Yoga: Balancing the excitation-inhibition equilibrium in psychiatric disorders
Urvakhsh Meherwan Mehtaa,*; B.N. Gangadharb a Department of Psychiatry, National Institute of Mental Health & Neurosciences (NIMHANS), Bengaluru, India
b National Institute of Mental Health & Neurosciences (NIMHANS), Bengaluru, India
* Corresponding author: Tel.: + 91-80-26995805, + 91-9448208167 email address: [email protected]
Abstract
Social behavioral disturbances are central to most psychiatric disorders. A disequilibrium within the cortical excitatory and inhibitory neurotransmitter systems underlies these deficits. Gamma-aminobutyric acid (GABA) and glutamate are the most abundant excitatory and inhibitory neurotransmitters in the brain that contribute to this equilibrium. Several contemporary therapies used in treating psychiatric disorders, regulate this GABA-glutamate balance. Yoga has been studied as an adjuvant treatment across a broad range of psychiatric disorders and is shown to have short-term therapeutic gains. Emerging evidence from recent clinical in vivo experiments suggests that yoga improves GABA-mediated cortical-inhibitory tone and enhances peripheral oxytocin levels. This is likely to have a more controlled downstream response of the hypothalamo-pituitary-adrenal system by means of reduced cortisol release and hence a blunted sympathetic response to stress. Animal and early fetal developmental studies suggest an inter-dependent role of oxytocin and GABA in regulating social behaviors. In keeping with these observations, we propose an integrated neurobiological model to study the mechanisms of therapeutic benefits with yoga. Apart from providing a neuroscientific basis for applying a traditional system of practice in the clinical setting, this model can be used as a framework for studying yoga mechanisms in future clinical trials.
Keywords
Yoga; GABA; Cortical inhibition; Oxytocin; Mechanism
1 The role of GABAergic neurotransmission in psychiatric disorders
1.1 Gamma-aminobutyric acid (GABA), glutamate, and brain function
GABA is an abundant inhibitory neurotransmitter in the central nervous system, and along with glutamate, an excitatory neurotransmitter, is responsible for a wide array of brain functions (Reis et al., 2009). It is a neutral amino acid, synthesized from glutamate by a cellular cytoplasmic enzyme called glutamate decarboxylase (Young and Chu, 1990) and plays an important role in maintaining both phasic as well as tonic inhibition in the brain (Sigel and Steinmann, 2012).
GABA, when released from synaptic vesicles, acts postsynaptically via GABAA receptors, which are ligand-gated anion-selective channels producing hyperpolarization of neuronal membranes and hence brief lasting, but fast and phasic neuronal inhibition. In addition, lower concentrations of ambient extrasynaptic GABA are known to activate different subunits of the GABAA receptor and hence produce persistent “tonic” neuronal inhibition (Farrant and Nusser, 2005). Any endogenous or exogenous processes that reduce this tonic neuronal inhibition are likely to contribute to heightened anxiety (Maguire et al., 2005). Interestingly, the phasic and tonic GABA inhibitions demonstrate a competitive homeostatic regulation of the total cortical inhibition (Wu et al., 2013). In addition, these inhibitory processes are under the regulatory control of neurosteroids—these are steroids synthesized in the brain (e.g., allopregnanolone) from circulating steroid hormone precursors like progesterone and deoxycorticosterone—via their actions on the GABAA receptor (Reddy, 2010). Likewise, GABAA-mediated tonic inhibition also regulates the hypothalamic response to stress by the release of cortisol peripherally (Lee and Maguire, 2014).
In contrast, GABAB neurotransmission is a slow synaptic inhibition, but more robust and is mediated via metabotropic receptors that produce both, post-synaptic neuronal inhibition as well as pre-synaptic autoreceptor-driven fatigue of synaptic inhibition—these are mediated by different G proteins (Motohashi et al., 1989; Sigel and Steinmann, 2012). The downstream effects of GABAB receptor activation are complex, partly owing to different isoforms (GB1a and GB1b) of the receptor and site of action (Doly et al., 2016). Nevertheless, it is clear from animal models and human in vivo experiments, that GABAB-mediated neurotransmission plays a role in reward processing, social motivation, and mood regulation—important ingredients of adequate social functioning (Jacobson et al., 2018). For instance, GABAB receptor-mediated tonic inhibition regulates the spontaneous firing of locus coeruleus neurons that release noradrenaline, a neurotransmitter critical in maintaining arousal and mood (Wang et al., 2015).
Glutamate, on the other hand, is an excitatory neurotransmitter and the most abundant neurotransmitter in the brain; glutamatergic neurons utilize ~ 60–80% of brain metabolic activity (Rothman et al., 2003). It is synthesized when the enzyme glutaminase facilitates deamidation of glutamine. Glutamate is responsible for a broad range of cortical functions that include but are not restricted to neuronal differentiation, neuronal migration, and synaptic plasticity, which support diverse cognitive abilities (Tapiero et al., 2002). Glutamate also exerts regulatory control over GABA-mediated inhibitory neurotransmission (Bak et al., 2006). Its multitude of pre-synaptic and post-synaptic effects are mediated by both metabotropic and ionotropic receptors. N-methyl-d-aspartate (NMDA) glutamate receptors are a specific class of ionotropic receptors that play a critical role in the development, as well as, the functioning of GABA interneurons (Cohen et al., 2015). A hypofunctioning NMDA-receptor system can potentially result in diminished GABA-interneuron function, an imbalance in the large-scale brain network balance maintained by the excitatory and inhibitory neurotransmitter systems, impaired functional connectivity and impaired synaptic plasticity leading to deficits in learning, memory, and predictive coding (Friston et al., 2016). This model of a GABA/glutamate imbalance resulting in social deficits is posited to underlie major psychiatric disorders including depression (Sanacora et al., 2012) and schizophrenia (Howes et al., 2015).
1.2 The clinical relevance of GABA and glutamate
The very abundance of these two neurotransmitters (GABA and glutamate), their overarching presence across diverse brain regions and their antagonistic functional properties, provides a mechanistic basis with which the brain executes control over behaviors. The best illustration of this is perhaps from the fact that GABA and glutamate are units of analysis (molecular level) for all the five behavioral domains within the Research Domain Criteria (RDoC) framework (Insel et al., 2010) to study psychiatric disorders. The RDoC is a novel initiative under the National Institute of Mental Health to study five psychological domains relevant to human behavior and functioning (negative and positive valence systems, cognitive systems, social processes systems, and arousal or regulatory systems) in a transdiagnostic manner from genomics to cellular physiology and finally behavior. The ultimate aim of this approach to studying behavioral abnormalities seen across various psychiatric disorders will be to develop precisional and individualized treatments (Insel, 2014). Any disparity in the optimal cortical excitation-inhibition balance can, therefore, express as behavioral abnormalities across the five RDoC domains. A common neurobiological template that illustrates the role of this GABA/glutamate balance is the prefrontal-amygdala fronto-limbic circuit, where GABAergic neurotransmission plays a central regulatory role over processing of emotions in the limbic cortex (Delli Pizzi et al., 2017). Various methods of investigating in vivo GABA functions in humans have indicated GABAergic neurotransmission abnormalities across a broad range of psychiatric disorders—schizophrenia (Benes and Berretta, 2001), depression (Brambilla et al., 2003), anxiety (Long et al., 2013), post-traumatic stress disorder (Rosso et al., 2014), autism (Cochran et al., 2015), and dementia (Bai et al., 2015). Moreover, it is well known that chronic psychological stress can lead to a blunting of the hypothalamo-pituitary-adrenal axis response and hippocampal tissue loss—this effect is also associated with an imbalance in the GABA/glutamate tone (Yang, 2014).
Electro/magnetoencephalography, transcranial magnetic stimulation (TMS), magnetic resonance spectroscopy (MRS), and positron emission tomography (PET) have been used to probe GABA functions at different time-space resolutions. While early and late prepulse inhibitions response to sensory or motor stimuli as assessed by EEG/MEG (Inui et al., 2018) or TMS (McClintock et al., 2011), can reflect GABAA and GABAB receptor-mediated neurotransmission with good temporal resolution, MRS measures of GABA concentrations (Egerton et al., 2018) and PET profiling of GABA receptors (Bai et al., 2015; Frankle et al., 2015) provide good spatial resolution of GABAergic functions in the brain. These lines of in vivo measurements have been supplemented by postmortem brain tissue studies, in conjunction with animal model experiments, which consistently report of deficient glutamic acid decarboxylase (GAD-67) expression—a key enzyme in the biosynthesis of GABA—playing a role in a range of psychiatric disorders (Akbarian and Huang, 2006; Akbarian et al., 1995). A note of caution, there are inconsistencies and limited replicability in results from these novel, technologically intensive investigations characterizing GABAergic abnormalities in psychiatric disorders (Taylor and Tso, 2015). A multi-modal combination of different lines of GABA-measurement investigations, leveraging the pragmatic and temporo-spatial advantages of individual investigations, applied in longitudinal, large-scale dense time-series (repeated closely over a period of time) is likely to provide more consistent and reliable results across psychiatric disorders.
Nevertheless, one of the final common behavioral outcomes of these major psychiatric disorders is social withdrawal, often linked to poor quality of life and real-world functional disability. This could be a result of either the cognitive (Volk and Lewis, 2005) or affect processing deficits (Brambilla et al., 2003) triggered by the GABA-glutamate imbalance. It is well established by now that social information processing deficits and residual negative behavioral symptoms like amotivation and anhedonia predict real-world dysfunction in severe psychiatric disorders (Bhagyavathi et al., 2015; Green et al., 2012). Several lines of animal experiments demonstrate the effects of inefficient cortical GABAergic activity and social deficits (Chao et al., 2010). In patients with schizophrenia, where social deficits are a defining feature, GABAA-mediated intracortical inhibition measured using paired-pulse TMS demonstrated a significant association with social cognition performance (Mehta et al., 2014b). Preclinical pharmacological studies also show a reversal in social deficits by administration of diazepam (Mielnik et al., 2014) and R-baclofen (Silverman et al., 2015)—modulators of GABAA and GABAB receptor neurotransmission, respectively. Any interventions targeted toward optimizing this excitation-inhibition balance may potentially be studied as therapies for improving social deficits across a range of psychiatric disorders.
2 Modulating the GABAergic system for therapeutic gains
Novel optogenetic stimulation studies in live mammals indicate that elevating the neuronal excitation-inhibition balance in the medial prefrontal cortex resulted in profound social deficits (Yizhar et al., 2011). The importance of this cortical excitatory-inhibitory balance is also emphasized by many clinical studies examining GABA-glutamate functions (Ford et al., 2017; Horder et al., 2018). As a corollary, any method of decreasing the excitation-inhibition balance, via modulation of GABA (enhancing) and glutamatergic (inhibiting) neurotransmission in humans can potentially improve social deficits.
Not surprisingly, benzodiazepines, a major class of GABA-receptor facilitators, have been studied and approved for the treatment of anxiety disorders (Guina and Merrill, 2018). Shorter-acting benzodiazepines are also the first line of treatment for catatonic symptoms—extreme motoric manifestations of severe psychiatric disorders (Rosebush and Mazurek, 2010). Understandably, given the high abuse potential of these medications (Brunette et al., 2003), their widespread use for treatment of social deficits in patients with other psychiatric morbidities, that often share genetic vulnerabilities with addiction disorders, is limited. Zolpidem, eszopiclone, and other non-benzodiazepine GABA facilitators have also been used—though primarily in treating insomnias—in the treatment of catatonia (Peglow et al., 2013) and persistent negative symptoms of schizophrenia (Mehta et al., 2018). In addition, with the better characterization of the GABAergic receptors, newer molecules targeting specific GABA sub-receptor systems are being evaluated for the treatment of social deficits in autism and Down syndrome (Möhler, 2015).
Selective serotonin reuptake inhibitors (SSRIs) are the first-line treatment of depressive and anxiety disorders; these medications also influence GABA-mediated neurotransmission in an indirect manner. Human studies demonstrate a 35% increase in GABA concentrations in the occipital cortex as measured using MRS after acute administration of citalopram, an SSRI, as compared to administration of saline in healthy individuals (Bhagwagar et al., 2004). This GABA enhancement is also seen in patients with depression who were treated with SSRIs for 2 months (Sanacora et al., 2002). TMS when given in repetitive patterns for the treatment of depressive disorders, also results in elevation of prefrontal GABA on MRS experiments (Dubin et al., 2016). Similarly, electroconvulsive therapy, which is used in the treatment of severe or resistant depressive disorders, also increase peripheral (serum) GABA levels (Esel et al., 2008) and cortical GABA concentrations (Sanacora et al., 2003). Interestingly, alcohol use, similar to benzodiazepines, also facilitates GABA neurotransmission at both pre- and post-synaptic levels (Kelm et al., 2011). The resultant anti-anxiety effects are the primary reason for alcohol to be used as a self-medicating agent, sought after by many individuals with anxiety disorders, albeit at a higher risk of developing substance use disorders (Robinson, 2011). This self-medication process in at-risk individuals could either result from a social learning process (Gottfredson and Hussong, 2011) or is an expression of a more innate stress reduction response. Interestingly, all the treatment modalities discussed here (benzodiazepines, other GABA modulators, SSRIs, electroconvulsive therapy, and TMS) are used across a broad range of psychiatric disorders as adjuvant therapies with the final aim of improving the distressing behavioral symptoms and the social dysfunction thereof.
In keeping with these observations, the practice of yoga—a multidimensional process of coordinated physical postures, respiratory control techniques, deep relaxation, and meditation—has been studied intensely as a potential adjuvant therapeutic strategy to improve psychiatric symptoms and social behaviors. Given its likely beneficial effects on mental relaxation, a sense of reduced “stress” (Li and Goldsmith, 2012), and its consistent beneficial effects on quality of life in patients with severe psychiatric disorders (Cramer et al., 2013a,b), yoga therapy finds a mention in one of the recent expert consensus treatment guidelines for schizophrenia (NCCMH, 2014). It is therefore imperative to be able to identify the neurobiological mechanistic pathways that it engages, modulates, and harnesses in the process of its therapeutic gains. It is here that the potential engagement of the GABAergic system can conceivably be studied as a model for the mechanism of action of yoga therapy. In the following sections, the role of yoga therapy in psychiatric disorders and its putative mechanistic basis, from the perspective of regulating the cortical excitation-inhibition balance, will be discussed.
3 Role of yoga in psychiatric disorders
Over the last decade, there has been a threefold increase in the number of experiments examining therapeutic benefits of yoga for a range of medical diagnoses, psychiatric disorders being the commonest (Jeter et al., 2015). Among the various psychiatric disorders, yoga therapy has garnered evidence from randomized controlled trials for anxiety, depression, and psychotic disorders. While the therapeutic benefits vary, definitive short-term reductions of anxiety (Cramer et al., 2018) and depression (Cramer et al., 2013b), and short-term quality of life gains in individuals with schizophrenia (Cramer et al., 2013a) have been observed with little information on long-term outcomes. Most often, yoga is used as an adjuvant to conventional psychiatric therapies (Varambally and Gangadhar, 2016).
In schizophrenia, yoga has recently been used to examine its effects on the general (attention, memory, and executive functions) and social cognitive (theory of mind, emotion processing, and social cue perception) abilities, which are impaired. It is worth emphasizing that cognitive deficits in schizophrenia are clinically very relevant, given their role in determining real-world socio-functional outcomes that are consistently demonstrated via cross-sectional and longitudinal experiments (Couture et al., 2006; Fett et al., 2011; Green et al., 2000, 2004). These cognitive deficits span general impairments (neurocognition) of attention, speed of processing, working memory, cognitive control, and other executive functions, as well as, impairments specific to applying our cognitive abilities in interpreting and inferring social cues and social situations (e.g., theory of mind, emotion processing, social perception, and attributional styles), which are referred to as social cognition impairments (Mehta et al., 2013b). Over the last couple of decades, there have been several attempts at treating cognitive deficits with a wide range of interventions that rely on harnessing neuroplasticity and encompass cognitive remediation, pharmacotherapy, physical exercise, and non-invasive brain stimulation therapies (Keshavan et al., 2014, 2015; Wykes et al., 2011). However, the best of these treatments has been resource and time intensive, with the modest therapeutic gains (Choi et al., 2013; Wykes et al., 2011). It is here that the role of yoga therapy in enhancing general and social cognition is placed in perspective. The neurocognitive benefits of yoga therapy were found to be comparable to that with physical exercise and better than treatment as usual (Bhatia et al., 2017). However, yoga therapy resulted in significantly greater improvements in emotion recognition abilities as compared to physical exercise and the waitlist group in a trial on schizophrenia patients (Behere et al., 2011). This finding has been replicated in another trial comparing yoga therapy with a waitlist group (Jayaram et al., 2013). In addition, a recently concluded randomized controlled trial examined social cognition comprehensively (all four dimensions as mentioned above) and found 20 sessions of yoga produced substantial therapeutic gains in social cognition impairments and negative symptoms as compared to treatment as usual over a 6-week period (Naik, 2016). Overall, it appears from studies on patients with schizophrenia, that yoga might have a rather specific beneficial impact on social cognition deficits—this may contribute to the improvement in the quality of life observed in other studies (Cramer et al., 2013a).
Yoga therapy for residual symptoms of schizophrenia as an adjuvant therapy has been found to be useful in both acute inpatient (Manjunath et al., 2013) and stable outpatient (Duraiswamy et al., 2007) settings. While all the above observations are made from randomized controlled trials, there are inherent limitations in the study designs, with challenges in double blinding, monitoring of home-based yoga therapy, and the pragmatic challenges of delivering daily supervised yoga therapies. Nevertheless, despite limitations, the best quality studies do show consistent therapeutic benefits especially in the domains of social cognition and social behavior, resulting in a better quality of life. It may be intuitive to predicate the therapeutic gains of yoga on its status as a culturally accepted method of stress reduction (Cramer et al., 2015; Machleidt and Ziegenbein, 2008). However, it is critical to identify the neurobiological mechanistic basis driving these therapeutic gains. This will perhaps spur a new stream of yoga research with the aim to identify the best therapeutic ingredients of yoga, which can then be implemented in a more efficient and pragmatic manner in routine clinical practice.
4 A mechanistic understanding of the effects of yoga: The role of GABA and oxytocin
4.1 The clinical illustration of depression and GABA impairments
In pursuing an understanding of the mechanisms underlying the therapeutic benefits of yoga, we take the example of depression, a common psychiatric disorder with perhaps the largest global disability burden across medical disorders (Vos et al., 2012). Depressive disorders are characterized by symptoms of intense low mood, anxiety, a lack of interest in pleasurable activities, easy tiredness, thoughts of hopelessness, helplessness, and worthlessness and a wide range of disturbances in sleep, appetite, sexual, and social functioning—lasting for 2 weeks or longer (American Psychiatric Association et al., 2000). Among the various neurobiological models of mood disorders like depression, there is multi-level support for the GABA hypothesis of emotion dysregulation. This is evident from observations of depression-specific reductions in the cerebrospinal fluid GABA levels (Gerner and Hare, 1981), lower MRS-measured GABA concentrations in the occipital and anterior cingulate cortices of depressed patients (Rao et al., 2011), and reduced TMS-measured short interval intracortical inhibition—a measure of GABAA neurotransmission and cortical silent period—a measure of GABAA neurotransmission—in the motor cortex of patients with depressive disorders (Radhu et al., 2013). Interestingly, lower GABA levels in the subgenual anterior cingulate cortex of depressed individuals (Gabbay et al., 2012) complement findings of reduced blood flow and glucose metabolism in the same region during depression (Drevets et al., 1997), thus highlighting the role of an inefficient prefrontal default mode control over limbic and executive networks (Northoff and Sibille, 2014). Studies from human social experiments suggest that social stress increases peripheral (serum or salivary) cortisol levels; this is often associated with the symptoms of social isolation or anxiety—this being modulated by sex hormones like testosterone (Lozza et al., 2017) or the bonding hormone, oxytocin (Heinrichs et al., 2003). Interestingly, evidence from preclinical animal experiments, as well as, human experiments suggests a reciprocal regulatory relationship between prefrontal GABA-glutamate equilibrium, and the functioning of the hypothalamo-pituitary-adrenal axis that modulates cortisol release to stress (Mody and Maguire, 2012). Patients with depression are also likely to have increased peripheral cortisol levels as compared to healthy comparison subjects; this elevated cortisol is reduced by antidepressant therapy with medications and yoga (Thirthalli et al., 2013). This study also demonstrated a significant positive association between a drop in depression severity ratings and drop in peripheral serum cortisol levels in patients who received yoga therapy.
4.2 Connecting the dots: The mechanistic basis of yoga—Effects on GABA
Indeed, the most common biological mechanisms of yoga that have been studied over the years are physiological markers of perceived stress (peripheral cortisol levels and sympathetic nervous system activity). A meta-analysis examining the effects of yogasanas with or without mindfulness-based meditation techniques, on such physiological markers of stress, reports diminished peripheral cortisol levels, and regulation of sympathetic activity—diminished blood pressure and heart rate (Pascoe et al., 2017) after yoga.
It is in this context, that it would be critical to examine if the cortisol reducing effects of yoga are actually related to the effects of yoga on the GABA-glutamate equilibrium in the brain. This has been proposed as a putative mechanistic framework for the effects of meditation that can be empirically examined (Austin, 2013; Elias and Wilson, 1995) and extended to the practice of yoga. There is indeed emerging evidence from human studies that have employed magnetic resonance spectroscopy (MRS) to study cortical GABA responses to yoga therapy. A pilot MRS study revealed a 27% increase in whole-slab GABA levels in regular yoga practitioners after 60-min of yoga practice, compared to a similar duration of reading (Streeter et al., 2007). Subsequent analyses revealed that this increase was best observed in the thalamus. In an ensuing study by the same group, healthy individuals were randomized to receive 12 weeks of yoga therapy or metabolically matched intermittent walking. The yoga group demonstrated a significantly greater increase in left thalamus GABA levels on MRS, which correlated with their self-reported mood and anxiety state improvements (Streeter et al., 2010). This finding indeed highlights how yoga therapy is likely to drive improvement in mood and anxiety symptoms in clinical populations by engaging with the cortical GABAergic system. In a clinical proof-of-concept demonstration, the same group was able to replicate these GABA-lowering effects of 12 weeks yoga therapy in two patients with major depression and chronic low backache, in conjunction with clinical improvement (Streeter et al., 2012). Future studies may examine this “engagement” of GABAergic function with yoga in patients with depressive or anxiety disorders. Furthermore, it may be examined if increasing cortical GABA levels are associated with a decrease in peripheral cortisol levels with yoga therapy.
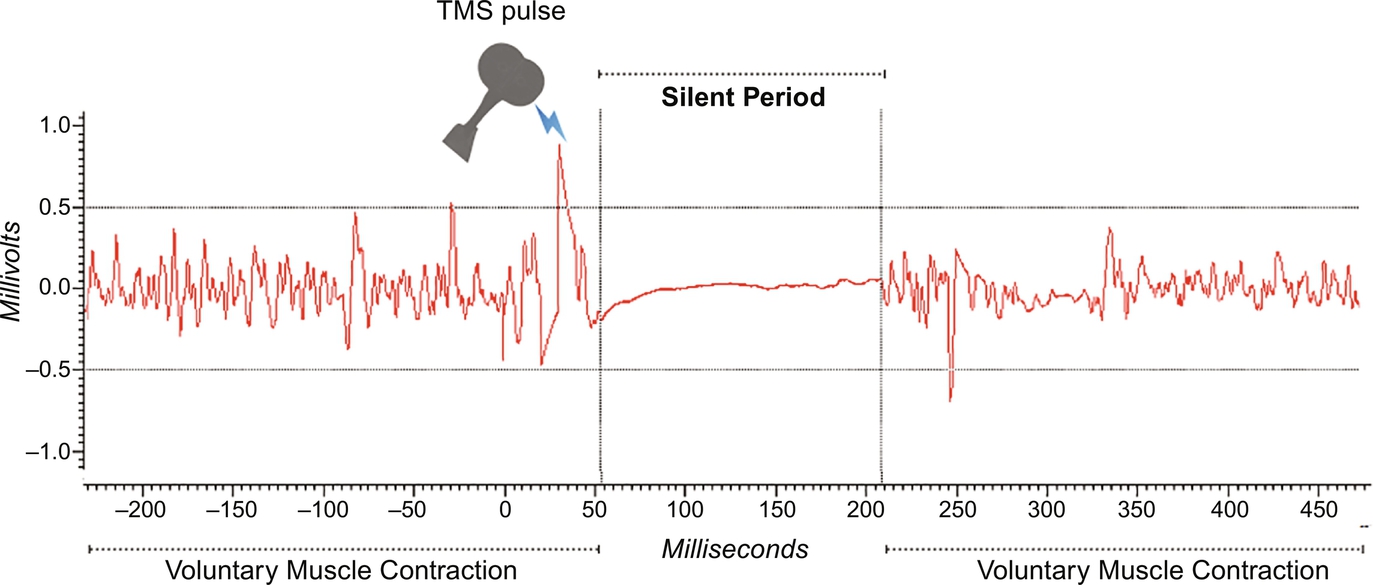
Cross-validating these MRS findings of elevated cortical GABA levels post-yoga, with other in vivo measurements of GABA function can further strengthen this hypothesis of yoga driving the GABA-glutamate equilibrium for optimal social functioning. TMS-derived cortical silent period (CSP) is the GABAB-mediated isoelectric electromyograph obtained secondary to suppression of motor activity when a suprathreshold stimulus is delivered to the motor cortex hand area when the contralateral hand is voluntarily held in contraction (Kobayashi and Pascual-Leone, 2003). Fig. 1 gives an illustration of this phenomenon. In a pilot observation of healthy volunteers recruited for a TMS experiment (Mehta et al., 2014c), we identified six participants who were experienced-yoga practitioners (average of 10-years yoga practice). We compared their TMS-derived cortical inhibition parameters with 10 age, gender, and education-matched healthy individuals who had not practiced yoga regularly. The two groups (n = 6 experienced-yoga practitioners; n = 10 non-practitioners of yoga) were comparable on TMS-derived resting motor threshold and short and long interval cortical inhibition (Mann-Whitney U > 31; P > 0.3). However, the yoga group had significantly longer CSP compared to the non-yoga group (Mann-Whitney U = 72; P = 0.016; see Fig. 2). We examined these effects in a prospective study, where TMS was used to measure cortical inhibition (short interval intracortical inhibition and CSP) before and after 1 month of yoga training (average of 18 sessions of 60-min duration yoga training) in beginners attending a yoga appreciation course. We found significantly enhanced CSP following the yoga training; this also had a positive correlation with the number of yoga training sessions attended (Govindaraj et al., 2018). Despite the absence of a control arm, this study reaffirmed our initial pilot observations of enhanced GABAB-mediated cortical inhibition after yoga training in a dose-dependent manner. In an independent study (Guglietti et al., 2013), a similar effect of enhanced GABAB-driven cortical silent period duration was found in experienced meditators after single session meditation practice compared to television viewing (control task) in a group of individuals who were non-meditators. Interestingly, there was no effect of meditation practice on GABAA-mediated short interval intracortical inhibition in this study as well. Another study examining brief (5 days) yoga therapy versus intermittent walking in a randomized controlled experiment on 40 patients with depression, demonstrated a significant enhancement of the diminished CSP in the yoga group but not in the walking group (Jakhar, 2017). This rather specific effect of yoga and meditation on GABAB-mediated neurotransmission may be studied as a neuromarker of treatment response to yoga therapy. However, not all in vivo human studies examining the effect of yoga on GABA have found a GABA enhancing effect. A randomized controlled trial examining the effects of 12 weeks of memory enhancement training (n = 11) or equal duration of yogic meditation (n = 14) in elderly individuals with mild cognitive impairment demonstrated no change in GABA levels in the dorsal anterior cingulate cortex and hippocampus as measured by MRS; there was no correlation between change in GABA levels and memory improvement in either group (Yang et al., 2016). The impact of clinical diagnosis, the age of the individuals, and site of examining GABA using MRS could explain the discrepancies observed in this study as opposed to earlier studies quoted above. Nevertheless, it is important to further contextualize these observed effects of yoga on GABAergic systems, elicited via MRS and TMS studies in keeping with other theories of the mechanism of yoga. An active component of yoga practice—om chanting, which may enable experiencing vibratory sensations were studied using functional imaging. When compared to rest states, om chanting produced deactivations in several limbic structures (cingulate, parahippocampal gyri, and amygdala)—such an effect was not observed with a control condition (Gangadhar et al., 2011). These observations also support the view of an enhanced GABA tone and hence inhibition of the prefrontal cortex regulation exerted on the lower limbic regions, thus resulting in limbic deactivations. This regulation of the excitation-inhibition equilibrium in the frontal cortex can have beneficial effects on social cognition, bonding, connectedness, and behaviors. It is in this context that the GABA regulation via yoga can be understood as a mechanism that (a) reduces perceived stress by regulating the release of cortisol and (b) increases pro-social cognitions and behaviors via its modulation of the neuropeptidergic system in the hypothalamus.

4.3 Connecting the dots: The mechanistic basis of yoga—Effects on oxytocin
Oxytocin is a social neuropeptide—secreted by the hypothalamus, that, along with vasopressin, regulates a broad range of social behaviors in humans. These behaviors include coping to stress (Neumann, 2002), empathy (Rodrigues et al., 2009), pair bonding (Carter et al., 2008), social anxiety, and social cognition (Heinrichs and Domes, 2008; Veenema and Neumann, 2008). Recent advances in biochemical estimations of peripheral oxytocin levels (Carter et al., 2007) and genotyping of the oxytocin receptor gene (Tost et al., 2010) have demonstrated important associations between peripheral oxytocin levels, oxytocin receptor gene polymorphisms and psychopathology in depression and schizophrenia. Similarly, in schizophrenia higher peripheral oxytocin is found to be associated with greater pro-social behaviors and less severe positive symptoms of delusions and hallucinations (Rubin et al., 2010). An inverse relationship between peripheral oxytocin and depression severity has been found consistently in pregnant women; however, this relationship varies in non-pregnant women and men, indicating other factors that regulate this relationship (Massey et al., 2016). Recent work has demonstrated how the practice of imitation and being imitated—important ingredients of yogasanas, especially in a group setting or a teacher-student setting—can act as an endogenous oxytocin nebulizer (Aoki et al., 2014; Delaveau et al., 2015). This is supported by observations from a randomized controlled trial that demonstrated significantly greater elevations of serum oxytocin levels after yoga therapy but not after a waitlisted period in patients with schizophrenia (Jayaram et al., 2013). Similarly, a mindfulness-based mind-body intervention demonstrated enhanced salivary cortisol in cancer survivors, compared to sleep hygiene education (Lipschitz et al., 2015). Interestingly, intranasal oxytocin administration is shown to enhance mu suppression—an indirect measurement of mirror neuron system activity (Perry et al., 2010). The mirror neuron system is a promising social brain substrate that is likely to be involved in the reflexive, embodied simulation-driven deciphering of intentions underlying actions, empathy, and related social cognitive processes (Carr et al., 2003; Iacoboni et al., 1999). In clinical populations, a deficient mirror neuron system activity, predominantly in untreated schizophrenia patients, was found to be associated with social cognition deficits in theory of mind and emotion processing (Mehta et al., 2014c). In contrast, a heightened responsivity (possibly secondary to frontal disinhibition) of this neural network is associated with hyper-imitative behaviors (Mehta et al., 2013a), hallucinations (McCormick et al., 2012), and affective instability or mood dysregulations (Mehta et al., 2014a). Given its role in social information processing and social behavior determination, it is intuitive to surmise the role of mirror neuron system activation in yoga interventions, which involve a range of imitative exercises, that are often performed in group-settings. Based on these observations, we have earlier proposed that this property of yogasanas, where imitation and being imitated are integral, can potentially facilitate optimal activations of the mirror neuron system via oxytocin release, and hence result in the improvements in social cognition and behavior performances, especially in patients with schizophrenia (Mehta et al., 2016). Studies examining the impact of group-settings of yoga practice versus individual or solo yoga practice on contemporary measurements of putative mirror neuron system activity in the premotor cortex, inferior frontal gyrus, and inferior parietal lobule, will help in refining our understanding of mechanisms underlying yogasanas.
4.4 The GABA and oxytocin synchrony
This brings the focus to the inter-dependent relationship between GABA and oxytocin neurotransmission. From a neurodevelopmental perspective, it has been demonstrated that maternal oxytocin plays a central role in switching the fetal immature excitatory GABA actions to mature inhibitory functions (Tyzio et al., 2006). Blocking this important neuroprotective process of the oxytocin-induced switch in GABA functions in rodents resulted in behaviors of social isolation, much like the autistic-like phenotype (Tyzio et al., 2014). In addition, we also now understand that oxytocin perhaps acts via GABA receptors (Gough, 2015), in addition to its effects via the oxytocin receptors—both these receptors are found in abundance in the central nucleus of the amygdala, an important center in the social brain. Recent animal experiments have emphasized the inter-dependent role of oxytocin and GABA in the amygdala to regulate social responses. For instance, treatment of prairie voles with oxytocin after a stress-inducing protocol results in recruitment of GABAergic neurons, which in turn inhibit stress-related activation of the hypothalamo-pituitary-adrenal axis (Smith et al., 2016). This cascade of neurochemical events is also likely to confer neuroprotection against inflammation and oxidative stress, common mechanisms of stress-induced neuronal damage (Kaneko et al., 2016). Despite limited evidence for this interactive, inter-dependent relationship between oxytocin, GABA, and cortisol in humans, indirect evidence from human clinical trials suggests that oxytocin administration improves sleep architecture just like benzodiazepines (Braga et al., 2014).
In summary, the practice of yoga is associated with the often self-reported feelings of enhanced social connectedness (Kinser et al., 2013) and the congruent objective improvements in mood (Cramer et al., 2013b), anxiety (Cramer et al., 2018), social behaviors of schizophrenia (Duraiswamy et al., 2007), and autism (Radhakrishna, 2010) and social cognition (Behere et al., 2011) in clinical populations. These “therapeutic” gains are likely to be driven by the GABA and oxytocin enhancing effects secondary to a broad range of components within yoga therapy described above. Oxytocin-dependent or independent GABA enhancement in these clinical conditions can improve social behaviors via its actions on cortisol. Independently, the oxytocin enhancing effects of yoga can modulate the mirror neuron system, thus driving pro-social behaviors. This integrated neurobiological model of therapeutic gains of yoga (Fig. 3) can be studied at several levels of analyses outlined by the RDoC criteria.
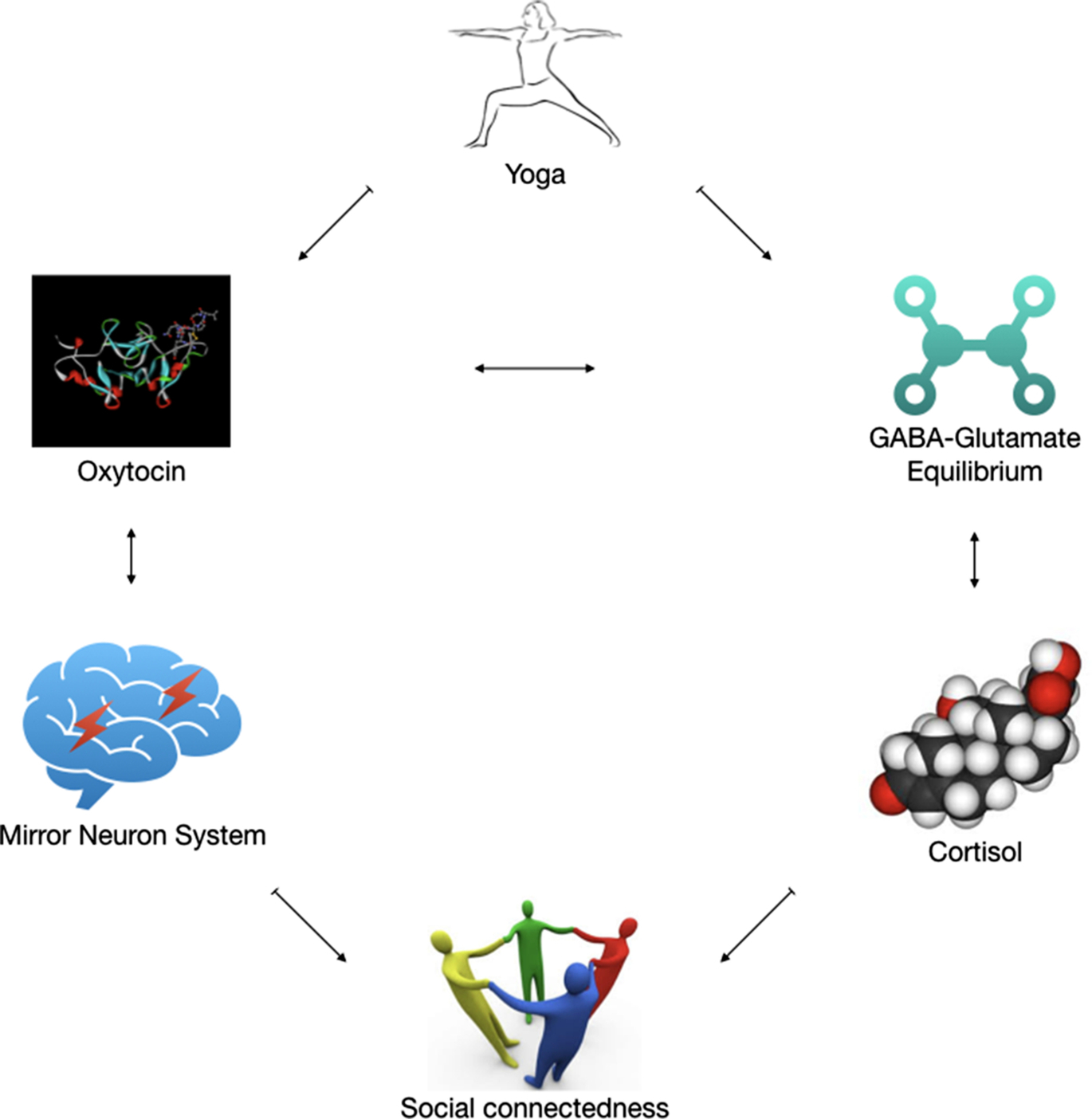
However, we concede that not many components of this model have been thoroughly examined with yoga therapy. Although emerging evidence supports the oxytocin and GABA enhancing effects of yoga, these findings need to be replicated, more so in clinical settings. The subsequent chain of physiological effects from oxytocin and GABA elevation to improve social functioning, via regulated cortisol response and mirror neuron system activation is yet to be examined empirically. The influence of independent components of yoga therapy—focused attention, posture-based exercises, chanting, breathing regulation, etc., on individual components of this model are also not teased out; most of the evidence for biological changes with yoga comes from the use of integrated yoga modules. Last, there might be other potential mechanisms (e.g., altering functional connectivity, synaptic plasticity, regulating autonomic brain functions) how yoga might exert its therapeutic gains in clinical conditions, which have not been alluded to in this model. It is likely that the active pathways mentioned in our model are related to some of these processes. These can be therefore added to the model based on the accumulation of stronger and consistent scientific evidence.
5 Clinical implications of this model
In keeping with recommendations of the National Institute of Mental Health funding policies (NIMH, 2018), it is critical that future trials of yoga for clinical conditions also consider additional investigations to address the mechanistic basis of this intervention. This requires researchers to (a) identify specific hypothesis-driven models that explain the benefits of yoga for a particular clinical condition and (b) examine the engagement of specific biological systems predefined in these hypothesis-driven models, in the course of any clinical gains reported. This exercise will not only provide a neuroscientific basis for applying a traditional system of practice in the clinical setting but also increase the acceptance of such a practice across cultures. The integrated neurobiological model that we have proposed here can be a useful hypothesis-driven model to be examined in such studies. It can also trigger the development and evaluation of other treatment avenues that engage these important social brain biological systems and bring about therapeutic gains that will not only be relevant to the severe psychiatric disorders like schizophrenia and depression but also developmental disorders like autism spectrum disorders. Given its role in engaging social brain systems in bringing about change in social cognition and behavior, the clinical application of yoga can expand to diverse psychiatric disorders that present with severe social impairments beyond schizophrenia and depression. These include its application as an adjunctive intervention in autism spectrum disorders, borderline personality disorder, and frontotemporal dementias. In this context, there have already been proof-of-concept studies in children with autism that demonstrates the feasibility and acceptability of prescribing yoga therapy for children with autism (Radhakrishna, 2010; Radhakrishna et al., 2010). Similarly, there have been calls to examine the therapeutic benefits of yoga in patients with frontotemporal dementias, that present with marked irreversible social behavioral impairments (Chandra et al., 2016; Kortte and Rogalski, 2013).
Among patients with schizophrenia, it is all the more important to examine and implement yoga therapy as a treatment of social cognitive deficits and residual negative symptoms like avolition and anhedonia, since these deficits are largely resistant to conventional psychopharmacological treatments (Kucharska-Pietura and Mortimer, 2013) and there are only partial or moderate gains with other newer treatments like cognitive retraining (Wykes et al., 2011) and non-invasive brain stimulation (Wolwer et al., 2014). In addition, based on this proposed model, it could also be examined if improvement in social cognition and behavior are mediated by reduction of anxiety—an important determinant of GABA function efficacy.
Biomarkers for psychiatric disorders and their role in predicting treatment outcomes have been limited because of the inherent challenges with respect to the heterogeneity and our limited understanding about the genetic and environmental factors that determine diagnoses and treatment outcomes. It is therefore important to combine efficacy studies with measurement of biological processes associated with these therapeutic benefits, based on existing theoretical frameworks. Replicability of these findings in independent datasets within and across diverse socio-cultural settings is equally important. Based on the oxytocin and GABA enhancing effects of yoga highlighted in this model, cost-effective and non-invasive in vivo monitoring tools like TMS-measured cortical silent period can be used as a clinical investigation to monitor treatment response and prognosticate.
Last, if the mechanisms proposed in this model are found to be true, then yoga might actually also be neuroprotective. Hence, it would be clinically relevant to initiate yoga therapy at an earlier stage in the course of illness, rather than await resistant symptoms to emerge. There should be a specific focus to, therefore, examine yoga therapies early during the course of schizophrenia or depression and study its impact on the long-term course and outcome of these disorders. Large-scale, multi-site clinical trials will be best suited to answer this very critical and challenging clinical question. If found to have a clinical role during the early course of illness, its potential clinical impact can also be examined in individuals with prodromal symptoms or those who are “at-risk” for developing severe psychiatric illnesses. Despite tremendous advances in understanding the neurobiological disturbances and developing accurate clinical definitions of these prodromal states, there are no standard treatment recommendations that are as yet applied in clinical practice for prodromal states. One of the apprehensions in this group of individuals is the potential harm to expose prodromal subjects to antipsychotic pharmacotherapy, knowing very well that not all prodromal states convert to a definitive psychiatric disorder like schizophrenia or depression. The role of yoga therapy in such instances of treating prodromal symptoms—milder quasipsychotic or subsyndromal mood changes in thoughts, emotions, and behaviors has not been systematically examined. This opens up forays for yoga to be explored as a preventive psychiatric intervention, a field with rather limited empirical evidence base, but tremendous clinical, public health, and humanitarian impact (Khalsa, 2013). In summary, the clinical implications of adapting and testing this model of yoga-driven improvements in social behaviors range from preventive psychiatry and prodromal pre-conditions to the more resistant residual negative and cognitive deficits of severe psychiatric disorders.
Acknowledgments
U.M.M. is supported by the Wellcome Trust/DBT India Alliance Early Career Fellowship, Grant/Award Number: IA/E/12/1/500755.
Funding
The funding agency had no role to play in the content and drafting of this chapter.
Conflict of interest
U.M.M. is one of the Associate Editors at Schizophrenia Research and receives honorarium from Elsevier for this service. None of the other authors report any potential conflict of interest.
