WHY HOGWARTS IS SO DARK
3.1 MAGIC VERSUS TECHNOLOGY
Many fantasy novels are set in preindustrial worlds, such as the Conan novels by Robert E. Howard, or in parallel worlds of quasi-medieval setting in which modern technology doesn’t exist, such as the Lord of the Rings and Earthsea trilogies. Others are set in worlds where magic coexists with the mundane world. As an example of the latter, the Harry Potter books provide a means to examine the distinctions between the magical worlds and the mundane.
Hermione Granger in The Goblet of Fire mentions that most modern electronics won’t work in Hogwarts because of the high concentration of magic there. This is a common feature in many works of the “urban fantasy” subgenre set in the modern world. Harry Dresden of the Dresden Files novels can’t own a computer or a car with a modern electronic system because they routinely break when near him. Both Dresden’s apartment and the houses of all wizarding families in the Harry Potter books are lit by candles. Most of the wizards in the Harry Potter books are improbably unfamiliar with the ways of modern-day muggles, to the point of not being able to operate a “fellytone.” Given the lifestyle, why would anyone want to be a wizard? There are a lot of ways in which we could pursue this theme, but one way in particular has been overlooked by most writers: how dark a castle like Hogwarts or Harry Dresden’s apartment would be if illuminated only by candles.
3.2 ILLUMINATION
Much of the action in the Harry Potter novels is set in a huge castle, Hogwarts, somewhere in Scotland. The castle is described in loving detail, with ghosts, moving staircases, and quasi-living pictures. The Great Hall at Hogwarts is one of the main settings in the castle. It is where the students eat, take important exams (such as their O.W.L.s), and learn disapparition. It is described thus when Harry Potter sees it for the first time in the novel Harry Potter and the Sorcerer’s Stone [201]:
Harry had never imagined such a strange and splendid place. It was lit by thousands and thousands of candles that were floating in midair over four long tables, where the rest of the students were sitting.
Even with the myriad candles, I suspect that the Great Hall would be a pretty dark place. One of the big but seldom remarked-on benefits of living in the modern world is the ability to light a dark room using electric lights. Herein hangs a tale.
Before Edison invented the electric lamp, all means of artificial illumination involved fire—burning something. If you’ve ever had to do your homework during a power outage you know that doing homework by candlelight is not the romantic, nostalgic affair it is painted as in historical novels. Much of the time you are maneuvering your book to catch as much light as possible from the flickering flames. No matter how bright the candle is, it is still pretty dim and very hard to read by.
This is a subtle issue that movies get wrong most of the time. Almost any historical movie will present a candlelit room as being nearly as bright as one lit by electric lamps. The reason for this is obvious: having characters stumbling around in what appears to be almost pitch blackness to modern viewers will detract from the story. However, the reason why candlelight and torchlight are dark is subtle.
To the human eye, not all light sources are created equal. Do the following experiment: laser pointers are available in both red and green varieties. Take two of equal power and shine both against a white screen or a light-colored wall. The green diode will seem much brighter than the red diode even though the power is the same. This is because the eye’s rods and cones are most sensitive near the wavelength of green light and less sensitive to the red. These retinal cells can detect light over only a very small spectral range; everything outside that range is invisible to the human eye.
To discuss this, we need to talk about how light works. Light is an electromagnetic wave: think ripples on a pond, not ocean breakers. I’ve made a picture of how a wave “looks” in figure 3.1.
The wave is moving to the right: it is a ripple of something, that something depending on what the wave is. If it’s a water wave, it’s a little hill on the water. If it’s a sound wave, it’s a compression of the air. If it’s a light wave, it’s a ripple in the electromagnetic field, which is a bit more complicated to explain. Here goes:
• Normal matter is composed of three different types of particles: protons, electrons, and neutrons.
• Electrons and protons have charge; by convention, the proton charge is positive and the electron charge is negative. The neutron, as the name implies, has no charge (it is neutral).
• Like charges repel, unlike charges attract.
So far, this is what most of you learned in basic chemistry class. However, what they didn’t teach you was this:
• Take a charge over here and wiggle it up and down somehow. A charge over there, some distance away, will begin to wiggle up and down in sympathy.
• However, it takes a while for the second charge to begin to wiggle up and down. If the distance is 1 meter, the second charge begins to wiggle up and down 1/299,792,458 of a second later.
• The time it takes is greater for bigger distances, lesser for smaller ones: the relation between the time it takes, t, and the distance, x, is given by
![]()
where c is the speed of light. By definition,
![]()
As far as anyone knows, this is the fastest speed anything can travel at in our universe.1
We like to say that there is a something that travels between the two charges. We call this something a light wave. What we measure is simple: charge wiggles up and down over here, charge over there wiggles in response after a time delay. The charge we wiggle here loses energy, the charge over there gains energy. Because we like to think energy is conserved, we say the light wave carries the energy from the first charge to the second charge. This is what light is, in a fundamental way: when you look at a star, charges in the star are wiggling up and down. Many years later, charges in the retina in your eye wiggle up and down in response, causing electrical signals to be sent to the visual centers of your brain, producing the sensation of sight.
However, the eye is not sensitive to all light. To understand this, we need to introduce two key concepts:
• The frequency of the light wave, f, is the number of times per second we wiggle the charge up and down. It is measured in cycles per second, or Hertz (Hz).
• The wavelength, λ, of the light wave is the distance between adjacent crests of the wave (see fig. 3.1). The letter λ is the Greek lowercase lambda, corresponding to lower case “l” in the Roman alphabet.
Wavelength and frequency are related in a simple way:
![]()
Thus, a light wave with a frequency of one cycle per second will have a wavelength of 300 million meters. This is a very long wavelength, much longer than the eye can see. In principle, and in practice as well, light can have any wavelength.
Most sources of light contain a mixture of different frequencies. (Lasers are an exception to this rule.) How bright a given light source appears depends not on the total power that the source puts out but on how much it puts out in the spectral region in which the eye can see. An infrared laser may have a total power of several kilowatts, but since the light isn’t visible to the eye, it wouldn’t work at all as a source of illumination. The eye is sensitive to light over a very small spectral region, wavelengths very roughly from 350 nm to 700 nm. A nanometer (nm) is a very small distance—1 nm equals one-billionth of a meter, or roughly 10−5 the diameter of a human hair! Short wavelength means high frequency: a wavelength of 600 nm means a frequency given by
![]()
Light of this wavelength will appear orange to the eye. In general, light of wavelengths 350–500 nm will appear purple or blue, light of wavelengths 500–600 nm will span the gamut from green to yellow-orange, and light of wavelengths 600–700 nm will appear orange or red. In this spectral region the eye is most sensitive to light of wavelength 555 nm, which is green light.
Sources of light such as candles or torches or light bulbs rely on heat to create light; in physics terms, they are blackbody radiation sources. Blackbody radiation sources are very important in this book; for example, the stars are all essentially blackbody radiators. A thermal source can be characterized by two factors, its radiating area and its temperature. The Stefan-Boltzmann formula tells one how much power (energy per second) a thermal source emits:
![]()
The terms appearing in the equation are:
• P: the emitted power (units of J/s, or watts);
• A: the emitting area (units of square meters, m2);
• T: absolute temperature (i.e., measured from absolute zero) (units of Kelvin, K); and
• σ: the Stefan-Boltzmann constant: σ = 5.67 × 10−8 W/m2K4.
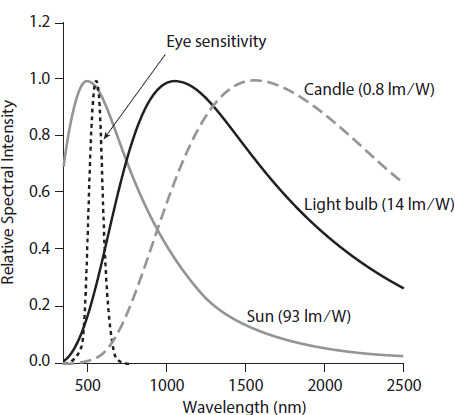
This light is distributed over a wide range of wavelengths. Figure 3.2 shows the blackbody spectrum due to several different sources of light. In particular, it can be seen that the curves are small at both short and long wavelengths but go through a peak in the middle. The peak is determined only by the temperature:
![]()
This isn’t the fundamental form of the equation but a version that makes it easy to calculate peak wavelengths. For example, the sun’s temperature is 5,800 K, implying a peak wavelength given by
![]()
This says that the higher the temperature of the light source, the shorter the peak wavelength will be. In addition to blackbody spectra, figure 3.2 also shows the relative sensitivity of the eye to light of different wavelengths; note that the wavelength where the eye is most sensitive (550 nm) is very close to where the peak of the Sun’s spectrum is (500 nm). The other light sources are much cooler than the Sun, meaning that the overlap isn’t as good. This is the point of this section.
Engineers use the unit of a lumen (lm) to measure how bright a light source is: because the eye is not uniformly sensitive to all wavelengths, there is no direct conversion of lumens to watts. The conversion and the luminous efficacy of the light source depend on the properties of the given source. There is an online appendix for this book (press.princeton.edu/titles/10070.html) describing how to calculate the brightness of a given light source from the blackbody spectrum. Readers who are interested in it can read it, but in general, the better the overlap of the spectrum with the eye’s sensitivity, the brighter the light source will be. The luminous efficacy characterizes how bright a given source of light will be:
![]()
where
• B is the brightness of the light source (in lumens);
• L is the luminous efficacy (in units of per watt, lm/W); and
• P is the total power radiated by the light source (in units of watts).
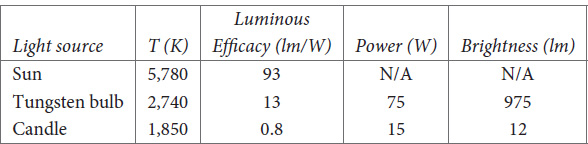
The highest possible value for L is 683 lm/W; you can only get a value this high if you are using a laser that emits light with a wavelength of 555 nm. The efficacy of any blackbody source, even the Sun, will be much lower. The key parameter is the temperature T of the different sources. My friend Dr. Raymond Lee is an expert on natural and artificial illumination. At my request he measured the spectral emissivity and blackbody temperature of two artificial light sources, a 75 W tungsten bulb and a candle. From his measurements, we determined that the bulb had an effective blackbody temperature of 2,740 K and the candle a temperature of 1,850 K. It was also possible to determine the total power radiated by the candle in all spectral regions. Using the data, we calculated a total radiated power of 15 W, with an accuracy of about ±15%.
Table 3.1 shows the luminous efficacy of various different light sources.
The candle will be much dimmer than the bulb for two reasons:
1. It radiates away only one-fourth the power the light bulb does.
2. Much less of that power is in the visible region of the spectrum.
The second point is the more important one. Because the blackbody temperature of the candle is so low, most of its light will be emitted in the infrared region, where it is invisible and useless for illumination. Only about 1% of the light from the candle is radiated away in the visible region of the spectrum, compared to about 10% of the light from the bulb. In figure 3.2 I’ve graphed how well the spectrum from each light source overlaps the eye’s sensitivity. The overlap with the solar spectrum is extremely high. It is less so for the tungsten bulb, and almost nil for the candle. Thus, a candle will appear 80 times dimmer than a light bulb!2
Let’s return to the question that inspired this section, how brightly illuminated is the Great Hall? How well a light source can brighten an area depends on the illuminance of the light, which is the total luminance divided by the total area it is illuminating. That is to say, a huge stadium with a single candle suspended above the middle of it will be much dimmer than a broom closet illuminated with an arc lamp. So we need the total area of the Hogwarts Great Hall. As I write this, I am sitting in the main reading room of the Library of Congress; it seems about the size of the Great Hall, although a different shape. Pacing it off, it is about 30 m in diameter (i.e., about 100 feet), for an area of about 700 m2. The illuminance of the hall is the total luminant flux divided by the area it is illuminating. To get an exact value would be very tricky, but we can reason as follows. Assuming that the candle is a uniform illuminator, equal amounts of light will be going off in all directions. Half of it will be going upward and will be wasted for purposes of illumination. Of the rest, some part will be going off to the side, although some of that will be reflected off the walls and not entirely lost. I’m going to estimate that 40% of the light will be useful for seeing with. Therefore, the total illumination (I) is given by

This is an average value: some areas will be somewhat brighter, others somewhat dimmer. However, it’s not going to vary by an enormous amount. This illumination is inadequate for reading and writing, which usually require a minimum value of 200 lm/m2. The Great Hall is used for eating, but even for simple repetitive tasks a minimum of about 100 lm/m2 is required.
Of course, the Great Hall is bewitched, so that the ceiling shows the sky outside; during the day it’ll be pretty bright. Maybe the reason why the four founders bewitched it in the first place is the dimness of artificial illumination in medieval times. Solar illumination can reach values in excess of 105 lm/m2, or over a thousand times brighter than the candlelight available in the Great Hall. However, at night the candles will provide most of the light, as the nighttime sky in the country can get very dark. Even the full Moon provides an illuminance of only 0.2 lm/m2. Please note that this is not a measure of how bright the full Moon will appear when, say, Ron looks at it; this is a measure of how much lighting the full Moon can provide for tasks such as eating and reading. More or less, the illumination of the Great Hall at night by the candles will be about the same as that experienced by someone standing outside during the middle of what we in the light-and-color biz refer to as civil twilight: the period of time right around when the sun is setting below the horizon.
Thinking about this issue, one starts to wonder about a few things more. First, candles have to be held upright to burn correctly, but there is a shadow region directly under the flame (where the candle is). This is going to block even more light from getting to the tables.
Second, the cost of using candles in this fashion is going to be horrific: generally speaking, they’re probably going to have to replace each candle each day. If we assume a cost of $1 per candle, this represents a bill of $5,000 per day, or (assuming a 200-day school year) a $1 million annual lighting bill for the Great Hall alone. Conservatively, it’ll cost about ten times more for the entire castle, or about $10 million per year. In magical terms, this is about one million golden galleons per year, or perhaps 1,000 galleons per student per year. By comparison, lighting a building using electricity is much cheaper. Under the same assumptions as above, we can provide an illumination of 200 lm/m2 to the Great Hall using a total power of about 10 kW using tungsten bulbs. This is enough for eating, writing, or reading. Assuming electricity costs of $0.1/kW-hr, lighting the hall costs about $10 per day for ten hours of illumination, or fully 500 times less than using candles. (How much do those students pay to go school?) Finally, as these candles burn, hot wax should drip onto the students eating below. I’m a little surprised Draco never complained about this.
One response to this entire chapter is to say, “Well, they’re magic candles, so they burn brighter than ordinary ones, and don’t drip, and cost nothing.” This is perfectly acceptable, although J. K. Rowling never writes that they are. It doesn’t detract anything from the story to take this view. However, another response is to say that this is one detail of a fascinating book, and it is part of “the Game” to examine such details and see where they lead us. I feel that the latter approach is far more interesting and more fun than leaving the books unexamined.
NOTES
1. We will usually round this to c = 3 × 108 m/s, which is almost always close enough for any calculation we make.
2. Of course, tungsten bulbs themselves aren’t ideal illuminators, which is why people are switching to compact fluorescent bulbs. The fluorescents have higher effective temperatures, so even more of their light is in the visible spectrum.