K, Ando M, Ito K, et al. Hypersensitivity pneumonitis of a mushroom worker due to Aspergillus glaucus. Arch Environ Health 1990; 45:245–247.
- 3. Zuk J, King D, Zakhour HD, et al. Locally invasive pulmonary aspergillosis occurring in a gardener; an occupational hazard? Thorax 1989; 44:678–679.
- 4. Land C, Hult K, Fuchs R, et al. Tremorigenic mycotoxins from Aspergillus fumigatus as a possible occupational health problem in sawmills. Appl Environ Microbiol 1987; 58:787–790.
- 5. Reijula K, Sutinen S. Immunohistochemical identification of Aspergillus fumigatus in farmer’s lung. Acta Histochem 1984; 75:211–213.
- 6. Mehta S, Sandhu R. Immunological significance of Aspergillus fumigatus in cane-sugar mills. Arch Environ Health 1983; 38:41–46.
- 7. Palmas F, Cosentino S, Cardia P. Fungal airborne spores as health risk factors among workers in alimentary industries. Eur J Epidemiol 1989; 5:239–243.
- 8. Anonymous. Fatal pulmonary aspergillosis following a farm accident (Letter). Chest 1984; 85:448–449.
- 9. Riddle H, Channell S, Blyth W, et al. Allergic alveolitis in a maltworker. Thorax 1968; 23:271–280.
- 10. Huuskonen M, Husman K, Jarvisalo J, et al. Extrinsic allergic alveolitis in the tobacco industry. Br J Ind Med 1984; 41:77–83.
- 11. Wiszniewska M, Tymoszuk D, Nowakowska-Swirta E, et al. Mould sensitization among bakers and farmers with work-related respiratory symptoms. Ind Health 2013; 51:275–284.
- 12. Hagemeyer O, Bunger J, van Kampen V, et al. Occupational allergic respiratory diseases in garbage workers: relevance of molds and actinomycetes. Adv Exp Med Biol 2013; 788:313–320.
- 13. Vincken W, Roels P. Hypersensitivity pneumonitis due to Aspergillus fumigatus in compost. Thorax 1984; 39:74–75.
- 14. Alvanja MC, Malker H, Hayes RB. Occupational cancer risk associated with the storage and bulk handling of agricultural foodstuff. J Toxicol Environ Health 1987; 22:247–254.
- 15. Autrup JL, Schmidt J, Autrup H. Exposure to aflatoxin B1 in animal-feed production plant workers. Environ Health Perspect 1993; 99:195–197.
- 16. Peraica M, Radic B, Lucic A, et al. Toxic effects of mycotoxins in humans. Bull World Health Organ 1999; 77:756–766.
- 17. Lawniczek-Watczyk A, Golofit-Szymczak M, Cyprowski M, et al. Exposure to harmful microbiological agents during the handling of biomass for power production purposes. Med Pr 2012; 63:395–407.
- 18. Quirce S, Cuevas M, Diez-Gomez M, et al. Respiratory allergy to Aspergillus-derived enzymes in bakers’ asthma. J Allergy Clin Immunol 1992; 90:970–978.
- 19. Selim MI, Juchems AM, Popendorf W. Assessing airborne aflatoxin B1 during on-farm grain handling activities. Am Ind Hyg Assoc J 1998; 59:252–256.
- 20. Cornet M, Levy V, Fleury L, et al. Efficacy of prevention by high-efficiency particulate air filtration or laminar airflow against Aspergillus airborne contamination during hospital renovation. Infect Control Hosp Epidemiol 1999; 20:508–513.
- 21. Topping MD, Scarisbrick D, Luczynska C, et al. Clinical and immunological reactions to Aspergillus niger among workers at a biotechnology plant. Br J Ind Med 1985; 42:312–318.
- 22. Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43:850–873.
- 23. Schwietz L, Gourley D. Allergic fungal sinusitis. Allergy Proc 1992; 13:2–6.
- 24. Robb P. Aspergillosis of the paranasal sinuses: a case report and historical perspective. J Laryngol Otol 1986; 100:1071–1077.
- 25. Conrad DJ, Warnock M, Blanc P, et al. Microgranulomatous aspergillosis after shoveling wood chips: report of a fatal outcome in a patient with chronic granulomatous disease. Am J Ind Med 1992; 22:411–418.
- 26. Hayes GE, Denning DW. Frequency, diagnosis and management of fungal respiratory infections. Curr Opin Pulm Med 2013; 19:259–265.
- 27. Reinwald M, Spiess B, Heinz WJ, et al. Diagnosing pulmonary aspergillosis in patients with hematological malignancies: a multicenter prospective evaluation of an Aspergillus PCR assay and a galactomannan ELISA in bronchoalveolar lavage samples. Eur J Haematol 2012; 89:120–127.
- 28. Leon EE, Craig TJ. Antifungals in the treatment of allergic bronchopulmonary aspergillosis. Ann Allergy Asthma Immunol 1999; 82:511–517.
- 29. Anonymous. Aflatoxins. IARC Monogr Eval Carcinog Risks Hum 1993; 56:245–395.
- 30. Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327–360.
BASIDIOMYCETES (INCLUDING MERULIUS LACRYMANS, LYCOPERDON, AND MUSHROOMS)
Common names for disease: Mushroom spore asthma, hypersensitivity pneumonitis, extrinsic allergic alveolitis, mushroom worker’s lung, mushroom compost worker’s lung, lycoperdonosis.
Occupational setting
Basidiomycetes are common in nature but they are rarely associated with human disease. Allergic reactions (asthma, rhinoconjunctivitis, and hypersensitivity pneumonitis) to inhaled spores have been described for a number of species.1 Basidiomycetes are also rare causes of allergic bronchopulmonary mycosis.2,3 Contact dermatitis has also been described, but infection is very rare.4 Certain basidiospores (e.g., Merulius lacrymans) contaminate wood with 20–25% water content, and mycelia typically extend in sheets over timber and adjacent brickwork.5,6 Mushroom workers may develop asthma, allergic rhinitis, or hypersensitivity pneumonitis from inhalation of several species of mushroom spores.7–11 Mushroom soup workers have been known to develop asthma and allergic rhinoconjunctivitis from inhaled mushroom dusts.12 Removal of stored spent mushroom compost may lead to the release of hazardous concentrations of hydrogen sulfide gas, a respiratory irritant.13,14
Exposure (route)
Inhalation of basidiospores causes hypersensitivity lung disease. Contact dermatitis after skin exposure to Hericium erinaceum has been reported.4
Pathobiology
The Basidiomycetes are known as club fungi because, after mycelial growth, a fruiting body is formed and club-shaped structures called basidia develop where basidiospores are produced. Many diverse forms are included in this class, including puffballs, common rusts and smuts, mushrooms, and certain yeasts. Spores may be discharged in bursts during times of high humidity.
Atopic asthmatics may demonstrate IgE-mediated reactivity to Basidiomycete aeroallergens. Merulius lacrymans, a basidiomycete found in buildings in cool temperate climates, has been associated with both asthma and hypersensitivity pneumonitis.5,6,15 In one report, a teacher developed insidious onset of symptoms of dyspnea, cough, malaise, fever, and weight loss. He also had rales on physical exam. His chest radiograph showed diffuse micronodular infiltrates. Pulmonary function tests showed low diffusing capacity for carbon monoxide. Serum precipitins to M. lacrymans present in his home were positive, as was inhalation challenge with the fungus. Clinical recovery progressed slowly following cessation of antigen exposure.
Inhalation of dried powder from the fleshy basidiomycete lycoperdon (puffball) has been associated with lycoperdonosis, a form of hypersensitivity pneumonitis that developed following treatment of epistaxis.16
In another report, eight workers exposed to dried mushroom soup developed symptoms of asthma and rhinoconjunctivitis.9 A Type I hypersensitivity reaction was suggested by clinical history, positive immediate skin prick test reactivity to mushroom extracts, and immediate response to inhalation challenge.
Mushroom worker’s lung typically occurs during the first few months of employment, though sensitization after many years of exposure is also described.11 Seven workers at a mushroom farm in Florida developed episodic dyspnea, cough, fever, chills, and myalgias. Pulmonary function tests and chest radiographs showed evidence of hypersensitivity pneumonitis. The workers were subsequently removed from exposure, but a specific causative antigen was not identified.7 In a similar case series from Japan, specific precipitating antibodies were found to mushroom spore extracts.11
Diagnosis
Diagnosis of sensitizing occupational asthma relies on the symptom and exposure histories, findings on pulmonary function testing (including positive methacholine challenge), and results of peak flow monitoring at and away from work. Allergy skin prick testing to mushroom antigen is often (but not always) positive. Diagnosis of hypersensitivity pneumonitis relies on a constellation of clinical findings, including a careful symptom and occupational history.17 Physical examination may be normal or show basilar lung crackles; the chest radiograph may be normal or show diffuse alveolar or interstitial opacities; pulmonary function tests show restriction, obstruction, or a mixed picture. Fiber-optic bronchoscopy with bronchoalveolar lavage and transbronchial biopsies may be helpful when the clinical suspicion is strong but routine tests are normal. Exercise physiology testing may be helpful in patients who have dyspnea but normal resting pulmonary function. Serum precipitating antibodies to mushroom extracts may be found in asymptomatic exposed workers and may be negative if the wrong antigen preparation is used, limiting their clinical utility.
Treatment
The treatment of hypersensitivity lung diseases begins with early recognition and prompt removal from exposure to the immunogen. Following removal, inhaled corticosteroids are useful as first-line pharmacotherapy for asthma. The efficacy of oral corticosteroids in the treatment of hypersensitivity pneumonitis is unclear, but treatment is probably indicated in patients with severe symptoms, radiographic, or functional abnormalities.
Prevention
Once sensitization has occurred, the prognosis for recovery from occupational asthma is directly related to the duration of exposure. If removal from exposure is delayed, individuals with hypersensitivity pneumonitis are at risk for disease progression. A high index of clinical suspicion for workers in at-risk environments is therefore crucial. Reduction of workplace exposure to high fungal concentrations through engineering and process controls reduces disease risk.
References
- 1. Rivera-Mariani FE, Nazario-Jimenez S, Lopez-Malpica F, et al. Sensitizationto airborne ascospores, basidiospores, and fungal fragments in allergic rhinitis and asthmatic subjects in San Juan, Puerto Rico. Int Arch Allergy Immunol 2011; 155:322–334.
- 2. Singh PK, Kathuria S, Agarwal K, et al. Clinical significance and molecular characterization of nonsporulating molds isolated from the respiratory tracts of bronchopulmonary mycosis patients with special reference to basidiomycetes. J Clin Microbiol 2013; 51:3331–3337.
- 3. Ogawa H, Fujimura M, Takeuchi Y, et al. A case of sinobronchial allergic mycosis; possibility of basidiomycetous fungi as a causative antigen. Intern Med 2011; 50:59–62.
- 4. Maes MF, van Baar HM, van Ginkel CJ. Occupational allergic contact dermatitis from the mushroom White Pom Pom (Hericium erinaceum). Contact Dermatitis 1999; 40:285–290.
- 5. Herxheimer H, Hyde H, Williams D. Allergic asthma caused by basidiospores. Lancet 1969; 2:131–133.
- 6. O’Brien I, Bull J, Creamer B, et al. Asthma and extrinsic allergic alveolitis due to Merulius lacrymans. Clin Allergy 1978; 8:535–542.
- 7. Sanderson W, Kullman G, Sastre J, et al. Outbreak of hypersensitivity pneumonitis among mushroom farm workers. Am J Ind Med 1992; 22:859–872.
- 8. Michils A, DeVuyst P, Nolard J, et al. Occupational asthma to spores of Pleurotus cornucopiae. Eur Resp J 1991; 4:143–147.
- 9. Mori S, Nakagawa-Yoshida K, Tsuchihashi H, et al. Mushroom worker’s lung resulting from indoor cultivation of Pleurotus osteatus. Occup Med 1998; 48:465–468.
- 10. Helbling A, Gayer F, Brander KA. Respiratory allergy to mushroom spores: not well recognized, but relevant. Ann Allergy Asthma Immunol 1999; 83:17–19.
- 11. Akizuki N, Inase N, Ishiwata N, et al. Hypersensitivity pneumonitis among workers cultivating Tricholoma conglobatum (Shimeji). Respiration 1999; 66:273–278.
- 12. Symington I, Kerr J, McLean D. Type I allergy in mushroom soup processors. Clin Allergy 1981; 11:43–47.
- 13. Velusami B, Curran TP, Grogan HM. Hydrogen Sulfide gas emissions during disturbance and removal of stored spend mushroom compost. J Agric Saf Health 2013; 19:261–275.
- 14. Velusami B, Curran TP, Grogan HM. Hydrogen sulfide gas emissions in the human-occupied zone during disturbance and removal of stored spent mushroom compost. J Agric Saf Health 2013; 19:277–291.
- 15. Horner WE, Helbling A, Lehrer SB. Basidiomycete allergens. Allergy 1998; 53:1114–1121.
- 16. Strand R, Neuhauser E. Lycoperdonosis. N Engl J Med 1967; 277:89–91.
- 17. Rose C, Lara A. Hypersensitivity pneumonitis. In: Murray JF, Nadel JF (eds.), Textbook of Respiratory Medicine, 5th edn. Philadelphia: Saunders, 2010:1587–1600.
BLASTOMYCES DERMATITIDIS
Common names for disease: Blastomycosis. North American blastomycosis, Gilchrist’s disease, Chicago disease, Namekagon fever
Occupational setting
Exposure to the fungus Blastomyces dermatitidis can cause the infection blastomycosis. Blastomycosis is most prevalent in the southeastern United States and Ohio-Mississippi River Valley area. However, the geographic range may be broader than previously believed as blastomycosis has been reported in Colorado following prairie dog relocation.1,2 An African form of blastomycosis has been reported. Disease is much more common in men than in women (9 : 1).
Epidemiologic studies suggest that patients often work outdoors and have intimate contact with soil. A horticulturist developed progressive blastomycosis from exposure to contaminated fertilizer.3 A tobacco worker in Switzerland and a packing material handler in England developed the illness after handling fungal fomites.4 There have been occasional reports of small clusters or disease outbreaks in many areas of the United States and Canada.5 In Virginia, four hunters were infected while raccoon hunting at night in swampy, wooded areas. In a Minnesota outbreak, four cases developed in three families constructing a cabin in a wooded area near a lake.6 In a 1979 Wisconsin outbreak, seven of eight individuals camping near a river developed acute pneumonia.7 In a larger outbreak in Wisconsin in 1984, numerous elementary schoolchildren and several adults who visited a beaver pond at a campground developed symptomatic blastomycosis with an incubation period of 21–106 days after exposure to the presumed point source.8,9 A technician working for several years in a small, dusty, wooden petroleum filtering shed in southwest Ontario developed systemic blastomycosis with meningeal involvement; B. dermatitidis was isolated from the earthen floor of the shed.10
These data suggest that B. dermatitidis survives in wet soil of acid pH with a high organic content and probably exists in point sources close to rivers, streams, or swamps. Environmental conditions during cool months may be more favorable for the saprophytic growth and survival of the fungus; disturbance of these sites either through occupational or avocational activities may lead to airborne dispersal of the spores.
Several cases of laboratory-acquired disease have been reported; the majority resulted from finger inoculation with the yeast form during autopsy by pathologists who developed primary cutaneous blastomycosis.11–13 Primary pulmonary blastomycosis also can be a laboratory-acquired infection.14
Exposure (route)
The most important route of exposure leading to infection from B. dermatitidis is disturbance of contaminated point sources leading to airborne dispersal of spores. Accidental skin inoculation of the yeast form has been reported, as has transmission via dog bites. Venereal transmission of genitourinary infection and in utero transmission are rare.
Pathobiology
Blastomyces dermatitidis is a dimorphic fungus that grows as a mycelial form at room temperature and as a yeast form at 37°C (Figure 24.3).
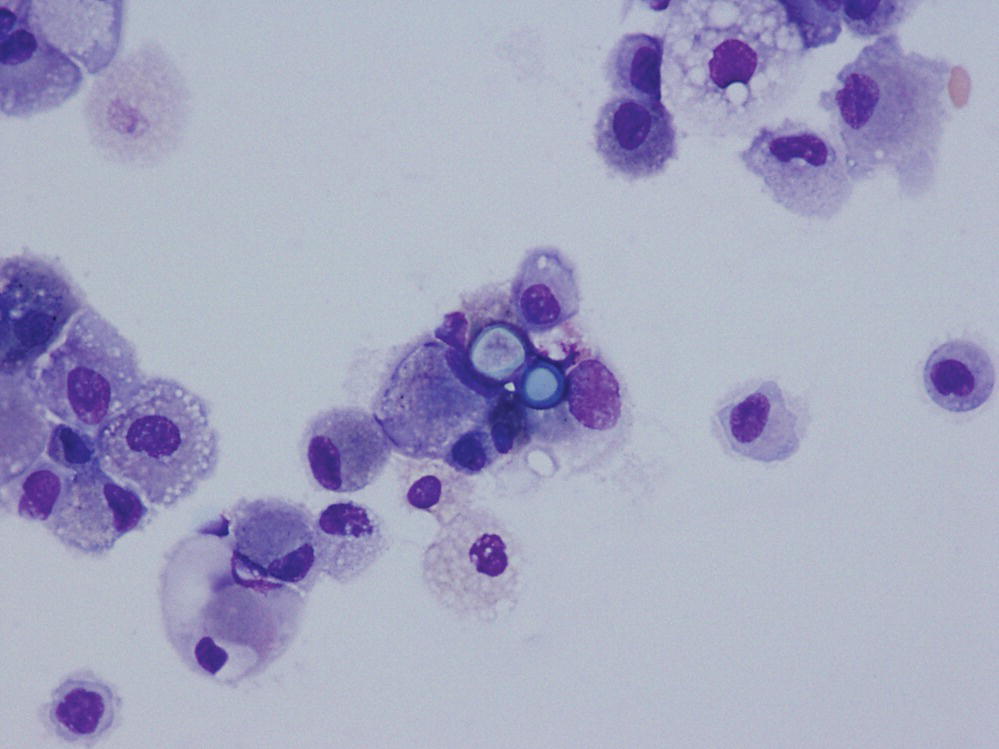
FIGURE 24.3 Bronchoalveolar lavage sample demonstrating typical yeast forms of Blastomycosis.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
The lung is the organ most commonly infected by B. dermatitidis; the resulting illness is usually indolent in onset and course. Symptoms may be present for weeks, months, or even years before diagnosis. Symptoms typically include cough, weight loss, chest pain, skin lesions, fever, hemoptysis, and localized swelling. In almost half the patients with pulmonary infection, respiratory symptoms are mild or absent. It is usually the systemic symptoms or extrapulmonary lesions that lead to medical attention.
A number of chest radiographic patterns have been described, including a patchy alveolar airspace process with air bronchograms, fibronodular densities, miliary nodules, linear interstitial infiltrates, and cavitation. Pleural effusions and hilar adenopathy are uncommon. Laryngeal, tracheal, or endobronchial lesions are seen occasionally. The rate of progression of indolent disease may be gradual or sudden and rapid. Occasionally, patients present with acute symptoms of fever, productive cough, and pleuritic chest pain. The chest radiograph typically shows single or multiple nodular or patchy infiltrates. Spontaneous improvement of acute blastomycotic pneumonia may occur after 2–12 weeks of symptoms.
Blastomycotic skin lesions involving the face, extremities, neck, and scalp are common and provide ready access for biopsy and culture. Most lesions arise from hematogenous seeding from the lung, although local inoculation may occur in researchers, pathologists, and morticians who handle infected tissue.
Osteomyelitis involving vertebrae, skull, ribs, and distal extremities are found in 14–60% of cases. Osseous lesions may produce symptoms from abscess development in adjacent soft tissue, by spread to contiguous joints, or by vertebral collapse. Radiography shows a sharply defined area of osteolysis.
Blastomycotic arthritis, typically monoarticular, is not rare and first appears as swelling, pain, and limited range of motion in an elbow, knee, or ankle. Infection of the prostate, epididymis, or kidney can be documented in cultured urine in one-fourth of cases. Hematogenous spread to the brain occurs in 3–10% of cases and may present as meningitis, brain abscess, spinal epidural lesions, or cranial lesions. Blastomycotic lymphadenitis and intraocular infection have been reported.
Diagnosis
Diagnosis of blastomycosis requires isolation of the fungus in culture or the demonstration of characteristic yeast-like cells in pus, sputum, or tissue. Mycelial growth is usually evident within 3–14 weeks of incubation at 25–30°C, but cultures should be kept for at least 4 weeks before recording them as negative.
Treatment
Oral itraconazole is the treatment of choice for patients with indolent extracranial blastomycosis. Amphotericin B remains the treatment of choice for patients with severe, rapidly progressive, or CNS infection.15
Prevention
Since environmental point sources in soils close to rivers and swamps are difficult to identify and since occupational inhalational exposures are rare, few preventive methods have been identified. Careful handling of laboratory specimens using BSL2 practices and procedures16 and wearing impermeable gloves will minimize the risk of aerosol exposure and hand inoculation.
References
- 1. De Groote MA, Bjerke R, Smith H, et al. Expanding epidemiology of blastomycosis: clinical features and investigation in Colorado. Clin Infect Dis 2000; 30:582–584.
- 2. Anonymous. From the Centers for Disease Control and Prevention. Blastomycosis acquired occupationally during prairie dog relocation—Colorado, 1998. JAMA 1999; 282:21–22.
- 3. Sarosi G, Serstock D. Isolation of Blastomyces dermatitidis from pigeon manure. Am Rev Respir Dis 1976; 114:1179–1193.
- 4. Anonymous. Blastomycosis. In: Rippon JW (ed.), Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. Philadelphia: WB Saunders, 1982:428–458.
- 5. Dwight PJ, Naus M, Sarsfield P, et al. An outbreak of human blastomycosis: the epidemiology of blastomycosis in the Kenora catchment region of Ontario, Canada. Can Commun Dis Rep 2000; 26:82–91.
- 6. Vaaler A, Bradsher R, Davies S. Evidence of subclincal blastomycosis in forestry workers in northern Minnesota and northern Wisconsin. Am J Med 1990; 89:470.
- 7. Cockerill F, Roberts G, Rosenblatt J, et al. Epidemic of pulmonary blastomycosis (Namekagon fever) in Wisconsin canoeists. Chest 1984; 86:688–692.
- 8. Klein B, Vergeront J, Weeks R, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Eng J Med 1986; 314:529–534.
- 9. Klein B, Vergeront J, DiSalvo A, et al. Two outbreaks of blastomycosis along rivers in Wisconsin: isolation of Blastomyces dermatitidis from riverbank soil and evidence of its transmission along waterways. Am Rev Respir Dis 1987; 136:1333–1338.
- 10. Bakerspigel A, Kane J, Schaus D. Isolation of Blastomyces dermatitidis from an earthen floor in southwestern Ontario, Canada. J Clin Microbiol 1986; 24:890–891.
- 11. Larson C, Eckman M, Alber R, et al. Primary cutaneous (inoculation) blastomycosis: an occupational hazard to pathologists. Am J Clin Pathol 1983; 79:523–525.
- 12. Larsh H, Scharz J. Accidental inoculation blastomycosis. Cutis 1977; 19:334–337.
- 13. Kantor G, Roenigk R, Mailin P, et al. Cutaneous blastomycosis. Report of a case presumably acquired by direct inoculation with carbon dioxide laser vaporization. Cleve Clin J Med 1987; 54:121–124.
- 14. Baum G, Lerner P. Primary pulmonary blastomycosis: a laboratory-acquired infection. Ann Intern Med 1970; 73:263–269.
- 15. Champman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosisi: 2008 update by the infectious diseases society of America. Clin Infect Dis 2008; 46:1801–1812.
- 16. Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical laboratories, 5th edn. HHS Publication no. (CDC) 21–1112. New York: U.S. Department of Health and Human Services, 2009.
CANDIDA SPECIES
Common names for disease: Candidiasis, candidosis, thrush, moniliasis
Occupational setting
Candida species are found in soils, especially those with heavy organic debris, and have been recovered from hospital environments and inanimate objects. The intact integument is the most important defense against cutaneous candidiasis. Environmental factors that lead to increased moisture, such as prolonged immersion of hands in water or tight clothing worn in hot climates, increase the risk for cutaneous candidiasis by compromising the tissue.1 Candida paronychia often arises after continued immersion and mechanical irritation of the hands. Homemakers, dishwashers, bartenders, cannery workers, and nurses are at risk for cutaneous candidiasis.2 Nonoccupational iatrogenic factors (antibiotics, immunosuppressants, barrier breaks, prostheses) and chronic disease such as diabetes are the most common causes of systemic candidiasis. Sensitization to candida antigens has been demonstrated in both farmers and bakers with respiratory symptoms, indicating the potential for candida to cause occupational rhinitis and occupational asthma.3
Exposure (route)
Organisms are normal commensals and the vast majority of human infections are of endogenous origin. Person-to-person transmission has been described.
Pathobiology
Candida albicans and the other Candida species are budding yeasts that produce mycelia with continued growth (Figure 24.4). Candida invasion of the moist areas of the skin produces a red, “scalded skin” lesion with a scalloped border. Satellite pustular lesions surround the primary lesion, and dry scaly lesions may also occur.

FIGURE 24.4 Grocott’s methenamine silver stain showing Candida yeast forms.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Diagnosis
Skin scrapings examined microscopically in potassium hydroxide (KOH) show budding yeast and mycelial hyphae.
Treatment
Nystatin ointment, topical amphotericin, gentian violet, and a number of other topical treatments are effective in the treatment of Candida paronychia and intertriginous candidiasis.
Prevention
Avoidance of tight clothing in tropical climates, tight boots that macerate the skin, and prolonged immersion of hands will prevent tissue compromise and diminish the risk of cutaneous candidiasis.
References
- 1. Campbell M, Stewart J. The Medical Mycology Handbook. New York: John Wiley & Sons, Inc., 1980:244–252.
- 2. Hunter P, Harrison G, Fraser C. Cross-infection and diversity of Candida albicans strain, carriage in patients and nursing staff on an intensive care unit. J Med Vet Mycol 1990; 28:317–325.
- 3. Wiszniewska M, Tymoszuk D, Nowakowska-Swirta E, et al. Mould sensitization among bakers and farmers with work-related respiratory symptoms. Ind Health 2013; 51:275–284.
CLADOSPORIUM SPECIES
Common names for disease: asthma, allergic rhinoconjunctivitis, and hypersensitivity pneumonitis (HP)
Occupational setting
Cladosporium spp. are ubiquitous in nature. Peak ambient air levels generally occur in summer and early fall.1,2 Farmers, agricultural workers, and occupants of water-damaged buildings are at risk for exposure and associated hypersensitivity lung diseases.3 Reports in sawmills have also described allergic disease due to cladosporium exposure.4 Workers cleaning mold off of salami during production developed hypersensitivity pneumonitis attributed to cladosporium.5 Cladosporium was one of the molds associated with hypersensitivity pneumonitis in a saxophone player from contamination of the instrument.6 Rarely, skin, corneal, CNS, and pulmonary infections may occur.
Exposure (route)
Exposure occurs primarily through inhalation of airborne conidia or spores (Figure 24.5). Skin infection may occur with direct inoculation.

FIGURE 24.5 Photomicrograph of Cladosporium conidia.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Pathobiology
Cladosporium sensitization occurs with a prevalence of about 3%7 and is associated with allergic rhinitis and eczema.8 Cladosporium sensitization has also been associated with increased bronchial hyperresponsiveness and is a risk factor for both the development of asthma and fatal asthma attacks.7,9,10 Occupational asthma related to Cladosporium sensitization is rarely reported. Other hypersensitivity reactions to Cladosporium include allergic bronchopulmonary mycosis and hypersensitivity pneumonitis (HP).11,12 HP developed in a 48-year-old woman after exposure to a contaminated indoor hot tub, and Cladosporium was confirmed as the cause by positive specific challenge.12
There are approximately 30 reports of brain abscess caused by Cladosporium trichoides in the literature, primarily in immunocompromised hosts.13 Skin and pulmonary infections also occur but are uncommon.
Diagnosis
Diagnosis of asthma relies on symptom and exposure histories, findings on pulmonary function testing (including positive nonspecific bronchial challenge), and the results of peak flow monitoring. Allergy skin prick testing and specific IgE to Cladosporium are often but not always positive. Diagnosis of hypersensitivity pneumonitis relies on a constellation of clinical findings, including a careful symptom and exposure history.14 Physical examination may be normal or show basilar lung crackles; the chest radiography may be normal or show diffuse alveolar or interstitial opacities; and pulmonary function tests show restriction, obstruction, or a mixed picture. Fiber-optic bronchoscopy with bronchoalveolar lavage and transbronchial biopsies may be helpful when the clinical suspicion is strong but routine tests are normal. Exercise physiology testing may be helpful in patients who have dyspnea but normal resting pulmonary function.
Diagnosis of infectious disease related to Cladosporium requires positive culture or the demonstration fungal forms in histological specimens.
Treatment
As with all hypersensitivity lung diseases, prompt removal from exposure is essential. Inhaled corticosteroids are useful as first-line pharmacotherapy for asthma. Oral corticosteroids may be indicated in patients with hypersensitivity pneumonitis manifested by severe symptoms and radiographic or functional abnormalities.
Fluconazole at a dose of 400 mg/day in combination with surgery has successfully treated CNS infection.15 Progressive disease is generally treated with intravenous amphotericin B; however, this has not been shown to alter the outcome.13
Prevention
Rapid remediation of water damage in homes and office buildings will prevent fungal growth and minimize exposure. Proper attention to building practices during new construction will help prevent subsequent leaks and water damage. In agricultural settings, engineering and process controls can reduce exposure to high fungal concentrations. Workers in at-risk industries should be educated regarding exposure risks and encouraged to seek early medical attention for persistent respiratory and systemic symptoms.14
References
- 1. Mediavilla MA, Angulo RJ, Dominguez VE, et al. Annual and diurnal incidence of Cladosporium conidia in the atmosphere of Cordoba, Spain. J Invest Allerg Clin Immunol 1997; 7:179–182.
- 2. Ren P, Nakun TM, Leaderer BP. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county. J Expos Analysis Environ Epidemiol 1999; 9:560–568.
- 3. Kotimaa MH, Terho EO, Husman K. Airborne moulds and actinomycetes in work environment of farmers. Eur J Respir Dis Suppl 1987; 152:91–100.
- 4. Klaric MS, Varnai VM, Calusic AL, et al. Occupational exposure to airborne fungi in two Croatian sawmills and atopy in exposed workers. Ann Agric Environ Med 2012; 19:213–219.
- 5. Marvisi M, Balzarini L, Mancini C, et al. A new type of Hypersensitivity Pneumonitis: salami brusher’s disease. Monaldi Arch Chest Dis 2012; 77:35–37.
- 6. Metzger F, Haccuria A, Reboux G, et al. Hypersensitivity pneumonitis due to molds in a saxophone player. Chest 2010; 138:724–726.
- 7. Chinn S, Jarvis D, Luczynska C, et al. Individual allergens as risk factors for bronchial responsiveness in young adults. Thorax 1998; 53:662–667.
- 8. Bundgaard A, Boudet L. Reproducibility of early asthmatic response to Cladosporium herbarum. Eur J Resp Dis Suppl 1986; 143:37–40.
- 9. Abramson M, Kutin JJ, Raven J, et al. Risk factors for asthma among young adults in Melbourne, Australia. Respirology 1996; 1:291–297.
- 10. Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 2000; 55:501–504.
- 11. Moreno-Ancillo A, Diaz-Pena JM, Ferrer A, et al. Allergic bronchopulmonary cladosporiosis in a child. J Allergy Clin Immunol 1996; 97:714–715.
- 12. Jacobs RL, Thorner RE, Holcomb JR, et al. Hypersensitivity pneumonitis caused by Cladosporium in an enclosed hot-tub area. Ann Int Med 1986; 105:204–206.
- 13. Dixon DM, Walsh TJ, Merz WG, et al. Infections due to Xylohypha bantiana (Cladosporium trichoides). Rev Infect Dis 1989; 11:515–525.
- 14. Rose C, Lara A. Hypersensitivity pneumonitis. In: Murray JF, Nadel JF (eds.), Textbook of Respiratory Medicine, 5th edn. Philadelphia: Saunders, 2010:1587–1600.
- 15. Turker A, Altinors N, Aciduman A, et al. MRI findings and encouraging fluconazole treatment results of intracranial Cladosporium trichoides infection. Infection 1995; 23:60–62.
COCCIDIOIDES IMMITIS
Common names for disease: Coccidioidomycosis, valley fever, desert rheumatism, valley bumps, California disease, Posada’s mycosis
Occupational setting
Coccidioides immitis is a soil fungus that is endemic in the semiarid or desert-like regions of the United States, Mexico, Guatemala, Honduras, Colombia, Venezuela, Bolivia, Paraguay, and Argentina.1 Coccidioides immitis may be dispersed by wind or by disruptions from construction work, farming, or archeological digs. Outbreaks after natural disasters (e.g., earthquakes) have been reported.2 Occupations at risk for developing coccidioidomycosis include agricultural workers, construction crews, telephone post diggers, archeologists, and military personnel traveling to endemic areas.3,4 Other outdoor activities in arid endemic regions also carry risk. A recent outbreak occurred in the cast and crew of a television program filming outdoors.5 Outbreaks in armadillo hunters in Brazil have also been reported.6 Laboratory workers are at risk for infection from inhalation of the arthroconidia.7 Although there are no racial, gender, or age differences in susceptibility to primary coccidioidomycosis, dark-skinned races and pregnant women are more prone to severe primary illness and disseminated disease.1
Cases of occupational person-to-person transmission are very rare. Six medical staff members were infected after inhaling arthrospores that had grown on the plaster cast of a patient with coccidioidal osteomyelitis. An embalmer developed disease after accidentally piercing his skin with a needle during preparation of the body of a victim of disseminated coccidioidomycosis.8 There have been occasional reports of coccidioidomycosis in Georgia, Virginia, and North Carolina among textile workers who inhaled dust particles from wool or cotton shipped from the San Joaquin Valley.
Exposure (route)
The primary route of exposure is inhalation. Skin inoculation and transplacental infection from mothers with disseminated disease have rarely been reported.9
Pathobiology
Coccidioides immitis exists in the mycelial phase in soil, where it matures to form arthroconidia that can be inhaled. In the host, these spores swell to form thick-walled, nonbudding, round cells that contain endospores (Figure 24.6).
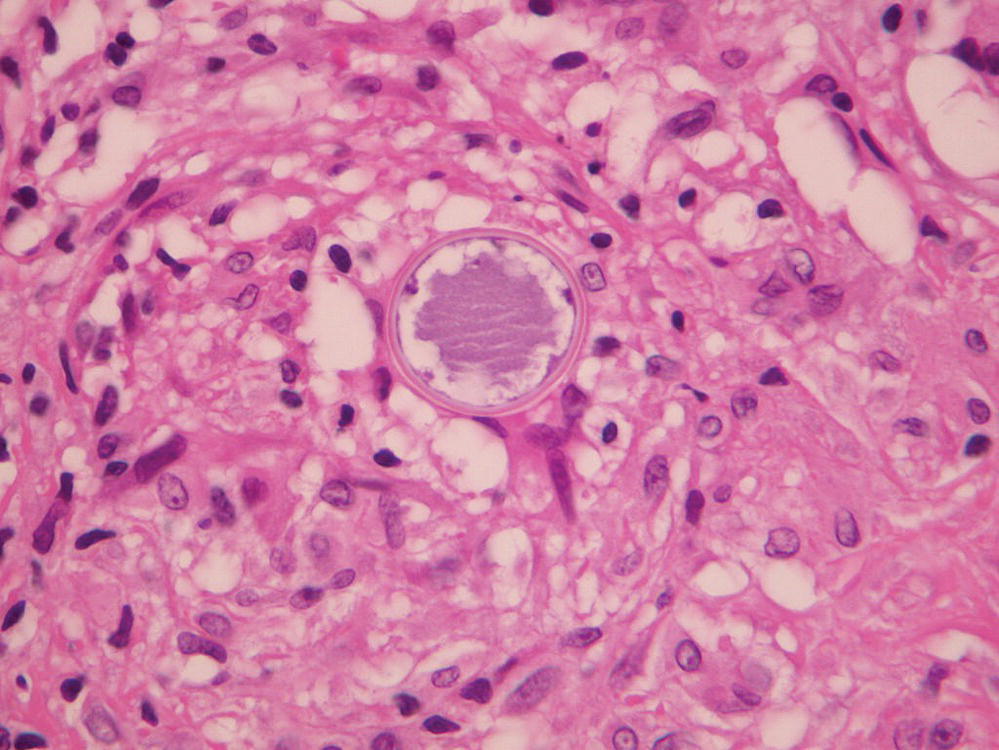
FIGURE 24.6 Tissue sample hematoxylin and eosin stain revealing a typical spherule of Coccidioidomycosis.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
In most cases, coccidioidomycosis is a mild respiratory infection or is completely asymptomatic. Primary coccidioidomycosis occurs in ~40% of patients with positive coccidioidin skin tests. Symptoms typically begin 7–21 days after exposure; they include fever, pleuritic or dull chest pain, cough, white or blood-streaked sputum, and constitutional symptoms of malaise, headache, anorexia, myalgia, and fever. A fine, diffuse, erythematous skin rash often occurs within the first few days of illness. Erythema nodosum and erythema multiforme are more common in Caucasian women with primary coccidioidomycosis; they are accompanied by arthralgias of the knee or ankle in a third of cases. The rash, arthralgias, and mild conjunctivitis that often occur are probably all manifestations of exuberant delayed-type hypersensitivity reactions to C. immitis antigens. The chest radiograph typically shows alveolar infiltrates, with or without hilar adenopathy. Paratracheal or mediastinal adenopathy suggests that infection may be spreading beyond the lung. Small pleural effusions may occur, but large effusions are uncommon. Laboratory studies often show a mild leukocytosis, elevated sedimentation rate, and eosinophilia. Conversion of the coccidioidin skin test to positive occurs 2–21 days after onset of symptoms. The appearance of complement fixing antibodies in serum is often delayed.
Coccidioidal pneumonia may resolve by forming dense spherical nodules (coccidioidomas) in the area of infiltrate. The mass may cavitate, leaving a single thin-walled cavity; approximately half of these cavities close spontaneously within 2 years. Though hemoptysis can occur, most are asymptomatic. Extension of the cavity to the pleura can cause bronchopleural fistula, pneumothorax, and coccidioidal empyema.10 Acute coccidioidal pneumonia may disseminate rapidly; it is potentially fatal. Some individuals develop a chronic progressive form of pulmonary coccidioidomycosis that mimics tuberculosis, with apical fibronodular lesions and cough, weight loss, fever, and chest pain of many months duration.
Extrapulmonary dissemination occurs in <5% of cases, most often in dark-skinned men. Pregnancy also appears to increase the risk of dissemination. Infection in later stages of pregnancy results in increasing morbidity and mortality for the mother. In disseminated infection, skin and subcutaneous lesions are the most common manifestations. Bone lesions occur in 20% of patients with disseminated disease. Meningitis occurs in one-third to one-half of cases of disseminated coccidioidomycosis, usually with a subacute or chronic presentation including headache, lethargy, confusion, or decreased memory. Anorexia, nausea, weight loss, and ataxia may occur. The most valuable diagnostic test for meningitis is the complement fixation test for cerebrospinal fluid (CSF) antibody to C. immitis, which is positive in 75–95% of cases. Multiple organ systems may be involved in disseminated coccidioidomycosis. Patients are at risk for anterior and posterior uveitis, lymphadenitis, cystitis, renal abscess, orchitis, epididymitis, peritonitis, urethroscrotal fistula, laryngitis, and otitis.
Diagnosis
Together with the typical clinical manifestations, a positive coccidioidin skin test is suggestive for disease in someone who has recently traveled to an endemic area. Coccidioidal serology on acute and convalescent sera is a reliable means of diagnosis. Current tests that use enzyme-linked immunoassay test kits with proprietary antigens are more sensitive than traditional serologies and can convert to positive at an earlier disease phase.1 Recovery of C. immitis from sputum, urine, or bronchial washing is definitive, but a negative culture does not exclude the diagnosis. Microscopic identification of C. immitis spherules on wet smear can aid in diagnosis. In disseminated coccidioidomycosis with meningitis, CNS serologic tests are helpful. Demonstration of C. immitis by culture, smear, or biopsy is the most definitive diagnostic test in disseminated disease. Fungemia is detected in ~12% of disseminated cases; it is an extremely grave prognostic sign.
Treatment
Because spontaneous cure is common, treatment is not usually necessary for acute pulmonary coccidioidomycosis.11 Intravenous amphotericin B may be indicated in some very ill patients with primary illness without proof of dissemination in an effort to prevent extrapulmonary foci. The only effective treatment for cavitary disease is surgical resection, with intravenous amphotericin B serving an adjunctive role. Repeated bacterial superinfection and the presence of an expanding cavity near the pleural surface are indications for resection of the cavities. Treatment of patients with chronic, indolent apical coccidioidal pneumonia is difficult and requires at least 1 year of therapy. Initial treatment with azoles is generally preferred. For those patients who fail initial therapy, options include switching to an alternative azole or to intravenous amphotericin B. When infection is confined to one lobe, combination treatment with intravenous amphotericin B and resection may be useful. Adjunctive interferon gamma may also be useful in refractory disease.12
In the treatment of disseminated coccidioidomycosis, therapy with an oral azole is begun unless the patient is severely ill, in which case intravenous amphotericin B is used. When amphotericin B is used initially, an oral azole is usually substituted once the patient has stabilized.11 Oral ketoconazole, itraconazole, or fluconazole following a course of treatment with intravenous amphotericin B can help in management of skin lesions, subcutaneous abscesses, and joint effusions. Complete cure is elusive, and improvement often takes many months. Oral fluconazole is the preferred treatment for coccidioidal meningitis, often beginning at high doses of 800 mg/day. Intrathecal amphotericin B may be added initially.11 For those patients who respond, lifelong therapy with oral azoles is required.11,13
Prevention
Given the considerable danger of laboratory infection by C. immitis, precautionary measures in handling cultures should be emphasized. The organism should be handled using BSL2 procedures and practices in clinical laboratories.14 Petri dishes should not be used for isolation of the organism from clinical specimens. Subculturing and harvesting of the arthrospores should be carried out under a laminar flow hood or other isolation hood using BSL3 practices and facilities. Viable plate cultures should never be discarded or sent through the mail. For outdoor work in endemic areas, dust control procedures should be followed; the California Department of Public Health has published guidelines.15
References
- 1. Nguyen C, Barker BM, Hoover S, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 2103; 26:505–525.
- 2. Schneider E, Hajjeh RA, Spiegel RA, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA 1997; 277:904–908.
- 3. (a)Johnson W. Occupational factors in coccidioidomycosis. J Occup Med 1981; 23:367–374. (b)EI-Ani A, Elwood C. A case of coccidioidomycosis with unique clinical features. Arch Intern Med 1978; 138:1421–1422.
- 4. Stander SM, Schooner W, Galgiani JN, et al. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J Infect Dis 1995; 171:1672–1675.
- 5. Wilken JA, Marquez P, Terashita D, et al. Coccidioidomycosis among cast and crew memebers at an outdorr television filming event – California 2012. MMWR Morb Mortal Weekly Rep 2014; 63:321–324.
- 6. Brillhante RS, Moreira Filho RE, Rocha MF, et al. Coccidioiomycosis in armadillo hunters from the state of Ceara, Brazil. Mem Inst Oswaldo Cruz 2012; 107:813–815.
- 7. Kohn G, Linne S, Smith C. Acquisition of coccidioidomycosis at necropsy by inhalation of coccidioidal endospores. Diagn Microbiol Infect Din 1992; 15:527–530.
- 8. Canoil F, Haley K, Brown J. Primary cutaneous coccidioidomycosis: a review of the literature and a report of a new case. Arch Dermatol 1977; 113:933–936.
- 9. Charlton V, Ramsdell K, Sehring S. Intrauterine transmission of coccidioidomycosis. Pediatr Infect Dis J 1999; 18:561–563.
- 10. Shekhel TA, Ricciotti RW, Blair JE, et al. Surgical pathology of pleural coccidioidomycosis: a clinicopathological study of 36 cases. Hum Pathol 2014; 45:961–969.
- 11. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis: IDSA guidelines. Clin Infect Dis 2005; 41:1217–1223.
- 12. Duplessis CA, Tilley D, Bavaro M, et al. Two cases illustrating successful adjunctive interferon-gamma immunotherapy in refractory disseminated coccidioidomycosis. J Infect 2011; 63:223–228.
- 13. Dewsnup DH, Galgiani JN, Graybill JR, et al. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med 1996; 124:305–310.
- 14. Centers for Disease Control and National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories, 3rd edn. Washington, DC: U.S. Government Printing Office, 1993:79.
- 15. Das R, McNary J, Fitzsimmons K, et al. Occupational coccidioidomycosis in California: outbreak investigation, respirator recommendations, and surveillance findings. J Occup Environ Med 2012; 54:564–571.
CRYPTOCOCCUS NEOFORMANS AND CRYPTOCOCCUS GATTII
Common names for disease: Cryptococcosis, torulosis, European blastomycosis
Occupational setting
The most important natural source of Cryptococcus is weathered droppings from pigeons and soil contaminated with avian droppings. The organism is most likely to be found in old pigeon droppings that have accumulated over years in roosting sites such as towers, window ledges, hay mows of barns, and upper floors of old buildings. Cryptococcus neoformans has also been isolated from the droppings of other birds, from dairy products, soil, wood, rotting vegetables and fruits, and swallows’ nests.1 Cryptococcal antibodies are detected more commonly in pigeon breeders than in other occupational groups,2 but the infection rate is no greater because the disease mainly affects immunocompromised hosts (including patients with AIDS, sarcoidosis, lymphoma, and those requiring chronic steroids). For example, a recent report describes a case of cryptococcosis secondary to exposure to contaminated chicken manure in a patient with Crohn’s disease on immunosuppression.3 The organism was cultured from bagpipes played by a patient with leukemia who developed pulmonary cryptococcosis.4 Cases of cryptococcosis rarely occur in clusters, and there is no clear occupational predisposition. Histories of exposure to pigeons or dust are usually unhelpful. Cryptococcus gattii is responsible for an unexplained outbreak of disease among immunocompetent individuals in the Pacific Northwest.5
Exposure (route)
Inhalation of fungal spores is the major route of entry.
Pathobiology
Cryptococcus is an encapsulated yeast-like fungus that reproduces by budding into 4–6 µm diameter cells (Figure 24.7) that can cause disease when they are aerosolized and inhaled.

FIGURE 24.7 Gram stain of fluid obtained from lumbar puncture demonstrating encapsulated Cryptococcus yeast forms.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
The most common clinical manifestation of cryptococcosis is infection of the cerebral cortex, brain stem, cerebellum, or meninges.6 Symptom onset may be insidious (with headache, dizziness, irritability, subtle altered mental status, personality change, and visual symptoms) or explosive (with rapid deterioration and death within 2 weeks of onset).
Pulmonary cryptococcosis has a variety of clinical manifestations and an unpredictable course. A self-limited pneumonia with indolent onset and symptoms of dry cough, chest pain, and little or no fever can occur. The chest radiograph typically shows one or more well-circumscribed areas of pneumonitis, occasionally with central cavitation. Pleural effusions, hilar adenopathy, and calcification are rare. Resolution often requires months of treatment and occasionally progresses to chronic pneumonia. The most serious outcome in cryptococcal pneumonia is silent dissemination to the central nervous system. Patients with underlying lung disease may develop asymptomatic colonization of the bronchial tree with C. neoformans.
Papular skin lesions from hematogenous dissemination occur in ~10% of patients with cryptococcosis; such lesions are more common in immunocompromised patients. Local cutaneous cryptococcosis as a result of direct inoculation may occur in immunocompetent individuals.7 Bone and joint involvement may also occur following hematogenous dissemination; vertebral lesions are the most common site. Ocular lesions of cryptococcosis include chorioretinitis, papilledema, optic atrophy, scotomata, and ocular motor palsies. Rarely, cryptococcosis may involve the genitourinary tract, heart valves, liver, sinuses, and GI tract.
Diagnosis
The most important procedure in the diagnosis of cryptococcal meningitis is lumbar puncture, which characteristically shows pleocytosis, elevated protein, and hypoglycorrhachia. India ink smear of CSF shows the encapsulated yeast in 50% of cases, and cryptococcal antigen is detected in 94% of cases. Diagnosis of cryptococcal pneumonia is often challenging, since cryptococci are scanty in sputum except in cases of cavitary lung disease or widely disseminated infection. Cultures of sputum and bronchoalveolar lavage (BAL) are occasionally helpful. Cryptococcal antigen can be measured in BAL fluid. Early studies showed high diagnostic sensitivity of BAL cryptococcal antigen at 98%; however, follow-up investigations indicate a sensitivity closer to 70%.8,9 Positive serum cryptococcal antigen is suggestive, but it occurs only in cases with extensive pulmonary infiltrates or extrapulmonary dissemination. Transbronchial biopsy is occasionally helpful, but surgical lung biopsy is often necessary to confirm the diagnosis. Central punch biopsy of cutaneous cryptococcosis with culture and histology has a high diagnostic yield.
Treatment
An aggressive search for disseminated disease (including cerebrospinal fluid culture, multiple urine cultures, and blood cultures) is necessary in patients with pulmonary cryptococcosis. If results are negative, chemotherapy can be withheld except in patients at risk for dissemination, such as those with steroid therapy, diabetes, HIV infection, or underlying malignancy. However, careful follow-up of immunocompetent patients with suspected illness is required. Therapy for pulmonary cryptococcosis is recommended if the serum level of cryptococcal antigen is greater than 1 : 8.5 For immunosuppressed patients or patients with severe involvement, pulmonary cryptococcosis should be treated the same as meningeal disease.5 Fluconazole at a dose of 400 mg/day for 6–12 months is the recommended therapy for mild to moderate pulmonary disease in normal hosts.5 Itraconazole, voriconazole, and posaconazole are acceptable alternatives. For CNS or severe disseminated disease, guidelines recommend induction therapy with 2 weeks of amphotericin B plus flucytosine followed by at least 8 weeks of fluconazole at 400 mg/day and then 6–12 months of 200 mg/day of fluconazole for maintenance.5 For nonimmunosuppressed patients, a longer induction of 4 weeks is recommended if no neurologic complications are present, with an additional 2 weeks if neurologic complications are present.5 Intrathecal amphotericin B has been used for refractory CNS disease. All patients with meningitis should be evaluated for elevated intracranial pressure. Daily large volume lumbar puncture to reduce intracranial pressure until a normal opening pressure is achieved on several consecutive days is recommended. Symptomatic hydrocephalus should be treated by ventriculoperitoneal shunt even if viable cryptococci are still present in CSF. Treatment for cryptococcosis in AIDS patients is beyond the scope of this discussion.
Prevention
Since cryptococcosis is typically a disease of the immunocompromised host and since occupational cases are rare, few preventive strategies have been identified. In the laboratory, BSL2 procedures and practices should be followed.10 In endemic areas, pigeon dropping controls should be utilized.
References
- 1. Gordon M. Cryptococcosis: a ubiquitous hazard. Occup Health Saf 1980; 49:61–63.
- 2. Tanphaichitra D, Sahaphongs S, Srirnuang S. Cryptococcal antigen survey among racing pigeon workers and patients with cryptococcosis, pythiosis, histoplasmosis and penicilliosis. Int J Clin Pharmacol Res 1988; 8:433–439.
- 3. Fraison JB, Guilpain P, Schiffmann, A, et al. Pulmonary cryptococcosis in a patient with Crohn’s disease treated with prednisone, azathioprine and adalimumab: exposure to chicken manure as a source of contamination. J Crohns Colitis 2013; 7:e11–e14.
- 4. Cobcroft R, Kronenberg H, Wilkinson T. Cryptococcus in bagpipes [Letter]. Lancet 1978; 1:1368–1369.
- 5. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322.
- 6. White P, Kaufman L, Weeks R, et al. Cryptococcal meningitis: a case report and epidemic- logic study. J Med Assoc Ga 1982; 71:539–542.
- 7. Micalizzi C, Persi A, Parodi A. Primary cutaneous cryptococcosis in an immunocompetent pigeon keeper. Clin Exp Dermatol 1997; 22:195–197.
- 8. Baughman RP, Rhodes JC, Dohn MN, et al. Detection of cryptococcal antigen in bronchoalveolar lavage fluid: a prospective study of diagnostic utility. Am Rev Respir Dis 1992; 145:1226–1229.
- 9. Kralovic SM, Rhodes JC. Utility of routine testing of bronchoalveolar lavage fluid for cryptococcal antigen. J Clin Microbiol 1998; 36:3088–3089.
- 10. Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories, 5th edn. HHS Publication no. (CDC) 21–1112. New York: U.S. Department of Health and Human Services, 2009.
CRYPTOSTROMA CORTICALE
Common names for disease: Maple bark disease, maple bark stripper’s disease
Occupational setting
Wood and sawmill workers engaged in the debarking of logs prior to cutting are at risk for developing hypersensitivity pneumonitis or asthma from a variety of fungi that contaminate wood, including Cryptostroma corticale.1,2
Exposure (route)
Inhalation of respirable spores can cause sensitization and subsequent occupational lung disease.
Pathobiology
Maple bark disease is a rare disorder that can affect both trees and humans. Both sensitizing asthma and hypersensitivity pneumonitis from the fungus contaminating maple bark have been described.1,2 In one report, five workers in the wood room of a paper mill, where logs were debarked and cut, developed cough, dyspnea, chest tightness, fever, and weight loss during the winter months, when workplace ventilation was minimal.3 Physical examination showed pulmonary crackles; chest radiographs showed a diffuse reticulonodular infiltrate, occasionally with patchy alveolar infiltrates; and arterial oxygen saturations were reduced in three of the five patients. Lung histology demonstrated granulomas and scattered fibrosis; fungal spores were detected by methenamine silver staining of lung tissue in four individuals.
Diagnosis
Diagnosis of sensitizing occupational asthma relies on symptom and exposure histories, findings on pulmonary function testing (including positive nonspecific bronchial challenge), and results of peak flow monitoring at and away from work. Diagnosis of hypersensitivity pneumonitis relies on a constellation of clinical findings, including a careful symptom history.4 Physical examination may be normal or show basilar crackles; the chest radiograph may be normal or show diffuse alveolar or interstitial opacities; and pulmonary function testing may be normal or show restriction, obstruction, or a mixed picture. Fiber-optic bronchoscopy with bronchoalveolar lavage and transbronchial biopsies may be helpful when the clinical suspicion is strong but routine tests are normal. Exercise physiology testing may be helpful in patients with dyspnea but normal resting pulmonary function. Serum precipitins are often found in asymptomatic exposed workers and may be negative if the wrong antigen preparation is used.
Treatment
A strong index of clinical suspicion and prompt removal of a sensitized worker from the antigen-containing environment are the mainstays of therapy. Treatment with inhaled steroids for asthma and with oral corticosteroids for severe hypersensitivity pneumonitis may be indicated in some patients.
Prevention
Removal of symptomatic individuals from the spore-laden environment and changes in the manufacturing process to reduce spore concentrations should lead to eradication of the disease.
References
- 1. Towey J, Sweany H, Huron W. Severe bronchial asthma apparently due to fungus spores found in maple bark. JAMA 1932; 99:453–459.
- 2. Emanuel D, Lawton B, Wenzel F. Maple-bark disease; pneumonitis due to Cryptostroma corticale. N Engl J Med 1962; 266:333–337.
- 3. Emanuel D, Wenzel J, Lawton B. Pneumonitis due to Cryptostroma corticale (maple-bark disease). N Engl J Med 1966; 274:1413–1418.
- 4. Rose C, Lara A. Hypersensitivity pneumonitis. In: Murray JF, Nadel JF (eds.), Textbook of Respiratory Medicine, 5th edn. Philadelphia: Saunders, 2010:1587–1600.
FONSECAEA AND OTHER AGENTS OF CHROMOMYCOSIS
Common names for disease: Chromomycosis, chromoblastomycosis
Occupational setting
Chromomycosis is a chronic cutaneous and subcutaneous fungal infection that occurs most commonly in the tropics and subtropics among barefoot agricultural workers.1 Corneal infections may also occur.2 These opportunistic fungi are common in soil, decayed vegetation, and rotting wood. Handling lumber and sitting on wooden planks in Finnish saunas are additional documented sources of infection.3
Exposure (route)
Traumatic inoculation of fungi into the skin is the main mode of infection. Person-to-person transmission has not been documented.
Pathobiology
Fonsecaea pedrosi is the most commonly isolated agent of chromomycosis, accounting for the majority of cases in Brazil and 61% of the cases in Madagascar1,4 ; Cladosporium carrionii is the major pathogen in South Africa, Venezuela, and Australia.5 All species produce slow-growing, 4–12 µm round, brown, thick-walled cells, often in clumps; hyphae may be seen in crusts from lesions (Figure 24.8). The infections are slow growing, with an average duration of illness prior to therapy of 11 years.6

FIGURE 24.8 Tissue sample hematoxylin and eosin stain revealing a cluster of brown thick walled cells of Fonsecaea.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
The most common manifestation is verrucous cutaneous infection. Over 90% of patients are male, and the lower limb is the site of infection in approximately 80%. Verrucous lesions are secondary to suppurative granulomas with large numbers of fungal cells.7 Early ulcerated nodules develop into cauliflower-like masses. Small ulcerations (“black dots”) are seen on the warty surface; they may be pruritic but are rarely painful. The second most common lesion is a well-delimited erythematous plaque or cicatricial lesion that on histology features tuberculoid-type granulomas with fewer fungal organisms.7 Scarring can cause lymphostasis and lymphedema of the involved extremity. Hematogenous spread to brain, lymph nodes, and other organs is rare.
Diagnosis
Characteristic pigmented sclerotic bodies (“copper pennies” when seen microscopically) are present in tissue and exudate in all types of chromomycosis. Fungi appear as long, septate, and branched hyphal forms in crusts and exudate. Serologic testing is unhelpful, and culture is only positive in a minority of cases.4
Treatment
In early stages, when lesions are small, wide, and deep, surgical excision is the treatment of choice. Medical therapy for chromomycosis has been disappointing. Topical antifungals, potassium iodide, amphotericin B, 5-fluorocytosine, ketoconazole, and local thermotherapy, alone or in combination, have all had varying degrees of success. Treatment with terbinafine has shown promise, with cure rates of 82% at 1 year in open trials; however, randomized controlled trials are still needed.8 Itraconazole, voriconazole, or combination therapy consisting of itraconazole with terbinafine or amphotericin plus terbinafine have reported success. The newer azoles have the best in vitro activity.9 Photodynamic therapy has also shown good clinical responses in refractory cases.10
Prevention
Since close contact with soil is the most prevalent predisposing condition, appropriate protective clothing is recommended to prevent cutaneous inoculation.
References
- 1. Silva JP, de Souza W, Rozental S. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic region. Mycopathologia 1998–1999; 143:171–175.
- 2. Barton K, Miller D, Pflugfelder SC. Corneal chromoblastomycosis. Cornea 1997; 16:235–239.
- 3. Sonck CE. Chromomycosis in Finland. Dermatologia 1975; 19:189–193.
- 4. Esterre P, Andriantsimahavandy A, Ramarcel ER, et al. Forty years of chromoblastomycosis in Madagascar: a review. Am J Trop Med Hyg 1996; 55:45–47.
- 5. McGinnis M. Chromoblastomycosis and phaeophyphomycosis: new concepts, diagnosis and mycology. J Am Acad Dermatol 1983; 8:1–16.
- 6. Pires CA, Xavier MB, Quaresma JA, et al. Clinical, epidemiological and mycological report on 65 patients from the Eastern Amazon region with chromoblastomycosis. An Bras Dermatol 2012; 87:555–560.
- 7. Avelar-Pires C, Simoes-Quaresma JA, Moraes-de Macedo GM, et al. Revisiting the clinical and histopathological aspects of patients with chromoblastomycosis from the Brazilian Amazon region. Are Med Res 2013:44:302–306.
- 8. Esterre P, Inzan CK, Ramarcel ER, et al. Treatment of chromomycosis with terbinafine: preliminary results of an open pilot study. Br J Dermatol 1996; 134(suppl 46):33–36.
- 9. Najafzadeh MJ, Badali H, Illnait-Zaragozi MT, et al. In vitro activities of eight antifungal drugs against 55 clinical isolates of Fonsecaea spp. Antimicrob Agents Chemother 2010; 54:1636–1638.
- 10. Lu S, Lu C, Zhang J, et al. Chromoblastomycosis in Mainland China: a systematic review on clinical characteristics. Mycopathologia 2013; 175:489–495.
HISTOPLASMA CAPSULATUM
Common names for disease: Histoplasmosis, Ohio Valley disease, cave disease, Tingo Maria fever, Darling’s disease, reticuloendotheliosis
Occupational setting
Working on or under structures that have been habitats for birds or bats can lead to histoplasmosis.1,2 Epidemics of acute pneumonia due to Histoplasma capsulatum result from group exposures to inhaled particulates containing high concentrations of the fungus.3 For example, an outbreak occurred among 13 of 24 students on a biology field trip to an endemic area. The exposure source was a hollow bat-infested tree.4 Common sites of outbreaks are bat-infested caves, starling roosts, and old chicken houses with dirt floors.5 Outbreaks related to disposal of bird droppings also occur.6 The endemic areas with the highest concentrations of disease are located in the eastern United States (Ohio River Valley) and Latin America. Activities such as exploring bat-infested caves, clearing bird roosts, or cleaning chicken houses are associated with the disease.7 Cleanup, construction, or demolition activities in urban areas may be associated with inhalation of airborne conidia.2,8 Cases of laboratory-acquired pulmonary histoplasmosis have been reported. Accidental inoculation in a hospital worker assisting on an autopsy and in a laboratory worker who accidentally pricked his thumb with a contaminated needle led to primary cutaneous histoplasmosis.
Infection caused by H. capsulatum variant duboisii occurs in tropical Africa. Human cases of duboisii infection have been associated with bat-infested caves and chicken roosts, suggesting that the var. duboisii shares the same ecological niche as the var. capsulatum.
Exposure (route)
The major route of exposure is inhalation of airborne conidia. Cutaneous inoculation has been reported.
Pathobiology
Histoplasma capsulatum is a yeast-like fungus with oval, budding, uninucleate cells measuring 1.5–2.0 µm × 3.0–3.5 µm that are often found within macrophages in viable tissue (Figure 24.9). The mycelial form is found in soil and bears infectious spores called microconidia (2–6 µm) and macroconidia (8–14 µm).
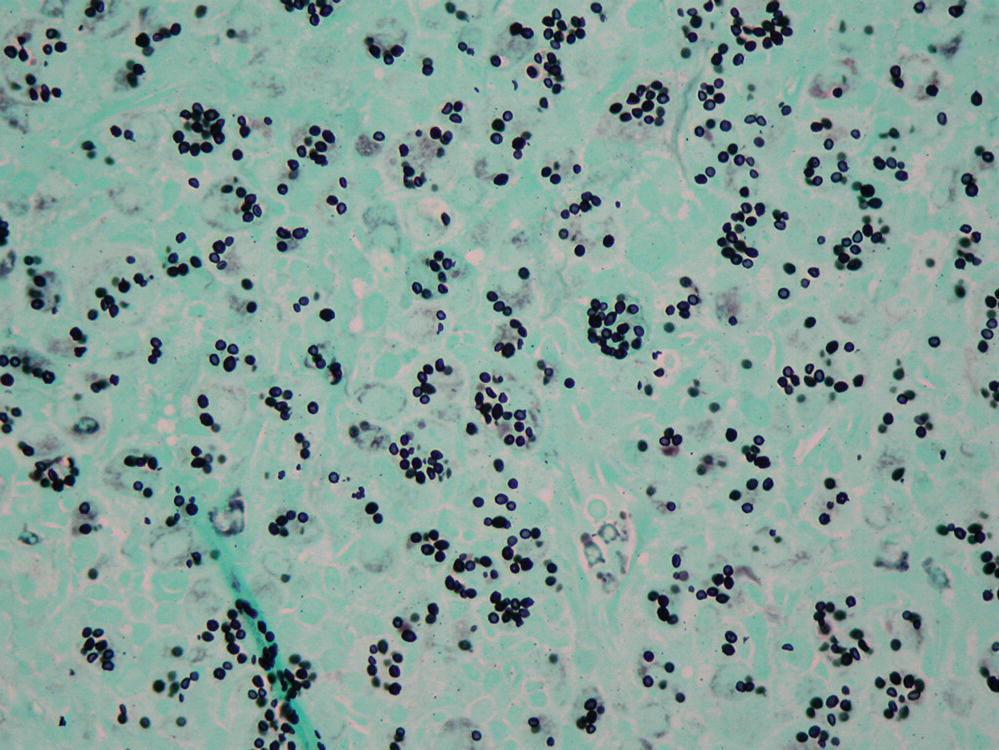
FIGURE 24.9 Grocott’s methenamine silver stain showing Histoplasma yeast forms.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Most infections are mild or subclinical. They are diagnosed in retrospect by a positive skin test or small, scattered pulmonary calcifications. Acute pulmonary histoplasmosis typically presents with symptoms of cough, pleuritic chest pain, fever, chills, myalgias, malaise, nausea, anorexia, and weight loss. The chest radiograph shows pulmonary infiltrates, usually patchy, finely nodular, and involving both lungs, often with hilar adenopathy. There are often slight elevations in peripheral blood leukocyte count and erythrocyte sedimentation rate. Illness may be mild or severe, accompanied by hypoxemia. Pleural effusions resolve over several weeks. Scattered calcification throughout the lung fields is a hallmark of healed acute pulmonary histoplasmosis. Microcalcifications of the spleen may be seen on the chest radiograph. Healing of a localized pulmonary infiltrate may lead to formation of a pulmonary nodule called a histoplasmoma.
Acute pulmonary histoplasmosis may result in lymphatic spread, leading to hilar or mediastinal lymphadenitis. In severe cases, granulomatous inflammation with central areas of caseation may replace entire mediastinal structures, causing fibrosing mediastinitis. Massive adenopathy typically appears in the hilar or right paratracheal area on chest radiograph. Obstruction of pulmonary veins causes hemoptysis and dyspnea. Heart failure and tracheobronchial hemorrhage are lethal complications of pulmonary venous obstruction. Histoplasmosis is the most common nonmalignant cause of superior vena cava syndrome, another potential sequela. Compression of the recurrent laryngeal nerve can cause hoarseness. Pericarditis may also occur, leading to potentially lethal complications such as tamponade and constrictive pericarditis. Esophageal complications of fibrosing mediastinitis may include ulceration, tracheoesophageal fistula, or traction diverticulum.
Chronic pulmonary histoplasmosis occurs most commonly in middle-aged men with underlying emphysema or chronic bronchitis. Symptoms include cough and sputum production, chest pain, dyspnea, malaise, weakness, fever, weight loss, and easy fatigability. Hemoptysis may also occur. The chest radiograph shows interstitial infiltrates, predominantly in the upper lobes. Nodular areas may slowly shrink or cavitate and expand. The course is marked by progressive hypoxemia, dyspnea, hemoptysis, bacterial pneumonia, and cor pulmonale. Laboratory abnormalities may include anemia of chronic disease, mild leukocytosis, and elevated alkaline phosphatase.
Hematogenously disseminated histoplasmosis is a rare but often lethal complication that occurs most commonly in immunosuppressed patients (e.g., those with hematologic malignancies, AIDS, or on high-dose corticosteroids). The clinical presentation may vary from acute to indolent. A variety of organ systems may be affected, causing meningitis, endocarditis, ulcerated lesions of the intestinal tract, hepatitis, and mucous membrane lesions of the oropharynx, face, and external genitalia. Ocular histoplasmosis occurs rarely. Laryngeal involvement can also occur.9,10
Diagnosis
A point source exposure to H. capsulatum should be suspected when several individuals develop respiratory illness two weeks following a common outdoor exposure. Serodiagnosis is presumptive but should be sought by immunodiffusion testing early in the disease course and by fourfold elevation of complement fixation titer between acute and convalescent sera. Cultures of blood, bone marrow, and urine should be obtained in hospitalized patients to rule out disseminated infection. Urinary antigen testing has a sensitivity approaching 90% for disseminated disease. Unfortunately, sensitivity is less than 50% for localized pulmonary infection. Specificity of urinary antigen testing is excellent although false positive reactions in patients with paracoccidioides can occur.11 Serum and bronchoalveolar lavage (BAL) fluid testing for antigen are promising additions. In one study, the sensitivity of serum antigen testing was 83% for acute and 88% for chronic pulmonary histoplasma.12 BAL antigen testing also has a higher yield than urine.13
In mediastinitis and pericarditis, Gomori methenamine silver (GMS) staining of biopsied lymph node sections has the best chance of demonstrating organisms. Sputum culture is recommended for diagnosis of chronic pulmonary histoplasmosis. If sputum is inadequate, bronchoalveolar lavage may be indicated. In disseminated histoplasmosis, taking a smear or biopsy or urinary antigen testing often leads to a faster diagnosis than awaiting culture result. Transbronchial biopsies may be useful in patients with diffuse radiographic infiltrates. In AIDS patients, GMS staining of bronchoalveolar lavage fluid has a high yield. Liver biopsy is useful in the setting of hepatic enlargement or abnormal liver function tests. Cultures of blood, bone marrow, and focal lesions have the highest yield for positive results.
Treatment
In most patients with acute pulmonary histoplasmosis, spontaneous improvement has begun at or before diagnosis, so no therapy is necessary. However, symptom persistence for more than 1 month is an indication for therapy.14 Some immunocompetent patients will develop severe diffuse pneumonia and respiratory failure after high spore exposures, requiring therapy with liposomal amphotericin B for 2 weeks, followed by 3 months of itraconazole. Corticosteroids are recommended in the first 2 weeks of acute fulminant histoplasmosis in those with significant respiratory complications to attenuate the inflammatory response.14 Early surgery to relieve pericardial tamponade and to confirm the diagnosis may be useful in pericarditis; however, antifungal therapy is often not required in this setting.14 The role of surgery in early mediastinal infection has not been adequately evaluated, but late in the course of fibrosing mediastinitis, complications including massive hemoptysis from venous obstruction may require surgical management. Neither high-dose steroid therapy nor antifungal chemotherapy appears to be beneficial in fibrosing mediastinitis.
For chronic pulmonary histoplasmosis, itraconazole is the drug of choice at a dose of 200 mg 3 times daily for 3 days, followed by twice daily. The duration of therapy is typically 1 year but some experts treat for up to 2 years to reduce the relapse rate. Blood itraconazole levels should be obtained after 2 weeks. Ketoconazole is an effective alternative, but fluconazole has a higher rate of treatment failure. In patients where compliance is a problem or in those who have contraindications to azole therapy, intravenous amphotericin B may be useful.
In patients with disseminated histoplasmosis, liposomal amphotericin B is recommended for moderate to severe disease for 1–2 weeks, followed by itraconazole at the above dosing. Itraconazole is the treatment of choice for mild disease. The total duration of therapy is at least 1 year, but immunosuppressed patients may require lifelong suppressive therapy.14
Prevention
Avoidance of circumstances in which H. capsulatum is likely to be found in high concentrations is the best approach to prevention. Although there are no data on efficacy, fit-tested negative pressure respirators with HEPA filters or powered air purifying respirators probably decrease the risk for inhalation exposure when cleaning or bulldozing bird roosts or chicken houses, activities that tend to increase the number of airborne spores. BSL2 practices and facilities are recommended for handling and processing clinical specimens and for identifying cultures and isolates in diagnostic laboratories. BSL3 practices and procedures should be used for manipulating identified cultures and for processing soil or other environmental materials that contain infectious spores.15
References
- 1. Sorley DL, Levin ML, Warren JW, et al. Bat-associated histoplasmosis in Maryland bridgeworkers. Am J Med 1979; 67:623–6.
- 2. Anonymous. Case records of the Massachusetts General Hospital. N Engl J Med 1991;325:949–56.
- 3. Goodwin R, Loyd J, Des Prez RM. Histoplasmosis in normal hosts. Medicine (Baltimore) 1981; 60:231–66.
- 4. Cottle LE, Gkrania-Klotsas E, Williams HJ. A multinational outbreak of histoplasmosis following a biology field trip in the Ugandan rainforest. J Travel Med 2013;20:83–7.
- 5. Stobierski MG, Hospedales CJ, Hall WN, et al. Outbreak of histoplasmosis among employees in a paper factory – Michigan, 1993. J Clin Microbiol 1996;34:1220–3.
- 6. Tosh F, Doto I, Beecher S, et al. Relationship of starling-blackbird roosts and endemic histoplasmosis. Am Rev Respir Dis 1970;101:283–6.
- 7. Furcolow M. Environmental aspects of histoplasmosis. Arch Environ Health 1975;10:4–8.
- 8. Dean A, Bates J, Sorrels C, et al. An outbreak of histoplasmosis at an Arkansas courthouse with five cases of probable reinfection. Am J Epidemiol 1978;108:36–46.
- 9. Teoh JW, Hassan F, Yunus M. Laryngeal histoplasmosis: an occupational hazard. Singapore Med J 2013;54:e208–10.
- 10. Durkin MM, Connolly PA, Wheat LJ. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J Clin Microbiol 1997;35:2252–5.
- 11. Taylor ML, Perez-Mejia A, Yamamoto-Furusho JK, et al. Immunologic, genetic and social human risk factors associated to histoplasmosis: studies in the State of Guerrero, Mexico. Mycopathologia 1997; 138:137–42.
- 12. Hage CA, Ribes, JA, Wengenack NL et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–54.
- 13. Hage CA, Knox KS, Davis TE, et al. Antigen detection in bronchoalveolar lavage fluid for diagnosis of fungal pneumonia. Curr Opin Pulm Med 2011;17:167–71.
- 14. Wheat JL, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807–25.
- 15. Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in microbiological and biomedical laboratories, 5th edn. HHS Publication no. (CDC) 21-1112, 2009.
MADURELLA SPECIES AND OTHER AGENTS OF MYCETOMA
Common names of disease: Mycetoma, Madura foot, maduromycetoma, maduromycosis
Occupational setting
Cases of this chronic indolent infection are most often seen in tropical and subtropical countries, such as India, Mexico, sub-Saharan Africa, and Venezuela. In some cases, saprophytic soil fungi enter the hands or feet after local trauma such as a thorn prick, or they enter the chest wall and back from soil-contaminated sacks carried on the shoulders; in other cases, mycetomas form on the head and neck as a result of carrying bundles of wood.1 Mycetomas (Figure 24.10) occur most frequently in male farmers and other rural laborers exposed to penetrating wounds from thorns and splinters. Inadequate nutrition and hygiene are probably contributory factors.

FIGURE 24.10 Tissue sample hematoxylin and eosin stain revealing a fungal mycetoma from Madurella.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Exposure (route)
The route of infection is through skin inoculation. Hematogenous spread has been rarely reported. Recent phylogenetic research indicates Madurella lives nested with other fungi in animal dung and enriched soil.2
Pathobiology
Causal fungi include Pseudallescheria (Petriellidium) boydii, Madurella mycetomatis, Madurella grisea, Acremonium (Cephalosporium) species, Fusarium species, Exophiala (Phialophora) jeanselmei, and a number of others.
A triad of signs—indurated swelling, multiple sinus tracts draining grain-filled pus, and localization to the foot (the most common site of infection)—characterize mycetomas.3 The primary lesion is a locally invasive, indolent, and painless subcutaneous swelling that slowly enlarges, causing subsequent distortion, pain, and disability. The radiographic findings include necrosis, osteolysis, and bone fusion.
Diagnosis
In addition to the classic clinical triad, characteristic grains in draining sinuses can be seen on hematoxylin–eosin staining. Gomori methenamine silver or periodic acid–Schiff staining will detect hyphae in tissue. Speciation requires culture of the grain and isolation of the organism.
Treatment
Surgical resection of a localized mycetoma may be necessary. Medical therapy is often unsuccessful, but newer triazoles may be of benefit. In vitro studies show isavuconazole has strong activity against Madurella.4 Likewise, posaconazole has been used with reported success.5 In a larger series, itraconazole at high dose improved the lesions and appeared to enhance encapsulation of the organisms making surgical resection easier.6 Madurella is not susceptible to the echinocandins.7
Prevention
Since the major predisposing factor is inoculation through bare feet in contact with soil, adequate shoes and clothing are recommended. Improvements in nutrition and hygiene would undoubtedly diminish the risk for infection as well. Reducing contact with cow dung or cow dung enriched soil may also be beneficial.2
References
- 1. Sugar AM. Agents of mucormycosis and related species. In: Mandell GL, Douglas RG, Bennett JE (eds.), Principles and Practice of Infectious Disease, 3rd edn. New York: Chruchill Livingstone, 1990:1962–1972.
- 2. De Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PloS Negl Trop Dis 2013; 7:e2229.
- 3. Butz W, Ajello L. Black grain mycetoma. Arch Dermatol 1971; 104:197–201.
- 4. Kloezen W, Meis JF, Curfs-Greuker I, et al. In vitro antifungal activity of isavuconazole against Madurella mycetomatis. Antimicrob Agents Chemother 2012:56:6054–6056.
- 5. Difonzo EM, Massi D, Vanzi L, et al. Madurella mycetomatis mycetoma treated successfully with oral posaconazole. J Chemother 2011; 23:243–244.
- 6. Fahal AH, Rahman IA, El-Hassan AM, et al. The safety and efficacy of itraconazole for the treatment of patients with eumycetoma due to Madurella mycetomatis. Trans R Soc Trop Med Hyg 2011; 105:127–132.
- 7. Van de Sande WW, Fahal AH, Bakker-Woudenberg IA, et al. Madurella mycetomatis is not susceptible to the echinocandin class of antifungal agents. Antimicrob Agents Chemother 2010; 54:2738–2740.
PARACOCCIDIOIDES BRASILIENSIS
Common names for disease: Paracoccidioidomycosis, South American blastomycosis, Brazilian blastomycosis, paracoccidioidal granuloma, Lutz’s disease
Occupational setting
Paracoccidioidomycosis is a chronic granulomatous disease that is geographically restricted to areas of Central and South America. The etiologic agent, Paracoccidioides brasiliensis, is found in soil in humid mountain forests. Over 5000 cases have been reported, the majority from Brazil.1 Disease is much more common in men than women (7–70:1) and typically occurs between the ages of 20 and 50. Women appear to acquire the disease at a younger age than men.2 Most cases occur in rural workers such as tree cutters (46% of all cases in one region of Brazil occurred in rural occupations2 ), and most patients are at least moderately malnourished.
Exposure (route)
The primary route of entry is by fungal inhalation into the lungs. Local trauma leading to inoculation with the organism is less common.
Pathobiology
Paracoccidioides brasiliensis is a dimorphic fungus that forms 2–30 µm round budding cells that are released when small.
The pulmonary infection is usually subclinical. It then disseminates to form ulcerative granulomata of the buccal, nasal, and occasionally gastrointestinal mucosa. In clinically evident lung disease, the alveolitis is manifested as patchy bilateral radiographic infiltrates with hilar adenopathy. Occasionally, chronic progressive pulmonary disease can occur, leading to diffuse cavitary and alveolar involvement. Symptoms and signs include dyspnea, productive cough, chest pain, fever, and rales. Hematogenous and lymphatic dissemination without lung involvement is more common, especially to mucous membranes and mucocutaneous junctions. Papules first become vesicles, then granulomatous ulcers. The spleen, liver, CNS, bones, lymph nodes, and intestine may be involved.
Diagnosis
Sputum, crusts, material from the granulomatous bases of ulcers, biopsies of lesions, and pus from draining lymph nodes contain fungal elements, typically budding yeast forms. Serologic studies (complement fixation, immunodiffusion) are usually positive,3 although there is some concern about interlaboratory variability.4 Antigen tests for gp43 and gp70 are available and can be used for diagnosis and to monitor treatment.5,6
Treatment
Most cases are self-limiting. Itraconazole is the drug of choice for cases requiring treatment.4 Amphotericin B may be required for extensive pulmonary and severe disseminated forms of infection.
Prevention
Disease is limited to endemic areas in Central and South America, where prevention of malnutrition and other diseases (such as Chagas’ disease and tuberculosis) may decrease the risk for paracoccidioidomycosis in rural workers.
References
- 1. Franco M, Montenegro M, Mendes R, et al. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop 1987; 20:129–132.
- 2. Blotta MH, Mamoni RL, Oliveira SJ, et al. Endemic regions of paracoccidioidomycosis in Brazil: a clinical and epidemiologic study of 584 cases in the southeast region. Am J Trop Med Hyg 1999; 61:390–394.
- 3. Restrepo A, Robledo M, Giraldo R, et al. The gamut of paracoccidioidomycosis. Am J Med 1976; 61:33–42.
- 4. Vidal MS, Del Negro GM, Vicentini AP, et al. Serological diagnosis of paracoccidioidomycosis: high rate of inter-laboratorial variability among medical mycology reference centers. PLoS Negl Trop Dis 2014; 8(9):e3174.
- 5. de Camargo ZP. Serology of paracoccidioidomycosis. Mycopathologia 2008; 165:289–302.
- 6. Dos Santos PO, Rodrigues AM, Fernandes GF, et al. Immunodiagnosis of paracoccidioidomycosis due to paracoccidioides brasiliensis using a latex test: detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl Trop Dis 2015; 9(2):e0003516.
PENICILLIUM SPECIES
Common names for disease: Humidifier lung, suberosis (Penicillium frequentans), cheese washer’s disease (Penicillium casei, Penicillium roqueforti), cheese worker’s lung, woodman’s disease, allergic bronchopulmonary mycosis (ABPM), Penicilliosis (Penicillium marneffei), Peat moss worker’s lung (Penicillium citreonigrum).
Occupational setting
Because the blue-green Penicillium molds (Figure 24.11) are ubiquitous in nature, they are common contaminants of indoor environments. Exposure to Penicillium spp. has been associated with hypersensitivity lung disease in cork workers,1 cheese workers,2,3 laboratory workers, farmers,4 tree cutters,5 sawmill workers, other handlers of mold-contaminated wood,6 peat moss workers,7 salami factory workers,8 and sausage production workers.9 Contaminated humidifier water and moldy HVAC (heating, ventilation, and air conditioning) systems have been associated with Penicillium-induced hypersensitivity pneumonitis.10,11 Penicillium spores are also encountered in poultry farming and coconut production.12,13
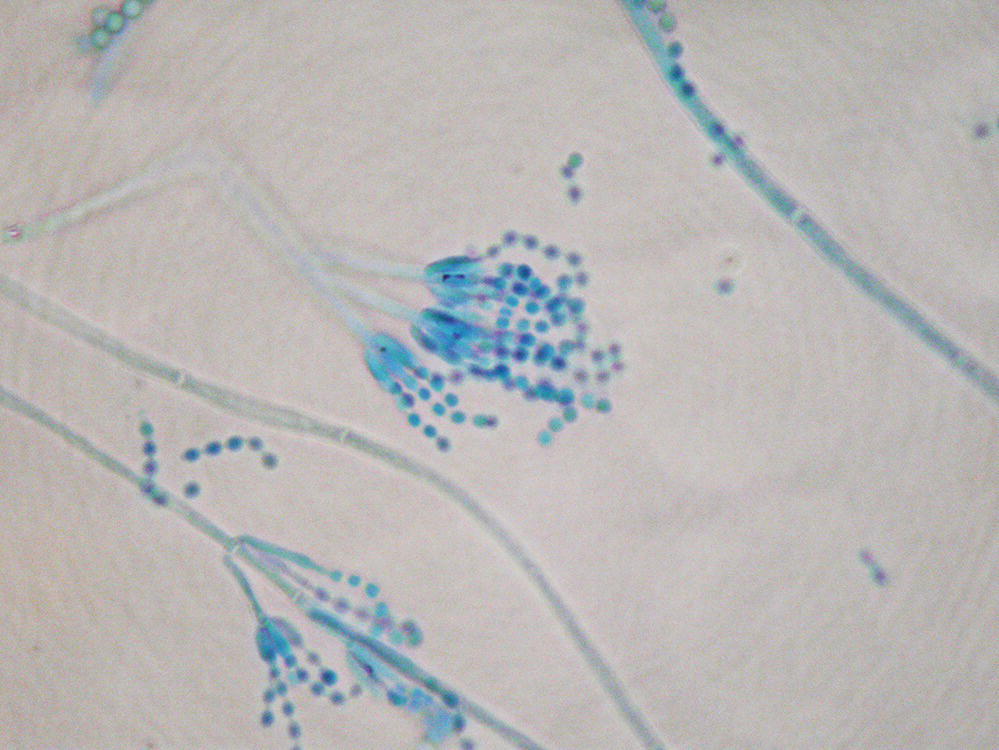
FIGURE 24.11 Photomicrograph of Penicillium conidia.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Exposure (route)
Inhalation of airborne spores is the major route of entry.
Pathobiology
A variety of Penicillium species have been associated with hypersensitivity lung diseases; the most common is hypersensitivity pneumonitis, although asthma has also been described. Penicillium frequentans spores in the air of factories where cork bark is processed can cause suberosis, a form of hypersensitivity pneumonitis. Penicillium casei and P. roqueforti have been associated with hypersensitivity pneumonitis in cheese workers exposed to Penicillium-contaminated cheese. Several Penicillium species isolated from contaminated humidifier water were shown to induce a precipitating antibody response in an entomologist exposed to mists generated by a reservoir type of humidifier. Woodworkers, including those exposed to mold-contaminated fuel chips, debarking of live trees, and sawmill particulates, are at risk for Penicillium-induced hypersensitivity pneumonitis. Workers exposed to Penicillium-contaminated peat moss have also developed hypersensitivity pneumonitis.
The clinical presentation of hypersensitivity pneumonitis is variable, ranging from severe, acute respiratory, and systemic symptoms to subtle, chronic symptoms.13,14 Acute illness is manifested by fevers, chills, cough, dyspnea, abnormal chest radiograph, and leukocytosis; improvement is seen within a few days following removal from exposure. The more subacute or chronic illness is manifested by insidious onset of cough and progressive dyspnea on exertion. Pulmonary physiology may be normal, show isolated obstruction, or show the more classic restrictive or mixed restrictive and obstructive pattern. Exercise physiology often demonstrates gas exchange abnormalities. The chest radiograph may be normal or show inhomogeneous, patchy alveolar infiltrates, or interstitial opacities High-resolution CT scans typically show diffuse, fine, and poorly defined centrilobular micronodules. Serum precipitating antibodies to Penicillium species are often positive.
Rare cases have been reported of allergic bronchopulmonary penicilliosis causing intermittent airways obstruction, transient pulmonary infiltrates, blood and sputum eosinophilia, a positive dual skin test (Types I and III), and precipitating antibodies in serum.15 Proximal saccular bronchiectasis is often found in the segment of lung containing the infiltrate. Bronchial hygiene alone or in combination with inhaled or oral corticosteroids is usually effective treatment.
Penicillium infections of clinical importance are very rare. There have been case reports of Penicillium infection of the ear, foot, urinary bladder, and lung. Penicillium presenting as a solitary pulmonary nodule in a nonimmunocompromised host has also been reported. Penicillium marneffei is endemic in Southeast Asia and is also found in Africa. It has been associated with recurrent episodes of hemoptysis attributed to bronchitis and bronchiectasis. Histopathologically, lung tissue shows granulomata with central areas of necrosis and neutrophilic infiltration with many yeast-like tissue-forming cells of P. marneffei. In addition, P. marneffei has caused disseminated infection manifested by fever, weight loss, anemia, and skin lesions (Penicilliosis) in immunocompromised hosts.16,17 Penicillium marneffei can cause peritonitis in peritoneal dialysis patients.18
Treatment
Treatment of Penicillium-induced hypersensitivity pneumonitis relies on removal of the affected individual from exposure to the contaminated environment. Systemic steroids have been used in severely ill patients with interstitial pneumonitis, but controlled clinical trials are lacking. Treatment of Penicillium-induced asthma involves elimination of antigen exposure and use of inhaled steroids and bronchodilators. Allergic bronchopulmonary penicilliosis is rare, but treatment should be similar to that for ABPA, with inhaled or oral corticosteroids to prevent recurrent pneumonitis and subsequent bronchiectasis. Treatment of P. marneffei generally includes induction with amphotericin B followed by itraconazole therapy.19
Prevention
Attention should be paid to safe handling of all types of solid fuel (wood, chips, and peat) and other materials in which mold may grow. Dry storage, prevention of mold growth, and use of appropriate respiratory protection are important.
Avoidance or elimination of water damage to HVAC systems is important in preventing significant indoor mold contamination. Regular HVAC maintenance and inspection, appropriate filtration of outside air, and provision of indoor environments free from water intrusion are crucial to prevent fungal amplification and dissemination. Hard surfaces supporting fungal growth in indoor environment should be cleaned with dilute bleach (1 : 10–1 : 50 dilution); then, the surface should be rinsed with clean water and dried. Mold-contaminated materials such as furniture, draperies, and insulation material should be discarded.
A variety of measures have been introduced in the cheese production industry to reduce contamination with airborne molds. These measures include wrapping cheese in foil or plastic film before entering the aging room, thus preventing surface mold formation; careful temperature and humidity control in aging rooms; and removal of surface mold contamination before it becomes an aerosolized dust.
References
- 1. Avila R. Lacey T. The role of Penicillium frequentans in suberosis (respiratory disease in workers in the cork industry). Clin Allergy 1974; 4:109–117.
- 2. Campbell J, Kryda M, Treuhaft M, et al. Cheese worker’s hypersensitivity pneumonitis. Am Rev Respir Dis 1983; 127:495–496.
- 3. Schlueter D. “Cheesewasher’s disease”: a new occupational hazard? Ann Int Med 1973; 78:606.
- 4. Nakagawa-Yoshida K, Ando M, Etches RI, et al. Fatal cases of farmer’s lung in a Canadian family. Probable new antigens, Penicillium brevicompactum and P. olivicolor. Chest 1997; 111:245–248.
- 5. Dykewicz M. Laufer P, Patterson R, et al. Woodman’s disease: hypersensitivity pneumonitis from cutting live trees. J Allergy Clin Immunol 1988; 81:455–460.
- 6. Van Assendelft A, Raitio M, Turkia V. Fuel chip-induced hypersensitivity pneumonitis caused by Penicillium species. Chest 1985; 87:394–396.
- 7. Cormier Y, Israel-Assayag I, Bedard G, et al. Hypersensitivity pneumonitis in peat moss workers. Am J Respir Crit Care Med 1998; 158:412–417.
- 8. Marvisi M, Balzarini L, Mancini C, et al. A new type of hypersensitivity pneumonitis: salami brusher’s disease. Monaldi Arch Chest Dis 2012; 77:35–37.
- 9. Morell F, Cruz MJ, Gomez FP, et al. Chacinero’s lung – hypersensitivity pneumonitis due to dry sausage dust. Scand J Work Environ Health 2011; 37:349–356.
- 10. Baur X, Behr J, Dewair M, et al. Humidifier lung and humidifier fever. Lung 1988; 166:113–124.
- 11. Bernstein K, Sorenson W, Garabrant D, et al. Exposures to respirable, airborne Penicillium from a contaminated ventilation system: clinical, environmental and epidemiological aspects. Am Ind Hyg Assoc J 1983; 44:161–169.
- 12. Richerson H, Bernstein I, Fink J, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. J Allergy Clin Immunol 1989; 84:839–844.
- 13. Rimac D, Macan J, Varnai VVM, et al. Exposure to poultry dust and health effects in poultry workers: impact of mould and mite allergens. Int Arch Occup Environ Health 2010; 83:9–19.
- 14. Nascimento Mdo D, Leitao VM, Neto Silva MA, et al. Eco-epidemiologic study of emerging fungi related to the work of babacu coconut breakers in the State of Maranhao, Brazil. Rev Soc Bras Med Trop 2014; 47:74–78.
- 15. Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012; 129:280–291.
- 16. Rose C, Lara A. Hypersensitivity pneumonitis. In: Murray JF, Nadel JF (eds.), Textbook of Respiratory Medicine, 5th edn. Philadelphia: Saunders, 2010:1587–1600.
- 17. Chang HR, Shu KH, Cheng CH, et al. Peritoneal-dialysis-associated Penicillium peritonitis. Am J Nephrol 2000; 20:250–252.
- 18. Lo Y, Tintelnot K, Lippert U, et al. Disseminated Penicillium marneffei infection in an African AIDS patient. Trans R Soc Trop Med Hyg 2000; 94:187.
- 19. Kurup A, Leo YS, Tan Al, et al. Disseminated Penicillium marneffei infection: a report of five cases in Singapore. Ann Acad Med Singapore 1999; 28:605–609.
SPOROTHRIX SCHENCKII
Common name for disease: Sporotrichosis
Occupational setting
Although sporotrichosis occurs worldwide, it is found mainly in temperate, warm, and tropical areas. Sporothrix schenckii is isolated most often from soil and living plants or plant debris. Cutaneous infection develops where the organism is introduced to sites of skin injury. Subsequent nodular lymphangitic spread is common. Pulmonary sporotrichosis is a rare condition caused by inhalation of fungal spores. Infection of bones and joints also rarely occurs in immunocompetent hosts.1
Occupations that predispose to infection include gardening, farming, masonry, outdoor work, floral work, and other activities with exposure to contaminated soil or vegetation such as sphagnum moss, prairie hay, salt marsh hay, or roses.2–4 Outbreaks have occurred among miners, nursery workers, and forestry workers who handle contaminated timbers, seedlings, mulch, hay, or other plant materials.5–7 In one study, risk for infection was related to exposure to moss and to seedlings from a particular nursery.8 Arm and finger infections have been reported in laboratory workers through contact with experimentally infected animals or contaminated material.9 Two laboratory workers developed conjunctival and eyelid infections after mycelial elements were accidentally splattered into the eyes. Transmission to humans from infected cats has also been reported.10 Armadillo hunting in Uruguay has been associated with sporotrichosis, presumably from exposure to the fungus isolated from the dry grass used by armadillos and rodents to prepare their nests. An outbreak of the illness in South African gold miners was traced to contaminated mine timbers.11
Exposure (route)
Sporothrix schenckii usually enters the body through traumatic implantation,12 but inhalation of fungal conidia is occasionally associated with pulmonary infection.
Pathobiology
Sporothrix schenckii is a dimorphic fungus with 4–6 µm round, oval, or cigar-shaped cells.
Cutaneous disease arising at sites of minor trauma begins as a small, erythematous papule that enlarges over days or weeks. It usually remains painless, but it may secrete a clear discharge. Typically, discrete nodular lesions spread along lymphatic channels. Skin lesions may also result from hematogenous dissemination. Such lesions may herald the onset of osteoarticular sporotrichosis, which is manifested by stiffness and pain in a large joint, particularly the knee, elbow, ankle, or wrist. Radiologic evidence of osteomyelitis develops slowly, and additional joints may become involved in untreated patients. An indolent tenosynovitis of the wrist or ankle with pain, limitation of motion, and nerve entrapment can occur. Endophthalmitis, brain abscess, chronic meningitis, and other manifestations of disseminated disease are rare. Patients with a history of alcoholism or immunosuppression are at increased risk for dissemination.13
Pulmonary sporotrichosis typically presents with cough, low-grade fever, weight loss, hemoptysis, and an upper lobe single cavitary lesion with or without surrounding parenchymal infiltrate. Pleural effusion, hilar adenopathy, and calcification are rare. In the absence of treatment, the lung lesion gradually progresses to death. Chronic obstructive pulmonary disease (COPD) is a risk factor for pulmonary infection.13
Diagnosis
Accurate diagnosis requires detection of the fungus in clinical specimens including skin biopsy, joint aspirate, or sputum, either through culture or by fluorescent antibody staining. Recent data suggest PCR techniques may be valuable for more rapid diagnosis.14,15
Treatment
The key to appropriate diagnosis and treatment of sporotrichosis is a high index of clinical suspicion combined with culture to confirm results. Itraconazole is the drug of choice for lymphocutaneous infection. 200 mg/day for 3–6 months results in a >90% cure rate.16 Fluconazole at a dose of 400 mg/day is second-line treatment; ketoconazole should not be used. Terbinafine may be as effective as itraconazole.17 Intravenous amphotericin B is reserved for treatment failures. Itraconazole at a dose of 200 mg twice daily can be used for mild pulmonary disease. However, surgery combined with antifungal therapy is more effective than medical therapy alone in cavitary disease.18
In osteoarticular sporotrichosis, itraconazole at a dose of 200 mg twice daily is recommended, as accompanying systemic illness is unusual. Cure rates of 60–80% have been achieved with this regimen. Amphotericin B is indicated for severely ill patients or for those in whom itraconazole fails. Amphotericin B is also the drug of choice for disseminated or meningeal sporotrichosis.13
Prevention
Use of heavy, impermeable gloves and long-sleeved shirts in at-risk occupations has been shown to limit traumatic fungal implantation.8 The use of alternative packing materials for plant products, such as shredded paper or cedar wood chips, has been recommended. Laboratory workers who handle contaminated material should work under appropriate hoods and utilize BSL2 work practices and facilities that minimize inhalation of conidia.19 Early recognition and prompt treatment of disease limit morbidity and mortality.
References
- 1. Bariteau JT, Warysz GR, Mcdonnell M, et al. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg 2014; 22:390–401.
- 2. Centers for Disease Control. Sporotrichosis among hay-mulching workers—Oklahoma, New Mexico. MMWR 1984; 33:682–683.
- 3. Dixon D, Salkin I, Duncan R, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J Clin Microbiol 1991; 29:1106–1113.
- 4. Cox R, Reller L. Auricular sporotrichosis in a brickmason. Arch Dermatol 1979; 115:1229–1230.
- 5. Grotte M, Younger B. Sporotrichosis associated with sphagnum moss exposure. Arch Pathol Lab Med 1981; 105:50–51.
- 6. Powell K, Taylor A, Phillips B, et al. Cutaneous sporotrichosis in forestry workers. Epidemic due to contaminated sphagnum moss. JAMA 1978; 240:232–235.
- 7. Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis. A point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted-house. Arch Intern Med 1997; 157:1885–1887.
- 8. Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis 1997; 176:499–504.
- 9. Cooper C, Dixon D, Salkin I. Laboratory- acquired sporotrichosis. J Med Vet Mycol 1992; 30:169–171.
- 10. Reed K, Moore F, Geiger G, et al. Zoonotic transmission of sporotrichosis: case report and review. Clin Infect Dis 1993; 16:384–387.
- 11. Einstein H. ACCP Committee Report: occupational aspects of deep mycoses. Chest 1978; 73:115.
- 12. Tan T, Field C, Faust B. Cutaneous sporotrichosis. J La State Med Soc 1988; 140:41–45.
- 13. Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the management of patients with sporotrichosis. Clin Infect Dis 2000; 30:684–687.
- 14. Liu X, Zhang Z, Hou B, et al. Rapid identification of Sporothrix schenckii in biopsy tissue by PCR. J Eur Acad Dermatol Venereol 2013; 27:1491–1497.
- 15. De Oliveira MM, Sampaio P, Almeida-Paes R, et al. Rapid identification of Sporothrix Specis by T3B fingerprinting. J Clin Microbiol 2012; 50:2159–2162.
- 16. De Lima Barros MB, Schubach AO, de Vasconcellos Carvalhaes de Oliveira R, et al. Treatment of cutaneous sporotrichosis with itraconazole – study of 645 patients. Clin Infect Dis 2011; 52:e200–e206.
- 17. Francesconi G, Francesconi do Valle AC, Passos SL, et al. Comparitive study of 250 mg/day terbinafine and 100 mg/day itraconazole for the treatment of cutaneous sporotrichosis. Mycopathologia 2011; 171:349–354.
- 18. Aung AK, Teh BM, McGrath C, et al. Pulmonary sporotrichosis: case series and systematic analysis of literature on clinic-radiological patterns and management outcomes. Med Mycol 2013; 51:534–544.
- 19. Centers for Disease Control and Prevention, National Institutes of Health. Biosafety in Microbiological and Biomedical Laboratories, 5th edn. HHS Publication no. (CDC) 21–1112. New York: U.S. Department of Health and Human Services, 2009.
STACHYBOTRYS CHARTARUM
Common name for disease: None
Occupational setting
Stachybotrys is a rare fungal contaminant of water-damaged cellulose materials. High exposure levels have been reported among farmers, woodworkers, and composting workers exposed to moldy plant products.1–4 High concentrations have also been described in water-damaged office buildings, courthouses, and homes.5–8
Exposure (route)
Exposure occurs through inhalation of mycotoxin-containing spores. Disease in animals has been reported after ingestion of contaminated plant feeds.
Pathobiology
Stachybotrys chartarum is a rare contaminant of nitrogen-poor straw and cellulose-based water-damaged materials.6 Pathogenic effects were first described in the veterinary literature of the 1920s when cattle and horses developed anemia and gastrointestinal hemorrhage after ingesting contaminated grain.9
Stachybotrys is believed to cause disease through the production of powerful trichothecene mycotoxins. The mechanism of trichothecene toxicity is potent inhibition of DNA, RNA, and protein synthesis. These mycotoxins act as powerful immunosuppressants and can induce hemolysis and bleeding in animal models.10,11
Many possible human health effects have been described but none definitively proven. Occupational exposure to Stachybotrys in farmers and agricultural workers handling contaminated straw has been associated with epistaxis, hemoptysis, skin irritation, and alterations in white blood cell counts.12 Stachybotrys has been implicated (along with many other toxigenic fungi) in sick building syndrome,5,6,8,13,14 although causation has not been definitively proven.15 A possible association has been described between Stachybotrys contamination of homes and idiopathic pulmonary hemorrhage/hemosiderosis in infants.7,16 A cluster of 10 infants suffering from idiopathic pulmonary hemorrhage/hemosiderosis occurred in Cleveland between January 1993 and December 1994. Only three cases had been reported in the prior 10 years. Symptoms began with a prodrome featuring abrupt cessation of crying, limpness, and pallor. The prodrome was followed by hemoptysis, grunting, and respiratory failure. Chest radiographs revealed diffuse, bilateral alveolar infiltrates, and laboratory examination revealed decreased hematocrits and hemolysis on blood smears. Fifty percent of the cases recurred after returning home, and one infant died. Initial epidemiologic investigations implicated water-damaged homes as a significant risk factor.17 Follow-up industrial hygiene studies revealed increased levels of Stachybotrys in case versus control homes. However, reexamination by the Centers for Disease Control and Prevention (CDC) concluded the data were not strong enough to definitively support an association between pulmonary hemorrhage in infants and exposure to S. chartarum.18
Diagnosis
Clinical evaluation requires careful occupational and environmental histories to elicit circumstances of water damage and environmental sampling to confirm the exposure.
Treatment
Removal of contaminated materials and prevention of circumstances leading to moisture intrusion in indoor moisture environments are keys to management of Stachybotrys exposure.
Prevention
Rapid remediation of water damage in homes and office buildings will diminish exposure risks. Proper attention to building practices during new construction will help prevent subsequent leaks and water damage. In agricultural settings, engineering and process controls can reduce exposure to high fungal concentrations.
References
- 1. Croft WA, Jarvis BB, Yatawara CS. Airborne outbreak of trichothecene toxicosis. Atmos Environ 1986; 20:549–552.
- 2. Mainville C, Auger PL, Smoagiewica W, et al. Mycotoxins and chronic fatigue syndrome in a hospital. In: Andersson K (ed.), Healthy Buildings Conference. Stockholm: Swedish council of Building Research, 1988:1–10.
- 3. Auger PL, Gourdeau P, Miller JD. Clinical experience with patients suffering from a chronic fatigue-like syndrome and repeated upper respiratory infections in relation to airborne molds. Am J Ind Med 1994; 25:41–42.
- 4. Johanning E, Auger PL, Reijula K. Building-related illnesses. N Eng J Med 1998; 338:1070.
- 5. Johanning E, Landsbergis P, Gareis M, et al. Clinical experience and results of a sentinel health investigation related to indoor fungal exposure. Environ Health Perspect 1999; 107(suppl 3):489–494.
- 6. Hodgson MJ, Morey P, Leung WY, et al. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. J Occup Environ Med 1998; 40:241–249.
- 7. Etzel RA, Montana E, Sorenson WG, et al. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med 1998; 152:757–762.
- 8. Sudakin DL. Toxigenic fungi in a water-damaged building: an intervention study. Am J Ind Med 1998; 34:183–190.
- 9. Hinitikka EL. Stachybotryotoxicosis as a veterinary problem. In: Rodricks JV, Hesseltine CW, Mehlman MA (eds.), Mycotoxins in Human and Animal Health. Park Forest South: Pathotox Publishers, 1977:277–284.
- 10. Pang VF, Lambert RJ, Felsburg PJ, et al. Experimental T-2 toxicosis in swine following inhalation exposure: clinical signs and effects on hematology, serum biochemistry, and immune response. Fundam Appl Toxicol 1988; 11:100–109.
- 11. Ueno Y. Trichothecene mycotoxins – mycology, chemistry and toxicology. Adv Nutr Res 1980; 3:301–353.
- 12. Hintikka EL. Human stachybotrytoxicosis. In: Wyllie TD, Morehouse LG (eds.), Mycotoxigenic Fungi, Mycotoxins, Mycotoxicoses. New York: Marcel Dekker, 1987:87–89.
- 13. Johanning E, Biagini R, Hull D, et al. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water-damaged office environment. Int Arch Occup Environ Health 1996; 68:207–218.
- 14. Mahmoudi M, Gershwin ME. Sick building syndrome. III. Stachbotyrs chartarum. J Asthma 2000; 37:191–198.
- 15. Hardin, BD, Kelman BJ, Saxon A. Adverse human health effects associated with molds in the indoor environment. J Occup Environ Med 2003; 45(5):470–478.
- 16. CDC. Update: pulmonary hemorrhage/hemosiderosis among infants – Cleveland, Ohio, 1993–1996. MMWR 1997; 46:33–35.
- 17. Montana E, Etzel RA, Allan T, et al. Environmental risk factors associated with pediatric idiopathic pulmonary hemorrhage and hemosiderosis in a Cleveland community. Pediatrics 1997; 99:117–124.
- 18. CDC. Update: pulmonary hemorrhage/hemosiderosis among infants – Cleveland, Ohio, 1993–1996. MMWR 2000; 49:180–184.
TRICHOPHYTON AND OTHER DERMATOPHYTES
Common names for diseases: Dermatophytosis, tinea corporis (ringworm), tinea glabrosa, Majocchi’s granuloma, tinea cruris (jock itch), tinea pedis (athlete’s foot).
Occupational setting
Cutaneous infections occur from exposure to the fungal dermatophytes, most commonly species of Trichophyton. The primary sources of dermatophytes are animals (zoophilic), humans (anthropophilic), and soil (geophilic). Dermatophytes originating from soil are occasionally responsible for outbreaks of human disease in exposed occupational groups such as gardeners and farm workers. Zoophilic outbreaks among cattle workers and rabbit farmers have also been reported.1,2 Coaches, trainers, and professional athletes are another high-risk occupational group.3,4 Spread of the anthropophilic organisms that infect glabrous skin is typically through contact with infected desquamated skin scales, such as in bathing or shower facilities in military barracks or factories.5 As many as 30–35% of British coal miners were found in one study to have dermatophyte infections of the feet.6 Trichophyton tonsurans was responsible for an outbreak of dermatophytosis in hospital personnel exposed to an infected patient.7 Trichophyton verrucosum, the cause of cattle ringworm, and Microsporum canis, in cats and dogs, are the most common zoophilic dermatophytes that cause human infection in temperate countries.8
Podiatrists exposed to toenail dust generated when drills and burrs are used to reduce the thickness of hyperkeratotic nails can develop hypersensitivity reactions to T. rubrum (including nasal and eye symptoms, restrictive changes on pulmonary function tests, and specific IgG-precipitating antibodies).9,10
Exposure (route)
Dermatophytes invade the stratum corneum of the skin, most commonly of the feet, groin, scalp, and nails. Hypersensitivity reactions of the nasal mucus membranes and lungs can occur from exposure to Trichophyton dusts.
Pathobiology
The three genera of pathogenic dermatophyte fungi are Trichophyton (Figure 24.12), Microsporum, and Epidermophyton. The classic lesion of dermatophytosis is an annular scaling patch with a raised edge and a less inflamed central area.

FIGURE 24.12 Lactophenol cotton blue wet mount revealing Trichophyton tonsurans.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Tinea refers to dermatophyte infection and is followed by the Latin word for the affected site. Trichophyton rubrum is the most common cause of tinea cruris, the dermatophyte infection of the groin. Scaling and irritation are the usual presenting findings. The disease is most common in young adult males. The leading edge extending onto the thighs is prominent and may contain follicular papules and pustules. Tinea pedis usually begins in the lateral interdigital spaces of the foot. The main symptom is itching; the skin usually cracks and may macerate. Tinea corporis usually involves the trunk or legs. Tinea capitis involves the scalp and can cause alopecia.11
Diagnosis
Fungal hyphae are easily observed as chains of arthrospores in wet mount preparations from skin scrapings. It is important to sample the edge of skin lesions and to allow the material to soften in potassium hydroxide before microscopic examination.
Treatment
Topical treatment with keratolytics and compounds with specific antifungal activity is usually successful. Nail, hair, and widespread skin infections may require oral agents such as griseofulvin. Trichophyton tonsurans infection may respond to oral ketoconazole. Griseofulvin and terbinafine are effective for tinea capitis.12,13
Prevention
To prevent tinea cruris, cool and loose-fitting clothing should be worn in hot and humid environments, where perspiration and irritation of skin are contributing factors. Avoidance of contact with contaminated clothing and towels is helpful. The floors of locker rooms or showers contaminated with dermatophytes should also be avoided. Sanitization of contaminated footwear and contaminated equipment can also help prevent the spread of infection.4,14
References
- 1. Lehenkari E, Silvennoinen-Kassinen S. Dermatophytes in northern Finland in 1982–90. Mycoses 1995; 38:411–414.
- 2. Weigl S, Figueredo LA, Otranto D. Molecular identification and phylogenesis of dermatophytes isolated from rabbit farms and rabbit farm workers. Vet Microbiol 2012; 154:395–402.
- 3. Collins CJ, O’Connell B. Infectious disease outbreaks in competitive sports, 2005–2010. J Athl Train 2012; 47:516–518.
- 4. Bassiri-Jahromi S, Sadeghi G, Paskiaee FA. Evaluation of the association of superficial dermatophytosis and athletic activities with special reference to its prevention and control. Int J Dermatol 2010; 49:1159–1164.
- 5. Korting H, Zienicke H. Dermatophytoses as occupational dermatoses in industrialized countries. Report on two cases from Munich. Mycoses 1990; 33:8609.
- 6. Gugnani H, Oyeka C. Foot infections due to Hendersonula toruloidea and Scytalidium hyaline in coal miners. J Med Vet Mycol 1989; 27:167–179.
- 7. Arnow P, Houchins S, Pugliese G. An outbreak of tinea corporis in hospital personnel caused by a patient with Trichophyton tonsurans infection. Pediatr Infect Dis J 1991; 10:355–359.
- 8. Chmel L, Buchvald J, Valentova M. Ringworm infection among agricultural workers. Int J Epidemiol 1976; 5:291–295.
- 9. Davies R, Ganderton M, Savage M. Human nail dust and precipitating antibodies to Trichophyton rubrum in chiropodists. Clin Allergy 1983; 13:309–315.
- 10. Abramson C, Wilton T. Nail dust aerosols from onychomycotic toenails. Part II. Clinical and serologic aspects. J Am Podiatr Med Assoc 1992; 82:116–123.
- 11. El-Khalawany M, Shaaban D, Hassan H, et al. A multicenter clinicomycological study evaluating the spectrum of adult tinea capitis in Egypt. Acta Dermatovenerol Alp Pannonica Adriat 2013; 22:77–82.
- 12. Grover C, Arora P, Manchanda V. Comparative evaluation of griseofulvin, terbinafine and fluconazole in the treatment of tinea capitis. Int J Dermatol 2012; 51:455–458.
- 13. Tey HL, Tan AS, Chan YC. Meta-analysis of randomized controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol 2011; 64:663–670.
- 14. Gupta AK, Brintnell WC. Sanitization of contaminated footwear from onychomycosis patients using ozone gas: a novel adjunct therapy for treating onychomycosis and tinea pedis. J Cutan Med Surg 2013; 17:243–249.
ZYGOMYCETES
Including the order Mucorales, and Hyphomycetes (Verticillium, Fusarium, and Neurospora)
Common names for diseases: Hypersensitivity diseases: Paprika splitter’s disease (M. stolonifer), Wood trimmer’s disease (Rhizopus, Mucor), Tomato grower’s asthma (Verticillium albo-atrum), Sinus fusariosis (Fusarium) Infections: Mucormycosis, phycomycosis, hyphomycosis, zygomycosis
Occupational setting
Hypersensitivity pneumonitis can occur in workers exposed to respirable Mucorales in paprika and from contaminated wood bark in sawmills.1,2 In addition to occupational hypersensitivity lung disease, various genera and species of the class Zygomycete, order Mucorales, can cause infectious mucormycosis. The Zygomycetes grow in the environment and in tissue as hyphae. They are thermotolerant fungi that are commonly found in decaying organic debris. Despite their ubiquity, human infection is infrequent. Typically, it is associated with severe immunocompromise, malnutrition, iron chelation therapy, diabetes mellitus, or trauma.3,4 The hyaline hyphomycete V. albo-atrum has been associated with cases of occupational asthma in tomato and tobacco growers exposed to crop outbreaks.5–7 Fusarium species are very common soil organisms. Maxillary sinus fusariosis has been described in agricultural workers exposed to Fusarium.8 Occupational asthma from immune sensitization to Neurospora spp. occurred in a plywood factory worker exposed to moldy wood products.9 Occupational sensitization was demonstrated by allergy prick skin testing, the presence of specific serum IgE antibodies, and inhalation challenge with the Neurospora mold growing on plywood. Suggested preventive strategies included sealing of the wood drying machine to minimize dust concentrations and shorter outdoor storage times for the plywood to prevent fungal growth.
Exposure (route)
For the hypersensitivity diseases (asthma and hypersensitivity pneumonitis) and for respiratory tract infection, the route of exposure is inhalation of respirable airborne hyphae. Cutaneous and subcutaneous mucormycosis can occur by direct implantation from “barrier breaks” or by hematogenous dissemination. Several cases have been associated with contaminated bandages and surgical dressings. Rarely, gastrointestinal transmission may occur.4
Pathobiology
All of the Zygomycetes grow as 4–15 µm wide hyphae in the environment and tissue and are identified microscopically by their morphology (Figure 24.13). As with other forms of hypersensitivity pneumonitis (HP), the symptoms of hypersensitivity diseases caused by these organisms may be acute and flu-like or subtle, chronic, and predominantly respiratory in nature. Acute illness is manifested by fevers, chills, cough, dyspnea, abnormal chest radiograph, and leukocytosis; improvement follows within a few days after removal from exposure. The more subacute or chronic forms of HP are typically manifested by insidious onset of cough, progressive dyspnea on exertion, and weight loss. Pulmonary physiology may be normal, show isolated obstruction, or show the more classic restrictive or mixed restrictive and obstructive pattern. Exercise physiology often shows gas exchange abnormalities. The chest radiograph may be normal or show inhomogeneous, patchy alveolar infiltrates, or interstitial opacities. Serum precipitating antibodies to Zygomycete antigens species are often positive.
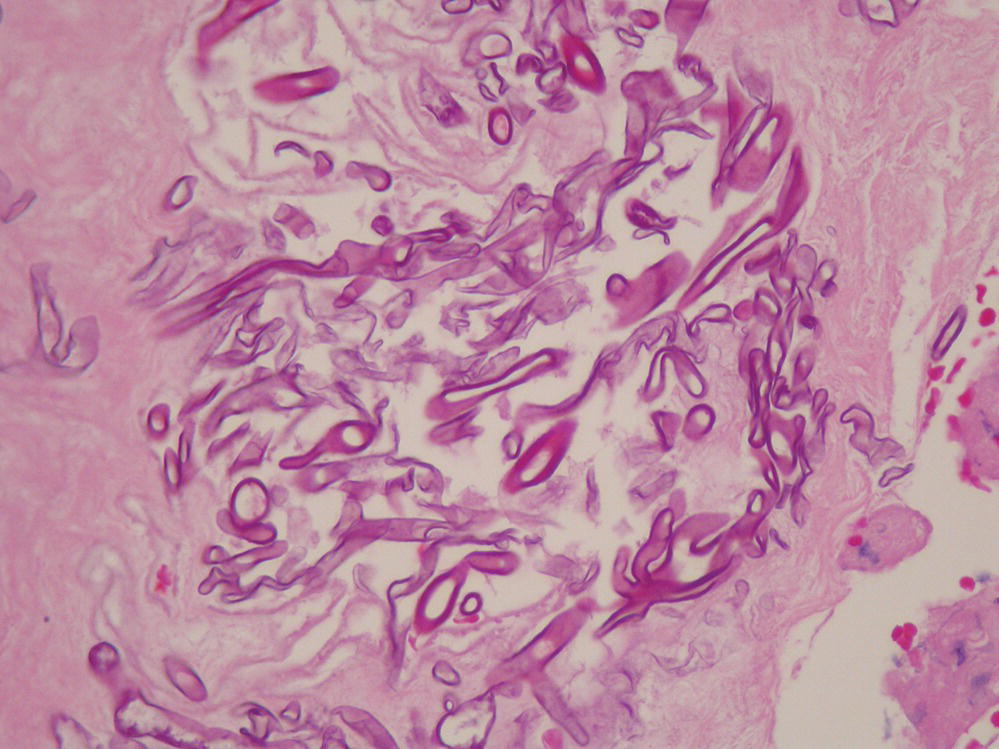
FIGURE 24.13 Tissue sample hematoxylin and eosin stain showing Zygomycetes hyphae.
Courtesy of Dr. Dominick Cavuoti and Dr. Francesca Lee.
Many different species in the order Mucorales have been implicated in infectious disease, including Rhizopus, Mucor, Mortierella, and Absidia species. Mucormycosis is the most acute, fulminant fungal infection known. Organisms invade arterial vessels and may infect the face and cranium, lungs, GI tract, or skin. Rhinocerebral disease typically occurs in the acidemic patient with uncontrolled diabetes; it begins in the nasal turbinates, paranasal sinuses, palate, pharynx, or ears and spreads to the central nervous system. Renal dialysis patients treated with deferoxamine may also be at increased risk as are immunocompromised patients (those with chronic steroid use, posttransplant, and hematologic malignancies). Presenting signs usually include a thick, dark, and blood-tinged discharge that often reveals hyphal strands on KOH mount. Rapid invasion of surrounding tissue with sloughing and cranial nerve dysfunction follows, and death usually occurs in a few days. Pulmonary mucormycosis occurs after inhalation of spores or from aspiration of nasopharyngeal secretions, leading to bronchitis and pneumonia with subsequent arterial invasion, often massive cavitation, and rapid death. Primary gastrointestinal and pelvic mucormycosis are uncommon, and malnourished patients or those with hematologic malignancies are most at risk. Cerebral zygomycosis has been described in intravenous drug abusers.10 This infection most likely spreads through the bloodstream following the intravenous injection of infectious organisms.
Treatment
Treatment of hypersensitivity lung disease focuses on early disease recognition and elimination of further exposure to the offending antigen. Oral corticosteroids may be useful for patients with severe symptoms or physiologic abnormalities.
Treatment of the underlying disease (e.g., diabetes) is the most effective method to control infectious mucormycosis. The prognosis is poor (80–90% mortality rate). Treatment requires a combination of surgical debridement of necrotic tissue and antifungal therapy with intravenous amphotericin B.4 Recently posaconazole has emerged as a potential salvage therapy.11
Prevention
Prevention of occupational sensitization to these organisms involves the provision of adequate ventilation, process controls, and respiratory protection to limit exposure to high fungal bioaerosol levels.
References
- 1. Eduard W, Sandven P, Levy F. Relationships between exposure to spores from Rhizopus, Microsporus and Paecilomyces variotii and serum lgG antibodies in wood trimmers. Int Arch Allergy Appl Immunol 1992; 97:274–282.
- 2. Hedenstierna G, Alexandrsson R, Belin L, et al. Lung function and Rhizopus antibodies in wood trimmers. A cross-sectional and longitudinal study. Int Arch Occup Environ Health 1986; 58:167–177.
- 3. Gordon G, Indeck M, Bross J, et al. Injury from a silage wagon accident complicated by mucormycosis. J Trauma 1988; 28:866–867.
- 4. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000; 13:236–301.
- 5. Eaton K, Hannessy T, Snodin D, et al. Verticillium lecanii. Allergological and toxicological studies on work-exposed personnel. Ann Occup Hyg 1986; 30:209–217.
- 6. Anonymous. Occupational asthma in tomato growers. Occup Health (Lond) 1989; 41:70–71.
- 7. Davies P, Jacobs R, Mullins J, et al. Occupational asthma in tomato growers following an outbreak of the fungus Verticillium albo-atram in the crop. J Soc Occup Med 1988; 38:13–17.
- 8. Kurien M, Anandi V, Raman R, et al. Maxillary sinus fusariosis in immunocompetent hosts. J Laryngol Otol 1992; 106:733–736.
- 9. Cote J, Chan H, Brochu G, et al. Occupational asthma caused by exposure to neurospora in a plywood factory worker. Br J Ind Med 1991; 48:279–282.
- 10. Stave GM, Heimberger T, Kerkering T. Cerebral zygomycosis in intravenous drug abuse. Am J Med 1989; 86:115–117.
- 11. Cronely OA, Vehreschild JJ, Ruping MJ. Current experience in treating invasive zygmycosis with posaconazole. Clin Microbiol Infect 2009; 15(suppl 5):77–81.
