DNA Fingerprinting
RFLP
PCR
A common phrase in the vocabulary of many geneticists is molecular markers. Molecular markers are DNA fragments that can be used as a fingerprint in the identification or characterization of individuals. These markers have become an increasingly helpful tool in genetic research and applications to biotechnology. The basic premise behind molecular markers is that there is natural genetic variation in individuals, and many genetic sequences are polymorphic, meaning they differ among individuals. Molecular markers seek to exploit this variation to identify individuals, traits, or genes on the basis of genetic differences.
Working with the human genome, Botstein proposed the use of DNA fragments as genetic markers for monitoring segregation. The first molecular markers to be used were fragments produced by digestion of DNA with restriction enzymes. The variation in fragment size obtained from different individuals after the digestion created the class of markers called restriction fragment length polymorphism (RFLP).
One of the quickest ways to discover the location of a gene is through reverse genetics; that is, starting from the trait of interest, one would identify the protein involved. By knowing that genes code proteins, one can try to locate the actual gene. If the sequence of the amino acids of the protein is known, the genetic code can be used to establish the sequence of corresponding nucleotides, which is at least a part of the gene. From this sequence, a complementary sequence of nucleotides can be built and used as a probe. These synthetic sequences, a single DNA strand, can be used to detect genes within the billions of nucleotide bases. The probes can be radioactively labeled to facilitate identification. After the probes hybridize with the corresponding genes in the chromosomes, it is possible to identify their location by the detection of radioactivity, revealed on X-ray film. Each probe with the complementary gene can be observed on the film as a dark spot or band. Increasingly, fluorescent dyes are used instead of radioactive probes.
DNA probes are used for mapping genes in the chromosomes and for genetic tests, as in the case of the diagnosis of breast cancer. These probes are also used in the characterization of individuals at a molecular level, a process called DNA fingerprinting.
DNA fingerprinting is also known as a DNA profile. This technique is useful in many areas: paternity tests, criminal cases, evolution studies, evaluation of biodiversity, mapping of genes, and genetic tests. Additionally, there are several methods for accessing the DNA profile of an individual, depending on the type of test being done.
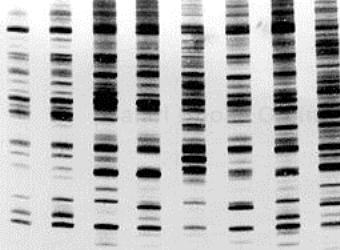
Developed by Alec Jeffreys in England, in the beginning of the 1980s, this technique is based on the distance between restriction sites in the DNA. The RFLP technique uses special enzymes called restriction enzymes to cut DNA into fragments. These enzymes recognize short, specific sequences of DNA and cut the DNA at those sites. After the DNA is treated with a restriction enzyme, it is cut into fragments of various sizes. The number and size of the fragments is unique to each individual. The restriction (cut) sites of a person, a corn variety, or a sheep are as unique as a fingerprint, allowing unequivocal identification of the individual. If the DNA of two individuals are cut with the same enzyme, EcoR V for example, two patterns of DNA fragments are produced, making it possible to distinguish them on the basis of the variation in the length of the fragments because each pattern of fragments is unique to each individual. The occurrence of many patterns of fragments with different lengths is called RFLP (Figure 9-1).
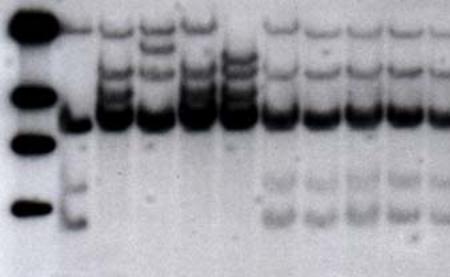
Figure 9-1. Autoradiograph of a DNA cut with the enzyme Hind III and revealed by a radioactive probe (RFLP).
The relative position of bands, like a bar code, reveals the fragment sizes. The pattern of bands can then be used reliably to identify the individual source of the DNA.
The procedure for analyzing RFLPs consists of isolating the DNA from an individual, digesting it with a restriction enzyme, and separating the DNA fragments through electrophoresis in agarose gel. The fragments are transferred to a cellulose membrane by the technique of Southern blotting. The detection of the polymorphic fragments is accomplished through hybridization with radioactive probes that can be viewed in autoradiograms.
For RFLP analysis, it is necessary to extract and purify the DNA. Purification eliminates carbohydrates, lipids, and proteins. The extraction buffer in general has a pH level of 8.0, a salt to dissociate the proteins from the DNA, a solvent to break down the lipid membranes, and a DNase-inactivating agent like EDTA (ethylenediamine tetraacetic acid).
As mentioned, DNA is digested by a restriction enzyme. These enzymes have the ability to cut DNA at precise locations in the DNA sequence. There are many possible cut sites within the genome of an organism. Most of these enzymes are derived from bacteria, which use these enzymes as part of their defense mechanism. The choice of the restriction enzymes is based on their cost and their effectiveness in producing polymorphism. Several restriction enzymes have frequently been used in RFLP analyses, including EcoR I, EcoR V, Hind III, BamH I and Pst I, among others.
After digestion, 2 to 15 µg of DNA are placed in an agarose gel and submitted to electrophoresis. Electrophoresis uses electrical charges to separate DNA fragments on the basis of size. After a sufficient period of time, the smaller fragments are separated from the larger ones, as the small fragments move more quickly in the electrical field. The DNA fragments are then transferred, by capillary action, to a nylon or cellulose membrane, where they are fixed in their location.
Nucleotide probes, short sequences of DNA conveniently labeled with radioactive P32, hybridize to DNA fragments attached to the membranes, allowing them to be viewed after developing film exposed to the radiation.
The RFLP procedure requires a relatively large amount of DNA, which prohibits its use in many situations. More recently, a DNA profile generated by polymerase chain reaction (PCR) has been used to analyze samples with a much smaller DNA content, as in saliva droplets in a telephone handset or a single hair from an individual.
Kary Mullis, who won the Nobel Prize in 1993, developed the PCR technique using bacterial enzymes involved in the DNA replication for in vitro amplification of DNA. Today, PCR is being used in research as well as in laboratories, clinics, and hospitals for genetic tests, and in forensic laboratories for the analysis of DNA evidence. Many modifications have made this procedure more or less specific, as appropriate, and it is important in many genetics applications.
PCR makes copies of specific DNA sequences. A PCR reaction requires the following materials:
DNA template: The DNA to be sampled.
Nucleotide bases: A, T, C, and G.
DNA polymerase: The enzyme for DNA replication.
Primer pairs: These are short specific sequences of DNA that flank the area of DNA to be amplified. This also aids in the binding and proper function of the polymerase enzyme.
Reaction buffer.
Salt solution.
The procedure begins with the heating of the DNA sample in the presence of nucleotides (A, T, C, and G), a pair of primers, the polymerase enzyme, and the buffer and salt solution. Heating promotes the denaturation of the DNA molecule, separating the two strands of the DNA sample. The sample is then cooled so the primers are able to hybridize with each strand, allowing the DNA polymerase to begin the replication of DNA. As the polymerase moves along the template DNA strand, the nucleotide bases are added to create a complementary copy of the DNA template. Subsequently, the sample is heated up to again promote the denaturation of the DNA, thereby beginning a new cycle. This cycle is repeated 20 to 30 times in an apparatus called a thermocycler (Figure 9-2). The DNA area flanked by the primers is amplified exponentially.

Source: Thermocycler image courtesy of Bio-Rad Laboratories, Inc.
Figure 9-2. PCR reaction scheme and a thermocycler, the apparatus used for PCR.
Due to its flexibility and specificity, several other methods have been and are being developed using PCR for the characterization of individual genetic variability. These include amplified fragment length polymorphism (AFLP) and SNP, as well as others. These different markers use different methods or amplify different portions of the DNA, but are used in similar PCR reaction protocols, and seek to distinguish the variation in different individuals. Some of these methods have become completely automated, such as the detection of SNP, by mass spectrometry and by hybridization on DNA microchips, which have become important tools for the development of pharmacogenomics (see Chapter 8, “Pharmacogenomics”).
PCR-based molecular markers are unlike RFLP markers because they require a very small amount of DNA for analysis. The regions of interest are amplified using specialized machines and enzymes that are able to make copies of the desired DNA regions. These markers have revolutionized DNA analysis by increasing speed and reducing the labor involved for the procedures. To understand use of these molecular markers it is necessary to understand the principle of the PCR (Figure 9-2).
A series of molecular markers has been developed based on the PCR technique. Some of the markers include short tandem repeats (STR) or microsatellites, random amplified polymorphic DNA (RAPD), SNP or point mutations, AFLP, and sequence characterized amplified regions (SCAR). They are used in studies of human, animal, plant, and microorganism genetic variability.
In the chromosomes of several organisms are areas containing short repeated sequence of DNA. The short sequences can be two to six nucleotides long, and they can be repeated many times. Geneticists have found them to be valuable as molecular markers and have developed PCR-based techniques to study them. They are called STR, short sequence repeats (SSR), or microsatellites. Some of the more common repetitive sequences are the dinucleotide CA and the trinucleotide CAT. These markers group in certain areas of the genome, in blocks of tandem (one behind the other) repetitions of at least five units. These repetitive blocks are highly variable and polymorphic, with the presence of several alleles due to the number of different repeated units (Figure 9-3). These areas can be easily amplified by PCR, and due to their polymorphism they are used in identification tests in criminal investigations (see Chapter 10, “Forensic DNA”) and analysis of genetic variability. After PCR amplification, the products are separated by size through electrophoresis on a polyacrylamide gel (Figure 9-4). Each microsatellite can present up to two bands, in cases where there are two different alleles in the individual (one from each parent).
Two research groups independently proposed the use of small random primers in the PCR to generate polymorphic markers in 1990. This technique is known as RAPD. It uses a synthetic oligonucleotide as a primer in the amplification process to produce a polymorphism detected by the presence or absence of a band. This technique consists of extracting the DNA of the individuals to be analyzed and using this DNA in the amplification reactions (PCR), which can be done using a different primer for each reaction. The products of each reaction are a result of the amplification of different chromosomal areas flanked by the pair of primers. The amplified fragments are separated by electrophoresis in a gel. Different individuals produce different patterns of amplified fragments, or DNA profiles (Figure 9-5).
Among the markers currently in use (see Figure 9-6), the RAPD technique is simpler and quicker than RFLP. It uses smaller amounts of DNA, does not involve the use of radioactive probes, and is less labor intensive. It has been used mainly in plants because it does not detect variation in humans and other animals.