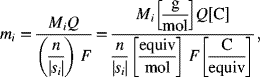Charles W. Tobias was born on November 2, 1920 in Budapest, Hungary and died in 1996 in Orinda, California. He describes his family as an “engineering family” and realized that he would be an engineer from the age of 4 or 5. Music was a lifelong passion, but engineering was his career. Tobias was inspired to select chemical engineering by his high school chemistry teacher. He attended the University of Technical Sciences in Budapest, where he obtained his Ph.D. in 1946. He was part of the Hungarian diaspora, fleeing Hungary in 1947 as the Communists were taking over the government. He traveled to Berkeley to pursue postdoctoral studies at the University of California where his brother was already working for Ernest O. Lawrence at the University's radiation laboratory. W.M. Latimer, a pioneer in the thermodynamics of electrolytes and Tobias's new boss, directed him to “…build up engineering electrochemistry here, and it should be the best in the world.” By all measures, he succeeded. He began as an instructor in the newly formed Department of Chemical Engineering and then became an Assistant Professor in 1950 and Professor in 1960. He also chaired the department from 1967 until 1972.
Charles Tobias is considered by many to be the father of modern electrochemical engineering. He added discipline and rigor, putting many of the principles on a sound theoretical footing. He studied fluid flow, electric potential fields, thermodynamic and materials properties, and mass transfer, linking his findings into a new discipline. Tobias was instrumental in moving the field from largely empirical approaches to a quantitative science that addressed scale-up and optimization. Professor Tobias is also credited with founding the electrochemical research program at Lawrence Berkeley National Laboratory, which for decades was the most influential electrochemistry group. He is reported to have started the first “electrochemical engineering” course. Professor Tobias joined with Paul Delahay in 1961 to edit a new monograph series entitled Advances in Electrochemistry and Electrochemical Engineering.
Professor Tobias had a long and productive involvement in the Electrochemical Society. He won several awards, including the Society's highest honor, the Acheson award. He served as President of the Electrochemical Society from 1970 to 1971 as well as an editor for the Journal of the Electrochemical Society for many years. As a result, he had a large influence on the structure and operation of the Society. Tobias brought the same philosophy of increasing the scientific rigor and quantitative engineering to the Society. He was also President of the International Society of Electrochemistry (1977–1978). In 2003, the Tobias Award of the Electrochemical Society was established to recognize outstanding scientific and/or engineering work in fundamental or applied electrochemistry by a young investigator.
Although Tobias had an immeasurable impact on the field of electrochemical engineering and was elected a member of the National Academy of Engineering, he was not particularly aggressive in publishing. One of his most significant findings was the use of propylene and ethylene carbonates for lithium batteries, but it was never published beyond a laboratory report. Nonetheless, Dr. Tobias believed that knowledge should always be shared—only ignorance needs secrecy. Much of the information here was derived from the chemical heritage foundation's oral history, which delivers an excellent portrait of his life.
http://www.chemheritage.org/discover/collections/oral-histories/details/tobias-charles-w.aspx
Image Source: Courtesy of UC Berkeley College of Chemistry.
Chapter 1
Introduction and Basic Principles
Welcome to this introductory text about electrochemical engineering. If you are like most people, you probably have little idea of what “electrochemical engineering” is and why it is important. That's okay. This book is to help you answer those questions and to prepare you to work in this exciting area. Electrochemistry is a branch of chemistry that studies the chemical changes that occur due to the flow of electrical current or, conversely, the production of electricity from chemical changes. As engineers, we are interested in the application of scientific knowledge, mathematical principles, and economic analyses to the design, manufacture, and maintenance of products that benefit society. It's not surprising, then, that electrochemical engineering has strong historical ties to the profession of chemical engineering.
Almost invariably, application of the principles of electrochemistry to develop products or devices requires consideration of an electrochemical system. An engineering system consists of multiple components that work together in a concerted manner. In addition to electrochemistry, other topics such as heat transfer, structural analysis, and materials science are critical to the design and operation of electrochemical systems. Today, we find engineers and scientists of all disciplines engaged in electrochemistry and collaborating in the design of electrochemical systems.
As with any topic, electrochemical engineering includes a new set of terminology that must be learned. There are also several conventions of practical importance. Hence, this chapter will introduce you to the key vocabulary of the discipline, as well as to some of the central aspects of electrochemical systems. In order to do this, we begin by looking at an electrochemical cell.
1.1 Electrochemical Cells
Electrochemical cells, such as the cell illustrated in Figure 1.1, lie at the heart of electrochemical systems. A typical electrochemical cell consists of two electrodes: an anode where oxidation occurs and a cathode where reduction takes place. Electrons move through an external circuit via an electronic conductor that connects the anode and cathode. The liquid solution that is between the two electrodes is the electrolyte. The electrolyte does not conduct electrons and does not contain any free electrons. It does, however, contain a mixture of negatively charged ions (anions) and positively charged ions (cations). These ions are free to move, which allows them to carry current in the electrolyte.

Figure 1.1 A Daniell cell is an example of an electrochemical cell. During steady operation, a constant current flows throughout the cell. For any given volume, the current entering and leaving must sum to zero since charge is conserved.
The reactions take place at the electrode surface and are called heterogeneous electron-transfer reactions. For example, the electrodeposition of copper in the cell shown in Figure 1.1 can be written as
Copper ions in solution accept two electrons from the metal and form solid copper. The reaction is described as heterogeneous because it takes place at the electrode surface rather than in the bulk solution; remember, there are no free electrons in the solution. Importantly, then, we see that electrochemical reactions are surface reactions. The metal that accepts or supplies electrons is the electrode. As written, copper ions gain electrons and therefore are reduced to form copper metal. When reduction occurs, the electrode is called the cathode.
In the same cell, the reaction that takes place on the other electrode is
The zinc metal is oxidized, giving up two electrons and forming zinc ions in solution. When oxidation occurs at the surface, the electrode is called the anode.
Both the copper and zinc reactions written above (Equations 1.1 and 1.2) are half-cell reactions, meaning that they describe a reaction that takes place at one of the electrodes. Half-cell reactions always have electrons as either a reactant or a product. Also, charge is always balanced in half-cell reactions. Charge balance means that the net charge on one side of the equation must equal the net charge on the other side of the equation. For example, in the above equation, the net charge on the Zn (left side of the equation) is zero. This value is equal to the net charge for the other side, which is (+2) + 2(−1) = 0. Charge balance is no surprise since electrochemical reactions simply add or remove electrons.
The cell illustrated in Figure 1.1 is called a Daniell cell and is named after John Frederic Daniell, who invented the cell in 1836. In this cell, the electrodes themselves (solid Zn and Cu) participate in the electrochemical reactions as either a reactant or a product. This is not always the case, as we shall see later. The electrons for the reduction of Cu2+ come from the other electrode where the oxidation of Zn takes place. The electrons that are liberated from zinc oxidation at the anode travel through an external circuit to participate in the reduction reaction at the cathode. The movement of electrons corresponds to an electrical current flowing through the external circuit. The anode and cathode reactions can be combined to give an overall cell reaction, also called the full-cell reaction. Note that there are no electrons in the overall reaction, but charge is still balanced.
In other words, the exact number of electrons released from oxidation is used for reduction. Similarly, for each copper ion that is reduced, one zinc ion is formed. Because there is a production of charge at the anode and a consumption of charge at the cathode, there is a net movement of charge in solution from the anode to the cathode. Thus, there is also a current flowing in the solution, but the net charge in the solution is unchanged.
The flow of current in an electrochemical cell is shown in Figure 1.1. Note that, by definition, current in the external circuit is opposite (cathode to anode) to the direction that electrons flow. As illustrated in the figure, current flows counterclockwise in a continuous circuit that includes the solution and the external circuit. The electrochemical reactions are the means by which current flows from the electrode to the solution (anode) and from the solution to the electrode (cathode) as part of this circuit. These reactions will not take place if the circuit is broken. This circuit is most commonly “broken” by disconnecting the external connection between the electrodes. In the absence of this external connection, the cell is at open circuit, and there is no net reaction at either electrode. Finally, we note that the name “electrochemical” refers to the fact that the chemical changes are connected to the flow of electric current. Some of the additional aspects of electrochemical reactions are discussed in the next section.
1.2 Characteristics of Electrochemical Reactions
Electrochemical reactions are reactions where electrons are transferred through a conductor from the species being oxidized to that being reduced. Most of the unique and important properties of electrochemical reactions are the result of the way that these electrons are transferred. These characteristics include the following:
- Separation of the oxidation (anodic) and reduction (cathodic) reactions. Because electrons are transferred through a conductor, the oxidation and reduction can take place on two different electrodes. This situation is the most common type of configuration for an electrochemical cell, as already illustrated.
- Use of electrons to perform work. In cells like the Daniell cell introduced in Section 1.1, electrons move spontaneously from the anode to the cathode when the circuit is closed (completed), because the energy of electrons in the anode is higher than the energy of electrons in the cathode. Some of that extra electron energy can be used to perform work by passing the electrons (current) through a load (e.g., an electronic device). Perhaps the most familiar application of this concept is in the battery that you use to power your phone or computer, where you are using electron energy to send messages to your friends or to complete your homework.
- Direct measurement of reaction rates. Typically, it is difficult to measure directly the rates of chemical reactions. However, since electron transfer in electrochemical reactions takes place through a conductor, the reaction rate is easy to measure—you just measure the electric current passing in the wire between the electrodes. That current is directly related to the reaction rate at each of the two electrodes. We will explore this later in the chapter when we discuss Faraday's law.
- Control of the direction and rate of reaction. Because electrons participate in both the oxidation and reduction reactions as either a product or a reactant, we can change the rate of reaction by changing the difference in potential between the electrodes. The electric potential is a measure of the electron energy. By adjusting the potential, we can speed up the reactions, slow them down, or even make them go in the reverse direction.
This textbook will help you to understand and to use the powerful properties of electrochemical reactions to design useful products. In the application chapters of the book, you will have the opportunity to see what others have done with electrochemical systems to create devices and processes that benefit humankind. In preparation, the next section of this chapter provides a brief overview of the applications that will be considered in order to illustrate the importance of electrochemical systems. However, before doing that, let's consider some additional aspects of electrochemical reactions.
In the Zn/Cu cell considered previously, the electrodes participated directly as either a reactant (Zn) or a product (Cu). In many instances, the electrodes do not participate beyond supplying or removing electrons. In these instances, the electrodes are called inert. Each of the electrochemical reactions considered in the Daniell cell involved one solid species and one aqueous species. This is by no means a requirement or even typical. Reactants and products in electrochemical reactions can be solids, liquids, dissolved species, or gases. The evolution of hydrogen gas, for instance,
is a reduction (cathodic) reaction that occurs at an inert electrode. Here, the reactant is dissolved in solution and the product is a gas. The oxidation of iron(II) to iron(III) provides another example:
In this case, both the reactant and product are dissolved species.
In looking at the reactions for the Daniell cell, we note that copper ions gained two electrons, zinc lost two electrons to form zinc ions, hydrogen ions gained one electron each to produce hydrogen gas, and a ferrous ion lost an electron to form a ferric ion. You may be wondering how we know how many electrons will be transferred and the direction in which the reaction will go. The direction depends on potential as we will see in the next two chapters. However, the oxidation and reduction products are determined by the electronic structure of the participating species. You undoubtedly learned about oxidation states in your beginning chemistry class, and those same principles apply here. Let's consider a couple of simple examples just to illustrate the concept. Sodium is an alkali metal and has a single electron in its outer electron shell. Whenever possible, sodium tends to give up that electron, leaving it with a +1 charge and a full outer shell. Thus, the stable state for a sodium ion is Na+. Similar logic can be used for the other alkali metals. In the second column of the Periodic Table you have alkaline-earth metals, such as magnesium or calcium. These elements have two electrons in their outer shell and, therefore, yield ions with a +2 charge such as Mg2+ or Ca2+, using rationale similar to that used for the alkali metals. Moving to the other side of the Periodic Table, we find chlorine that has seven electrons in its outer shell. Chlorine likes to pick up an extra electron in order to complete that shell. Therefore, it forms Cl−. Finally, the electronic structure of some elements (e.g., most transition metals) allows them to form ions with different oxidation states; for example, iron can form Fe2+ or Fe3+.
Fortunately, you don't need to memorize the Periodic Table or to look up the electronic structure of an element every time to determine the relevant oxidation state or half-cell reaction. Tables of known electrochemical reactions are available, and a short list appears in Appendix A of this book. These tables not only show the reactions, but also give the standard potential for each reaction. This standard potential is extremely important as we will see in the next chapter. Note that, by convention, the reactions in Appendix A are written as reduction reactions, but we can treat them as being reversible.
1.3 Importance of Electrochemical Systems
Electrochemical systems are not only essential for our society but are also common in everyday life. Imagine a world without batteries to power your personal electronic devices. How would travel change without low-cost aluminum that is essential for aircraft? What if corrosion of the steel in bridges, the hulls of ships, superstructures of buildings, and pipelines couldn't be controlled? These examples, electrochemical energy storage, industrial electrolysis, and corrosion, are some of the major applications of electrochemical engineering. Following development of the fundamental principles of electrochemical engineering, these and other applications are covered in separate chapters.
We all know that the storage of energy using batteries (Chapters 7 and 8) is an essential feature for countless technologies. The size of the global battery market is more than 50 billion dollars annually. Applications of batteries are widespread in society, ranging from basic consumer electronics, to automobiles, implanted medical devices, and space travel. Energy storage is needed where portability is required, emergency power is desired, or simply when electrical demand and supply are not matched. Batteries store energy chemically and convert that energy to electricity as needed. For many applications, the conversion between chemical and electrical energy must be extremely efficient and, in some cases, nearly reversible in order for batteries to be practical. Electrochemical double-layer capacitors (Chapter 11) can also be used to store energy.
The purpose of industrial electrolysis is to use electrical energy to convert raw materials into desired products. This transformation of raw materials takes place in an electrochemical reactor. Industrial electrolytic processes (Chapter 14) consume about of 6% of the U.S. electricity supply. Although a number of metals and other materials can be produced electrochemically, the main products of electrolysis are aluminum, chlorine, and sodium hydroxide (caustic soda). Low-cost electricity and electrochemical routes for production transformed aluminum from an expensive and rarely used metal to a commodity material that is essential for society. Chlorine and sodium hydroxide, produced simultaneously on a huge scale by electrolysis, are required for the production of a variety of materials, including plastics, paper, soaps, solvents, and a large number of other chemicals.
The many desirable properties of metals have led to their widespread use in industry and, frankly, in nearly every aspect of our lives. Corrosion (Chapter 16) is the unwanted attack of metals by their environment. The manufacture of metals is sometimes done electrochemically as described above for the production of aluminum. We can think of corrosion as the reverse process, by which metals are converted back into their oxide form. This cycle is illustrated in Figure 1.2. The process of corrosion is electrochemical in nature. The global economic cost of corrosion has been estimated to be several trillion dollars annually. Therefore, it is important that engineers understand the conditions under which corrosion is likely to occur. They should also be able to measure, predict, and mitigate the negative impacts of corrosion. Avoidance of corrosion is incorporated into the appliances found in our homes. Additionally, corrosion prevention is essential for maintaining the integrity of the bridges over which we travel, and an integral part of the design of natural gas and oil pipelines that supply our energy. These are just a few of the areas where understanding corrosion is important.

Figure 1.2 Life cycle of metals. Many of the processes are electrochemical.
Electrodeposition is the electrochemical deposition of a metal onto a surface (Chapter 13). Electrodeposition is used for decoration, to provide protective coatings, and is essential in the fabrication of interconnects for large-scale integrated circuits. Most metal fasteners (screws, nails, clips, and bolts to name but a few) are coated for appearance, to improve their corrosion resistance, or to reduce friction. Many of these fasteners use electrochemical processes to provide these coatings.
Roughly 10% of the world's population is afflicted with diabetes. Since treatment requires regular monitoring of blood glucose levels, the measurement of glucose concentration is one of the most frequent tests conducted. Did you know that most of the billions of these tests are done electrochemically? These sensors work by measuring the current generated from electrochemical oxidation of glucose. Modern automobiles use oxygen sensors to allow efficient operation of the fuel injection system. These sensors are also electrochemical devices.
We hope that this section has helped you gain an increased appreciation of the importance of electrochemical systems. In addition to the applications noted above, there are other promising technologies in development. These include fuel cells (Chapters 9 and 10), which convert clean fuels into energy at efficiencies much higher than that of a combustion engine. Fuel cells have been essential for manned space flight, but have yet to achieve widespread commercial success. Semiconductor electrochemistry (Chapter 15) offers opportunities for photoelectrochemical processes to convert sunlight into electricity or to produce useful chemicals through artificial photosynthesis. The list of developing applications extends well beyond these two examples, and electrochemistry will undoubtedly continue to provide new products and processes that will improve our quality of life and benefit humankind.
Before we can examine applications in detail, some fundamental principles must be mastered. These fundamental principles provide the foundation for all of the applications mentioned above. We begin by introducing some basic terminology, as well as reviewing scientific units and the conventions adopted in this text.
1.4 Scientific Units, Constants, Conventions
There are seven base units within the International System of Units (SI system), as shown in Table 1.1 for quick reference. Except for luminous intensity, all of the quantities are used extensively in this text. Among the base units is electric current, I, which is critical for the examination of electrochemical systems. Electric current is measured in amperes [A].
Table 1.1 The Seven SI Base Units; All Except Candela Are Used Frequently in This Book
| Quantity | Name of unit | Symbol for unit | Nomenclature used in text |
| Length | meter | [m] | L |
| Mass | kilogram | [kg] | m |
| Mole | mole | [mol] | ni |
| Time | second | [s] | t |
| Temperature | kelvin | [K] | T |
| Current | ampere | [A] | I |
| Luminous intensity | candela | [cd] | – |
Table 1.2 presents derived units that are used widely in this text and are associated with electrochemical engineering. For each quantity, the name and the SI symbol for the quantity is presented. Our general practice will be to place units in brackets, for example, [Pa-s]. When working problems, dimensional consistency is critical; it will also help you to avoid calculation errors. Therefore, for convenience, the table provides the dimensions of these derived quantities written in terms of both the base units and in an alternative form convenient for calculations. For instance, the units for capacitance [m−2·kg−1·s4·A2] can be written more compactly in terms of other derived units [C·V−1], which will usually be more intuitive and easier to remember when checking for dimensional consistency. Finally, the last column provides the variable symbol used in this text to represent the physical quantity. Where possible, we have used the most common nomenclature to represent these quantities. A detailed list of the nomenclature is provided at the beginning of the book. It is important to note that sometimes the nomenclature for a variable may be the same as the unit. To minimize confusion, remember that variables and fundamental physical constants are in italics, whereas the units will not be italicized. Thus, V represents the potential of an electrochemical cell, whose units are volts [V]. Similarly, A is area [m2], whereas [A] is the unit for current in amperes. Most often we will be dealing with scalar quantities. However, occasionally we will encounter vector quantities, ones that have both magnitude and direction. These quantities will be written in bold face. For example, velocity, a vector quantity, is written with the symbol v.
Table 1.2 SI Derived Units That Are Important for This Text
| Quantity | Name of unit | Symbol for unit | Expressed in SI base units | Alternative expression | Nomenclature used in text |
| Electric charge | coulomb | [C] | A·s | [F·V] | Q |
| Potential | volt | [V] | m2·kg·s−3·A−1 | [A·Ω], [J·C−1] | V, ϕ |
| Capacitance | farad | [F] | m−2·kg−1·s4·A2 | [C·V−1] | C |
| Resistance | ohm | [Ω] | m2·kg·s−3·A−2 | [V·A−1] | RΩ |
| Electrical conductance | siemens | [S] | m−2·kg−1·s3·A2 | [A·V−1], [Ω−1] | S |
| Force | newton | [N] | m·kg·s−2 | – | Fx |
| Pressure | pascal | [Pa] | kg·m−1·s−2 | [N·m−2] | p |
| Energy | joule | [J] | m2·kg·s−2 | [N·m] | E |
| Power | watt | [W] | m2·kg·s−3 | [J·s−1] | P |
| Frequency | hertz | [Hz] | s−1 | – | f |
The coulomb [C] is an SI-derived unit of electrical charge—simply the amount of charge that passes with a current of 1 ampere for 1 second. The elementary charge, denoted with q, is a fundamental physical constant. The value of q is 1.602177 × 10−19 C and equal to the charge of a single proton. Additional essential fundamental constants are provided in Appendix B.
1.5 Faraday's Law
In this section, we examine Faraday's law, which is the relationship between the amount of current that flows through the external circuit and the amount of material that is either consumed or produced in a half-cell reaction. To explore how this works, let's return once again to the zinc reaction:
From the half-cell equation, it is apparent that two electrons are produced for every zinc atom that reacts. Typically, it is more convenient to work in terms of moles rather than atoms. Therefore, two moles of electrons are produced for every mole of zinc atoms that is oxidized. As you can see, we can easily relate the moles of electrons to the moles reacted or produced for any species in a given half-cell reaction.
The next step is to relate the moles of electrons to the current that we measure through the external circuit. To do this, it is customary to introduce a new unit of charge—an equivalent. An equivalent is defined as a mole of charge (either positive charge or negative charge, it does not matter). The number of equivalents of a compound is simply the amount of the substance (in moles) multiplied by the absolute value of its charge, zi. For instance, one mole of Na+ is 1 equivalent, whereas one mole of Ca2+ would be 2 equivalents. Because the sign of the charge does not matter, one mole of electrons is equal to 1 equivalent of charge.
The external current is expressed in amperes [A or C·s−1]. Therefore, we need a relationship between coulombs [C] and equivalents. That relationship is called Faraday's constant, F, which has a value of 96,485 C/equivalent. Faraday's constant can also be expressed in terms of two other constants: the fundamental unit of charge, q, and Avogadro's number, NAV,
This expression is another way of stating that an equivalent is a mole of charge, since q is the amount of charge on a proton in coulombs. We also need a relationship between the current, I [A], and the total charge passed in coulombs. That relationship is
where Q is the charge in [C], I in [A], and t in [s]. In situations where the current is constant, Equation 1.8 simplifies to
We now have the pieces that we need to write the relationship between the current in the external circuit and the amount of a substance that is either reacted or produced. Let's apply what we have learned to the zinc electrode.
Let's now generalize what we have done to this point. Any half-cell reaction can be expressed in the following form:
where ![]() is the stoichiometric coefficient for species i, which can be either positive or negative, Ai is the symbol for the specific species (e.g.,
is the stoichiometric coefficient for species i, which can be either positive or negative, Ai is the symbol for the specific species (e.g., ![]() ), and n is the number of electrons involved in the reaction. The mass of species i that is either consumed or produced is equal to
), and n is the number of electrons involved in the reaction. The mass of species i that is either consumed or produced is equal to
where ![]() is the molecular weight of species i. This equation is known as Faraday's law. We have been careful to include the stoichiometric coefficient with Equation 1.11a. However, most often Faraday's law is written as
is the molecular weight of species i. This equation is known as Faraday's law. We have been careful to include the stoichiometric coefficient with Equation 1.11a. However, most often Faraday's law is written as
We will also use this form. When using Equation 1.11b, you need to recognize that n represents the number of electrons per species i. Look carefully at the examples that follow.
Although Equation 1.11 is useful, the most important aspect of this section is the process that we used to develop the equation. We started with charge, Q, in standard units of [C]. This charge is directly related to the current that we measure in [A] as a function of time. We then converted that charge to moles of electrons (equivalents). The moles of electrons were then related through the half-cell reaction to the moles of species i that were either reacted or produced. Finally, we used the molecular weight to convert moles to the desired mass of species i.
A similar procedure can be used to calculate quantities such as the total moles reacted, the molar rate of reaction, or the molar flux due to reaction at the surface. The use of Faraday's law and the more general procedure used to derive it are demonstrated in Illustration 1.3.
Finally, we note that the reactants and products may be charged or neutral. They can also take several forms, such as a species dissolved in the electrolyte, a solid that deposits on the electrode, or a gas. Illustration 1.3 involved a solid reaction product, PbSO4(s). Now let's consider the oxidation of chloride ions in solution to form chlorine gas:
Two chloride ions from solution each give up one electron and they combine at the metal surface to form the gas.
1.6 Faradaic Efficiency
The electrochemical reactions that we have been discussing are called faradaic reactions since they involve electron transfer that is directly related to the consumption of reactants and the formation of products as described by Faraday's law. To this point, we have only considered one reaction at each electrode. However, it is possible, and in fact common, for multiple reactions to take place at a single electrode in a real system. Frequently, one of the reactions is the desired reaction, and the other reactions are referred to as side reactions. In such cases, not all of the current goes into the desired reaction. We can define a faradaic efficiency to characterize the fraction of the total current that drives the desired reaction.

Use of this efficiency is demonstrated in Illustration 1.5.
1.7 Current Density
To this point in the chapter, we have considered the total current, I. However, as you know from your experience, some electrochemical devices are small like a hearing aid battery. Others are significantly larger, such as the battery used to start your car. You would not expect the total current from these devices to be similar. Because electrochemical reactions take place on surfaces, we frequently normalize the total current by the surface area in order for us to better understand and characterize the system. Current density is defined as the current divided by the area of the electrode and will be used extensively throughout this book. Similarly, the molar rate of reaction is often expressed as a flux, [mol·m−2·s−1]. These molar fluxes are used extensively in studying mass transfer. Let's illustrate these concepts with an example.
Strictly speaking, molar flux, and therefore current density, are vector quantities—ones that have both a magnitude and direction. The current density, i, is defined as
where Ni is the molar flux [mol·m−2·s−1] and zi is the electrical charge of species i. This equation indicates that for current to flow, there needs to be a net movement of charged species. We will only occasionally need to treat these quantities as having more than one directional component; therefore, our practice will be to not make any special effort to identify these quantities as vectors unless needed. However, even for one-dimensional current flow, we need to pay attention to the direction, which of course depends on how our coordinate system is defined. Referring back to Illustration 1.4, the flux of chloride ions is from right to left, which is a negative flux (in the negative x direction). The charge of the chloride ion, zi, is also negative. Therefore, current flow is positive in that example.
1.8 Potential and OHM'S Law
Another important quantity in the study of electrochemical systems is the potential. We can define an electrostatic potential, ϕ, in terms of the work required to move a unit (positive) charge from infinity to a specific position in the metal or in solution. This work can also be thought of in terms of energy. The unit for potential is a volt [V], which is also [J·C−1]. Since we are frequently interested in the potential of the electrodes, and since these are generally metals where electrons are the carriers of current, we can think of potential as a measure of the energy of the electron in the metal. Thus, the cell potential measured with a voltmeter in Figure 1.1 represents the difference in electron energy in the two electrodes.
As you may have guessed, the flow of current in metals and in electrolyte solutions is critical to the understanding and analysis of electrochemical systems. A potential difference in a metal or in an electrolyte leads to the flow of current. The difference is often expressed in terms of a potential gradient, which is the rate of change in potential with distance. By convention, electric current is defined as flowing from high potential to low potential, which is the direction that a positive charge would move due to the difference in the potential. This situation is illustrated in Figure 1.3. Current in a metal (e.g., wire) is due to electron flow. Since electrons are negatively charged, electrons travel from low potential to high potential. Ohm's law relates the current and potential. For one-dimensional conduction (current flow),
where κ is the electrical conductivity of the electrolyte. The electrical conductivity is a property that characterizes how well the material moves electrical charge; it has units of [S·m−1]. If the current density is constant, Equation 1.15 can be integrated to give
This equation applies to one-dimensional current flow in an electrolyte of constant composition. A similar expression also applies to metals in general.
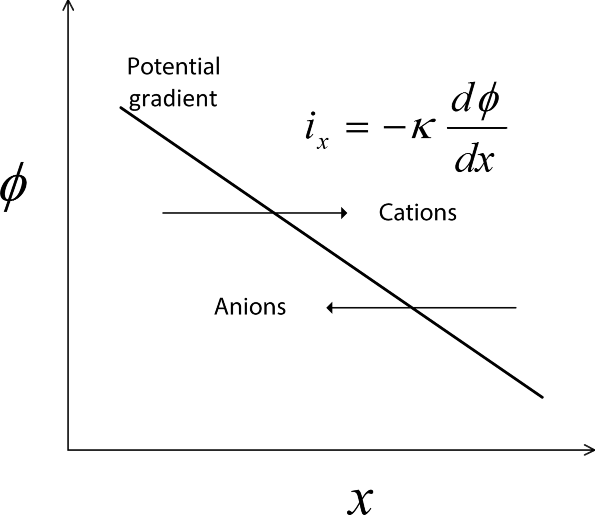
Figure 1.3 Gradient in potential and flow of current. Current flow is from left to right.
1.9 Electrochemical Systems: Example
We end this chapter with an example of an industrial electrolytic process—chlorine production by the chlor-alkali route. Our objectives in this section are twofold. First, to review principles and terminology within the context of an important electrochemical system. Second, to introduce the concept of the current–voltage relationship, also called the I–V curve. The connection between current and voltage is essential to any analysis of electrochemical systems. Finding and understanding this I–V relationship is a persistent theme of the book.
A simple representation of the electrochemical system for the chlor-alkali process is shown in Figure 1.4. The overall reaction can be written as
In order to operate without interruption, reactants are supplied and products removed continuously. The reactant stream for the process is a purified brine of sodium chloride (NaCl), essentially a concentrated solution of salt water. The solution flows at a steady rate into the electrochemical cell, also called the electrochemical reactor. In addition to chlorine gas, sodium hydroxide and hydrogen are produced simultaneously in the process. Despite the appearance from the above equation, this reaction does not occur homogeneously. Instead, two heterogeneous electron-transfer reactions take place on separate metal surfaces:
The two metals on which the reactions occur are the electrodes, which are inert since they do not participate in the reactions. The electron-transfer reactions are faradaic processes and are governed by Faraday's law. Oxidation takes place at the first electrode of our example, where two chloride ions lose electrons to form chlorine gas. Therefore, this electrode is the anode. The electrons enter the metal and travel through an external circuit. At the other electrode, the cathode, water is reduced to form hydrogen gas. The electrons consumed at the cathode are supplied by the external circuit, and the number of electrons produced at the anode is the same as the number consumed at the cathode. The electrolyte that is between the two electrodes contains ions of Na+, Cl−, and OH−. In this instance, there is a membrane separator that serves two functions: It keeps the two gaseous products apart and it allows sodium ions to move from the anode compartment to the cathode compartment, but excludes transport of anions. It is this movement of sodium ions that carries the current in the electrolyte. Note that there are three phases: solid (electrodes), liquid (electrolyte), and gas (reaction products). Furthermore, not all species are present in every phase. For instance, free electrons are found only in the metals, ions are confined to the electrolyte, and hydrogen gas is limited to the cathode. This heterogeneity is one of the defining characteristics of electrochemical systems—Almost all of the action occurs at interfaces between the electrode and the electrolyte.

Figure 1.4 Electrochemical cell for the chlor-alkali process.
As you are probably beginning to appreciate, electrochemical systems are inherently complex. We've already seen that both electrodes are necessary and must be considered together. Many things happen at once: electron transfer, adsorption and desorption, transport of reactants and products, current flow in the electrolyte, and surface-mediated combination. In order to understand and analyze electrochemical systems, we'll need to simultaneously apply our knowledge of thermodynamics, kinetics, and transport. These fundamental topics will be considered systematically in the chapters that follow.
Electrochemical cells are sometimes broken into two categories: galvanic and electrolytic. The two types have more similarities than differences, and are governed by the same engineering principles. The main distinction between them is that energy or electrical work is an output of a galvanic cell (e.g., a fuel cell or a battery discharging), whereas electrical energy is an input to electrolytic cells. We see in Figure 1.4 that a direct current (DC) power supply adds electrical work in the chlor-alkali process; therefore, this process is electrolytic.
An important feature of electrochemical systems is that current is an expression of the rate of reaction. In our example, the electrical current through the cell is directly related to the formation and consumption rates of hydrogen, chlorine, and sodium chloride through Faraday's law. We can control the rate of reaction by controlling current. How much work is associated with this current flow? To answer this question, we need to know the potential of the cell. The potential is an expression of energy for charged species (electrons and ions), and provides the driving force needed for electrochemical reactions and for the flow of current in both metals and electrolytes.
We cannot simultaneously control the potential and current of an electrochemical system. If the potential applied to our electrolysis cell is increased, then the rate of hydrogen/chlorine production will also be increased. Alternatively, we might establish the rate of production by setting the current, but we would need to accept the resulting potential. These are the two basic modes of operation for electrochemical cells: potentiostatic (constant potential) or galvanostatic (constant current).
As mentioned above, a key objective in the analysis of any electrochemical system is to find the relationship between current and potential. The relationship can be complex, but with careful examination of the thermodynamics, kinetics, and mass transport, it can be readily understood. In the first chapters of this book, we will develop the fundamental principles needed. These will systematically be applied to a number of electrochemical systems, and we will return repeatedly to the relationship between the current and voltage as characterized by the I–V curve. Much of this textbook will be dedicated to the development and application of the relationships that describe the current (the reaction rate) as a function of potential (the driving force). You already know one such relationship, the resistor. Using an equivalent form of Ohm's law (Equation 1.15), we can relate the current and potential for a resistor. If the current density is constant, Equation 1.16 can be written in terms of resistance to give
This equation shows a linear relationship between potential and current density. However, the current–voltage relationship is generally more complex for an electrochemical system, and we will need to use thermodynamics (Chapter 2), electrode kinetics (Chapter 3), and mass transfer (Chapter 4) to describe it. Here, we simply want to introduce some of the basic features of such a curve.
An example of a current–voltage curve is shown in Figure 1.5 for steady-state operation. First, note that when the current density is zero, the potential of the cell is not zero. If the external circuit is open (disconnected), no current flows and we say that the cell is at open circuit. This open-circuit potential is largely determined by thermodynamic considerations and depends on the type of electrode and the composition of the electrolyte near the electrodes. For the chlor-alkali cell, it is about 2 V, as indicated in the figure. This equilibrium potential is the subject of Chapter 2. If the external circuit were simply closed (connected) without a DC power supply, current would flow. However, the reactions would be in the direction opposite to that indicated by Equation 1.17. In other words, chlorine, hydrogen, and hydroxyl ions would spontaneously react to form chloride ions and water, and the potential of the cell would be lower than the equilibrium potential. This situation corresponds to using the chemical energy of the reactants to perform work, and corresponds to a galvanic cell. The difference between the equilibrium or open-circuit potential and the actual cell voltage is called polarization (see Figure 1.5).
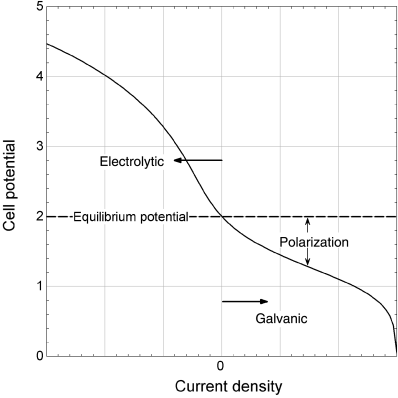
Figure 1.5 Representative relationship between current and potential at steady state. The dividing line between galvanic and electrolytic operation is at a current density of zero.
Of course, this is not how a chlor-alkali cell is intended to operate. We want to produce chlorine gas and caustic soda. By the addition of work, we can drive the reaction in the opposite direction, the direction indicated in Equation 1.17. To do this, the cell potential must be greater than the equilibrium potential. Because we are adding work to the cell, we refer to the cell as an electrolytic cell. As shown in Figure 1.4, a voltage source is added to the chlor-alkali cell in order to provide the electrical work needed to drive the reaction in the desired direction. The difference between the cell potential and the equilibrium potential is a measure of how much work or energy must be added to drive the reaction at a particular rate.
Returning to Figure 1.5, which shows the I–V curve for the complete electrochemical cell, we have seen that an electrochemical cell has a nonzero potential at open circuit, where the current is zero. This potential is described by thermodynamics and represents the difference in the energy of electrons in the two electrodes. Operation of the cell in a galvanic mode takes place at cell potentials below the equilibrium potential, and corresponds to the conversion of chemical energy into electrical energy. In contrast, operation of the cell in an electrolytic mode occurs at potentials greater than the equilibrium potential, and requires that energy be added to the cell. In both cases, the difference between the equilibrium potential and the cell potential during current flow is called the polarization. As shown in the figure, the relationship between current and cell potential is, for the most part, nonlinear. The details will be covered in subsequent chapters. Also note that the curve is not symmetric. Although the basic shape of the curve under galvanic operation is similar to that of the electrolytic curve, the details are different.
Closure
In this chapter we have introduced the electrochemical cell and electrochemical systems. Common terminology for electrochemical systems has been presented, and will be used throughout the book. One of the most fundamental concepts, Faraday's law, was discussed. This law relates electrical charge to the amount of reactants consumed or products produced in an electrochemical reaction, and is essential for the analysis of electrochemical systems.
Further Reading
- LeFrou, C., Fabry, P., and Poignet, C. (2012) Electrochemistry: The Basics with Examples, Springer Berlin.
- Pletcher, D. (2009) A First Course in Electrode Processes, Royal Society of Chemistry, Cambridge.
- West, A.C. (2013) Electrochemistry and Electrochemical Engineering: An Introduction. CreateSpace Independent Publishing Platform.
Problems
1.1. The original “International Ampere” was defined electrochemically as the current required to deposit 1.118 mg of silver per second from a solution of silver nitrate. Using this definition, how does the international ampere compare to the SI version? (Note that the SI version is based on the Ampere force law).
1.2. Molybdenum is deposited from a molten salt. 12.85 g are deposited in 1 hour using a current of 7 A. How many electrons are consumed per mole of Mo reacted? Given that Mo is present in the molten salt as an ion, what is its oxidation state in the molten salt?
1.3. How much hydrogen is needed to operate a 50 kW hydrogen/oxygen fuel cell for 3 hours? The potential of the cell is 0.7 V (remember that Power = IV). The reaction at the anode is
1.4. Calculate the daily aluminum production of a 150,000 [A] aluminum cell that operates at a faradaic efficiency of 89%. The cell reaction is
1.5. The annual production of Cl2 is about 45 million tons per year. Assume that a typical plant is operational 90% of the year. The operating voltage of a cell is 3.4 V (considerably higher than the equilibrium voltage).
- Write down the half-cell reaction for the oxidation chloride to form chlorine.
- Determine the total current worldwide needed to generate the global supply of Cl2.
- Calculate the electrical power needed to produce the global supply of chlorine using electrolysis.
1.6. A 25 A current is passed through a molten aluminum chloride melt. What are the likely reactions at the two electrodes? How long must this current be in place to deposit 50 g of Al. During this same time, what is the volume of gas that is evolved at STP (standard temperature and pressure: 273 K, 100 kPa).
1.7. An industrial aluminum cell is operating at 4.2 V with a current of 200 kA. The faradaic efficiency is 95 %, which means that 95% of the current goes to Al production. What is the rate of production of Al in kg per day? The starting material is Al2O3. As an aside, a Boeing 747 aircraft is made from over 60,000 kg of Al.
1.8. Continuous sheet copper is made by electrodeposition from a solution containing CuSO4 onto a rotating drum of lead. For the conditions given below, what should be the rotation speed of the drum (revolution per hour)?
- Cathode current density = 1750 [A·m–2]
- Current efficiency = 95%
- Desired “thickness” (mass per area) = 1.22 [kg·m–2]
- Angle of cathode immersion = 165°
1.9. How many grams of lithium are in a 1320 [mAh] cell phone battery? Note that [mAh] is a unit of charge.
1.10. A plate of steel has lost 50 g to corrosion over the past year. Determine the current that would be associated with this rate of corrosion?
1.11. Corrosion of stainless steel in concrete is an important engineering problem. Because of the long times associated with the low rates of natural corrosion, accelerated testing is often used. One form of accelerated testing is the application of a potential to the metal of interest. However, under the accelerated conditions, the current efficiency may be low. In competition with the oxidation of iron to Fe(II), oxygen can be evolved at high pH:
A current of 1.4 mA is passed for 100 hours and the mass loss is 0.11 g. What is the faradaic efficiency for the iron oxidation reaction? How many moles of oxygen are evolved?
1.12. Consider a nickel–zinc battery operating at a current density of 4500 A·m−2. The space between the electrodes is filled with an alkaline electrolyte. If the conductivity of the electrolyte is 60 S·m−1 and the distance between the two electrodes is 2 mm, what is the potential drop across the cell due to ohmic losses?