Principles of Genetic Transformation
Methods of Genetic Transformation
Bottlenecks in Transformation
Genetic Engineering Products
Potential of Transformation
Gene Expression
Transgenic Locus
Genetic transformation in its most basic form is the introduction of transgenes (foreign genes) into an organism in a way that they might be expressed. This technique, also called genetic engineering, allows for the transformation of bacteria with genetically engineered plasmids that possess resistance to antibiotics as well as the transformation of a bovine reproductive cell with a transgene for hormone production.
The final objective of genetic engineering, or recombinant DNA technology, is the stable and inheritable expression of a new trait in a different organism or individual. This is done through proper construction of a vector to carry the transgene. Plasmids, retroviruses (RNA virus), and bacteriophages are especially important as vectors (means to deliver the transgene) in the process of transformation. In this process, genetic engineers cut and rearrange DNA fragments to build a genetic construct (transgene), which is finally inserted into a vector (Figures 3-1 and 3-2).
Hebert Boyer and Stanley Cohen achieved the first successful genetic transformation in 1973, when they constructed a gene with portions of DNA from bacteria and an amphibian. These scientists wanted to express antibiotic resistance in an organism that lacked the trait. With the successful use of enzymes and vectors, these men pioneered the use of genetic engineering and transformation. Their work is the basis of much of the current work in biotechnology.
The term genetically modified is frequently used to describe organisms that were genetically transformed or engineered. The science of genetic engineering was developed with the objective of building genes for genetic transformation. Genetic transformation systems possess three main components:
A mechanism for introduction of the foreign DNA into the target cell.
A cell or tissue suitable for transformation.
A method for the identification and selection of transformed cells or individuals.
Success in transformation for any species depends on these three components. Obviously, each one must be optimized and, therefore, as technology develops, transformation should become a more routine activity. The final objective in transformation is the introduction of a new trait in an individual. When the desired trait exists in any other sexually compatible individual, the first alternative should be to transfer the trait through crossing and selection, as has been done in conventional breeding since the 19th century. Modern soybean, corn, cotton, and wheat varieties, as well as swine, cattle, and poultry lines used in agriculture to feed the world, were initially obtained by traditional methods of crossing and selection.
One of the main limitations of conventional genetic improvement is that the breeder is limited to traits among species that are sexually compatible. For instance, the field bean is a species rich in sulfur-containing amino acids. However, beans are naturally deficient in lysine. On the other hand, rice is naturally rich in lysine, but deficient in sulfur-containing amino acids. It is not possible to naturally cross these species, so the conventional plant breeder is unable to develop a new field bean variety with elevated lysine levels or a rice cultivar rich in sulfur-containing amino acids. Genetic transformation allows the exchange of genes between organisms previously limited by sexual incompatibility. With genetic engineering and transformation, it is possible to transfer genes among bacteria, animals, plants, and viruses. In fact, one of the areas of research in biotechnology is the improvement of nutritional profiles in crops. New, more nutritional bean and rice varieties can now be developed through advances in genetic engineering.
The basic tools for genetic transformation are restriction enzymes, which are used to cut DNA at specific sites, and ligases, which catalyze the joining of DNA fragments, as seen in Chapter 2, “Genetic Engineering.” Using the right restriction enzymes, it is possible to cut the circular bacterial plasmid DNA, causing it to linearize. With a ligase, it is possible to add other DNA fragments containing the gene of interest and join them to the linearized plasmid. Under the right conditions, the ends of the plasmid, now with the added DNA fragments, rejoin to create a new circular plasmid with some DNA modifications. The new plasmid can be introduced into certain bacteria through a process called electroporation, and the bacteria can then be used to transfer the transgene to the target species. If the plasmid DNA is integrated into the genome of the recipient species and the transferred genes are expressed, the individual is considered to be transformed or transgenic.
Among the several methods of plant transformation, four have yielded the best results: Agrobacterium species-mediated transformation, microprojectile bombardment, microinjection, and direct transformation. Each of these methods has merits and limitations and is used in specific situations. At this time there is no single technique that is suitable for all species.
Tumors and uncontrolled cellular growth in plants can occur due to genetic factors or bacterial and viral infections. An example is crown gall in plants, where tumors are caused by bacteria that causes uncontrolled growth on the stem of the infected plants. This problem is caused by Agrobacterium tumefaciens, a soil bacterium that infects some plants because of a wound on the plant. Plasmids present in the bacteria are responsible for tumor growth after infection by A. tumefaciens. The bacteria are able to recognize wounds on the plant, and this induces the transfer of the bacterial plasmid into the plant. The plasmids are capable of integrating into the DNA of the host plant, causing uncontrolled plant growth and the formation of tumors. The ability of A. tumefaciens to efficiently transfer plasmid DNA into the host has made it important in early studies in genetic transformation.
Agrobacterium tumefaciens was the first vector used for introduction of foreign DNA in plant cells. Although Agrobacterium has only been used to infect dicot plant species, such as soybean, tomato, pea, and cotton, the protocol has been modified to allow the bacteria to infect some monocot (grass) species as well. Many research groups working with plants have found this to be the preferred transformation approach. Another soil bacteria, Agrobacterium rhizogenes, causes the growth of secondary roots after infection. This bacterial species has also been used for plant transformation.
The basis of this transformation method is the bacterial plasmid, which contains the genetic sequence that is integrated into the host genome. One of the most important parts of a plasmid is the region responsible for the translocation of its DNA into the host plant genome. This is called transfer DNA (T-DNA), and this area of DNA is key to the tumor growth in infected plants. The region is located between the right border and left border (RB and LB in Figure 3-3) of the plasmid. Plasmids also contain other important DNA sequences; some of them control the production of auxin and cytokinin, two important plant hormones involved in tumor formation. With the use of the restriction enzymes, a transgene can be introduced between the right border and left border of the plasmid, allowing the bacteria to transfer novel genes into the recipient plant.
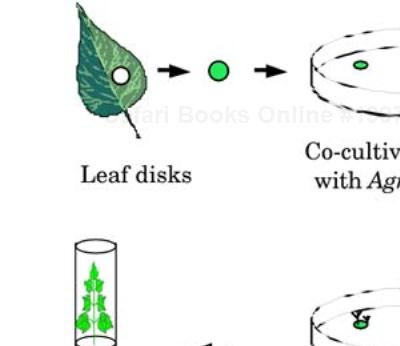
One of the techniques used for transformation mediated by A. tumefaciens uses leaf disks (Figure 3-4). Leaf disks of about 6 mm in diameter are cultured on a tissue-culture media containing A. tumefaciens with plasmids containing the transgene. After approximately a month of incubation in the tissue culture medium, seedlings start to develop on the leaf disks. Through selection methods, transgenic seedlings are identified for whole plant regeneration.
This technique has also been called microprojectile acceleration or biolistics, but microparticle bombardment is the formal name for the machine called a gene gun. This method, developed at Cornell University, was designated biolistic (biologic + ballistics = biolistic), because high-speed microscopic projectiles (microprojectiles) are accelerated into the cells to be transformed.
This transformation method consists of the acceleration of a macroprojectile loaded with millions of tungsten or gold microspheres about 1 µm in diameter (microparticle). The microspheres are coated with the transgene, or DNA of the gene of interest. Microspheres have a high specific mass, allowing them to acquire the needed momentum to penetrate the target cells. The macroparticle is propelled in the direction of the cells at high speed, but it is retained, after a small distance, on a steel mesh so that the microparticles continue in the direction of the target cells (Figure 3-5). Helium gas at high pressure is used to propel the macroparticle, and the acceleration chamber operates under a partial vacuum, which allows for improved microsphere movement. Once inside the target cells, the DNA coating the microspheres is released and can be integrated into the plant's genome.
Many of the commercial transgenic crop varieties on the market today were developed using the gene gun. However, due to its cost and the complex integration patterns resulting from this method, several research groups are reducing its use.
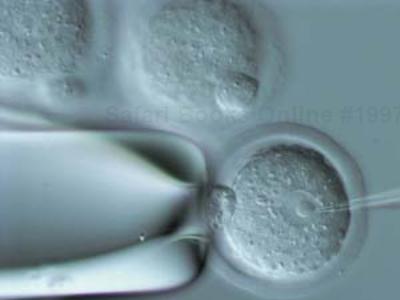
This method was developed for animal transformation but has also been extended to plants. Although very difficult and laborious, DNA microinjection has yielded positive results and has been used in several laboratories.
In this technique, microcapillary needles are used to introduce DNA directly into cells (Figure 3-6). Each cell to be transformed must be manipulated individually. One of the advantages of this method is that the optimum amount of DNA can be injected into the target cells, which helps to ensure optimal integration. Positive results have already been obtained in several crop species such as corn, wheat, soybean, tobacco, and rice, and in animals like salmon, cattle, and swine.
Transformation using direct methods was accomplished soon after the first Agrobacterium-mediated transformation. These methods use protoplasts (cells after the removal of the cellular wall) as targets for transformation. This is a simple method that consists of adding great amounts of transgenic plasmids to a protoplast culture, which guarantees that a small proportion of the protoplasts will be taken up (assimilated) by the plasmids. The assimilation rate can be increased with the addition of polyethylene glycol (PEG) or the use of an electric discharge (electroporation). No barrier to direct transformation has been detected, indicating that this method can be used with virtually any species. The problem with this method lies in the difficulty of regenerating a whole plant starting from protoplasts. Therefore, it has not been used as widely as the other methods.
Tissue culture has been identified as one of the largest obstacles in the development of transgenic plant products. It is necessary to develop protocols that allow the regeneration of whole individuals from the transformed cells or tissue. One of the difficulties faced by scientists is that regeneration methodologies work well with some, but not all species or germplasm within a species. This severely limits the spectrum of individuals that can be transformed. In many cases, the procedure has been the transfer of the transgene through classical genetics and breeding methods. An example of this is in the genetic transformation of wheat. Genetic transformation of most wheat varieties is very difficult because of problems in tissue culture. One variety, Bobwhite, is the exception, and protocols have been developed for the transformation of this wheat variety. Once a gene has been successfully transferred into Bobwhite, it can be moved into other varieties through traditional breeding methods.
Another difficulty associated with the use of tissue culture in transformation is somaclonal variations. Plants produced from tissue culture have higher mutation rates and the appearance of abnormal variation. This is due to the delicate environment in which cells are cultured. Many times, the cultured plants have problems associated with the cell cultures and not from the transgene integration.
Transformation methods currently in development promise to revolutionize the introduction of genes in plants. Some of these methods are already being used with the model plant Arabidopsis thaliana, commonly known as mouse ear cress. One of the methods involves the submersion of floral buds in a solution containing plasmids bearing the transgenes. Another alternative technique, still in development, is the transformation of seeds mediated by Agrobacterium tumefaciens. Although the methods have been used with success in Arabidopsis, the literature does not report its use in crop species. The key aspect of these two methods is that transformation is carried out without the need to regenerate plants through tissue culture. These methods are exciting because the transformation procedure works on the seeds that can then be planted to identify transgenic individuals.
Genetic transformation has developed several new products with impacts on society, from medicines to food products with better nutritional quality. The largest commercial success of genetic engineering was the production of human insulin in transgenic bacteria in 1980. Since then, many other products have been released.
The first genetically engineered crop variety to reach the market was the tomato variety Flavr Savr, developed by the Calgene Company, located in Davis, California. This product, introduced to the market on May 21, 1994, was developed with the introduction of two novel genes in a tomato plant. The first gene was a reverse copy of the poligalactonurase gene, which codes for an enzyme that breaks down cellulose. The introduction of this gene in the reverse form, also called antisense, resulted in low production of the poligalactonurase enzyme. Consequently, ripe tomato fruits do not lose their firmness because the cell wall of these fruits, which is made of cellulose, does not degrade as rapidly as it does in normal tomatoes. The second gene transferred in the development of Flavr Savr codes for resistance to the antibiotic kanamycin. This gene works as a reporter or marker to facilitate the identification of transformed individuals. Table 3-1 includes a partial list of transformed crop species. The introduced traits in those species were, for the most part, tolerance to herbicides, resistance to pests, and nutritional quality.
The objectives of variety development through biotechnology are the same as conventional genetic improvement. Most of the desired traits are improved yields, increased vigor, pest resistance, and nutritional quality. However, biotechnology allows the development of varieties with traits that cannot be developed by conventional breeding.
The introduction of genes coding for a structurally modified enzyme can result in its insensibility to certain chemicals or environmental conditions. For example, the herbicide glyphosate blocks the biosynthesis of aromatic amino acids. The introduction of a gene that codes for a modified form of the enzyme EPSP (5-enolpyruvyl-shikimate-3-phosphate synthase) results in resistance of the herbicide glyphosate.
The introduction of several copies of a gene or the use of a strong promoter can result in the overproduction of the protein. This can be used in some nutritional applications or for disease resistance when certain proteins are needed to fight disease.
Partial or total suppression of gene expression can be obtained by RNA antisense technology. This technology consists of the introduction of a gene in the reverse orientation in relation to the original sequence. When transcribed, this produces a polynucleotide complementary to the original gene. The mRNA of the gene of interest is complementary to the introduced gene, resulting in the formation of a double helix RNA that blocks the translation process. Theoretically, the antisense mRNA can be used to inhibit the expression of any gene.
Genes of other species can be introduced in the target species, making traits available in one species available to any other. Several possibilities exist, including these:
Metabolism: Transfer of genes from nitrogen-fixing species to grasses.
Biopesticides: The Bt gene was transferred from the bacterium Bacillus thuringiensis to corn, cotton, and other crops.
An example is barley resistance to Barley Yellow Dwarf Virus (BYDV), which resulted from the introduction of the gene that codes for the envelope protein of BYDV into barley.
The introduction of male sterility genes can increase the rate of cross-fertilization in a self-pollinated species.
Introducing genes that code for the capability of absorption of heavy metals or the capability of metabolizing pollutant residues could have important applications in biodegradation. This subject is also discussed in Chapter 11, “Bioremediation.”
The introduction of genes that code for production of substances with therapeutic properties can be used for the production of medicines.
All cells possess the typical number of chromosomes of their species. Therefore, root, epidermis, or pod cells of a soybean plant possess all 40 chromosomes typical of this species. However, not all of the genes are expressed in every cell. For instance, genes that code for chlorophyll production are expressed in the leaves and any other green part of the plant. However they are silenced in the roots, which is the reason these cells do not contain chlorophyll. Gene regulation is a complex process that is affected by a series of factors. A common occurrence in genetic engineering is a lack of expression after a gene has been transformed into an organism. Therefore, an understanding of mechanisms involved with gene expression is critical in genetic transformation.
In bacteria, some genes are activated while others are silenced, depending on the conditions in which these microorganisms are grown. For example, the bacteria Escherichia coli can use two different carbohydrates, lactose and glucose, as energy sources. The bacteria needs to synthesize specific enzymes that catalyze the breakdown of the carbohydrates into energy. The enzymes, like all other proteins, are coded by genes. When E. coli is cultivated in a medium with both glucose and lactose (preferably glucose), it metabolizes. The genes coding for the production of the enzymes that metabolize glucose are thus expressed preferentially. The metabolism of lactose requires an additional enzyme that is only synthesized, or activated, after the medium runs out of glucose and lactose is the only energy source available. This phenomenon is called gene regulation.
Gene expression in more complex organisms is still not completely understood. The complexity of gene regulation is a puzzle in the zygote, a cell formed by the union of sperm and egg cells, in which the genes coding for differing functions have to be activated in a precise and orderly manner. The same genetic information present in the zygote is also present in any other cell in the body, from muscles to skin. Obviously, different genes are activated or expressed in each organ in a different way.
Gene expression is not just a function of where the cell is, but also the result of environmental stimuli. Cells of a floral bud of soybeans differentiate into flowers when the plant is grown during long nights. If the soybean plant is grown during short nights, it continues vegetative growth and does not bloom. Another example of gene regulation occurs with animals, including humans. Testicle and ovary cells do not start the production of sexual hormones until the individual reaches puberty.
Another example of the complexity and importance of gene regulation can be observed in the metamorphosis and development of butterflies and moths. These insects take three forms during their lives: caterpillar, pupa, and adult butterfly or moth (Figure 3-7). The insect possesses the same genes and DNA during these three different developmental phases. Although the caterpillar, pupa, and adult have the same genes, it is interesting to observe that different genes are expressed in the three developmental phases. In the caterpillar phase, the genes for production of several legs and a stronger mouth capable of chewing leaves are expressed, but not the genes for production of wings. However, the genes for the formation of a delicate mouth apparatus, appropriate for nectar feeding, and genes for the formation of wings are active in the insect's adult phase. The gene expression pattern changes during insect development to allow for the correct progression of its life cycle.

Source: Courtesy USDA-ARS. Bottom photo by Juan Lopez.
Figure 3-7. Expression of different genes during the development of the corn earworm moth.
The mechanisms regulating gene expression involve regulatory genes. As opposed to the genes discussed up to this point, these DNA sequences do not code for any protein. Their function is to promote the activation or the silencing of genes.
An important part of gene regulation is the promoters. A promoter is a DNA sequence preceding the gene, which contains regulatory sequences to control the rate of RNA transcription. Promoters control when and in which cells a certain gene is expressed. Through the manipulation of promoters it is possible to induce superexpression, underexpression, or even gene silencing.
Some promoters are constitutive—that is, they induce gene expression continually—whereas others are inducible. Among these, there are some that are chemically inducible, and others are activated by heat, light, or hormones. Some promoters are active in certain tissues and organs, but not in others. In this case, they are considered tissue-specific promoters, as in the case of chlorophyll production. The promoters of the chlorophyll genes are not active in roots, but they are active in the leaves and in all green parts of plants.
Some of the promoters frequently used in genetic engineering of plants include the following:
Constitutive
UBI from corn
35SCaMV from a cauliflower virus
Tissue-specific
Phaseolina promoter, a seed-specific promoter from field beans
Vicillin promoter, a seed-specific promoter from peas
Glutamine promoter, an endosperm-specific promoter from wheat
Inducible
Rubisco 5S promoter, inducible by light
Aside from promoters, other genetic factors are important in proper gene expression. Although the genetic code is universal, it is also considered degenerate, as more than a single codon codes for a certain amino acid (see Chapter 1, “History: From Biology to Biotechnology”). Different organisms have acquired the preferential use of specific codons for certain amino acids during evolution; this can also have an impact in gene expression. That was the case of the Bt gene from Bacillus thuringiensis introduced in corn. Initially, the expression of that bacterial gene in corn was low; however, when a transgene was reengineered to favor the preferential use of certain codons by corn, gene expression occurred at normal levels.
Several other factors can affect the expression of transgenes, such as the presence of a peptide signal, the site of its integration in the genome, the number of copies integrated, and transgene rearrangements during the integration process. Integration of transgenes in the host genome, in general, happens at random; that is, it can occur in any chromosome of the cell and it can land in any part of the chromosome. However, most of the transgenic varieties have the transgene inserted close to the ends of the chromosome. Multiple copies of the transgene are typically introgressed together.
Gene constructs used in genetic transformation posses a promoter, coding region, and termination sequence (Figure 3-8). In Figure 3-8, the vicillin promoter, specific for expression in seeds, drives the expression of the gene UDP 6-glucose dehydrogenase in the antisense orientation. The construct also possesses the NOS (noplaine synthase) termination sequence, which marks the site for the end of transcription. Besides the transgene of interest, in general, reporter genes are introduced simultaneously to facilitate the identification and selection of transformed individuals.
Figure 3-8. Example of the genetic construct used for silencing the gene UDP 6-glucose dehydrogenase in soybeans.
For the selection of the transformed cells, the gene construct contains a gene sequence that codes for antibiotic or herbicide resistance. Frequently, neomycin or hygromycin antibiotic resistance genes or the phosphinotricim acetyl transferase herbicide tolerance gene is included under a strong constitutive promoter, such as 35SCaMV. The transformed cells would be the only ones possessing the capability to grow in a medium with a selective agent (antibiotic or herbicide), thereby facilitating their selection.
Frequently, a gene reporter is also included in the genetic construction. The function of this reporter is to allow the visual identification of transformed cells. Three genes have been used as reporters in plant transformation: Glucaronidase (GUS), Luciferase (LUC) and Green Fluorescent Protein (GFP). GUS allows the identification of transformed individuals by the expression of a blue color, because they become blue in the presence of the chemicals X-Gal and IPTG (isopropyl beta D-thiogalactoside). Luciferase, a protein in fireflies, turns the transformed individuals phosphorescent, and GFP, isolated from a species of jellyfish, codes for a fluorescent-green color in transformed individuals.
Genetic transformation of individuals is a difficult task. The science behind the methods is understandable on a basic level, but the results from the procedures do not always work out as planned. Specific gene sequences are needed to induce the expression of a transgene, and genes are needed to identify the transformed cells. Still, the use of transformation is being improved to more accurately express desired traits in different organisms. The comprehension of the intricacies of transformation is a key to understanding the broad applications of biotechnology.