2. Baby Science: How to Conceive a Tennis Star and Other Procreative Miracles
We can’t help ourselves but to get a bit personal with the baby chapter. After all, as two of your authors (Andy and Kay) began writing this book, they had conceived a baby. And during the writing, Kay carried and gave birth to him. And he was raised through his first two and half years of life.
When it came time to write this chapter, the new parents were already heavily invested in the topic, and the research was exciting and highly relevant to the little farty beast in the bassinet next to their bed.
With that, dear reader, please meet Carter Devon Walker (see Figure 2.1).
If you want to be a parent or have actually become a parent, you know that a longing to know what your baby looks like starts from the moment he or she is a twinkle in your eye.
As with any pregnant woman, Kay had emotional swings throughout her pregnancy. In the last two months or so she’d have tearful moments where she would say: “I can’t wait to meet our little man.” Andy would get a little steamy, too.
Who doesn’t want to meet their baby?
It’s natural for parents to wonder what their child will look like. Which parent or grandparent he might resemble? And whether he will have (in our case) Sasquatch feet like his father.
When asked in a 2007 Gallup poll of 1,000 American adults, respondents were split on if, after discovering they were pregnant, they would want to learn their baby’s sex. Here are the numbers:
• 51 percent said they would wait until the baby was born
• 47 percent said they would want to know before the birth
• 2 percent had no opinion
Curiously, 58 percent of those who already had a child wanted to know their baby’s gender before birth, as did two-thirds of younger adults aged 18 to 34.
As the great American baby room painter Benjamin Moore, once said, “Knowledge is power.” Ok, maybe it was philosopher Francis Bacon who said that. (And ok, Benjamin Moore is a company, not a person.) Anyway, you get the idea.
But what if you don’t just want to know what they look like? What if you can design how they look so when they come out of mommy’s belly, there are no surprises?
Sounds weird and creepy? Perhaps for some. For others it is a choice they’d make. How do we know? Because we talked to a doctor in Los Angeles who has a waiting list full of people who want to design their baby’s looks.
In this chapter we explore the history of baby hacking. Test tube babies became possible in the 1980s. And in recent decades, gender selection has become possible. New DNA procedures will soon allow for disease prevention at a genetic level.
We’ll tell you how to use the wonders of medical science to choose a boy or a girl and how designer babies can be made. How problems with making babies have been fixed. What is possible today, and what’s coming soon. Really soon.
Baby Technologies
Making and birthing babies, raising children, keeping them thriving as teens, and getting them safely into adulthood, where they can ride motorcycles and eat red meat, among other perilous activities, is a lot easier than it used to be thanks to progress in baby technologies.
As we mentioned in the previous chapter, infant mortality has dropped massively in the past century as a direct correlation to accelerating technology improvement. That said, let’s look at some of the trends.
Baby Food
Aside from baby poo, there are few things that new parents obsess over more than feeding their child. Parents are crazy. Andy and Kay included. (Sean is exempt here.) They obsess about pretty much anything relating to their baby. But what goes into their baby (or toddler, or child) and what comes out of the little offspring typically dominates parental brain cycles.
“Oh honey, it was very seedy this morning. Not like the runny stuff the other day.”
Dinner at Nobu will never be the same again.
As you probably know, a baby’s development is highly reliant on what you feed it. In the first five to six months or so, it is breastmilk or infant formula. Then it’s baby food. Until you go into full-on kid meals and the ensuing “eat your broccoli and then you can have dessert” battles.
The breast milk versus infant formula decision can be an emotionally charged one, especially for mom. Some mothers might encounter a lot of pressure from family, peers, and medical professionals to breastfeed. And a decision not to breastfeed can often result in feelings of guilt, anxiety, and uncertainty.
If you are raising a super baby, you’ll want to know the facts, and what is coming next.
The Invention of Infant Formula
Generations of nonbreastfeeding mothers can thank a man named Justus von Liebig for the miracle that is infant formula. In 1897, he invented Liebig’s Soluble Food for Babies, and was the first person to understand the nutritional makeup of mother’s milk.
Early infant formula was welcomed by mothers who couldn’t afford to hire a wet nurse, a lactating woman who can feed a baby when their mothers can’t. Formula feeding at the time was called dry nursing. It raised concerns about proper nutrition: Could a man-made scientifically derived infant formula really be okay for babies?
Thomas Morgan Rotch designed a more natural solution, which he called the “percentage method.” It combined cow’s milk, cream, milk, water, and honey. By 1907, his homemade formula was all the rage.
But there were problems. Babies who fed on the original recipe were prone to diseases such as scurvy, rickets, and bacterial infections. Orange juice and cod liver oil were added to the mix to remedy the issues. Then came the formula companies. They produced new evaporated milk versions of infant formula, and by the 1950s more than half of babies in the United States were reared on the powdered products.
Hard science paired with the invention of the icebox, the affordability, and the convenience, made formula feeding a trend. By the 1970s, 75 percent of North American babies were fed infant formula. These days, it’s the other way around. So what happened? The Nestlé boycott, that’s what.
The Nestlé Boycott
In 1977, a boycott was launched in the United States against the company’s products. At issue was Nestlé’s advertising of its infant-formula baby food as an alternative to breast-feeding, particularly in less-developed countries.
The argument is that mothers in underdeveloped countries lack the ability to read or understand directions, and this impairs their ability to mix the correct ratio of infant formula powder to water. Lack of clean water in some regions can also put formula fed babies at risk.
The U.S. Senate subsequently held a public hearing investigating the claims. An infant formula marketing code was developed then presented to Nestlé, which accepted it. The boycott was subsequently dropped. Since then, 84 countries have enacted legislation implementing all or many of the provisions of the Code, which makes healthcare providers responsible for promoting breast milk over substitutes. It also sets forth labeling requirements for infant formula brands, such as Similac and Enfamil. Manufacturers are required to say that breastmilk is better on all their baby formula products (see Figure 2.2).
Figure 2.2 The Similac baby formula label promotes breast milk over formula because of marketing regulations that date back to 1981.
Study: Breast Might Not Be Best?
In 2014, a controversial study published by Cynthia Colen, Assistant Professor of Sociology, was released by The Ohio State University. It suggests that breastfeeding might not be better than formula feeding, after all.
(That loud series of pops you might have just heard was the breastfeeding lobbyists’ brains exploding.)
The research study examined 8,237 children in total, 7,319 siblings and 1,773 pairs of siblings, where children came from the same family but one child was breastfed and one child was formula fed. (These are referred to as “discordant siblings.”) The discordant siblings represented 25 percent of the total sibling population tested.
The study included families with different ethnicities and from varying socio-economic situations. All the children were assessed between the ages of 4 and 14". This provided an appropriate amount of time for their bodies to develop and for potential long-term health outcomes to surface.
Colen’s team evaluated the children for 11 health issues. When breastfeeding was measured against formula feeding in members of the same family, the criteria collected suggested that breastfeeding was no better than formula feeding.
The one measure that stood out among the 11 examined was asthma. Breastfed babies were affected more negatively by asthma than their formula-fed contemporaries.
Superior Infant Formula
There are a number of reasons why breastfeeding proponents say human milk is the best way to feed your baby. In truth, infant formula is missing a critical ingredient essential for building a strong immune system. It’s called 2-fucosyllactose (2FL).
2FL describes one type of human milk oligosaccharides (HMO). HMOs are polymers—large molecules such as protein or DNA—found in breast milk. 2FL essentially protects the body from infection. The bottom line is that it defends babies against pathogens by strengthening their immune systems.
In 2012, food microbiologist Michael Miller and his team at the University of Illinois found a way to synthetically create large amounts of 2FL at a lower cost than was previously possible allowing scientists to study 2FL more readily. Without it, a single study would cost $1million for a supply of 2FL alone. The high manufacturing cost is also why 2FL is missing in current infant formulas.
There’s still more work to be done. An improved infant formula, including 2FL, could one day soon appear on the market and rival mother’s breast milk.
Ultrasound Technology
A key part of prenatal care is going for a mid-term checkup to get a peek at the baby inside mom. This gives you an opportunity to find out the gender of the child and gives your ob-gyn a tool to ensure the baby is developing well. This is done with a technology called ultrasound. The technology, sometimes referred to as sonography, sends high-frequency sound waves into the body from a probe called a transducer. The sound waves hit tissues and organs in the body. These structures bounce the sound back to the probe in different ways, based on what they are made of and how far away they are from the probe. This information is processed by a computer and a two-dimensional image is generated on an ultrasound screen. It’s a handy tool when a doctor needs visual guidance and is otherwise blindly navigating inside the body.
Ultrasound is most commonly used on pregnant women to monitor fetal development. However, it is also used to help diagnose issues that affect organs and tissues. It’s ideal for peering at soft tissue in the gut; however, it has some limitations. It’s not ideal for examining bones or parts of the body that hold air or gas, such as the bowel.
Ultrasound has improved drastically since its introduction. These days, it produces an image that looks a lot more like baby. If you want to get a sharper look at your baby, you might consider 3D ultrasound.
3D and 4D Ultrasound
Olaf von Ramm and Stephen Smith at Duke University invented the 3D ultrasound in 1987, but it took some time to make its way to the mainstream. Today, 3D ultrasound is available to any parent-to-be who is willing to pay for it. It is similar to a CT scan, which is a form of X-ray that collects virtual slices of a scanned region on the body and builds them into a three-dimensional structure (more on this in Chapter 5 “The Human Computer: How to Rewire and Turbo-Boost Your Ape Brain”).
In the case of a 3D ultrasound, sound waves create 2D image segments that are reconstructed with a powerful computer system, which results in a three-dimensional image.
For an even more profound visual experience, 4D ultrasound is the way to go. It captures more detail and does it in real-time. With 4D ultrasound, you can see a live video of the baby moving inside its mother. To see it in action, have a look at this Youtube video: http://superyou.link/4dultrasound.
The FDA hasn’t approved 3D and 4D ultrasounds yet. As is the case with any ultrasound, when the emitted sound waves enter the body, they heat the surrounding tissue. Although not illegal, the FDA recommends against them because the long-term effects on the baby and mother are unknown.
Smartphone-Based Ultrasound
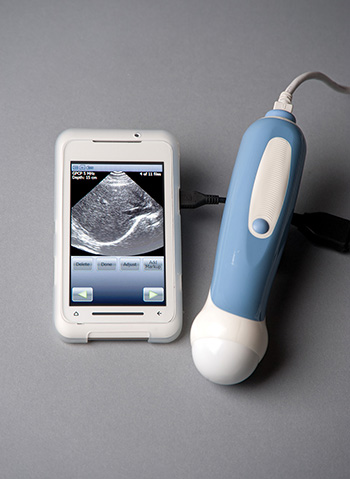
MobiUS SP1 Ultrasound Imaging System is the world’s first smartphone-sized system (see Figure 2.3).
(Permission to use image provided by Mobisante.)
Figure 2.3 The MobiUS SP1 Ultrasound Imaging System.
Medical professionals use it to provide remote care. A scan done in one country would be done locally—say on a pregnant mother—and the images would then be sent via the Internet to a medical facility for analysis anywhere in the world. The device has been approved by the FDA in the United States and costs $7,500 to $8,000.
The machine is small enough to fit in a pocket. It can capture eight different types of scans and store them in the device’s 8GB of memory. Images are shared via a Wi-Fi connection, a cellular data network, or a wired USB connection.
The Future of Ultrasound
Ultrasound technology is being explored for uses beyond imaging. And you might be able to use devices in your own home sometime in the near future.
High-Intensity Focused Ultrasound
Although ultrasound is traditionally used for imaging, doctors are looking at new ways to use it to destroy tissue in a highly targeted manner. It’s called high intensity focused ultrasound (HIFU) and is being tested on patients with various neurological disorders and cancers. However, it’s also being used in the baby world.
A 2014 article in New Scientist magazine reported the use of HIFU to treat twin fetuses that suffered from a rare disorder known as arterial perfusion. The twins were connected with an umbilical cord that passed through the placenta. This meant that only one of the twins had a heart. He had the big job of supporting the life of both him and his sibling. It’s too much work for only one heart, so in many cases both twins die.
For the first time ever, in 2013, doctors used HIFU to sever the connection and save one of the babies. The baby boy was delivered successfully at 37 weeks by Cesarean section.
More work needs to be done to fully understand HIFU, but this is one example of a treatment where it can save lives.
Magneto-Acoustic Imaging
Do-it-yourself ultrasound may be just around the corner. Pregnant women would be able to scan themselves with a home device and send the images to their doctors.
An experimental technology called magneto-acoustic imaging combines ultrasound with a magnetic field to produce resolution up to 1 mm (or 0.04 inches). This technology is exciting because it can be miniaturized. Ultrasound transducers, radiofrequency (RF) electrodes, and related electronics can all be produced on tiny silicon chips.
We can expect a working device in the next three to five years, and possibly a home medical imaging system within a decade.
Birth Control Technologies
Let’s turn to another society-bending technology that had a huge impact on parents.
Pop Quiz: Name three things when you think of the 1960s.
Woodstock? JFK assassination? Man on the moon? What else? Maybe you said: the pill. The introduction of oral contraceptives, combined with the women’s rights movement, gave women full control over their reproductive cycles for the first time.
Before the pill, various birth control methods had been around a long time, since 3000 b.c.e. to be more specific. The first condoms were made from materials such as fish bladders, linen fabrics, and animal intestines. It was not until 1838 that condoms were more popularly made from vulcanized rubber.
Although condoms were available, there was a lot of religious and political dialogue around birth control. In 1873, the United States government passed the Comstock Act, which was designed for the “suppression of trade in, and circulation of, obscene literature and articles of immoral use.” That included birth control technology, and as such, condoms. It also enabled the postal service to confiscate illicit items distributed by mail.
Then Margaret Sanger came along, and, in 1916, she opened the first birth control clinic in the United States. She was subsequently jailed for 30 days as a result.
In 1932, Sanger ordered a diaphragm from Japan to provoke a legal battle. The diaphragm was confiscated by the United States government, and Sanger challenged the action in court. It led to a 1936 court decision overturning an important provision of the Comstock laws, which prevented physicians from obtaining contraceptives. As a result, women gained a bit more freedom.
Sanger continued her work well into her 80s and was involved in some of the research that led to the discovery of the birth control pill.
In 1960, Enovid-10, the first birth control pill, was approved by the FDA. Today, there are many birth control technologies to choose from. And there are some exciting new methods in development that will soon come available.
Types of Birth Control
Birth control methods available today fall within four categories:
• Barrier methods that physically block sperm from contact with an egg.
• Hormone treatments that deliver hormones into a woman’s body and cause physiological changes that impair conception.
• Surgical procedures that provide a 100 percent birth control solution.
• The fourth is simple and sometimes overlooked. It’s abstinence.
Barrier Methods
• Condoms—Sleeves made from latex, polyurethane, or synthetic rubber that block sperm. The condom is the only method of contraception that can also prevent sexually transmitted infections. There are condoms for both men and women.
• Contraceptive sponge—This is made of foam and measures about 2 inches in diameter. It works internally in a woman by blocking sperm from entering the cervix and releases spermicide, a toxic solution that destroys sperm.
• Diaphragm—A diaphragm is a shallow silicone cup with a steel rim that creates an internal seal against the female cervix, which stops sperm from entering the uterus.
• Cervical cap—Similar to the diaphragm, the cervical cap covers the cervix and prevents the entry of sperm. Each cup is tailored to a woman based on whether she has previously given birth.
Hormone Treatment Methods
• Oral contraception—Widely referred to as “the pill,” there are many brands of oral contraception. Each works in a similar way. Pills must be taken daily so that they release hormones regularly to stop the release of a woman’s eggs.
• Contraceptive patch—The contraceptive patch is 2 × 2 inch sticker containing hormones that are released into the body through the skin. It prevents a woman from releasing eggs and makes implantation unlikely.
• Intrauterine device (IUD) or intrauterine system (IUS)—Small T-shaped devices that are inserted by a physician into a woman’s uterus. The IUD is partially made from copper and plastic, whereas the IUS is made of plastic only. The copper found on the IUD inhibits sperm and stops eggs from implanting. The IUS works in a similar way, but releases a synthetic hormone that inhibits pregnancy.
• Contraceptive implant—The contraceptive implant is similar to the IUS because it releases a hormone into the body. However, it is a 4-cm (1.6 inches) long plastic tube that is inserted under the skin in a woman’s arm. The hormone enters the body through the bloodstream and lasts for up to three years before it needs to be replaced.
• Vaginal ring—This is a small ring that is manually inserted and is similar to a diaphragm or cervical cup; however, the ring is not a barrier method. It works by releasing hormones.
Surgical Procedures
• Female sterilization—This surgery involves either blocking or sealing off the fallopian tubes so that the ovaries, which produce eggs, are no longer linked to the uterus. Eggs released are reabsorbed and sperm is unable to reach them.
• Male sterilization—This procedure is known as a vasectomy. It’s a 15-minute procedure where the tubes that carry sperm are blocked or cut by a surgeon. This procedure can usually be reversed.
“No sex equals no chance of pregnancy. The end.”
Abstinence
No sex equals no chance of pregnancy. The end. Unless you’re the Virgin Mary. But that puts you in a different bracket.
The Future of Birth Control
As you can see, a lot of technology options have been developed over the years to stop babies from being conceived, and to verify when they have been. Since the back half of the last century, they have been arriving at an accelerated rate, and they are becoming increasingly easier to access and use (and kinder to bunnies). What will be available next, however, is nothing short of extraordinary.
Remote Control Birth Control
Plans for the first remote-controlled contraceptive device for women are currently in development by a company called microCHIPS. Preclinical trials started in 2015 with the ultimate goal to have it hit the United States market by 2018. MicroCHIPS is a Boston-area startup, funded by Microsoft founder Bill Gates and his wife Melinda.
The remote control device is an implant the size of a thick postage stamp. It can be inserted under the skin by a physician in areas such as the buttocks, upper arm, or abdomen. Its lifespan is up to 16 years. Once installed, it administers a hormone called levonorgestrel. The hormone is currently found in many oral contraceptives and it stops ovulation, the process by which a woman’s body releases eggs. Once the device is inserted by a healthcare provider it can be toggled on and off with a remote control.
The implant has an air-tight seal made of titanium and platinum on the reservoirs that contain the drug. An electric current is passed through the seal daily by an internal battery which allows a dose of the hormone to be released into the body.
“The idea of using a thin membrane like an electric fuse was the most challenging and the most creative problem we had to solve,” said MicroCHIPS president Robert Farra in an interview with MIT Technology Review.
Although the prototype works well, researchers still need to figure out how to encrypt the information over a wireless connection. You don’t want your neighbor to flip the TV channel and turn off your wife’s birth control.
“You don’t want your neighbor to flip the TV channel and turn off your wife’s birth control.”
We can see a day when you might be able to manage your birth control with your smartphone. Yes, there’ll be an app for that, too.
Male Contraception
Most contraceptive devices are designed for women, with the exception of condoms. That could change if an injectable male contraceptive developed in India becomes available. It is currently undergoing trials in the United States and might become available in the next couple of years, likely 2018.
The innovation is called RISUG, which stands for Reversible Inhibition of Sperm Under Guidance. It is an injectable substance composed of two liquids that when combined, form a gel that blocks the passage of sperm in the vas deferens, the anatomical tube that transports them from a man’s testicle to his urethra. It’s the same conduit that gets tied off during a vasectomy. The compound also kills any sperm that come in contact with it.
The treatment is a 15-minute procedure where the solution is injected directly into the vas deferens. It takes only a few minutes for the entire inside of the tube to be coated.
The procedure was developed by Sujoy K. Guha, a professor of biomedical engineering at the Indian Institute of Technology in Delhi. In 2011, a technology transfer agreement was signed by an American organization, the Parsemus Foundation, for the rights to use the patent. Trials were conducted in the United States in 2014. When the product passes testing, it will be sold under the name VasalGel.
Trials in India have passed Phase I and Phase II trials are underway. In Phase I, volunteers who received the treatment were monitored for 15 years. There were no complications or pregnancies.
Phase II trials have shown to be equally successful. The only side effect so far is a swelling of the testes that can last up to two weeks following the initial procedure. This comes with no pain. (Authors’ tip: You might need bigger underpants, for a while.)
It looks like the procedure can be reversed, but this has only been tested in primates. They received the treatment for a year and a half and were given a reversal treatment, either an injectable with baking soda or a low electrical current. This removed the blockage. In two to three months, normal sperm flow resumed. It sounds awful, but we assume they will not be talking about zapping chimp privates in the ad campaign.
The United States product Vasalgel is currently in the animal testing phase (aka bunnies). Human testing is slated beyond that. If all goes well, the product will become available at a cost of no more than $800.
Curing Mr. Happy
Like on national TV, we can’t be too salacious here. So let’s talk somewhat clinically about male performance in bed. There’s a whole category of drugs that help men who have trouble in this department. Blue diamond pills. Yellow oval pills. Brands that rhyme with Niagara. If you are grown up, you know what we mean here. If you are not sure, go ask your mom.
It’s pretty self-evident that a man who has performance issues in the bedroom will have difficulty siring a child without some medical intervention.
Luckily, great strides have been made in the medical world to help men who have occasional or frequent trouble achieving this physiological state to make babies with a woman. It’s estimated that 1 in 10 men suffer from erectile dysfunction, or ED. And, men over 40 are typically the age group that has to deal with this issue. Though, dude, if you are younger and this is a problem for you, it’s cool. It happens to everyone sometimes.
ED can be caused by a series of issues. Typical causes include disease, blood circulation, injuries, surgery, or medications. Smoking, weight gain, and excessive drinking can contribute to the issue. Psychological or emotional issues can also be factors.
The latter can be treated with therapy and stress and anxiety management. Medication is typically prescribed for guys with physiological issues.
The big breakthrough in male erectile health arrived in 1991 with the discovery of the compound called sildenafil citrate. You probably know it as Viagra. The little diamond-shaped blue pill was first of a family of drugs to help men improve their sex life. Here’s a summary of the family these drugs by brand:
• Viagra: The original erectile treatment. Lasts about 4 hours.
• Cialis: Called the weekend pill. A 36-hour or longer potency.
• Levitra: Kicks in faster, in about 12 to 30 minutes. Lasts 24 hours.
• Staxyn: It’s Levitra, but in a form that dissolves quickly, so that it works faster.
• Stendra: The quickie pill. Kicks in about 15 minutes and lasts 6 hours.
The drugs are all PDE-5 inhibitors, which work by increasing blood flow to the penis during sexual activity. It works for about 80 percent of men.
Pfizer developed Viagra in the late 1990s in the UK. Their drug researchers were initially studying high blood pressure and stumbled on the surprisingly fun side-effect. We can only imagine what the human drug testers wrote on their forms.

If these drugs don’t help, then there are more extreme treatments that include the following: A medicated pellet inserted into the male urethra (the tube where urine flows from); medicine injected into the penis; or the use of a vacuum device on it.
For more severe cases, a penile prosthesis is an option. It involves two inflatable cylinders that are inserted surgically. A pump is also placed inside the body and, when activated, it pushes fluid from a hidden reservoir into the cylinders and that causes an erection. This equipment is under the skin so that it’s not evident in the locker room or the bedroom. Seems like a bit of a clumsy robo solution, but until there are other therapies it’s a valid fix for some men. Plus c’mon, robots are cool.
![]() We’re Not Making This Up
We’re Not Making This Up
The drug Viagra is also known to reduce jetlag in hamsters. You think we’re kidding? This is from research published by two Argentinian scientists from the Proceedings of the National Academy of Sciences.
Although there is a good selection of ED therapies available today, what’s coming in the future is quite, dare we say, exciting. Here’s a summary:
• Uprima—This cleverly named drug is a tablet that dissolves under the tongue. It works by stimulating the brain chemical dopamine, which heightens sexual interest and sensations. Sounds great. However, its major side effects are nausea and vomiting. Additionally, and perhaps unfortunately, a small number of people pass out after a dose. In late 2014, its release in the United States was (perhaps thankfully) put on hold. That said, it is currently available in Europe. Clinical trials are also currently being conducted on a nasal spray form of this drug, which might cause less nausea.
• Gene therapy—This technique in development by researchers will help treat and prevent a variety of diseases. It targets the genes (elements in the human biological blueprint) that cause the problems. The idea is to repair or modify the genes that might not be functioning properly in penile tissue. Replacement of these proteins would result in better erections. Gene therapy is expected to make great progress in the next decade. We’re forecasting sooner than ten years thanks to the logarithmic improvement of technologies.
• Melanocortin receptor agonists—These are a new set of medications being developed to treat both low sex drive in women and erectile dysfunction in men. They have been shown to produce an erection in animal studies. (There is probably a banana joke here, but we’ll leave it alone.) The medication impacts the nervous system instead of the vascular system. PT-141—also known as bremelanotide (or “brem”, to some)—contains a synthetic hormone that acts on the hypothalamus, a part of the brain. It appears to be effective alone or in combination with PDE-5 inhibitors (Viagra and the like). Some call it the libido drug, because it acts on the brain and not specifically blood flow to help male performance. It is also known to work to boost the female libido, an issue that thus far has limited known treatment options (although note that many sufferers have turned to off-label drug use to treat it). If you click around the web, you’ll find people who have tried it with great success. You can even order it online for about $30 for a small vial. Main side effects include facial flushing and nausea. Brem is not yet approved by the FDA for mass-market use, but it is being studied by Palatin Technologies, Inc., which expects to wrap up current research sometime in late 2016. It says it may then make a submission for approval to the FDA. More research is needed before the drugs can be proven to be safe.
Baby-Making Technologies
Couples, particularly single randy teenagers and young adults, spend a large chunk of their lives trying to avoid making babies, while still going through the rather fun motions.
However, when a couple is ready to make a baby, ironically, sometimes the process doesn’t work. Here’s good news and better news. Today we know so much more about sex, fertility, and the biological processes required to produce a healthy newborn baby. The expertise on this topic is growing at a remarkable and compounding rate.
The logarithmic improvement of technology is at play here. More than 2,000 years ago, Aristotle cracked open a few fertilized chicken eggs in his efforts to understand the nature of life.
We can thank the humble microscope for opening up the world of human fertility because human eggs are a lot smaller than chicken eggs. A microscope powerful enough for use in scientific investigation was produced in the early eighteenth century. But magnifying tools had been available long before that. Glass was invented in the first century and the Romans investigated viewing objects through it almost from day one.
In the thirteenth century, Italian Salvino D’Armate designed the first eye glass that allowed its wearer to see magnified objects through one eye. An early microscope came later as the magnifying glass was adapted to make objects bigger.
However, it wasn’t until 1677 that Dutch microscope innovator and scientist Antonie van Leeuwenhoek produced a microscope powerful enough to see sperm. In 1898, fertilization was described as the union of an egg and a sperm. And only eight decades later, in 1978, the first “test-tube” baby, Louise Joy Brown, was born in England. A year later, her sister was born, also with the aid of in vitro science.
You might see the logarithmic improvement of technology in the previous short historical account. If you did, we think you’re clever.
Aristotle had the curiosity but couldn’t get much further than what he could observe with his eye. But then the invention of glass begat magnifying technology, which in turn provided the technology for a microscope. That was in the first 1,700 years or so. With the invention of the microscope, it took scientists only 200 to 300 years to figure out how to make babies in a test tube.
The First Test Tube Baby
If you are 40 years old (or older), you might remember a news story from the late 1970s about Louise Joy Brown. Brown was the first test tube baby, born on July 25, 1978. Her genesis was aided by the first in vitro procedure, a process where egg and sperm are introduced and combined to produce a viable human embryo in the lab.
It all happens in a petri dish, a shallow receptacle where egg and sperm are mixed. The resulting embryo is then re-implanted into a healthy woman. It in turn embeds in the wall of her uterus and she becomes pregnant.
In Vitro Fertilization
The process to create Louise Joy Brown, developed by U.K. medical pioneers Dr. Patrick Steptoe and Dr. Robert Edwards, is called IVF or in vitro fertilization. Edwards received the Nobel Prize for his pioneering work on in vitro fertilization in 2010. Steptoe, Edwards’s partner, died in 1998. He was passed over by the Nobel judges because it is not awarded posthumously (somehow that seems unfair).
![]() Why “In Vitro?”
Why “In Vitro?”
In vitro, from Latin, means “in glass.” The first “test tube” baby was conceived in a petri dish, which is a flat-bottomed glass dish.
Here’s how a typical IVF procedure is done today.
A laparoscope, a probe with a light on it, is inserted through a small incision just below a woman’s belly button. It is used to reach her ovaries and extract her eggs. The eggs are mixed with sperm in a petri dish. Two days later, the fertilized egg is deposited inside the woman’s uterus where it can attach itself to the uterine wall.
Not all procedures work the first time. The success rate is 28 percent to 35 percent, which, as you can imagine, is frustrating for couples who are struggling to conceive. It can also be an expensive process. Treatment ranges from $10,000 to $15,000 and is not covered by medical insurance.
![]() Fertility Abroad
Fertility Abroad
About 20,000 to 25,000 couples travel outside of their home country each year to receive treatment elsewhere. It’s a trend known as fertility tourism. According to the website IVF-abroad.org, Dubai and Israel surprisingly have a good reputation for IVF. Couples with tighter budgets go to Mexico, Panama, Argentina, or Columbia for IVF treatment, where procedures are more affordable.
Candidates seeking IVF treatment are typically dealing with infertility problems, ranging from medical issues, such as fallopian tube damage or polycystic ovarian syndrome, to older couples who have passed their best reproductive years. As of 2013, 5 million IVF babies had been born worldwide since the procedure had been developed. That’s just slightly smaller than the population of Atlanta (5.5 million people in 2013).
A Swedish study, cited in Time magazine, found that 47 out of 100,000 infants born from IVF develop cognitive deficits, such as low IQ or problems in communicating or socializing with others, compared to 40 out of 100,000 naturally conceived children.
Artificial Insemination
Artificial insemination (AI) gives women the choice to have a baby with or without a partner. The fertility treatment is not quite as fun as the traditional baby-making process. No sex required! Sperm from a selected man—a donor or partner—is inserted into a woman’s uterus by a doctor with an unsexy medical instrument. It’s all very clinical and the man can be a continent away and anonymous to the mother.
This is a good option for single women wanting to have a baby, or lesbian couples. Donor sperm is usually acquired from a friend, acquaintance, or donor bank.
Male/female couples seeking this treatment are typically dealing with medical issues such as immune system rejection of sperm and dysfunctions of the cervix (such as cervical scarring) that make it difficult for the couple to get pregnant on their own.
The first AI procedure on humans dates as far back as 1770. A renowned Scottish anatomist and surgeon, Dr. John Hunter, advised a patient of his who had hypospadias (a condition where the opening of the penis is located on the underside of it, instead of the tip) to collect his ejaculate, put it in a syringe, and have his wife insert it into herself.
This was perhaps a primitive yet effective early AI operation. However, it led to more studies in fertility science. The big breakthrough arrived mid-century when Dr. Jerome K. Sherman, a then-doctoral candidate at the University of Iowa, discovered the ability to freeze sperm to preserve it for later use. In 1953 he pioneered the technique that allowed the use of frozen sperm to be used to create a viable and healthy pregnancy. The method was unveiled at the 11th International Congress of Genetics in 1963 and a decade later, the first sperm banks opened for business.
Today, there are three common AI procedures. The easiest and most common one is intracervical insemination which involves the use of raw (unwashed), fresh, or frozen semen which is deposited on the neck of the cervix to most closely mimic natural baby making.
Intrauterine insemination involves introducing washed semen (see related factoid) directly to the uterus. Women receiving this procedure should be under 30 years of age and the donor of the sperm should have a sperm count of more than 5 million per milliliter.
Lastly, there is a method called intrauterine tuboperitoneal insemination where both the fallopian tubes and the uterus are injected with 10 ml (about 2 teaspoons) of sperm. This procedure is often used for couples who are dealing with male infertility and mild forms of endometriosis, a problem where uterine cells grow outside the uterus causing pain and infertility.
The Future of Reproduction Technology
While IVF technologies increase the chances of producing a baby after natural methods have failed, it doesn’t always work or guarantee a healthy or disease-free baby. At least until now.
Next Generation DNA Sequencing
How about an IVF procedure that guarantees a woman will produce a 100 percent genetically healthy baby? Dr. Dagan Wells, a medical researcher at Oxford University in England, has pioneered a new method that not only does that, but also improves the chances that the egg will attach to the uterine wall so that a woman becomes pregnant.
With current IVF procedures it is easy for chromosomal abnormalities to be overlooked under the microscope. This can lead to problems with implantation and can cause complications in the pregnancy so that a mom could lose her baby.
In designing the new procedure, Wells and his team used existing DNA sequencing techniques, but improved them. DNA sequencing is a process whereby the blueprint inside a cell that describes a human being is checked to ensure it is genetically healthy and has no errors. It’s like a spell check on a document. Or, maybe it should be a called a cell check.
DNA sequencing, where one cell is checked, is less expensive than current IVF screening procedures, but here is the catch. Scientists can only safely remove one cell from each embryo at a time without the risk of destroying it entirely. As you can imagine, this is a delicate and time-consuming process.
Wells’s team solved the problem by creating a unique method that sequences cells from groups of embryos at the same time. They used a system of barcodes to keep them organized and to ensure that the correct cells are paired back with their parent embryo following the screening process.
When the cells are removed, 2 percent of each embryo’s genome are sequenced. This percentage is enough for them to identify the number of chromosomes. It does not however, provide additional information with regards to specific genes.
Wells’s trials were conducted at Main Line Fertility, a clinic outside of Philadelphia, where the parents of the first baby born using his technique were treated. They gave birth to a healthy baby boy named Connor Levy in 2013.
It’s easy to see why Wells’s method is an improvement. Prior to entering trials, the couple had produced a total of 13 embryos that developed properly, but the problem was, researchers had no idea which ones to transfer. Wells analyzed the embryos and his test selected 4 of the 13 that were actually healthy. Of that four he only needed to use one.
This is very different from current IVF procedures. They require the analysis of up to three embryos for best results. With more embryos introduced in the uterus, there is a 30 percent chance that the couple will produce multiples (twins, triplets, or high-order multiples).
Wells’s technique eliminates this. There is no chance of multiple births.
Current genomic procedures cost approximately $5,000. Wells’s procedure runs as little as $1,000 per treatment. And, with his accuracy rates, a genetically healthy baby can be produced on the first implantation with the first embryo.
If you’d like to learn more about the work of Dr. Wells and his team, visit Reprogenetics at superyou.link/reprogenetics
Baby-Saving Technologies
If you have a sick baby, as a parent you’ll do anything to make the little tyke better. Thank goodness these technologies are either here, or on the way. Of course, in some cases you have to act before the baby is born or ever gets sick.
Stem Cells from Cord Blood
Before your baby is born you should be asked if you want to save your child’s cord blood—that is, blood in the umbilical cord. This procedure allows stem cell-rich blood to be collected and preserved for therapeutic medical use by the baby’s family at a later date.
The process is relatively simple, it takes only ten minutes and the baby does not feel a thing. Once the baby arrives, the cord is clamped and cut as it normally would be 1 to 2 minutes following the birth. A needle is then inserted into the umbilical vein, the area of the cord that remains attached to the placenta, and 1 to 5 oz of blood is then drained from the cord into a collection bag. The bag is shipped to a blood bank where it is tested and processed using a controlled method of freezing.
The blood is stored in either a private cord blood bank for the exclusive use by family members, or can be donated to a public cord bank and used for blood transfusions. The blood from the umbilical cord is rich in stem cells, which are seed cells that can develop into many types of human cells. It can be used to help cure a myriad of diseases, such as leukemia. Without cord blood, treatment for leukemia would require a bone marrow transplant from a matching and often hard-to-find donor.
Other uses include therapies to treat these major illnesses: Aplastic anemia, thalassemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, cerebral palsy, autism, type 1 diabetes, and host of rare and fatal metabolic disorders.
According to Babycenter.com in 2012, 33,900 cord blood units were shipped from the United States worldwide. In total there are more than 1 million cord blood units stored in United States family blood banks.
The average cost for the first year of storage ranges from $1,400 to $2,300. In the ensuing years, you can be charged up to $1,800 per year for storage.
Only a small percentage of people will ever use their cord blood. At any point in time, it can be donated to a public blood bank. Most western countries have cord blood banks, including the United States, Canada, and the UK.
We asked medical futurist Dr. Bertalan Meskó (who you will hear more from in Chapter 4, “Lifesaving Hacks: Whirring Hearts, Printed Organs, and Miraculous Medicine”) about cord blood to help us decide whether authors Kay and Andy should save Carter’s blood.
He told us that if he were having a baby in 2014 he would probably opt for cord blood. In another five years he thinks cord blood will not be necessary. As soon as 2019, Meskó predicts that based on the developments he has been witnessing in medical science, “We will be able to convert skin cells into any type of tissue,” meaning that adults will be able to use their adult skin cells to treat their diseases.
Genome Sequencing
It’s not a mainstream procedure yet, but in June 2014 the first American baby had his genome sequenced at birth. The baby was born in California, and his father, Razib Khan, was a graduate student studying genetics at the University of California.
Genome sequencing is a process by which an individual’s genes are mapped. It is a detailed blueprint of what makes you you. This allows doctors to look at specific genes to determine if potential diseases will develop.
In an interview with the MIT Technology Review, Khan said sequencing his son in utero “was more cool than practical.”
![]() Razib’s Baby
Razib’s Baby
Read more about Razib Khan and his adventures in genomics here: http://www.superyou.link/dealwithit
To get his son’s genome sequenced, Khan had to first get a sample of his DNA, even though he had yet to be born. He did this by requesting a chorionic villus sampling (CVS) test, which is used on pregnant women to test for genetic abnormalities in unborn babies. Cells are taken from the placenta and analyzed to retrieve valuable information about the growing baby’s DNA.
His wife’s doctor sent the sample from the placenta to Signature Genomics, in Spokane, Washington, for analysis. When the results from the test came back normal Khan asked for the raw data. The request met a lot of resistance.
Britt Ravnan, one of the company’s lab directors, explained why in the MIT Technology Review: “What was unusual in this case was that it was not the patient or the physician asking for the sample, but the patient’s husband.”
After Khan’s wife and her doctors filled out the necessary paperwork, Signature Genomics released the information. Khan sequenced his son’s genome using a free online tool called Promethease. It’s a do-it-yourself genome sequencing program that is easy for anyone to use, although users have to agree to a stringent terms of use policy. They are asked to upload specific genetic information or export it from a personal genetics testing company, 23andMe.com. The software then generates a genome report. It was that easy. If Khan can do it, you can too.
However, genome sequencing is a controversial subject. If couples get their baby’s genetic information before their baby is born and the information suggests a genetic disorder, the couple could choose to abort the baby.
One other wild card. The genetic information isn’t guaranteed to be accurate. It’s more of a predictor. Genome sequencing has limitations. It predicts possible diseases, but that doesn’t mean a condition will necessarily manifest in an individual. Some outcomes, such as heart disease and stroke, don’t come from a single gene. Other factors are involved including lifestyle choices and environmental exposure.
The possibility of elective abortions motivated by genetic information are why anti-abortion lobbyists and some religious organizations will fight the future approval of genome sequencing for babies, prior to birth. Khan argues that personal genomic information is a personal right. “How dare the government question [our] right to know the basic genetic building blocks of who [we] are,” he wrote in one of his blog posts.
Nanotechnology for Babies
If there is one field of science that is going to have a major impact on our health and well-being, it’s nanotechnology. Nanotechnology is the ability to change, adjust, or repair elements at nanoscale. A nanometer is the diameter of 5 carbon atoms, which is at the molecular level. This is why developments in nanoscience are showing great promise for the field of biology. When it comes to humans, it means we’ll be able to manipulate not only tiny cells but their contents, too.
“If there is one field of science that is going to have a major impact on our health and well-being, it’s nanotechnology.”
The greater application of nanotechnology is that we can build tools to manipulate the building blocks of the universe at the atomic or molecular level. Theoretically if you wanted to build a cheese sandwich from its base elements, super advanced nanotechnology tools would let you do that.
Ray Kurzweil told Big Think, “We’ll be able to create devices that are manufactured at the molecular level by putting together molecular fragments in new combinations. I can send you an information file and a desktop nanofactory will assemble molecules according to the definition in the file and create a physical object.”
Not only will we be able to modify a cheese sandwich at its elemental level, we’ll be able to use nanotechnology in medicine specifically for babies. It will be useful in fertility and the actual processes that create and maintain life, ensuring that a couple can conceive and birth a healthy baby.
The University of Alberta in Canada is currently researching nanotechnology to improve the success of organ transplants for newborns.
The challenge with transplants is that the blood type of the donor often needs to be compatible with the blood type of the recipient; otherwise the recipient’s immune system will reject the donor organ within the first 24 hours. This is true for adults, who require an exact blood type match. Babies (and young adults) have a developing immune system so it’s possible that their body could accept an organ with a blood type that doesn’t match their own. In medical circles, organs incompatible with blood type are what’s called ABOi organs.
In 2014, 50 percent of babies who needed a new heart died while waiting to receive a heart that matched their blood type. This is an unfortunate statistic, especially when 60 percent of donor hearts for babies are discarded because they don’t get used in the window of time before they become unusable.
The Canadian research team is currently conducting trials to see if attaching blood antibodies to nanoparticles that are sent directly into the bloodstream can increase the likelihood that a baby’s body will accept an ABOi organ. In the future, vaccines could be developed that would make it possible for babies or children to receive the antibodies via an injection.
In June 2014, the University of Alberta received $1.1 million in funding from the Canadian federal government to develop this research, and studies are have started on laboratory animals. If successful, the research would transition into human testing.
If the proposed nanotechnology is effective, it could save the lives of 100,000 babies worldwide each year.
![]() Saving Lives, One Baby at a Time.
Saving Lives, One Baby at a Time.
Each year, 20-million premature babies die worldwide. In developing countries, the mortality rates are significant due to a lack of incubation equipment.
In 2011, a team at Stanford University, led by Jane Chen (CEO) and Rahul Panicker (CTO), launched their first product—the Embrace Infant Warmer. The affordable $25 baby warmer is a cocoon-shaped pod designed to keep a baby warm. No electricity is required because the device is warmed with a wax pouch insert.
Embrace has saved babies with the product in places such as South India and Nepal, where trials first began. The company’s aim is to help millions of babies in resource-constrained areas survive by developing low-cost healthcare technologies.
3D Printing To Save Babies
3D printing is an exciting prospect in the medical world. It was created in the mid-1980s by American inventor Chuck Hull who developed stereolithography, a process where an ultraviolet laser is used to heat plastic material so that it hardens into a number of horizontal layers that are piled vertically to create a specific object.
For example, in the world of engineering, machine parts can be printed on demand. Or, new inventions can be prototyped quickly without the need of a costly manufacturing process.
Because newborns are tiny, operating on them or finding donor organs or tissue to treat their diseases is not easy. Historically, organs come from donor babies—ones that haven’t survived. But, all that is changing with the advent of 3D printing.
In 2012, the first 3D printed splint saved a baby boy named Kaiba. It was surgically implanted into his body to repair his trachea, the pipe that delivers air from the month to the lungs.
The baby’s windpipe was too narrow, which restricted his ability to breathe. It’s a rare condition known as tracheobronchomalacia.
In 2012, Dr. Scott Hollister and Dr. Glenn Green, professors at the University of Michigan, performed the groundbreaking operation. They took an X-ray of the baby’s trachea and custom-designed a splint to fit his body. Then, they performed a surgery to replace the old trachea with the one they created. Immediately following the surgery, Kaiba could breathe normally.
Eventually, the splint will grow with his airways. It’s biodegradable so that it will completely dissolve in his body over a period of three years.
The doctors also received a FDA approval for a second similar surgery to treat another baby boy, Garrett Peterson, a year later. His surgery was performed on January 31, 2013. Today, he is a very healthy little boy.
They are now discussing plans to start a formal study so that they can test their method on more babies. One day soon there is hope that is will become a common medical, baby-saving, technique.
In 2015, doctors at C.S. Mott Children’s Hospital at the University of Michigan’s Health System used a 3D printer to build a model of an unborn fetus’ face.
An ultrasound revealed that the fetus had a (potentially cancerous) mass, and doctors were uncertain of the correct procedures required to deliver the baby without restricting its ability to breathe. So, they used an MRI to gather images to print a 3D composite.
The 3D model revealed that the unborn baby had a cleft lip and a cleft palette with no airway obstruction. With this knowledge, the team of doctors were able to safely deliver the baby.
Dr. Glenn Green, Associate Professor of Pediatric Otolaryngology at Mott Children’s Hospital, led the team of doctors. He told CBS News “by doing the 3D modeling, we were able to tell that the mass wasn’t cancerous and determined that it would be safe to not do the most advanced types of intervention.”
This was the first time 3D printing was used in utero. Green told CBS News, “we think this is a real game changer”. He suggests that in the future, 3D printing could help many more doctors: “OB’s could potentially have a 3D printer right by the ultrasound machine.”
![]() Print Your Fetus?
Print Your Fetus?
In 2016, 3D printed fetuses might be the hottest trend for expectant parents. Watch this video to learn more: http://superyou.link/3dfetus
Designer Babies
Two months into author Kay’s pregnancy with Carter, we obtained an image composite from Morphthing.com to get a sense of what Andy Walker Jr. might look like. Using the program, we uploaded a picture of both our faces and clicked a button, and got a sense of what our developing son looked like. As you can see from the photo in Figure 2.4, it’s hardly scientific, nor is it accurate. In fact, if you cross Kay’s picture with a picture of the poet William Butler Yeats you get a similar baby with black-and-white old fashioned glasses. He looks like he could write a heck of an Irish poem, though.
Figure 2.4 Morphthing.com composite of Andy and Kay (left), an actual picture of Carter at 5 months (center), and a Yeats/Kay combo from Morphthing (right).
Still, it was close as we could get to seeing what our little guy would look like before he was born.
Today, there’s no definitive way to know for certain what children are going to look like before they are born, but with advances in medical science, and movements in government policy, one day parents will be able to select their traits. Yes, it’s time to delve into the very controversial world of designer babies.
Gender Selection
What if you could choose to have a boy or a girl? For some, this might seem a bit unnatural. It was for Kay, who as we mentioned earlier, was pregnant while writing this book. Now that she has a boy she admits she wouldn’t mind if baby number two was a girl.
Before she started the research for this book project she wouldn’t have considered gender selection as an option. But, as she educated herself on the process, she became much more open to the possibility. Read the next section and see if your opinion changes, too.
Nature’s Method
Let’s start with the natural gender-selection process. It’s important to know how things work naturally, so you can better understand how intervention works to get the gender parents want for their baby.
Chromosomes are an important part of gender selection. They are thread-like structures found in the cell’s nucleus, which is the cell’s command and control center. These segments of DNA contain genes and genes carry valuable information from each parent to their offspring.
Genes map out what traits a person will get. It’s why baby Carter has Andy’s Sasquatch feet and Kay’s finger toes. Carter also has one second toe that’s longer than his big toe on one foot only and he got that from his maternal grandfather. (And, we’re sure, that one day he’ll be mad that we told his toe secrets to the world here. Sorry, Carter!)
Otto Bütschli, a German zoologist, was the first person to identify the chromosome in the 1880s, but it took many years for us to fully understand them. It was not until 1955 that cytogeneticist Joe Hin Tjio discovered that the normal number of chromosomes in each human cell is 46. A cytogeneticist, by the way, is someone who studies chromosomes specifically.
Tijo’s breakthrough was a big deal because it helped scientists understand what was normal and abnormal. They could study cells under a microscope and identify genetic abnormalities.
Now, there are two chromosomes that determine whether a human will become a boy or a girl. They are the X and Y chromosomes, and when they combine they produce a code that determines gender.
Carter is a boy, so he has XY chromosomes. Ellie, his little girlfriend down the street, has XX chromosomes, which makes her a girl.
Women carry only X chromosomes. Men carry two kinds of chromosomes (some sperm have the X chromosome, whereas some have a Y chromosome). This means it’s the father that determines the gender of his offspring. This process of sex differentiation happens during week six of a baby’s development. That week, the baby, now an embryo, has sex chromosomes from both parents that start to express themselves. The woman provides an X to the offspring and the man provides either his X or his Y. The result is an XX girl baby or an XY boy baby.
Based on the chromosomes, sex organs grow from an originating organ called the gonads that turn into testes for a boy or ovaries for a girl.
The whole sex differentiation process has always been left up to fate. That is, until scientists figured out the mechanics of it all and discovered new gene-screening technologies that would allow the process to be manipulated.
Before choosing the sex of a baby was even a possibility, the technology used to do it had to be established. Gene-screening technologies were originally established to screen embryos for genetic abnormalities, but once this became available it was possible to decipher the baby’s gender using the same techniques.
Amniocentesis
Amniocentesis was perhaps the first procedure used to determine gender. It was originally developed to screen for disorders such as Down syndrome, cystic fibrosis, sickle cell disease, muscular dystrophy, and Tay-Sachs.
By the 1970s, it became a profitable tool when marketed for gender selection. In Asia, where some cultures value male babies more than female babies, there was high demand.
Amniocentesis involves the extraction of amniotic fluid, the liquid that surrounds the fetus. This liquid protects the baby and helps it grow. The fluid is composed of water and electrolytes at first, but by weeks 12 to 14 it also contains proteins, carbohydrates, fats, and the baby’s urine.
The amniotic fluid is sampled with a syringe that penetrates the mother’s belly. Once the fluid has been extracted, fetal cells are separated from the fluid. The sex can be identified by examining the cells under a microscope.
The procedure is still in use today, but not commonly used for gender selection. It’s used primarily on three categories of mothers to be:
• Women who are older than 35
• Women who already have a baby with a genetic disorder
• Women who are carriers of potential genetic mutations
The Ericsson Method
In the 1970s, Dr. Ronald J. Ericsson created a new method of gender selection called appropriately, the Ericsson Method. It’s a procedure that divides sperm based on gender and uses it in high concentrations of the selected gender sperm to create a gender-specific baby. Simply put, to make a girl baby, doctors take the girl sperm and put it in the mom and vice versa for a boy.
Ericsson believed that the sperm with X chromosomes, female sperm, swim slower than sperm with Y chromosomes, male sperm. When a group of sperm are released in a test tube that contains albumin, a protein in human blood plasma, or more informally, the clear white substance you’d see around a yoke if you cracked an egg (the white), some will swim slower into the albumin than others. Those sperm, the slower swimmers, are the female ones and they’ll be used in artificial insemination for a female baby. The fast swimmers are used to make boys.
Overall, the method is only 70 percent to 72 percent effective for producing male babies and 69 percent to 75 percent effective for producing female babies. While it is still used in United States clinics, its lower success rates make it a choice for gender-selection treatments, but not the best one.
Sperm Spinning
Sperm spinning involves separating male and female sperm based on their genetic composition. Besides being slow swimmers, female sperm are larger, and they live longer than male sperm. Female sperm also look different when viewed under a laser. And they look great in heels.
“Female sperm are larger, and they live longer than male sperm.”
The sperm are placed inside a centrifuge where they are spun so that their contents separate. Immotile sperm (aka duds) and seminal fluid are separated from the healthy sperm and the remaining sperm are stained with a fluorescent dye so that the DNA in each cell can be viewed. The female sperm shine brighter under a laser because they are plumper. (But, warning: Never call a female sperm “fat”.)
The sperm-spinning technology is called MicroSort. It was established by the U.S. Department of Agriculture. In 1984, however, the rights to the MicroSort technology were sold to a company in Virginia named MicroSort and they are still in operation.
Preimplantation Genetic Diagnosis
The preimplantation genetic diagnosis (PGD) method is currently the most effective gender-selection technology. PGD is an embryo selection technique. It does two things. First, it allows for the embryo to be sorted—male versus female. It also is a process used for genetic screening. The results are a gender-specific and genetically healthy embryo for implantation. Once the selection process is complete, it can be used in either IVF or AI.
More specifically, this is how it works:
When a sperm and an egg meet, they merge—or fertilize—which means 23 chromosomes from the man combined with 23 chromosomes from the woman generate the 46 chromosomes necessary to create the first cell of their offspring. The newly formed single-celled entity is called a zygote. The zygote cell divides into two in a natural cell-division process known as mitosis.
During mitosis, the cells duplicate themselves entirely and multiply. This has to happen for the initial cell to eventually become a human being. So, one cell becomes two, two cells becomes four, four becomes eight, and so on until you get something that resembles either Kim Kardashian or Donald Trump, or someone in between. (We shudder at the thought.)
By the third day, it consists of eight cells. At this point, one or two cells can safely be removed and used for PGD without compromising the developing embryo.
Since PGD can screen for genes, it’s also been used to save potential offspring from developing a long list of genetic disorders.
In 2000, Adam Nash was the first American baby who was genetically engineered using PGD to save a sibling’s life. His sister Molly had been born five years earlier with a condition called Fanconi anemia, a fatal disease that affects bone marrow. She was dying, so Dr. John Wagner, the Director of Clinical Research at the University of Minnesota’s Marrow Transplant Progress, used PGD to select an embryo with the same genetic abnormalities as Molly, but one that wasn’t a carrier of her disease.
The selected embryo became her brother Adam. When he was born the stem cells from his umbilical cord blood were used to save her life.
However, engineering offspring based on traits such as gender is a bit more controversial because it’s not medically necessary. So, there aren’t many countries that sanction it. In places such as the United States, the technique is being used because there have been no policies established to prohibit it.
Nevertheless, an average of 4,000 to 6,000 PGD procedures are performed around the world each year.
The Fertility Doctor, the Media, and the Vatican
When the media is camped out on your front lawn and the Vatican comes calling, you know you are controversial and on the cutting edge of your field.
That’s the position Dr. Jeffrey Steinberg, a Los Angeles-based reproductive endocrinologist and founder of The Fertility Institutes, found himself in during early 2009.
Steinberg had been conducting research in albinism, people having little or no pigment in their eyes, skin, or hair. This is a result of inherited genes that don’t make the usual amounts of a pigment called melanin (1 in 17,000 people in the United States has some form of albinism).
Steinberg’s intention was to find ways to prevent eye issues suffered by people with the disorder. In the process, he isolated a gene for eye color, and that led to the discovery of additional genes related to a series of physical traits such as eye color, hair color, and hair texture. The findings opened up the possibility that parents-to-be would be able to select the physical traits of their unborn children.
Theoretically, doctors would use in vitro fertilization to create a series of embryos, and then would select one of them for genetic analysis. If this process was ever available, your fertility doctor would select the embryo that best genetically matches the traits you want. Taking things a step further, some researchers believe that as genetic science improves, specific genes could be engineered to produce the selected traits.
The media dubbed this “designer babies.” The New York Daily News ran the headline “Custom-made babies delivered: Fertility clinic doctor’s design-a-kid offer creates uproar.” ABC News ran the headline “Fertility Doctor Will Let Parents Build Their Own Baby.”
And there were plenty of phone calls.
“We had the usual outbursts from the radical right and radical left and even people in between. We had people that thought we were Nazi mongers (and) people who couldn’t wait to get on the list to get a girl with red hair,” Steinberg recalled.
“We had people that thought we were Nazi mongers (and) people who couldn’t wait to get on the list to get a girl with red hair.”
Then Steinberg got a call from The Vatican.
“That was the straw that broke the camel’s back,” he said. “They were very nice. They have scientists at the Vatican, and we discussed it for quite a while.”
Steinberg decided to stand down. “I said, Yeah let’s back off for now. No one is going to die if they don’t get these traits. We have a list hundreds of names long, and for every characteristic you could imagine. So we backed down. I just didn’t want to spend the time on it.”
Controversy is not new to Steinberg. Early in his career he attracted unwanted attention while practicing in vitro fertilization.
“Anything that is new is scary,” he explained.” When I did the first in vitro fertilization baby way, way back I came out of my office and someone had put a hand scrawled note on the windshield of my car that said ‘test tube babies have no soul.’ Now you go to a party and a third of the people there are test tube babies.”
The Fertility Institutes’s primary business these days is gender selection. Parents can preselect a boy or a girl. Even that had its initial controversy.
“My professional society heavily criticized it, but a third of those members are now participating in it and offering it,” Steinberg said.
By 2014, The Fertility Institutes had produced almost 10,000 gender-selected babies. The technique they use is PGD. Steinberg says his company does not guarantee gender, although, it has never gotten the gender wrong. However, the uncertainty couples face is in the implantation of the gender-selected embryo. Sometimes it doesn’t stick to the uterine wall of the mother, and so the procedure fails to result in a viable pregnancy.
It’s also a costly procedure and the majority of the research being done today in gender selection is focused on getting the cost down. In 2014, a couple who wanted the procedure would face a bill of $18,000. The cost to identify an egg as a boy or a girl is not expensive. However, the cost to ensure it is genetically healthy is.
Steinberg explained, “It doesn’t do us any good to know we’ve got a girl if the girl is missing a chromosome because she is never going to make a viable baby. People don’t want to know they have an XX embryo. They want to know they have a viable girl.”
To do this, they have to select an embryo that is the desired gender and has 46 chromosomes, which produces a healthy human.
During this selection process Steinberg’s team made an unexpected discovery.
In the process of gender selection, they found that some couples are “good boy-baby makers” and some are “good girl-baby makers.” It’s a process that is completely unique to the couple.
![]() Does Family Factor Into the Gender of Children?
Does Family Factor Into the Gender of Children?
If you are looking at your brothers and sisters or extended family to try to understand whether you’ll have boys or girls, don’t bother. Gender trends in your family tree won’t help. Good-boy-making couples or good-girl-making couples are determined uniquely by the genetic chemistry between you and your partner.
Steinberg says that couples who are good at making boys before they come to him tend to be good at making boys during the gender selection process (and vice versa).
“When people who already have a lot of boys at home ask for a girl, we get both types of embryo in the lab but we find that we get mostly boys, and the girls we do get are never quite the same quality. This explains why they are making boys at home.”
Whereas couples make both boy and girl zygotes (zygotes, remember, are fertilized eggs), the body only uses viable candidates. Women shed the nonviable zygotes through a natural selection process. Good boy baby-making couples produce more viable boy zygotes than girl zygotes, and those fertilized eggs stick more readily. This is what is happening when you encounter a couple that’s had a series of same-gender children.
The other challenge that fertility specialists deal with is the incidence of abnormal human eggs. When Steinberg and his team looked at the viability of eggs from young healthy women (egg donors aged 23 to 25), they discovered 25 percent to 30 percent were genetically abnormal. Steinberg called this finding “astonishing because we never thought humans were so inefficient as far as making normal genetic embryos.”
So what’s the future of designer babies? Work still has to be done to get there. And there has to be a big demand for it. Steinberg predicts designer babies will be available in some form in the next decade.
Today, 20 percent of Steinberg’s clients who ask for gender selection, also ask for other trait selection. He puts their name on a list and tells them “Not now, maybe later.”
In 2014, Steinberg had 700 to 800 couples on a list who said they’d pay to choose their baby’s eye color. The second trait most requested is hair texture. Number three? Athletic ability.
Qualities such as athletic ability and height are traits that require the isolation of multiple genes (they are called “polygenic” in scientific terms), so Steinberg said it will take a bit longer to figure those out. When athletes come in for gender selection, Steinberg requests blood samples (and permission) to study them later.
![]() Anyone for Tennis?
Anyone for Tennis?
If you could genetically select athleticism as a trait in your baby, what sport would you ask for? It turns out most moms-to-be that visit Steinberg’s clinic say, if it were available, they would ask for tennis players.
Genetically Engineered Babies
If selecting traits for your baby intrigues you, and you were disappointed that it’s not yet available, don’t worry. A lot of activity is going on in the world of genetics, especially around babies. It won’t be long before it is available. There are technologies available today that will allow parents-to-be to genetically screen for diseases.
Here’s the catch: The service is only (currently) available to women who want to use a sperm donor to get pregnant, because donors are genetically screened for quality before their sperm is admitted into the sperm bank.
What’s in the way is politics, policy, and social acceptability.
Maybe the wait will be longer than expected. The science is there, but the societal acceptance isn’t there yet.
The following option, however, is possible today.
GenePeeks is a New York-based company that has developed a technology called Matchright. This technology pairs the DNA of two hypothetical parents and virtually creates a hypothetical baby who has their combined genetic makeup. It uses scientifically designed algorithms and next-generation sequencing tools to match a woman’s DNA with more than 1,000 sperm donors in their catalogue.
That’s right. Women seeking to use a sperm bank can access the service.
The results from the test suggest the best genetic matches, meaning the ones that have the least chance of disease.
The company was created by Anne Morriss and Dr. Lee Silver. Silver is a biology professor from Harvard University with an interest in genetics, and Anne Morriss is a mother on a mission to save parents from ever having to deal with a chronically ill child. Her son was born with MCADD (medium-chain acyl-CoA dehydrogenase deficiency), a disease that prevents the body from converting fats to sugars. It can lead to seizures, breathing difficulties, brain damage, coma, and sudden death.
Here’s how it works. Each person is a carrier for multiple gene mutations, so everyone is at risk of having a child with a rare recessive disease. When two parents carrying the same recessive gene come together, there is a 25 percent chance they will produce a sick child. Each parent themselves carries one recessive gene and one healthy gene, which is why they are perfectly healthy. The disease goes undetected until it’s paired with DNA that carries the same recessive gene where it gets expressed.
Whereas most IVF clinics already screen donors for a dozen or so genetic disorders, perform regular health checks, and acquire each donor’s family history, the Matchright process evaluates the possibility of 500 genetic conditions.
The service is only currently available to women using a sperm bank who want to screen out donors that are not a good match in terms of their combined genetics. Women who are interested in using GenePeeks can order a home test kit that takes a sample of their saliva and is sent back to a lab for analysis. The analysis takes about 4 weeks, and then they receive a personalized catalogue of FDA-approved donors which have been selected for them as healthy sperm match candidates.
The technology is not yet ready for couples preplanning a pregnancy, but GenePeeks says it has intentions to make it available in the near future.
It’s currently only available in the United States, but if you live outside its borders, the company suggests on its website to call and inquire.
Testing 1, 2, 3 ... Is Your Baby Genetically Healthy?
If you are pregnant today, you might not know that your genes have been or will be screened while you carry your baby. Blood tests fall under the category of what are called “carrier tests,” which are prenatal tests that screen parental genes. The most common tests, called the Triple Screen and Quad Test, determine whether the baby will develop genetic disorders or brain defects.
There are additional genetic prenatal tests available, but they are much more invasive and involve the extraction and analysis of cells from the developing fetus and surrounding structures. The procedures are for women whose pregnancies are high-risk. This is because the procedures are invasive and pose a very small risk of miscarriage (about 1 in 100 women). Women whose pregnancies are deemed high risk are 35 years or older, have already had a baby with chromosomal defects, or have genetic markers that signal potential problems.
One common test is called amniocentesis. You may recall from earlier in this chapter, that amniocentesis involves the extraction and analysis of amniotic fluid. Another common test, performed at an earlier stage of pregnancy—10 to 13 weeks—is chorionic villus sampling (CVS). Cells are taken from specific cells in the placenta that match the cells of the baby that is being formed. But, if both amniocentesis and CVS don’t provide sufficient test results, a third method, cordocentesis can be used to extract cells from the umbilical cord for analysis.
These carrier tests and prenatal tests are what’s available today, but soon things might look very different. Correcting gene problems before they start is the future. But, it’s a very controversial topic because although the technology is available, it’s social policy and public opinion that are currently blocking its availability.
There are signs of progress though.
In October 2013, the U.S. Patent and Trademark Office awarded a controversial patent to 23andMe, a Mountain View, California company that specializes in genetic testing. Though it is best known for home DNA testing kits, the patent gave it exclusive ownership of a genetic calculation technology called “gamete donor selection.” If permitted, parents who use fertility clinics could select traits for their unborn children.
Traits would be selected by checking boxes on a long list of criteria and would include characteristics such as: gender, height, weight, and eye color.
When the media got all excited about the news, 23andMe.com responded—in part with a blog post that said, “We never pursued the concepts discussed in the patent and we don’t have any plans to do so.”
There was even more media attention when the FDA ordered 23andMe to stop selling its personal genome service in late 2013. The FDA said the $99 Personal Genome Service (PGS) that asks customers to send a saliva sample to a lab to receive their personal genome violated the Cosmetic Act.
Two years later, 23andMe resumed its operations, and this time with the FDA’s approval. Today, it offers a similar test but with zero information on personal health risks. The new test provides information on carrier status, which relates to the probability of genetic mutations and disease in potential offspring. The kits also come with a higher price tag of $199.
We asked them about designer babies, and a spokesperson responded by email: “23andMe does not play a role or have any plans to pursue any technology in the field of designer babies.”
Maybe not. But we think it could be offered at some point in the next few years as soon as the government gets out of the way, and public opinion shifts.
Daddy, Mommy, and Your Extra Mommy
One more pop quiz! Can three people have a baby together? Not without some scientific help. But yes, a three-parent baby is genetically possible. The procedure is intended to prevent a range of incurable mitochondrial diseases currently affecting 1 in 10,000 people.
The creation of this special baby involves three-parent in vitro fertilization (TPIVF), which uses the DNA from three people (two women, one man) to create one fertilized egg for implantation.
When a baby is made, DNA from the man and DNA from the woman come together to produce their offspring. What most people don’t know is that there is a type of DNA that’s given to the child exclusively by the mother. It is called mitochondrial DNA.
Mitochondrial DNA comes from the mitochondria, the “power centers” found in most of the cells in the human body. They convert chemical energy from food into energy that our bodies use to run.
Women who are carriers of the mitochondrial DNA mutation (the “MELA” A32A3G mtDNA mutation) will pass it on to their babies unknowingly because they are often perfectly healthy themselves.
Since the mitochondria perform so many critical functions in a number of tissues, there are hundreds of mitochondrial diseases. Each disorder comes with a list of different health problems. Common symptoms range from a loss of motor control, muscle weakness and pain, and gastrointestinal dysfunctions.
But that’s not all. Add to that difficulties swallowing, poor growth, cardiac disease, liver disease, diabetes, respiratory issues, and seizures, to name just a few.
Scientists have found a way to intervene in the fertilization process to eliminate the potential of genetically transferred mitochondrial disorders.
In the egg with the mitochondrial mutation, the cell’s nucleus is removed. Then they insert that nucleus into the second woman’s egg, which has had its original nucleus removed. This hybrid egg, which now houses the nucleus from the first egg but contains the healthy mitochondrial DNA from the second egg, is then fertilized with the sperm (see Figure 2.5).
(Illustration provided by Cornelia Svela.)
Figure 2.5 The process of three-parent baby making is shown here.
In 2015, Britain became the first country to legalize this procedure. (So, as you read this book, the first three-parent baby might be making its way into the world.)
In February of 2014, fertility rules were changed by the UK government to make Britain the first country in the world to allow mitochondrial replacement (MR) therapy. But the rules were subject to additional approvals from the British parliament.
A CNN February 2014 report suggested that the FDA was also considering the legalization of three-parent babies in the United States.
Art Caplan, the director of medical ethics at New York University’s Langone Medical Center, told CNN in an interview that the procedure to correct mitochondrial mutations is a “humane and ethical thing to do.”
But, he also suggested that there is a more controversial side to be considered: “Where we get into the sticky part is, what if you start to say, ‘While we’re at it, why don’t we make you taller, stronger, faster or smarter?’”
We are all for it. You might be also. Or maybe not.
Pregnant Men?
Though the technology exists to likely make it possible for a man to get pregnant, there would still be a lot of social, political, and legal hurdles to overcome, especially in the United States.
The First “Pregnant Man”
In 1974, Tracy Lehuanani LaGondino was born female, but by the time she reached the age of 10, she felt more male than female. Tracy started to use the pronoun “he,” and on his 23rd birthday he started the physical transition to become male. He renamed himself Thomas and became Thomas Trace Beatie.
It started with what is called “top surgery,” which is the surgical procedure on the breasts of transgender patients. In this case, their removal. After that, Thomas looked like a man on the outside, but his female reproductive organs remained intact.
In 2005 he married his wife Nancy and two years later the couple decided to have a baby. But, it was Thomas, not Nancy, who carried their child. Nancy was infertile and couldn’t become pregnant. So they decided that Thomas would become pregnant using donated sperm.
The publicity surrounding this extraordinary parenting scenario made him a media sensation and led to appearances on Saturday Night Live, The Late Show with David Letterman, and the Oprah Winfrey Show.
Thomas went on to birth two more children and then he had his female reproductive organs surgically removed. (He and Nancy also divorced in 2012.)
All three birth records mark Thomas’s gender as the mother with the letter M for male. And so, he remains the first man to give birth.
![]() Don’t Be Fooled
Don’t Be Fooled
Although the video at Malepregnancy.com seems like a true take on the first pregnant man, you can rest easy. It’s not. The procedure seems plausible, especially to anyone who has no formal medical training. Give a man some female hormones, use IVF to implant an embryo and placenta in his abdominal cavity so that he can grow the baby, and then deliver it with a Caesarean section, and we were almost fooled. But with a little poking around we discovered none of it is real.
The Future of Male Pregnancy
Whereas Thomas Beatie is the first man to birth a baby, he will not likely be the last. Beatie was born a woman, so it could be argued this is not a true case of a biologically male pregnant man.
Can men really become pregnant? If you look at the animal kingdom, then the answer is yes. Male seahorses and pipefish, a closely related species, carry fertilized eggs in their pouches and birth babies after a few weeks. Nature hasn’t provided the same facility for wannabe man-moms. But if we have learned anything while writing this book, it’s that if something is not possible now, it will be shortly. Gene therapy lets us change the human blueprint. Stem cells allow the growth or repair of any human tissue. Bioprinting makes it possible to create new organs from scratch. And if none of that works, nanotechnologies will let us push the building blocks of life around with tiny nanoscale bulldozers.
“... if something is not possible now, it will be shortly.”
The other ray of hope for Mr. Mom wannabes is this curious fact: A miracle baby born in Montreal in 2012 shows that a uterus might not be needed to bring a baby to term. Dionne Grant, a Jamaican tourist visiting Canada, gave birth to a healthy baby boy after the child grew outside of her uterus in Grant’s abdomen. This is a rare condition called an ectopic pregnancy, in which a fertilized egg implants itself outside the uterus, usually in the mother’s fallopian tubes. (Fallopian tubes are conduits for an egg to descend from the ovaries to the uterus.) Doctors believe the baby survived because the placenta—the meaty tube that provides nutrients to the baby during its development—glued itself to the top of the Grant’s uterus, accessing blood to nourish the baby.
So could a dad carry a baby in his belly? Yes, likely. There would have to be other interventions using some medical magic, but we say give it ten years and will all be possible. Probably much less.
For you doubters out there in Super You Land, consider this: Scientists can now grow vaginas. Read on.
Lab-Grown Vaginas
It’s taken two decades of work for Anthony Atala and his team at Wake Forest Institute for Regenerative Medicine in North Carolina to bioengineer and transplant a very delicate human organ—a vagina.
In 2014, a culmination of techniques used on rabbits that started in the 1990s led to the first successful vaginas grown in a lab. Four women suffering from Mayer-Rokitansky-Kuster-Hauser syndrome (MRKH) participated in the trial.
MRKH affects the development of the genitals in 1 in 5,000 women. Those women are born without a vaginal canal and are missing parts of their reproductive system. It affects their ability to have sex, menstruate, and reproduce.
All four women who participated in the study had abnormal uterus development but their vulva, clitoris, and labia were intact. They all received the operation at the ages of 13 to 18 years old, a time when the body is still maturing.
The procedure involves the removal of small part of the vulva, which is used to grow new cells. These are then layered into a scaffold made of collagen that disintegrates one month after the surgery. Each scaffold is formed using calculations made from MRI scans. This helps fit the new vagina to the shape and size of each recipient’s body. The scaffold is then placed into a cavity in the abdomen. A bit of stitching holds the new vagina in place and a stent makes sure it maintains the proper form as it grows.
Atala’s team waited four to eight years to publish the results of the trial to ensure its long-term success. All the women now have functional vaginas and two have uteruses. The big question is whether these women will be able to have children or whether they will experience side effects long-term.
From Birth to Forever
Reflecting on this chapter, it’s clear to us that the process of procreation and raising a child in the next century is going to become a very different process to what it was for our parents. The options for a yet-to-be born child that are about to become possible are quite astounding. As parents, we will be able to set them up for clean genetics, fix any problems in utero, and birth them into a life that will be very different from previous generations. Life expectancy of your child might become limitless, as you will see in our longevity chapter. A life free of disease might also be possible. And those children might grow into humans that will have access to all the wonders of technology found in science fiction and beyond.
There is evidence that with the realization of stem cell technology, gene therapy, and nanomedicine, not only will our children be able to repair themselves through technology, but perhaps they might even elect for an extended life and choose to delay or eliminate death as an option. And if we are all of the age where we are lucky enough to enjoy this too, we will be able to live to see their children grow and the generations beyond that.
That might seem like a wild and seemingly unrealistic statement to some, but the emerging science, and prognostication among most futurists, suggests it will all be possible within this lifetime. Certainly it will be possible in the next few decades and the next decade will bring the beginnings of all this to everyday life.
And like it or not, designer babies are on their way. It will be an option for the next generation of parents, provided there is social and political will. That is the wild card. The question is do we want that option? Would we design our children’s hair and their eyes and their proclivity toward certain physical skills or capabilities?
Your authors wouldn’t choose these designed aesthetics, but we would choose a clean genetic palette for our children, when it becomes possible.