Classification of Adhesives and Compounds
There are a large number of adhesives on the market. It is helpful to organize these adhesives in groups with common characteristics to facilitate their understanding and use. Adhesives can be classified in a number of ways, although no one classification is universally recognized. Classifications include source, function, chemical composition, physical form, and application. A unique class of adhesives includes those used in medicine and dentistry. These adhesives have been covered in a separate chapter.
Keywords
Adhesive base; solvents; diluents; fillers; adhesives
4.1 Introduction
There are a large number of adhesives on the market. It is helpful to organize these adhesives in groups with common characteristics to facilitate their understanding and use. Adhesives can be classified in a number of ways, although no one classification is universally recognized. Classifications include source, function, chemical composition, physical form, and application. A unique class of adhesives includes those used in medicine and dentistry. These adhesives have been covered in Chapter 5.
4.2 Adhesive Composition Formulation
Adhesives resemble paints in formulation in that they may contain a number of components in addition to the adhesive materials, which are also called the binders. Every component is not found in every adhesive. For example, not all adhesives contain a solvent or filler. The key components that may be found in commercial adhesives have been defined in this section.
4.2.1 Adhesive Base or Binder
This is the primary component and has the function of forming the bond, thus holding the substrates together. The binder is generally the component from which the name of the adhesive is derived. For example, an epoxy adhesive may have many components, but the main component is the binder, i.e., the epoxy resin.
4.2.2 Hardener (for Thermosetting Adhesives)
This is a substance added to an adhesive to promote the curing reaction by taking part through catalysis or cross-linking. Two-part adhesive systems generally have one part that is the base and a second part that is the hardener. Upon mixing, a chemical reaction takes place that causes the adhesive to solidify. A catalyst is sometimes incorporated in an adhesive formulation to speed up the reaction between the base and the hardener. Very small amounts of catalyst are required, compared to the principal components such as base and hardener.
4.2.3 Solvents
Solvents are sometimes needed to reduce the viscosity of the adhesive to enhance its spreadability. Solvents used with synthetic resins and elastomers are generally organic in nature. Often a mixture of solvents is needed to achieve the desired processability characteristics such as controlled solvent evaporation and removal. This can be accomplished by combining solvents with variable volatilities.
4.2.4 Diluents
These are liquid ingredients added to an adhesive to reduce the concentration of the binder component. Diluents are added principally to lower the viscosity and to modify the processing conditions of some adhesives. Reactive diluents do not evaporate, as would solvents. They react with the binder during the cure cycle and are incorporated in the cured adhesive.
4.2.5 Fillers
Fillers are relatively neutral substances added to the adhesive to improve its working properties, strength, permanence, or other qualities. Fillers are also intended to reduce materials costs. Considerable changes can be made in the properties of an adhesive by selective use of fillers. Fillers are used to modify adhesives to govern properties such as thermal expansion, electrical and thermal conductivity, shrinkage, and heat resistance.
4.2.6 Carriers or Reinforcements
These are usually thin web-type materials such as plastic film, fabric, or paper used to support the adhesive composition. The role of the web in an adhesive-coated web includes acting as a carrier, a release media, a tape, or a film. The carriers could also serve as a bond-line spacer and reinforcement for the adhesive.
4.2.7 Other Additives
In addition to the basic components, an adhesive may contain a number of other additives, each aimed at achieving a specific characteristic. They include plasticizers, accelerators, inhibitors, retarders, tackifiers, thickeners, film formers, antioxidants, antifungal agents, and surfactants.
Formulation of an adhesive is more of an art than a science. Little basic information has been published about the formulation of adhesives because of their proprietary nature. There are few references that provide recipes of adhesives and the resulting properties [1–4].
4.3 Classification of Adhesives
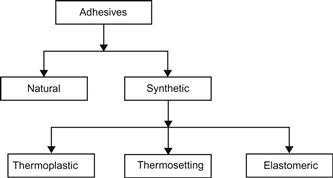
This section presents classifications of adhesives from a number of points of view including function, source, physical form, mode of application and setting, chemical composition, Society of Manufacturing system, and Rayner system. A simple classification scheme is depicted in Figure 4.1. Adhesives are either produced from a natural source such as starch glue or, as is the case with the majority of consumptions, they are synthesized from basic hydrocarbons. The synthetic group consists of thermoplastic and thermosetting adhesives, both of which follow the definitions used in plastics for thermoplastic and thermosetting polymers.
4.3.1 Source: Natural vs. Synthetic Adhesives
This classification is based on whether the adhesive is produced from natural sources or synthesized from basic hydrocarbons.
4.3.1.1 Natural Adhesives
This term is used to include vegetable- and animal-based adhesives and natural gums. These include organic materials such as casein, blood, albumin, hide, bone, fish, starch, resin, shellac, asphalt, chitosan, and inorganic adhesives like sodium silicate. Their use, except for the inorganic adhesives, is mostly limited to paper, paperboard, foil, and light wood. They are inexpensive, easy to apply, and have a long shelf life. These adhesives develop tack quickly but have low strength properties. Most are water-soluble and use water as a solvent. They are supplied as liquids or dry powders to be mixed with water. Some are dispersions in organic solvents [5]. Commercial natural adhesives are described in more detail [6].
Starch and Dextrin
These materials are derived from cereals or roots such as corn, wheat, tapioca, and sogo. The basic constituent is polysaccharide which on hydrolysis yields long chain glucose units. Variations can occur with the use of different food materials and with changes in hydrolysis methods.
The main use for these adhesives is the paper industry where they are used in multiwall bags, corrugated paper, etc. Dextrin, which is dry roasted starch, is used in remoistenable adhesives.
Gelatine (Animal, Fish, Vegetable Glues)
There is a wide range of products included in this overall category. Generally they are all proteins which are derived from the hydrolysis of either collagen or soya flour, or by separating casein from skim milk.
• Animal glues from bones and hides are used in gummed tape and textiles, and in the paper industry for book-binding and case making, for example.
• Fish glues manufactured from skins have been used in bonding rubber gasket to steel, paper to steel, etc.
• Caseins from skim milk are used mainly in wood bonding.
Asphalt and Bitumen
These high fractions of crude oil are more used as sealers rather than adhesives, except in the bonding of coarse grade papers to produce waterproof building papers.
Natural Rubber
Rubber is derived as a latex from the rubber tree (Hevea brasiliensis). The raw latex is collected and concentrated and either sold as a latex or coagulated and sold as solid for solvent dissolution. Adhesives made from natural rubber, which is essentially polyisoprene, are very tacky, and are used in pressure-sensitive applications or where long bond times and tack are required such as tapes, ceramic tile adhesives, and flooring adhesives.
Resins, Shellac
Natural resins have been used as adhesives. Shellac is used in bonding mica splittings to form micaboard and used in abrasives. Gum arabic is used in remoistenable adhesives. Copal gums are used in spirit-soluble lino cements.
4.3.1.2 Synthetic Adhesives
This term is usually applied to all adhesives other than natural adhesives (i.e., elastomeric, thermoplastic, thermosetting, and alloys). All structural adhesives are synthetic.
4.3.2 Classification by Chemical Composition
This classification describes synthetic adhesives as thermosetting, thermoplastic, elastomeric, or combinations of these types (alloys) [5,7].
4.3.2.1 Thermosetting Adhesives
These are materials that cannot be heated and melted after the initial cure. Curing takes place by chemical reactions at room temperature or at an elevated temperature, depending on the type of adhesive. Some thermosetting adhesives require considerable pressure, while others require only contact pressure. Solvents are sometimes added to facilitate application. These adhesives are usually available as solvent-free liquids, pastes, and solids [7].
Thermosetting adhesives are provided as one- and two-part systems. The one-part systems usually require elevated temperature cure and have a limited shelf life. The two-part systems have longer shelf lives and can usually be cured slowly at room temperature, or somewhat faster at moderately higher temperatures. A disadvantage is their need for careful metering and mixing to make sure that the prescribed proportions are blended and that the resultant mixture is homogeneous. Once the adhesive is mixed, the useful life is limited [7].
Because thermosetting resin adhesives, when cured, are densely cross-linked, their resistance to heat and solvents is good, and they show little elastic deformation under load at elevated temperatures. Bonds are able to withstand temperatures of 93–260°C and peel strength is fair. The major application is for stressed joints at somewhat elevated temperatures. Most materials can be bonded with thermosetting adhesives, but the emphasis is on structural applications [7]. Examples of thermosetting adhesives are given in Table 4.1.
Table 4.1
Major Thermosetting Adhesives [7]
| Cyanoacrylates | Epoxy |
| Polyester | Polyimide |
| Urea-formaldehyde | Polybenzimidazole |
| Melamine-formaldehyde | Acrylic |
| Resorcinol | Acrylic acid diester |
| Resorcinol-phenol-formaldehyde |
4.3.2.2 Thermoplastic Adhesives
These materials do not cross-link during cure and they can be melted without significant change in their properties. They are single-component systems that harden upon cooling from a melt state, or by evaporation of a solvent or water vehicle. Wood glues are thermoplastic emulsions that are common household items. They harden by evaporation of water from an emulsion.
Thermoplastic adhesives are not ordinarily recommended for use at above 66°C, although they can be used up to 90°C in some applications. These materials have poor creep resistance and fair peel strength. They are used mostly in stressed joints and designs with caps, overlaps, and stiffeners. The materials most commonly bonded are nonmetallic material, especially wood, leather, plastics, and paper [5,7]. With the exception of some hot-melt adhesives, thermoplastic adhesives are not generally used for structural applications. Examples of thermoplastic adhesives are given in Table 4.2 [7].
Table 4.2
Major Thermoplastic Adhesives [7]
| Cellulose acetate | Polyvinyl acetals |
| Cellulose acetate butyrate | Polyvinyl alcohol |
| Cellulose nitrate | Polyamide |
| Polyvinyl acetate | Acrylic |
| Polyvinyl chloride | Phenoxy |
| Polyvinylidene chloride |
4.3.2.3 Elastomeric Adhesives
These materials are based on synthetic or naturally occurring polymers. They have superior toughness and elongation. Elastomeric adhesives may be supplied as solutions in organic solvents, latex cements, dispersions, pressure-sensitive tapes, and single- or multiple-part solvent-free liquids or pastes. Curing varies, depending on the type and the form of adhesive. These adhesives can be formulated for a wide variety of applications, but they are generally used for their high degree of flexibility and superior peel strength [5,7].
Some elastomeric adhesives are supplied in film form. Most of these adhesives are solvent dispersions of water emulsions. Temperature environments up to 66–204°C are practical. Elastomeric adhesives never melt completely. Bond strengths are relatively low, but flexibility is excellent. These adhesives are used in unstressed joints on lightweight materials, so they cannot be considered structural adhesives. They are particularly advantageous for joints in flexure. Most of these adhesives are modified with synthetic resins for bonding rubber, fabric, foil, paper, leather, and plastic films. They are also applied as tapes [5,7]. Examples of elastomeric adhesives are given in Table 4.3.
4.3.2.4 Adhesive Alloys
Combining resins of two different chemical groups chosen from the thermosetting, thermoplastic, or elastomeric groups makes up these adhesives. The thermosetting resin, chosen for its high strength, is plasticized by the second resin, making the alloy tougher, more flexible, and more resistant to impact [5]. The adhesive alloys take advantage of the most important properties of each component. They are commonly available as solvent-based solutions and as supported and unsupported films [5].
Except for some epoxy compounds, heat and pressure are usually required for curing. Most alloy adhesives are solvent-based dispersions or 100% solids. These adhesives have a balanced combination of properties and are generally stronger over wider temperature ranges than other adhesives. They are suitable as structural adhesives and are used where the highest and strictest end-use conditions must be met (regardless of cost) such as in military applications [5,7].
Materials bonded include metals, ceramics, glass, and thermosetting plastics. Applications are primarily for high strengths and high temperatures [5,7]. Examples of alloy adhesives are given in Table 4.4.
Table 4.4
Major Alloy Adhesives [7]
| Epoxy-phenolic | Neoprene-phenolic |
| Epoxy-polysulfide | Vinyl-phenolic |
| Epoxy-nylon | Polyvinyl acetal-phenolic |
| Nitrile-phenolic |
4.3.3 Classification by Function
4.3.3.1 Structural Adhesives
These are materials (Table 4.5) of high strength and performance. Their primary function is to hold structures together and to be capable of resisting (bearing) high loads [7]. A more detailed discussion can be found in Chapter 1.
Table 4.5
| Adhesive Material | Modifier |
| Epoxy | |
| Modified (or alloyed) epoxy | Toughener, nylon, phenolic, polysulfide, resorcinol and phenol formaldehyde, melamine, and urea-formaldehyde |
| Modified (or alloyed) phenolic | Nitrile, vinyl, neoprene |
| Polyaromatics | |
| Polyester | |
| Polyurethane | |
| Anaerobic | |
| Cyanoacrylate | |
| Modified acrylic | |
| Neoprene (chloroprene) | |
| Nitriles (acrylonitrile-butadiene) | |
| Polysulfide |
4.3.3.2 Nonstructural Adhesives
These adhesives are not required to withstand substantial loads but merely hold materials in place. This group is sometimes called “holding adhesives.” Examples include adhesives/sealants that are primarily intended to fill gaps and rubber cements that are used to adhere paper to paper in office applications [7].
4.3.4 Classification by Physical Form [7]
Adhesives can be subdivided by their form such as liquid, powder, film, or paste. The physical state of the adhesive generally determines how it is to be applied.
4.3.4.1 Liquid Adhesives
These adhesives are easily applied by means of mechanical spreaders such as rolls, by spraying, or by brushing.
4.3.4.2 Paste Adhesives
Paste adhesives have high viscosities to allow application on vertical surfaces with little tendency to sag or drip. These bodied adhesives can serve as gap fillers and sealants.
4.3.4.3 Tape and Film Adhesives
These adhesives provide a bond line with uniform thickness and offer the advantages of no need for metering and ease of dispensing. Adhesive films are available as pure sheets of adhesive, or with film or paper reinforcement.
4.3.4.4 Powder or Granule Adhesives
It is usually not possible to apply adhesives in solid form. These materials must be heated or dissolved in a solvent to be converted into a liquid form, to enable their application to surfaces.
4.3.5 Classification by Mode of Application and Setting [7]
Adhesives are often classified by their mode of application. Depending on viscosity, adhesives can be coated, sprayed, or brushed. Adhesive pastes and mastics are applicable by extrusion and may be applied by syringe, caulking gun, or pneumatic pumping equipment.
Another way to distinguish between adhesive groups is the manner by which they flow or solidify. As given in Table 4.6, some adhesives solidify simply by evaporation of solvent, while others harden as a result of heat activation or chemical reaction. Pressure-sensitive systems flow under pressure and are stable when pressure is removed.
Table 4.6
Adhesives Classified by Activation and Cure Requirements [5,7]
| Requirement | Types Available | Forms Used | Remarks |
| Heat | Room temperature to 232°C types available; 121–177°C types most common for structural adhesives | Formulated in all forms; liquid most common | Application of heat will usually increase bond strength of any adhesive, including room-temperature-curing types |
| Pressure | Contact to 3.5 MPa types available; 0.17–1.38 MPa types most common for structural adhesives | Formulated in all forms; liquid and powder most common | Pressure types usually have greater strength, except for modified epoxies |
| Time (room-temperature curing) | Types requiring a few seconds to a week are available; 0.5–24 h types are most common for structural adhesives | Formulated in all forms | Time required varies with pressure and temperature applied and immediate strength |
| Catalyst (room-temperature curing) | Extremely varied in terms of chemical catalyst required; may also contain thinners, etc. | Two components: paste+liquid or liquid+liquid | This type may sometimes require elevated temperature (<100°C) and/or pressure instead of, or in addition to, a chemical agent |
| Vulcanizing | Varied types requiring addition of a chemical agent (usually sulfur); may also contain a catalyst | Two liquid components | Premixed types requiring temperatures of 121–177°C for vulcanization are available |
| Reactivation | Types requiring heat or solvent or second coating of adhesive | Dry film or previously applied liquid | Heat-cure adhesive is best for nonporous surfaces and/or maximum strength |
| Radiation | UV-acrylics, cationic epoxies, dual-curing adhesives | Liquids | UV-curing adhesives have shorter curing times than conventional adhesives and can therefore increase production speed and productivity |

4.3.6 Classification by Specific Adherends or Applications [7]
Adhesives are also classified according to their end use as follows:
4.3.7 Society of Manufacturing Engineers Classification
A Society of Manufacturing Engineers publication [8] provides a useful, in-depth classification of adhesives, as given in Tables 4.7 to 4.18 [9].
Table 4.7
Catalytic Plural Components—Chemical Cure
| Epoxy | Polysulfide |
| Phenolic | Polyurethane |
| Resorcinol-formaldehyde | Silicones |
| Polyester |
Table 4.8
Catalytic Plural Components—Moisture Cure
| Silicones | Cyanoacrylate |
| Polyurethane | Epoxy (one-container type) |
| Polysulfides |
Table 4.9
Heat-Activated Systems (One-Part, May Be Solid Film)
| Polybenzimidazole (PBI) | Polyvinyl acetates |
| Polyimide (PI) | Vinyl-phenolic |
| Epoxy | Vinyl-epoxies |
| Nylon | Urethanes |
| Phenolic |
Table 4.10
| Natural rubber | Acrylics |
| Reclaimed rubber | Miscellaneous |
| Synthetic rubbers | Cellulose esters |
| Nitrile rubber | Asphalt |
| Neoprene (polychloroprene) | Polyamides |
| Butyl rubber | Phenoxy resins |
| Styrene-butadiene rubber | Bisphenol-A polycarbonate |
| Phenolics | Polysulfone |
| Urethanes | |
| Vinyl resins | |
| Polyvinyl acetate | |
| Vinyl-phenolics | |
| Polyvinyl alkyd ethers | |
| Polystyrene |
Table 4.11
| Natural rubber | Miscellaneous adhesives |
| Reclaimed rubber Synthetic rubber Vinyl resins | Natural products (animal glue, starch, soya, blood glue, casein, cellulose derivatives) |
| Acrylics | Carboxylic-containing copolymers |
Table 4.14
| Vinyl-phenolics | Elastomer-epoxies (as nitrile-epoxies) |
| Epoxy-phenolics | Aromatic polymers (PI and PBI) |
| Nitrile-phenolics | |
| Nylon-epoxies |
Table 4.15
| Urea-formaldehyde | Polyesters |
| Melamine-formaldehyde | Silicones |
| Phenol-formaldehyde | Furanes |
| Resorcinol-formaldehyde | Acrylics |
| Epoxy | Soluble nylons |
| Polyisocyanate (polyurethane) | Polyaromatics (PI and condensed polycyclic aromatic hydrocarbons)a |
aThese are really thermoplastics but are often grouped with thermosets because of their high melting points.
Table 4.16
Cellulose adhesives (cellulosics)
Polyvinyl adhesives
Polyvinyl ester adhesives (especially polyvinyl acetate)
Polyvinyl acetal adhesives
Polyvinyl alcohol adhesives
Polyvinyl alkyl ether adhesives (some are elastomers)
Polystyrene adhesives
Acrylic resin adhesives
Acrylic esters
Acrylic acid diesters (including anaerobic sealants)
Cyanoacrylates
Acrylic copolymers
Polyamide resin and nylon adhesives (including nylon adhesives with traces of phenolic)
Miscellaneous thermoplastic adhesives
Polycarbonates
Polyacetals
Polyethylene
Polysulfide (sometimes considered thermoplastics, but these are really rubbers)
Table 4.17
Other Hot-Melt Thermoplastic Adhesives
| Ethyl cellulose | Polyisobutylene |
| Polyvinyl acetate | Hydrocarbon resins |
| Ethylene-vinyl acetate | Polypropylene |
| Ethylene-ethyl acrylate | Polyamides |
| Butyl methacrylates | Polyesters |
| Polystyrene and copolymers | Phenoxies |
Table 4.18
| Vinyl-phenolics | Nylon-epoxies |
| Epoxy-phenolics | Elastomer-epoxies |
| Nitrile-phenolics | Neoprene-phenolics |
| Epoxy-polysulfide |
4.3.7.1 Chemically Reactive Types
4.3.7.2 Evaporative or Diffusion Adhesives
4.3.7.3 Hot-Melt Adhesives
4.3.7.4 Delayed-Tack Adhesives
4.3.7.5 Tape and Film Adhesives
4.3.7.6 Pressure-Sensitive Adhesives
Pressure-sensitive adhesives are not discussed in this book.
4.3.8 Classification by Rayner [10]
This is another useful classification that somewhat resembles the “classification by chemical composition” described above.
4.3.8.1 Thermosetting Resin Adhesives
4.3.8.2 Thermoplastic Resin Adhesives
4.3.8.3 Two-Polymer Adhesives (Alloys)
4.3.9 Additional Classification
The following grouping has been found by the author to be convenient. Some of the adhesive types have already been listed. A list of natural glues is presented in Table 4.19.
Table 4.19
| Vegetable glues | Inorganic glues (adhesives, cements) |
| Starch | Soluble silicates |
| Dextrins | Phosphate cements |
| Soybean glue | Basic salt (Sorel cements) |
| Rosin | Litharge cements |
| Animal glues | Sulfur cements |
| Casein | Hydraulic cements |
| Blood adhesives (blood glues) | |
| Shellac | |
| Bone and hide glues | |
| Fish glues |
4.4 Other Classifications
There are still more ways to classify adhesives (Table 4.20).
Table 4.20
Classification of Adhesives by the Type of Cure [11]
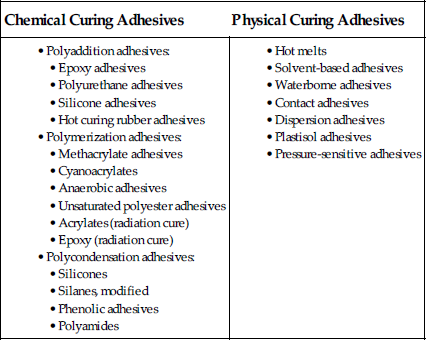
4.4.1 By the Type of Cure That Occurs in the Adhesive
4.4.2 By the Mechanical Properties of Adhesives
a. Elastic adhesives which have a high elongation before fracture occurs, e.g., silicone adhesives, silane modified adhesives, and one-component polyurethane curing by moisture
b. Rigid adhesives, which have high impact resistance but low elasticity, such as epoxy adhesives, anaerobic adhesives, and one-component polyurethane curing by heat.
4.4.3 By the Number of Packages or Components Needed to Produce the Solidification or Curing of the Adhesive
a. One-component adhesives are those adhesives that are presented in a single container or package, such as moisture curing polyurethane adhesives, cyanoacrylates, silicones, moisture curing adhesives, and modified silanes.
b. Two-component adhesives are those adhesives whose components are separated into two different containers or packages, it being necessary to mix them in the correct proportions to begin the process of solidification or curing, such as two-component polyurethane, acrylate adhesives, and 2-component epoxy.