3.2
PHYSICAL PROPERTIES
All substances have two major types of properties—chemical properties and physical properties. The chemical properties of a substance determine how it combines with other substances. For example, combustion is a chemical reaction between a fuel and oxygen. Physical properties involve changes in substances that don’t represent a chemical change in a substance. In general, the major difference between chemical and physical changes is that physical changes are reversible.
The physical state of a substance, called its phase, describes whether it is a gas, liquid, or solid. This state has a big effect on energy trading. The physical phase of fuel determines how it must be transported and what type of engine has to be built to burn that fuel. Controlled state changes, like the ones that occur in distillation or air conditioning systems, are the key to many processes in the energy markets. Uncontrolled state changes, like condensation, can be very destructive.
The reason that state changes are important is that solids, liquids, and gases each have different physical properties. Solids and liquids generally stay put—you can place either in a cup and they won’t immediately disappear. This isn’t the case with gas—any time a gas isn’t contained, it immediately dissipates. Also, when gas is compressed, it gets hot and radiates heat into the environment. When gas is allowed to expand, it cools down and absorbs heat. For example, gas will need to be cooled substantially if you try to compress it enough to turn it into a liquid. Even then, if liquefied gas isn’t kept cool and prevented from absorbing heat, it will start to expand again.
States of Matter and Separation
Energy products typically need to be purified prior to being used. This usually means separating out one piece of a raw material from everything else. Examples of separation processes are extracting gasoline from crude oil or removing ethanol from an ethanol/water mixture.
There are three primary states of matter under normal conditions—solid, liquid, and gas.1 As temperature of a solid material is increased, it does one of two things. Usually, it will transition sequentially from a solid to a liquid to a gas, or it will undergo a chemical transformation. Exactly what happens will depend on the substance being heated. A common example of physical state transformation is ice being melted to form water, and then further heated to turn into steam. An example of a chemical reaction is cooking a piece of meat. A state change can be reversed (water can be frozen to form ice), but a chemical reaction is generally a one-way transition (once one’s dinner is burnt, cooling it down won’t help).
The ability to transition certain substances between liquid, solid, and gas states plays an important role in energy trading. There are discrete temperatures at which a substance will transition from one phase to another. The two most important temperatures are the boiling point (where liquid/gas transitions occur) and the melting point (where solid/liquid transitions occur). By heating or cooling a mixture of certain substances, it is possible to separate them by carefully controlling the temperature. Each substance has a unique boiling point and melting point that aren’t shared with other substances. Because these points are unique, when a mixture is slowly heated, substances will melt off or vaporize one at a time.
In practice, it is fairly common for most mixtures to undergo both chemical reactions and physical state changes in response to changes in temperature. It is also possible under some conditions for substances to convert directly from solids into gases.
• Solid. In a solid state of matter, the molecules of a substance are closely packed and hold fixed positions relative to one another. As a result, the volume of a solid is constant unless altered through application of sufficient physical force. Application of sufficient physical force to deform a solid substance will result in a permanent deformation.
• Liquid. A liquid is a fluid where molecules are loosely arranged. A liquid is characterized by a distinct boundary at its edges that will hold molecules into the main mass of the substance. This surface tension is not sufficiently strong to prevent liquids from freely deforming in shape, but it will hold the bulk of the liquid in place. Liquids conform to the shape of the container in which they are stored but are not permanently deformed. When a liquid is moved to a new container, it will change to conform to that shape. The volume of a liquid is determined by its temperature and pressure.
• Gas. In a gas, molecules are almost completely independent. As a result, gases are much less concentrated and have a much lower density than either liquids or solids. Like a liquid, the volume of gas is determined by temperature and pressure. It is relatively easy to heat or cool gas by compressing it or allowing it to expand.
Distillation
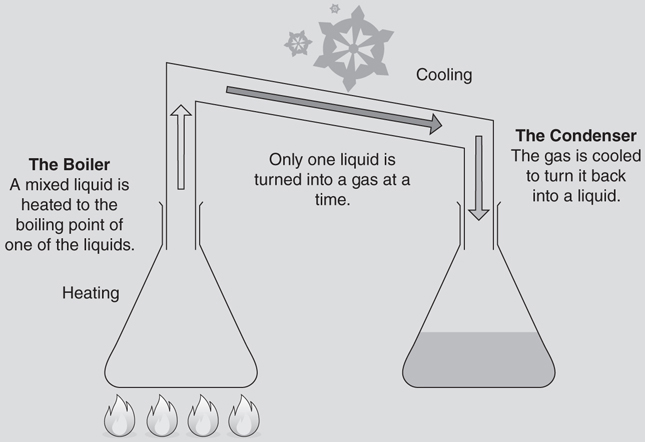
Distillation is the process of heating a liquid mixture until it starts to boil and then capturing and cooling the resulting gas. It is a common method of separating liquids and identifying unknown hydrocarbon liquids. Distillation is a physical process that does not rely on chemical reactions. Instead, it takes advantage of each substance having a unique boiling point to separate mixtures.
There are two major pieces in distillation units—a boiler and a condenser. The boiler is a container used to heat up the mixture to the boiling point of one of the liquids. At the proper temperature (the desired boiling point), one (and only one) of the liquids will turn into a gas and rise into the tubing to exit the boiler. At that point that gas (now a purified substance) is cooled in the condenser to be turned back into a liquid.
When a liquid reaches its boiling point, it will stay at that temperature until it is fully converted into a new state of matter. For example, if water is boiled, as long as the water vapor can escape, the liquid will stay at a constant temperature. Continuing to heat boiling water won’t make the water hotter; it will just cause a faster liquid-to-gas conversion. Distillation is important because this is the primary method for separating a mixture of fuels into pure products.
Physics of Gas
Another place that science affects trading is in the natural gas markets. Unlike solids or liquids, which have relatively constant volumes, the volume occupied by a gas can change dramatically in response to outside conditions. These changes in volume affect both the pressure and temperature of the gas. This can be very important since temperature could cause the fuel to change between gas and liquid states, spark a chemical reaction, or separate from some substance with which it is mixed.
Unless constrained, gases will expand to fill whatever is containing them. Gas spreads from areas of high pressure into areas of low pressure and will continue to move until the pressure is equalized. The primary way of moving gases is to create a pressure difference between two areas. Both a straw and a gas pipeline work by this phenomenon. For example, it is possible to create a vacuum by sucking on one side of a straw. Gas will move into and through the straw to balance out the pressure. The higher the difference in pressure, the faster the gas will move through the straw.
Things get more complicated when temperature is introduced into the equation. The temperature, pressure, and volume of a gas are all interrelated. It is impossible to change one quantity without affecting the other quantities. Compressing a gas will raise its temperature and allowing it to disperse will lower its temperature. Fuels like methane are rarely pure methane. There will be a large number of trace gases also in the mix. Temperature changes can be especially important if the temperature of the gas cools below the boiling point of some gas in the mixture. If that happens, liquids will start to accumulate in a pipeline.
At a layman’s level, the relationship between temperature, pressure, and volume of a gas can be approximately described by the ideal gas law (Figure 3.2.2). This law applies to gases where collisions between molecules are perfectly elastic, and is a reasonable approximation of the most important relationships described in the chapter. For example, if temperature rises (the right side of the equation gets bigger) and volume is held constant, the pressure needs to increase (because the law is an equality, if the right side of the equation gets larger, the left side needs to get larger too).
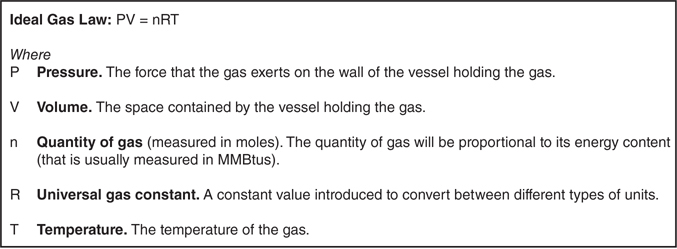
Figure 3.2.2 The ideal gas law
Gas Pipelines
Gas is often moved through pipelines through the use of compressors (Figure 3.2.3). These compressors operate like a fan—they create a vacuum on one side and a high pressure area on the other. Since compressors affect the pressure of the gas, they will also affect its temperature. A pipeline is a long chain of compressors linked by pipes and provides perfect conditions for corrosion.
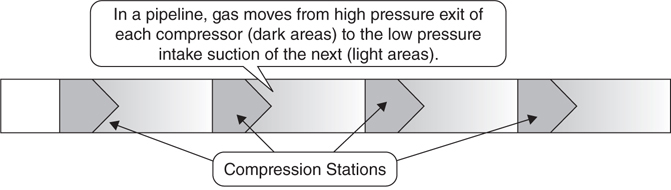
Figure 3.2.3 Compressor stations
When a gas moves between pipe segments, it passes through a compressor unit that alters the pressure of the gas. As a result of the increase in pressure caused by the compressor, the temperature of the gas will increase. If any part of the gas is chemically reactive, corrosion becomes more likely. When gas moves away from the high pressure area toward the next compressor, it will start to cool. This might cause some of the gas to condense into a liquid. This causes issues because pipes are air-tight and the gas won’t evaporate. Unless it is removed, a substantial amount of liquid might eventually build up in the pipe (Figure 3.2.4).

Figure 3.2.4 A simple pipeline
Air Conditioning and Refrigeration
One of the major consumer uses of energy is to maintain comfortable living and working environments. Heating techniques have existed for thousands of years. However, air conditioning didn’t come into popular use until the twentieth century.
Air conditioning and refrigeration have revolutionized modern culture—but they are incredible energy hogs. Cooling systems are based on physical state changes. The key to any cooling system, whether it is a household air conditioner or an industrial natural gas liquefaction facility, is finding a chemical that can easily transition between liquid and gas phases. This chemical (the working fluid) is turned into a gas to cool it down (so it can absorb heat from something) and then compressed (to heat it up) so that it can radiate the heat somewhere else.
As pressure increases, the temperature of a gas increases. When pressure drops, the temperature of a gas will decrease. The core of an air conditioner is a mechanical device that compresses the fluid before it goes outside (to raise its temperature) and decreases the pressure of fluid when it comes inside (to lower its temperature).
There are three main parts to a cooling unit—a compressor, a condenser, and an evaporator. The compressor and condenser are typically located outside and the evaporator inside. The working fluid circulates between these three units (Figure 3.2.5).
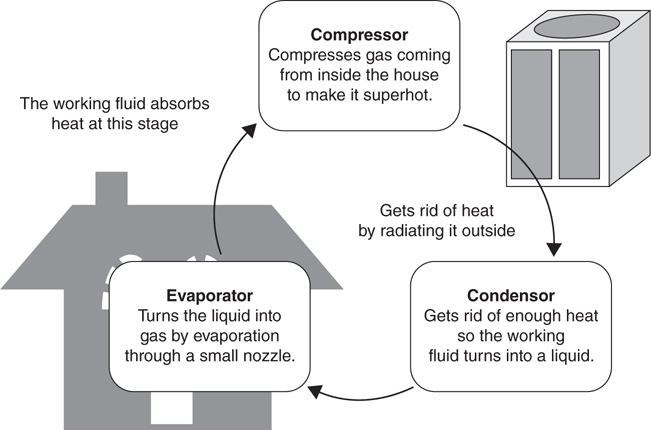
Figure 3.2.5 The air conditioning cycle
Stage 1: Compressor. When gas leaves the interior of the house, it is pulled into a compressor. The compressor takes the warm gas from inside the house and compresses it to make it into a very hot gas. Compressing the working fluid increases its temperature substantially. When the superhot fluid is exposed to outside air, it will cool down by radiating heat into the environment.
Stage 2: Condenser. The mechanism that actually radiates heat into the environment is called the condenser. The condenser is a set of metal fins that radiate heat into the outside environment. Typically, a fan forces outside air over the fins, cooling the working fluid. When the working fluid cools down enough, it turns into a liquid and is sent inside the house.
Stage 3: Evaporator. The liquid that is sent inside is still fairly warm—it’s at least as warm as the outside air temperature. It will need to be further cooled if it is to absorb heat from inside the building. To do this, the working liquid is forced through a narrow hole into a low pressure container called an evaporator. Liquids boil more easily at low pressures. As the liquid converts into a low pressure gas, it absorbs heat. The working liquid, now a cold gas, is then free to absorb heat from the interior environment. After warming up, the working liquid is sucked into the compressor to start the cycle again.
1 Under nonstandard conditions, there are a number of other states of matter that are not being included in this discussion. These can run from Bose-Einstein condensates, which exist in the center of black holes, to plastic crystals like glass, to superheated ionized gases called plasma. While these states are all important under some conditions, they are all much less common than the primary three phases.