Contingent negative variation and P3 modulations following mindful movement training
Stefano Lasaponaraa,b,c; Joseph Glicksohnd,e; Federica Mauroa,f; Tal Dotan Ben-Soussana,* a Research Institute for Neuroscience, Education and Didactics, Patrizio Paoletti Foundation for Development and Communication, Assisi, Italy
b Department of Neuropsychology, IRCCS Fondazione Santa Lucia, Rome, Italy
c Dipartimento di Scienze Umane, Università LUMSA, Roma, Italy
d The Leslie and Susan Gonda (Goldschmied) Multidisciplinary Brain Research Center, Bar-Ilan University, Ramat-Gan, Israel
e Department of Criminology, Bar-Ilan University, Ramat-Gan, Israel
f Department of Psychology, Sapienza Università di Roma, Roma, Italy
* Corresponding author: Tel.: + 39-3938897774 email address: [email protected]
Abstract
In the study of the electrophysiological correlates of attention, a phasic change in alertness has been classically related to a negative frontal-central shift called Contingent Negative Variation (CNV). Studies investigating the effects of meditation on the CNV in participants reporting frequent transcendental experiences (TE) reported reduced CNV in choice reaction time task (CRT), and increased CNV in simple reaction time task (SRT), suggesting that meditation can induce a more balanced attentional state. In the current study, we tested whether a similar effect could be obtained in healthy non-meditators using a single session of a specifically structured sensorimotor training (Quadrato Motor Training—QMT). In addition, in contrast to previous studies, we further examined the P3 component, reflecting cognitive load and novelty detection. We found that similar to previous studies, following a QMT session, CNV amplitude reduced in CRT and increased in SRT. Conversely, the P3 amplitude increased in CRT and decreased in SRT. Taken together, these results support the idea that QMT has attentional benefits in normal healthy participants, similar to those observed in experienced meditators.
Keywords
CNV; P3; Sensorimotor training; Attention; ERP
1 Introduction
In the study of attention, a warning stimulus (S1) preceding a second imperative target signal (S2) produces both a phasic change in alertness, i.e. the ability to increase and maintain attentional allocation toward an impending stimulus (for a review see Petersen and Posner, 2012), as well as a warning-induced reduction in reaction time (RT) for S2. At the electrophysiological level, this phasic change in alertness has been indexed by a negative shift occurring in the EEG on the frontal-central derivation, first reported by Walter et al. (1964), termed the Contingent Negative Variation (CNV). Typically, the early phase of CNV, measured in the 500–800 ms window after S1, is thought to reflect automatic orienting processes (Tecce, 1972; Tecce et al., 1982). Conversely, the late part of the CNV, measured 200–500 ms before S2, is considered to reflect preparatory processes (Brunia and Damen, 1988; Van Boxtel and Brunia, 1994a,b) including motor, perceptual, cognitive, and attentional resources (Jang et al., 2016; Kropp et al., 2013; Tecce et al., 1982).
In this classical formulation, the CNV is induced by simple reaction time (SRT) tasks in which the execution of a simple motor response presumably recruits low levels of executive functions. In these cases, it has been suggested that the preparation for S2 induced by S1, which generates the change in cortical activity indexed by CNV, positively modulates subsequent S2 processing and the related motor response by enhancing stimulus processing (Gomez et al., 2003).
Importantly, it is also possible to evaluate late-CNV activity as an index of phasic alertness, during an S1–S2 paradigm in which the imperative stimulus represented by the more demanding attentional task, the double-choice reaction time (CRT) task, is designed to increase the executive function requests by S2 processing. This kind of comparison has been addressed in a study investigating CNV modulations due to transcendent experiences (TE), i.e. experiences subjectively characterized by “silence” and “loss of boundaries of time, space and body sense” during Transcendental Meditation (TM; Travis and Tecce, 1998). In this case, the authors found that for participants exhibiting more frequent TE, late CNV in a CRT task was reported to be lower in amplitude as compared to participants with rare or absent TE (Travis and Tecce, 1998). Conversely, in subjects reporting frequent TE, the CNV amplitude in a simple RT paradigm was reported to be increased, together with a reduced sensitivity to distractor stimuli that were present in the task (Travis et al., 2000). Travis and Tecce (1998) suggested that lower CNV in the choice trials may reflect a more balanced attentional set, or in other words, it seems that subjects with frequent TE could achieve the ability to better focus their attention, excluding distracting stimuli and improving their signal-to-noise ratio. Early phase CNV was not sensitive to the experimental manipulations used in either of these two studies.
Interestingly, in a recent study (Pauletti et al., 2014) it was shown that simply repeating multiple times a cued CRT task in normal healthy participants reduced RT, but had no influence on CNV amplitude, that remained steady along the sessions. The authors suggested that the continuous recruitment of attentional resources does not undergo habituation when it is related to the brain activity required in the maintenance of working memory, when the mental model of the stimulus environment is updated. Taken together, these results lead to the hypothesis that the balanced attentional set observed by Travis et al. (2002) and its corresponding electrophysiological correlates are perhaps selectively associated with TM experience, and that healthy participants without the same experience in TM would not show similar effects.
However, positive modulations of attentional resources and the induction of particular receptive mental states have also been associated with other forms of meditation, such as mindfulness, which trains individuals to develop focused attention on the present (Ben-Soussan et al., 2013; Farb et al., 2007). Importantly, similar effects of facilitation are also associated with cognitive training as well as motor training (Elbert et al., 1995; Klados et al., 2016).
In this context, Quadrato Motor Training (QMT), a specifically structured mindful movement practice (Ben-Soussan et al., 2013, 2014), which involves a combination of attention with motor response for producing the correct sequences of movement, was found to improve coordination, attention and creativity (Ben-Soussan et al., 2013, 2015). More importantly for the purposes of the present study, it was also found that QMT increases attention in contrast to motor and cognitive control groups (Ben-Soussan et al., 2014), as well as compared to breathing meditation (Ben-Soussan et al., 2017), demonstrating that QMT reduces impulsivity and automatic responses (Ben-Soussan et al., 2017). Thus, if QMT has the potential to induce similar attentional facilitation, as previously observed in experienced TM meditators (Travis et al., 2002), then one can expect a decrease in CNV amplitude in a CRT task.
An important confirmation of attentional facilitator effects induced by QMT may be obtained by the modulation of the ERP component following S2. In fact, it is noteworthy that in a CNV paradigm wherein S2 involves a multiple-choice response, it is possible to detect a P300 component following S2 (250–350 ms post-target) reflecting the activation of selective executive frontal and parietal networks (Tecce, 1972).
In general, P300 is produced in parietal and frontal brain areas and is thought to reflect processes involved in stimulus evaluation or categorization (Donchin, 1981). P300 is further related to sustained attention, working memory, context updating and discrimination (Nair et al., 2016). It has usually been studied using the oddball paradigm, in which low-probability target items are mixed with high-probability non-target (or “standard”) items (Polich, 2003). It has been used as an indirect measure of the attentional demands of a task on cognitive workload (Donchin, 1981; for a review see Polich, 2007). In the specific case of SRT and CRT tasks, while preparatory activity is reflected electro-physiologically by the CNV, the P300 component that follows S2 serves as a marker of the perception and evaluation of S2 (Gomez et al., 2003).
It follows that together with the modulation of the CNV, an attentional set characterized by a greater ability to focus on the task, such as the one hypothesized by Travis and Tecce (1998), would result in higher cognitive load and consequently may influence the P300 component. In fact, one possible explanation for this attentional facilitation consists in a greater reverberation of neural activity induced by the CNV on the S2 response within the parieto-frontal areas.
Consequently, in the present study, we hypothesized that: (1) increased P3 in CRT, as compared to SRT, will be observed, due to the higher cognitive complexity and attentional demands of the task; (2) increased P3 should be observed together with CNV modulation following QMT, due to a facilitated attentional state (Travis and Tecce, 1998).
To test these hypotheses, normal healthy individuals participated in one session of QMT, soon after and immediately before performing both SRT and CRT tasks, which are highly similar to the ones used by Travis et al. (2000), during which we recorded the EEG, and computed the task-dependent event-related potential (ERP).
2 Methods
2.1 Participants
Twenty-three healthy right-handed subjects (15 females, 8 males; age: 19–41 years; mean age 26.5 years) participated in the study. All participants performed the SRT and CRT tasks immediately before and soon after a single QMT trial (the two sessions were about half an hour apart). All participants had normal or corrected-to-normal visual acuity and reported having normal color vision. They were all recruited in Bar-Ilan University and gave their informed consent to participate in the study, which was approved by the ethical committee of the institute.
2.2 Quadrato Motor Training
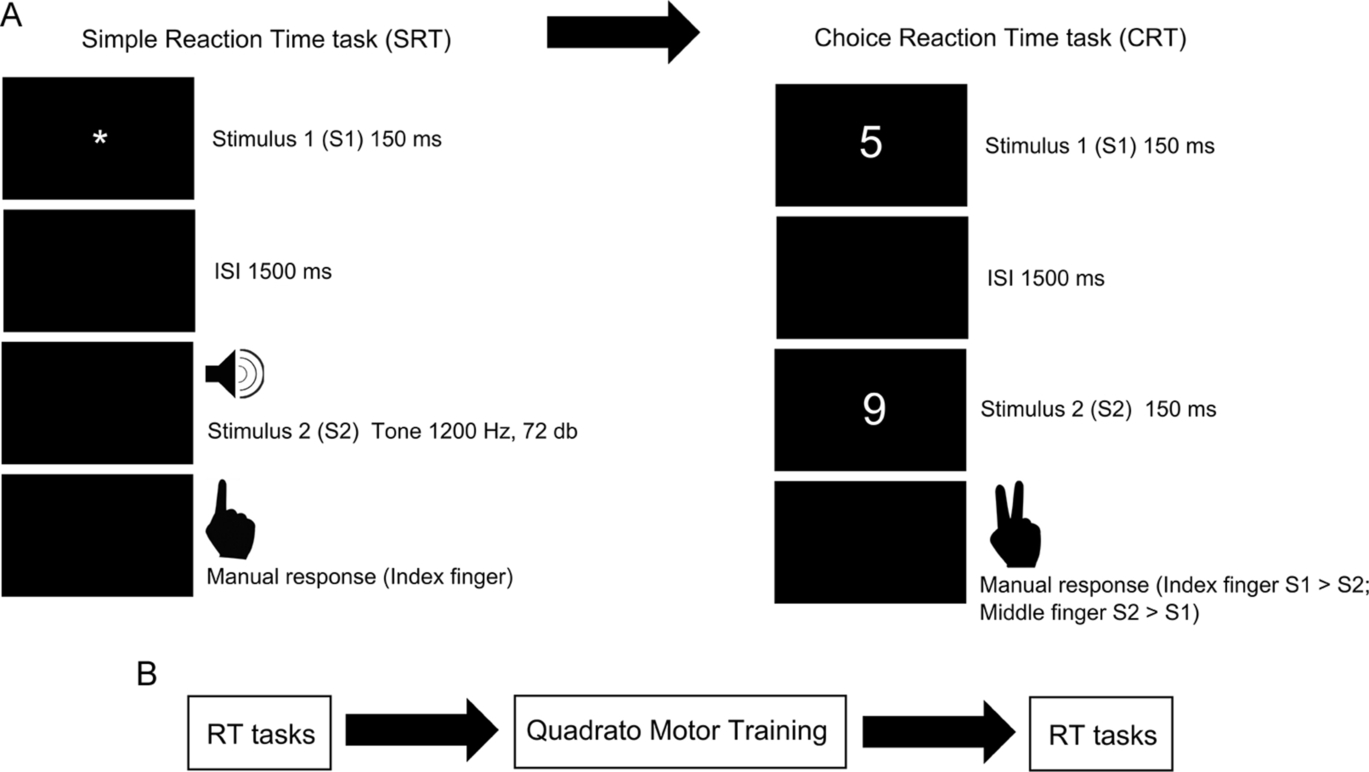
QMT requires standing at one corner of a 0.5 m × 0.5 m square and making movements in response to verbal instructions given by an audio tape recording (see Fig. 1). The participants are required to keep the eyes focused straight ahead, with hands loose at the side of the body, to continue with the next instruction and not to stop in the case of mistakes. At each corner of the square, there are three possible directions to move in; thus the training consists of 12 possible movements. In this experimental protocol, the training consisted of a sequence of 69 commands lasting 7 min. We used a movement sequence paced at a rate of an average of 0.5 Hz (similar to a slow walking rate) and instructed the participants to begin all movements with the leg closest to the center of the square.

2.3 Tasks
Two RT tasks were performed: SRT and CRT. Each task comprised 31 trials. In both tasks, responses from the subjects were provided via a response box (Electrical Geodesics Inc., Eugene, USA).
2.3.1 Simple RT task
The SRT task consisted of the presentation of an S1 stimulus (flashing asterisk, 150 ms duration and 1° in size) appearing at the center of a computer screen, which served as a warning signal for a second stimulus S2, appearing 1500 ms after the end of S1 (a continuous computer-generated tone, at 1200 Hz and 72 dB). During the trial, participants were instructed to focus their eyes on the screen (placed at 57.5 cm from participant) and to end the tone as quickly as possible, by pressing a button on a response box with the right index finger. A practice block of three trials was given prior to the task. Inter-trial interval was between 5 and 8 s.
2.3.2 Choice RT task
In the choice-task, there were two stimuli, S1 and S2. Each was a digit number (150 ms in duration, font—courier new, size 18, white on a black background). Similar to the SRT task, S2 appeared 1500 ms after the onset of S1 (see Fig. 2). Each participant was required to press with the index finger, one of two buttons (button number “1”) if the number presented as S1 was larger than the number presented as S2, and to press the second button (button number “2”) with the middle finger if the S2 number was larger. The number set was the same as in Travis et al. (2000, 2002) (given by personal communication, 2007).
2.4 RT recording and analysis
RTs were calculated and log transformed. CRT task accuracy was the percent of times that the subject correctly identified the larger number by pressing the appropriate button. In both tasks, trials in which RT exceeded > 2 SD from the mean were excluded from the analysis. RT was analyzed in a mixed repeated measure ANOVA with factors: Task (SRT, CRT) × Time (Pre, Post). CRT accuracy was analyzed with a one-way ANOVA with factor Time (Pre, Post).
2.5 EEG recording and analysis
The EEG recording was performed in an electrically shielded room, using a 64-channel geodesic sensor net GES 300 EGI system (Electrical Geodesics Inc., Eugene, USA) with Net Station software version 4.2.2. The head-coverage included sensors lateral to and below both eyes to monitor horizontal and vertical eye movements (EOG electrodes). Impedance for each channel was measured and kept below 50 kΩ as recommended for this system (Ferree et al., 2001). All electrodes were online referenced to Cz during the recording. Subsequently channels were rereferenced off-line to the average reference. The EEG was amplified with a band pass of 0.1–100 Hz (elliptic filter) and digitized at a sampling rate of 250 Hz. The continuous EEG was filtered offline by using a 0.3–40 Hz band-pass filter, and then segmented with a time window of 100 ms prior to S1 until 600 ms after S2 (to a total of 2200 ms). The 100 ms period preceding S1 onset was used to apply the baseline correction for the analysis of CNV (Picton et al., 2000; Travis et al., 2002), while the 200 ms period preceding S2 was used to apply the baseline correction for P3 analysis. Artifact detection was then performed as well as bad channel replacement. Rejection values for eye blinks and eye movement were set to higher than 140 and 55 μV, respectively, in the relevant eye channels. If any segment contained either a blink, one eye movement, or > 10 bad channels, the segment was marked as “bad,” and subsequently replaced by interpolation. Then, ocular artifact removal was performed, and artifact detection and bad channel replacement were performed again. The epochs were then averaged separately for each task and experimental condition. Under normal conditions, a clear averaged CNV derived from the grand-average of all the participants can be obtained in normal adults with 6–12 trials (Tecce, 1972). Here, CNV was analyzed from the whole sample of trials, which included 31 trials, separately for both SRT and CRT.
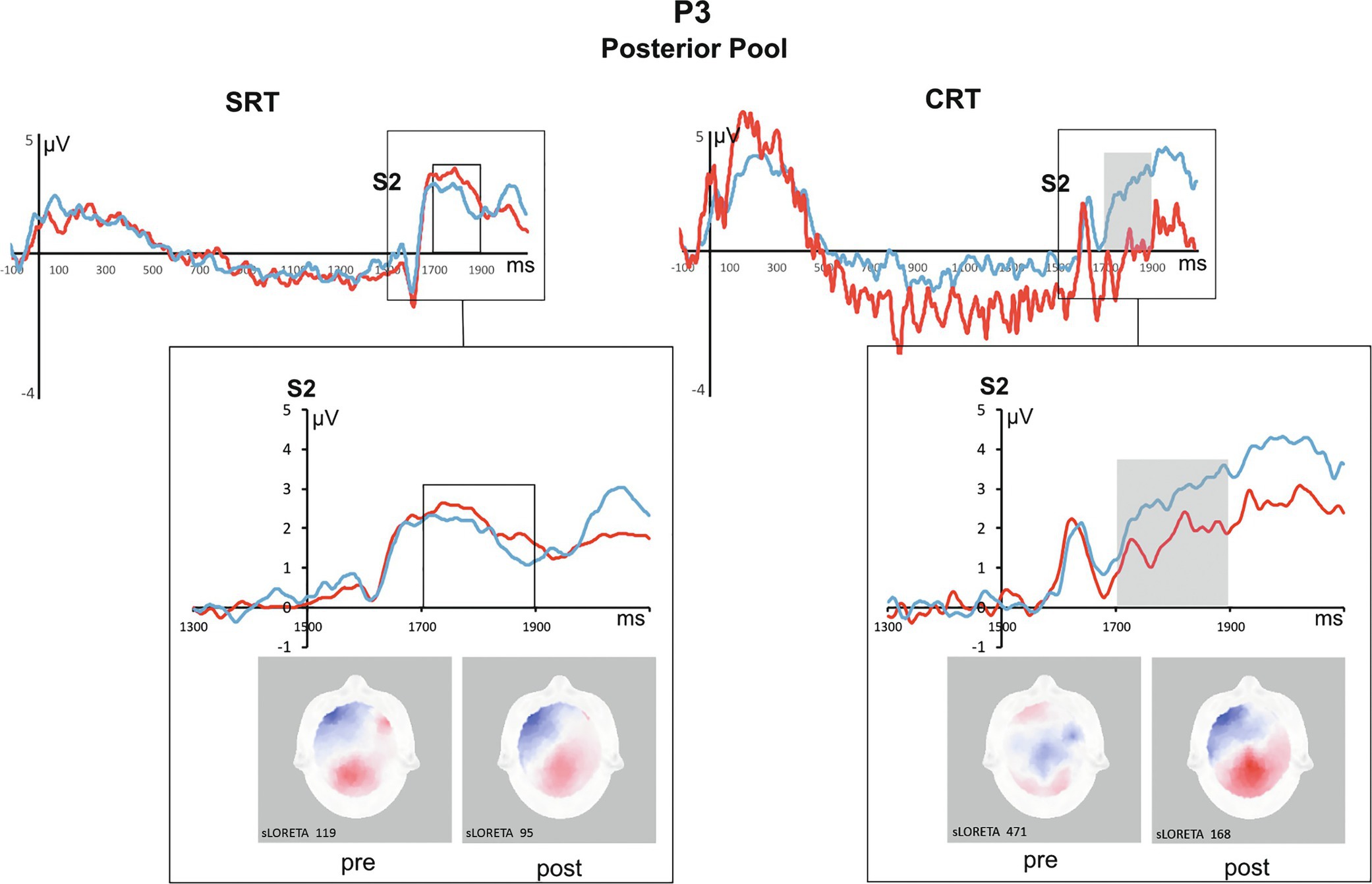
The analysis of S2-related (CNV, P3) ERP components was performed over selected time intervals at pooled electrode locations where these ERPs components were most evident in scalp topographies, based on the literature (Tecce, 1972; Travis et al., 2000). In particular, CNV was calculated as the mean amplitude over a 200 ms time interval before S2 (1300–1500 ms of the total epoch), in three different pool of derivations (Frontal pool: Fc1, Fcz, and Fc2; Central pool: C1, Cz, and C2; Parietal pool P1, Pz, and P2; Bender et al., 2004). A Task (SRT, CRT) × Time (Pre, Post) × Electrode Site (Frontal, Central, Parietal) repeated measures ANOVA was used for CNV analysis. Similarly, the P3 was calculated as the mean amplitude over a time window of 200 ms in a pool of derivations including P1, P2, Pz, Cpz (Correa et al., 2006; Hohnsbein et al., 1991). P3 normally starts around 250 ms post-target and could go on until 550 ms post-target. This was particularly observed in the CRT condition of the present work. Here, in order to compare similar periods between experimental conditions, we consider a window starting at 200 ms post-S2 target and finishing at 400 ms post-S2 target (1700–1900 ms of the total epoch), perfectly centered around the largest positive peak. Individual voltages were analyzed through a Task (SRT, CRT) × Time (Pre, Post) repeated measures ANOVA.
3 Results
3.1 Behavioral results
We found a main effect for Task [F(1,41) = 151.3, P < 0.001; see Fig. 3] showing faster RT in SRT as compared to CRT (206.4 ms vs. 591.6 ms; log-transformed: 2.3 vs. 2.7, Bonferroni post-hoc comparison: P < 0.001), and a main effect for Time [F(1,41) = 28.5, P < 0.001], resulting in a general improvement in RT following QMT (423.5 ms vs. 374.1 ms; log-transformed: 2.5 vs. 2.4, Bonferroni post-hoc comparison: P < 0.001). Finally, a significant Task × Time interaction indicated that improvement in RT was considerably greater for the CRT task as compared to the SRT task (22 ms vs. 76 ms; log-transformed: 0.04 vs. 0.1, Bonferroni post-hoc comparisons: P < 0.001). As concern CRT accuracy we found no difference between Pre- and Post-QMT experimental session (F < 1, P = ns) with both percentage of accuracy close to 100% (99.6 and 99.8 respectively).

3.2 Electrophysiological results
3.2.1 Contingent Negative Variation
The ANOVA conducted on the CNV mean amplitude indicated a significant Task × Time interaction [F(1,41) = 9.79, P = 0.003; see Fig. 4], resulting from an increased amplitude of the CNV for SRT (pre-QMT: − 0.39 μV vs. post-QMT: − 1.47 μV; P = 0.04) and a decreased amplitude for CRT (pre-QMT: − 2.14 μV vs. post-QMT: − 1.10 μV; P = 0.02). In addition, there was a main effect for Site, indicating that generally, CNV was stronger at Central (− 1.60 μV) as compared to both Frontal (− 0.96 μV) and Parietal (− 0.86 μV) derivations (all P < 0.02).

3.2.2 P3
For P3, a significant Task × Time interaction [F(1,41) = 6.1, P = 0.01; see Fig. 5] was found. This interaction indicates an opposite pattern of results, compared to that of the CNV: the P3 amplitude increased following QMT for CRT (pre-QMT: 1.68 μV vs. post-QMT: 2.65 μV; P = 0.03), while no difference in P3 amplitude was found between pre- and post-QMT for SRT (pre-QMT: 2.32 μV vs. post-QMT: 1.87 μV; ns), though the direction of change (reduction in amplitude) is as hypothesized.
4 Discussion
In the present study, we investigated whether QMT can induce attentional benefits in healthy non-meditator participants similar to those observed in a previous study with meditator participants experiencing frequent TE (Travis and Tecce, 1998; Travis et al., 2000).
Similar to previous investigations, in the present work such attentional benefits were evaluated through the recording of the CNV ERPs component, using a pre-post design for both SRT and CRT tasks. At variance with previous studies, we decided not to focus selectively on the CNV, but to take into account another important ERP component in addition: the P3. The presence of the P3 is, in fact, noteworthy, in a paradigm in which S2 requires a choice between targets or in general, cognitive effort in order to produce a correct answer (Pauletti et al., 2014; Tecce, 1972).
The main results we found are: (I) enhanced CNV following QMT, for SRT and conversely, (II) decreased CNV for CRT. These electrophysiological results matched at a behavioral level an improvement in RT following QMT, leading to faster RT for both SRT and CRT tasks, with a significantly faster CRT.
Although facilitation in RT could simply be due to the repetition of the task, the double dissociation concerning the two ERP components excludes this interpretation.
Indeed, the findings concerning the CNV are in strong agreement with the ones reported by Travis et al. (2000) and could be therefore initially interpreted using the models proposed by these authors. They suggest that such findings could be the result of a more balanced attentional set in which subjects waited for S2 before they initiated response preparation processes. However, such an interpretation may be limited. First, in their study Travis et al. compared CNV amplitude across participants having frequent and infrequent TE, with no control condition or a baseline evaluation of the CNV. Second, and more importantly, in order to be confirmed, the conclusions reached by the Travis et al. would need the evaluation of further ERP components, more related to attentional functions, such as the P3, as we have shown here. In fact, Travis et al. (2002, p. 300) had noted in a footnote that “future research can investigate the relation of P300 and CNV…,” and that is what we have accomplished in the present study.
In general, albeit with some differences concerning the paradigm in use, all previous studies have shown that in healthy non-meditator participants, simple task repetition produced a reduction in P3 amplitude (i.e., in an oddball paradigm with motor response; Rushby and Barry, 2007; Timsit-Berthier, 1984) or at most, resulted in its stability (i.e., in a discrimination task between target-tones and non-target-tones; Pauletti et al., 2014) between one repetition and the next. These results were maybe due to the fact that, in the case of a reduction in P3 amplitude, the updating of the neural model of the stimulus becomes automated in conjunction with reduced saliency of targets; while the elicitation of the P3 was maybe related to response inhibition, required each time a non-target-S2 occurred, thus resembling the P3 elicited during a Go/noGo task (Falkenstein et al., 1999).
Importantly, in the domain of mathematical cognition, other studies using magnitude judgments, i.e. identifying the greatest among two numbers, similar to the one used in the present work in the CRT task, have reported no P3 modulations due to magnitude comparison (i.e., distance effect; Dehaene, 1996; Libertus et al., 2007).
Although results for SRT seem to be in this direction, being stable between pre- and post-QMT, we found an increased P3 post-QMT, as compared to pre-QMT, for CRT. Such a result seems compatible with the idea of an enhanced attentional set, as hypothesized by Travis and Tecce (1998). It has further been shown that temporal and statistical uncertainty regarding upcoming stimuli favors the establishment of an increased attentional state in which awareness of near-threshold stimuli is increased. In turn this awareness can result in the enhancement of both P3 subcomponents, P3a and P3b, reflecting respectively the maintenance of visual memory trace through reverberation of activity in a parieto-frontal network (Lasaponara et al., 2015). Similarly, the same enhanced attentional state, induced by QMT in the present study, could result in an amplification of the cognitive facilitation following the CNV in a parieto-frontal network, which positively modulates the P3 recorded centrally.
Taken together, the current results are in line with those previously reported related to the ability of QMT to enhance reflectivity and conversely decrease impulsivity and automatic responses (Ben-Soussan et al., 2014, 2017), and advance current knowledge regarding the dynamic change in CNV and P3 following sensorimotor training. However, some potential limitation should be taken into account.
First, in the present study it was used only one training paradigm without a control group. Thus, it is difficult to draw final conclusions regarding the effect of non-specific physical exercise—of moving the feet in any pattern—as opposed to the suggested effects of moving them in a specific guided sequence. Yet, previous studies examining QMT have demonstrated that cognitive changes are QMT-specific, and are not observed in simple motor training with reduced choice requirements (Ben-Soussan et al., 2013, 2014, 2017), nor in walking training in which the participants were instructed to make successive steps using the same QMT pace, duration, and auditory cue, but their movement was free in space, not within a square (Venditti et al., 2015).
Second, even if according to previous paper by Polich (1991) and Tecce (1972), 31 trials should allow to record both reliable P300 and CNV, we must report on the fact that such a number of trials is lower than usual for recording ERPs components. Such a number was originally choose mainly to reproduce the original task by Travis et al. (2000) and secondary, to avoid fatigue and sustained attention contamination in participants.
Finally, as these ERP components, especially P300, are closely linked to impulsivity and intelligence (Houlihan et al., 1998; Stelmack et al., 1993) and since QMT has been previously found to elicit attention and reflectivity as opposed to several control groups (Ben-Soussan et al., 2014, 2017), a future study, with higher number of trials and the use of control group, should also examine the relationship between these parameters following QMT and other sensorimotor and mental training paradigms. We are currently working in this direction.
