Watch über-active fluorine gas form stable compounds so,
Like Teflon’s nonstick CF2s, but CFCs must go!
Let fluoride help strong teeth to make,
On “would-be drugs” watch F (so small!) new pharma-traits bestow.
Watch über-active fluorine gas form stable compounds so,
Like Teflon’s nonstick CF2s, but CFCs must go!
Let fluoride help strong teeth to make,
On “would-be drugs” watch F (so small!) new pharma-traits bestow.
THE FIRST MEMBER OF THE GROUP 17 HALOGENS
Fluorine surveys its fellow halogens from its vantage point atop group 17 of the periodic table. In elemental form fluorine is a highly toxic1, pale yellow gas (F2) that’s also the most reactive of all elements. Yet, after reacting, many of the compounds it forms are particularly stable.
see-eff-twos see-eff-sees
A prime example of a stable, fluorine-based compound is polytetrafluoroethylene (PTFE): a polymer that consists of long chains of CF2 units and is better known as Teflon. Teflon’s constituent C–F bonds are so inherently stable and inert that they make a highly thermostable, waterproof, and corrosion-resistant polymer. In fact, its stability is such that practically nothing will stick to it; small wonder, then, that it’s used as the non-stick coating on frying pans!
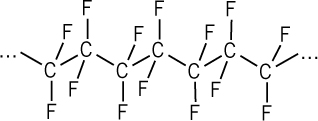
A segment of Teflon polymer showing the chain of CF2 units
Fluorine-containing chlorofluorocarbons (CFCs) are highly stable compounds. They were once ubiquitous as refrigerant gases in air conditioners and refrigerators and as propellant gases in spray cans. They’re now banned because they persist so long in the environment that they damage Earth’s fragile ozone layer (see “Oxygen, O” on page 23 for more information on the ozone layer).
Fluoride, F–, is the negatively charged (or anionic) form of fluorine. It helps prevent dental cavities by converting the calcium phosphate in tooth enamel (as hydroxyapatite, Ca5(PO4)3OH) to the harder, more acid-resistant fluorapatite, Ca5(PO4)3F. Fluoride is widely used in toothpastes and in some countries is added to municipal water supplies.
aytch-eff
Hydrofluoric acid, informally known as HF, is an aqueous solution of gaseous hydrogen fluoride (HF). Despite being a weaker acid than other halogen acids, such as hydrochloric and hydrobromic acid, hydrofluoric acid is extremely hazardous. Hydrogen fluoride’s small molecules can penetrate the skin, whereupon the fluoride ion (F–) promptly binds to the calcium and magnesium ions (Ca2+ and Mg2+, respectively) so essential to the normal function of the body’s cells. As a result, cells die, ultimately causing body tissue to liquefy and bone matter to waste away. In addition, the acute loss of calcium ions can cause the heart’s muscle to fail, triggering cardiac arrest. Hydrofluoric acid burns can be fatal, even when they cover as little as 2.5 percent of the body’s surface area (about the size of the sole of your foot). Treat hydrofluoric acid with the utmost respect and always handle it with extreme care!
eff
Fluorine is present in approximately 20 percent of all pharmaceuticals. Interestingly, the performance of some prospective drug candidates has been enhanced simply by strategically replacing one or more hydrogen atoms in their chemical structure with fluorine. The reason for this improvement varies. Sometimes the new fluorine atoms improve the drug molecule’s binding ability at the active site of some target enzyme; other times, they prevent the drug molecule from binding to the active sites of enzymes that would otherwise destroy it.