Tellurium, you’ve help’d improve steel’s work-ability,
But tell us ’bout your garlic breath . . . di-CH3 Te!
Your solar cells catch lots of rays,
Your minibars chill chardonnays.
Your weight is more than I next door; ’midst anode muds you’ll be.
Tellurium, you’ve help’d improve steel’s work-ability,
But tell us ’bout your garlic breath . . . di-CH3 Te!
Your solar cells catch lots of rays,
Your minibars chill chardonnays.
Your weight is more than I next door; ’midst anode muds you’ll be.
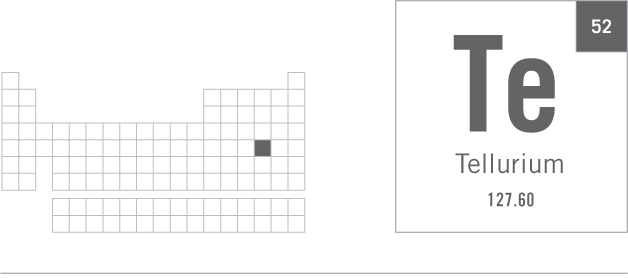
GROUP 16 METALLOID
Tellurium is a very rare element that exists as either a metallic-looking, silvery-gray solid or as a dark gray powder. One of its more prominent uses is as an additive to stainless steel, helping to make the steel more workable and amenable to being machined.
dye-see-aytch-three-tee-ee
Tellurium’s name comes from the Latin word tellus meaning Earth. Some of tellurium’s compounds are volatile and are even more malodorous than the corresponding foul-smelling compounds of its “upstairs neighbors,” selenium and sulfur. If you ingest tellurium, your body metabolizes some of the absorbed element to produce the volatile organometallic compound dimethyl telluride, (CH3)2Te, which smells like garlic when you exhale!
Tellurium is used to manufacture cadmium telluride (CdTe) solar panels. Thin films of CdTe provide the semiconductive layers that absorb and then convert sunlight to electricity.
Passing an electric current through an arrangement of semiconductor thermocouples made from bismuth telluride (Bi2Te3) produces a cooling effect without the need for refrigerant fluids or moving parts. This thermoelectric phenomenon (known as the Peltier effect) provides the refrigeration source for some of those cute little fridges you find in hotels.
eye
[’midst: preposition, short for “amidst” — in or into the middle of]
The atomic weight of a given element is usually less than that of the element immediately after it on the periodic table. Tellurium is one of the few elements to buck this trend: its atomic weight (127.60) is more than that of iodine (126.90), which comes immediately after it.7 The reason is that the abundance of tellurium’s eight naturally occurring isotopes is skewed in favor of tellurium-130 and tellurium-128. These account for 66 percent of all tellurium atoms. In contrast, most iodine atoms exist as the iodine-127 isotope.
Tellurium is a very rare element. It languishes in 72nd place on the list of natural abundances for elements and is present at only five parts per billion (ppb) in Earth’s crust. But it’s found at a concentration of up to 8 percent in the muds generated at the anode electrode during the electrolytic refining of copper, thus facilitating its commercial availability.