Characteristics of Adhesive Materials
This chapter discusses individual adhesive types in detail. As a matter of convenience, the adhesives have been arranged in alphabetical order without regard to classification. Certain general categories are also listed, such as alloys, aromatic polymer, conductive, delayed-tack, elastomeric adhesives, anaerobic, film and tape adhesives, hot-melt adhesives, inorganic glues, microencapsulated adhesives, rubber-based adhesives, solvent-based systems, thermoplastic resin adhesives, thermosetting-resin adhesives, and water-based adhesives.
Keywords
Adhesives; alloys; polymers; adherends; phenolics
This chapter discusses individual adhesive types in detail. As a matter of convenience, the adhesives have been arranged in alphabetical order without regard to classification.
Certain general categories are also listed, such as alloys, aromatic polymer, conductive, delayed-tack, elastomeric adhesives, anaerobic, film and tape adhesives, hot-melt adhesives, inorganic glues, microencapsulated adhesives, rubber-based adhesives, solvent-based systems, thermoplastic resin adhesives, thermosetting-resin adhesives, and water-based adhesives.
Practically all adhesives were derived from plant or animal sources prior to the twentieth century. The main classes included glue from animal bones, fish glue, and vegetable adhesives. Ancient Egyptians are known to have used casein for lamination of wood for construction of bows and furniture [1]. Progress in organic chemistry and an increase in demand for adhesives led to the development of synthetic compounds beginning with phenol-formaldehydes and casein adhesives. Developments in polymerization and adhesive chemistry proceeded in a near parallel fashion. An abbreviated chronological history of modern adhesive development is given below.
| 1920s: | Cellulose ester, alkyd resin, cyclized rubber in adhesives, polychloroprene (neoprene), soybean adhesives |
| 1930s: | Urea-formaldehyde, pressure-sensitive tapes, phenolic resin adhesive films, polyvinyl acetate wood glues |
| 1940s: | Nitrile-phenolic, chlorinated rubber, melamine-formaldehyde, vinyl-phenolic, acrylic polyurethanes |
| 1950s: | Epoxies, cyanoacrylates, anaerobics, epoxy alloys |
| 1960s: | Polyimide, polybenzimidazole, polyquinoxaline |
| 1970s: | Second-generation acrylic, acrylic pressure-sensitive, structural polyurethanes |
| 1980s: | Tougheners for thermoset resins, water-borne epoxies, water-borne contact adhesives, formable and foamed hot melts |
| 1990s: | Polyurethane-modified epoxy, curable hot melts, UV and light cure systems |
| 2000s: | Water-borne adhesives, reduced volatile organic compounds, solvent-free one- and two-part adhesives |
5.1 Acrylics
The most popular and most commercially successful structural acrylic adhesives in use are polymerizable mixtures of polymers dispersed or dissolved in methyl methacrylate (MMA) monomer. These adhesives are supplied as two separate components that are primarily mixed just prior to application. One component contains a peroxide compound (oxidizing agent) and the second component contains an amine or metal salt (reducing agent) that reacts with the peroxide component upon mixing to initiate the free-radical polymerization of the MMA monomer [2].
Acrylic resins are used for bonding cloth, plastics, leather, and, in some cases, metal foils. The acrylic monomers most commonly used in adhesives are ethyl acrylate, methyl acrylate, methacrylic acid, acrylic acid, acrylamide, and acrylonitrile. The polymers or copolymers are soluble in common organic solvents and can be supplied in much the same manner as other solvent-based systems. In addition, the polymers are soluble in the monomers. When a catalyst is added, monomers polymerize, thus providing good bonding to glass and to plastic surfaces of similar composition (e.g., polymethylmethacrylate) [3–5].
A variety of acrylic copolymers are prepared by emulsion polymerization. A number of acrylic adhesives that are called “reactive adhesives,” “modified acrylics,” “second-generation acrylics,” or “reactive-fluid adhesives” have become available over the years. These formulations polymerize in the glue line and become an integral part of an adhesive assembly.
The “first-generation acrylic adhesives” cover adhesives that used solutions of polymers, usually rubber, in methacrylate monomers, and involved polymerization of these monomers in the presence of a reinforcing resin. The cure system of first-generation products used benzoyl peroxide and tertiary amines. The newer compounds are based on a combination of different modifying polymers for acrylics and a surface activator. A modifying polymer reinforces and toughens the bond and provides a reactive chemical site, which acts as a catalyst in the presence of special activators. Adhesion takes place when the monomers and activators graft polymerize in modifying the polymer in the glue line.
Primary benefits of the second-generation acrylics included increased toughness and impact strength of metal-to-metal bonds, as well as the ability to bond metal surfaces, even oily metal surfaces, with little or no surface preparation. The products were also shown to be capable of effective performance as “100% solids” alternatives to solvent cements in application such as plastic pipe bonding and decorative lamination of vinyl and high-pressure laminates to metals and particle board [2].
In commercial form, these acrylic adhesive systems consist of two components, each being a 100% solids composition in fluid form, reacting to form an adhesive film. Curing takes place by a free-radical reaction scheme. As a result, these materials do not require careful metering and accurate mixing for full performance. Other advantages include [4–6]:
• Tolerance for oily and otherwise poorly prepared surfaces
• Rapid bonding at room temperature, which can be further accelerated by an increase in the temperature or the use of accelerators
• High peel and impact strength, combined with excellent shear strength
• Good environmental resistance and elevated-temperature properties (up to 177°C).
Excellent bonds to a wide variety of substrates can be obtained. Aluminum, brass, copper, stainless steel, and carbon steel are easily bonded to similar or dissimilar metals. Most plastics, including glass-reinforced grades, can also be bonded, along with wood, glass, cement-asbestos board, and hardboard.
Some adhesives will bond cured elastomers. Typically, a thin layer (0.0025 mm thickness) of the activator is applied to one of the adherends and a layer of the adhesive (0.026–0.26 mm) is placed on the other adherend. The two substrates are then pressed together and secured until adequate handling strength develops. Most acrylic adhesives cure to this point in 2–20 min, but some cure in as little as 10 s. In all cases, cure is completed within 24 h. These adhesives provide excellent shear, peel, and impact strengths at temperatures ranging from −107°C to +121°C. These adhesives can withstand short exposures up to 177°C [4–6].
Bonds made with acrylics typically resist immersion in isooctane, motor oil, aircraft hydraulic fluid, 10% sodium chloride solution, distilled water, ethyl alcohol, and dilute mineral acids and alkalies. However, they are not resistant to concentrated acids and alkaline solutions or acetone. Weathering resistance, including salt spray environments, is also excellent.
Acrylic adhesives can be used to replace spot welding where immediate handling of the joined metal parts is required. Another broad area of application is for bonding dissimilar substrates, including metals and other materials with different coefficients of expansion [4–6].
For additional information about acrylic adhesives, see the discussion on anaerobic adhesives (see Section 5.4) and cyanoacrylate adhesives (see Section 5.11).
5.2 Allyl Diglycol Carbonate (CR-39)
See Section 5.34 on polyester adhesives.
5.3 Alloyed or Modified (Two-Polymer) Adhesives
These adhesives are important as structural adhesives especially in metal bonding. They comprise a thermosetting and a thermoplastic polymer, including certain elastomers. Although each component has adhesive properties by itself, on the whole the conjoint system forms a stronger and more versatile adhesive. The two-polymer systems have been particularly successful as film and tape adhesives.
The physical properties of each component polymer are modified by the addition of the other, possibly increasing heat resistance of one component, while reducing that of the other. Similarly, the toughness of one component may be increased by sacrificing the flexibility of the other component. Therefore, it is possible to formulate a variety of adhesives with a wide range of characteristics by simply varying the ratio of one polymer to the other.
In most widely used two-polymer adhesives, the thermosetting component is phenolic. Phenolic resins are generally compatible, although not easily miscible, with a number of thermoplastic polymers. Particularly good compatibility is demonstrated between conventional alcohol-soluble phenolic resins and polyvinyl esters and acetals. Epoxies are important in two-polymer adhesive systems. The most important thermoplastic components are the polyvinyl acetals (polyvinyl formal and butyral) and synthetic rubber, particularly nitrile rubber. Soluble nylons are also an important class [7].
Five of the most important two-polymer adhesives used in films and tapes include vinyl-phenolics, epoxy-phenolics, nitrile-phenolics, nylon-epoxies, and elastomer-epoxies. Neoprene-phenolics are available in organic solutions and in supported and unsupported films. These adhesives are used to bond a variety of substrates. Curing takes place under heat and pressure at 150–260°C and 0.3–1.75 MPa for 15–30 min for film and at 90°C and contact to 0.7 MPa of pressure for 15–30 min for the liquid, after drying at 90°C. Because of their high resistance to creep and most service environments, neoprene-phenolic joints can withstand prolonged stress. Fatigue and impact strengths are excellent, but shear strength is lower than that of other modified phenolic adhesives [6,8,9].
Epoxy polysulfides [10] are available as two-part liquids or pastes that cure at room temperature or higher to rubbery solids that provide bonds with excellent flexibility and chemical resistance. These adhesives bond well to a number of substrates. Shear strengths and elevated-temperature properties are low, but resistance to peel and low-temperature properties are quite high.
Of the five alloy-tape adhesives, vinyl-phenolic is also available in solvent-based solution and emulsion, liquid, and co-reacting powder. Epoxy-phenolic is also available as a two-part paste. Solvent blends of this material are usually force-dried at 80–90°C for 20 min before assembly of adherends. Curing is generally for 30 min at 95°C with contact pressure, followed by 30 min to 2 h at about 165°C and 0.07–0.4 MPa pressure. The postcuring provides optimum strength at elevated temperatures.
Nitrile-phenolic and nylon-epoxy adhesives are also available as solvent solutions as well as in film form. The nitrile-phenolic film is cured at 150–260°C for 15–30 min with bonding pressures from 0.12 to 1.8 MPa. The liquid alloy is dried at 80°C and cured for 15–30 min at 90°C and contact at 0.7 MPa of pressure. The nylon-epoxy paste is cured for 3 days at 20°C to 1 day at 150°C under bonding pressure from 0.11 to 0.32 MPa. Cure temperatures for some formulations can be increased to 200°C with corresponding reduction in cure time (4 h). No volatiles are released during cure, so large areas can be bonded without venting [6,8,9].
Examples of commercial nitrile-phenolic adhesives include Henkel’s Plastilock® and 3M’s Scotch-Weld™ AF film adhesives. Features of these adhesives include fuel and chemical resistance and retention of strength after aging. Some of the properties of two grades of Plastilock® which retain flexibility and shear strength in a service temperature range of −55°C to 132°C can be found in Tables 5.1 and 5.2. Examples of performance data for Scotch-Weld™ are presented in Table 5.3.
Table 5.1
Properties of Some of Henkel’s Plastilock® Nitrile-Phenolic Film Adhesives [11]
| Typical Technical Data | PL 663 | PL 691 |
| Base | Nitrile rubber/phenolic resin | Nitrile rubber/phenolic resin |
| Form | Unsupported film on polyethylene separator | Liquid, 8–10% total solids |
| Color | Cream | Amber |
| Gauge | 0.010″ to 0.002″ or 0.020″ to 0.003″ (0.025 cm″ to 0.005 cm or 0.050 cm″ to 0.008 cm) | 6.87 lb/gallon (0.82 kg/l) |
| Film width/coverage | Available in widths up to 24″ | 600 SF for 0.2 milli inch dry film thickness |
| Cure | 135 min at 305°F (152°C), 100 psi (0.69 MPa) | |
| Storage life | 6 months at 55°F (13°C) or below | 6 months at 55°F (13°C) or below |
| Flash point | 20°F (−7°C) | |
| Brookfield viscosity at 25°C (−3.8°C) | 20 cps, #1 spindle at 20 rpm |
Table 5.2
Bond Strength of Plastilock® PL 633 Nitrile-Phenolic Film Adhesive to Aluminum 2024-T3 [11]
| °F | °C | PSI | MPa | |
| Lap shear | −67 | −55 | 2,700 | 18.6 |
| Lap shear | 75 | 23 | 3,880 | 26.8 |
| Lap shear | 180 | 82 | 2,595 | 17.9 |
| Lap shear | 270 | 132 | 1,520 | 10.5 |
| T-Peel | 75 | 23 | 184 lb/3″ | 819 N/75 mm |
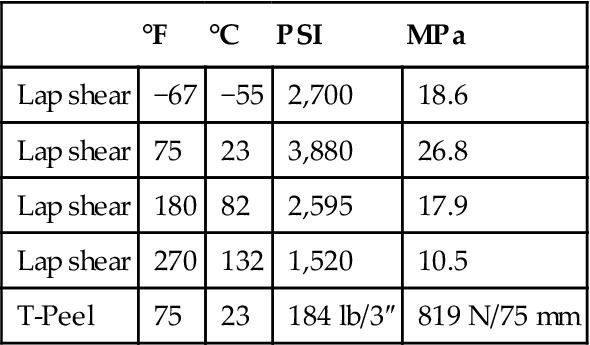
Table 5.3
Properties Test Results for Scotch-Weld™ AF 6 Bonded Specimens Prepared and Tested According to Military Specification MMM-A-132 Type 1, Class 2 [12]
| Test Condition | Film AF6 Unprimed | |
| 1. Normal temperature 75°F (24°C) | 3,400 psi (23.4 MPa) | |
| 2. 10 min at 180°F (82°C) | 1,600 psi (11.0 MPa) | |
| 3. 10 min at −67°F (−55°C) | 3,000 psi (20.7 MPa) | |
| 4. Normal temperature 75°F (24°C) after 30 days’ salt water spray | 3,200 psi (22.0 MPa) | |
| 5. Normal temperature 75°F (24°C) after 30 days’ at −120°F (49°C) and 95–100% RH | 3,300 psi (22.7 MPa) | |
| 6. Normal temperature 75°F (24°C) after 30 days’ immersion in tap water | 3,700 psi (25.5 MPa) | |
| 7. Normal temperature 75°F (24°C) after 7 days’ immersion in JP-4 fuel | 3,300 psi (22.7 MPa) | |
| 8. Normal temperature 75°F (24°C) after 7 days’ immersion in anti-icing fluid | 3,100 psi (21.4 MPa) | |
| 9. Normal temperature 75°F (24°C) after 7 days’ immersion in hydraulic oil | 3,600 psi (24.8 MPa) | |
| 10. Normal temperature 75°F (24°C) after 7 days’ immersion in type III hydrocarbon fluid | 3,200 psi (22.0 MPa) | |
| Creep rupture | ||
| 11. Normal temperature 75°F (24°C) for 192 h at 1600 psi | 0.0072 in. (0.1829 mm) | |
| 12. 180°F (82°C) for 192 h at 800 psi | 0.0065 in. (0.1651 mm) | |
| Fatigue | ||
| 13. Normal temperature 75°F (24°C) 750 psi at 106 cycles | No failures | |
| Other tests | ||
| 14. Tensile shear 75°F (24°C) blister detection | 3200 psi (22.0 MPa) | |
| 15. Tensile shear Scotch-Weld AF 6/3M™ Scotch-Weld™ Structural Adhesive Primer EC-1290 10% | Test temp. | Test results |
| −67°F (−55°C) | 2,400 psi (16.5 MPa) | |
| 75°F (24°C) | 3,400 psi (23.4 MPa) | |
| 180°F (82°C) | 1,600 psi (11.0 MPa) | |
| 16. T-Peel Scotch-Weld AF 6/Scotch-Weld EC-1290 10% | Test temp. | Test results |
| −67F (−55°C) | 7 piw (31.2 N/25 mm) | |
| 75°F (24°C) | 60 piw (267 N/25 mm) | |
| 180°F (82°C) | 25 piw (111 N/25 mm) | |
| 17. Scotch-Weld AF 6 unprimed overlap shear strength on chromic anodized aluminum | Test temp. | Test results |
| 75°F (24°C) | 3,800 psi (26.2 MPa) | |
| 180°F (82°C) | 1,700 psi (11.7 MPa) | |
| 250°F (121°C) | 1,200 psi (8.3 MPa) | |
| 300°F (149°C) | 1,000 psi (6.9 MPa) | |
| 350°F (177°C) | 900 psi (6.2 MPa) | |
| 400°F (204°C) | 800 psi (5.5 Mpa) | |
| Cure cycle: 60 min at 350°F, 90 psi, 10°F/min rise (60 min at 177°C, 0.62 MPa, 5.6°C/min rise.) | ||
| 18. Thermal conductivity: 0.062 btu/h sq. ft./°F; /ft | ||
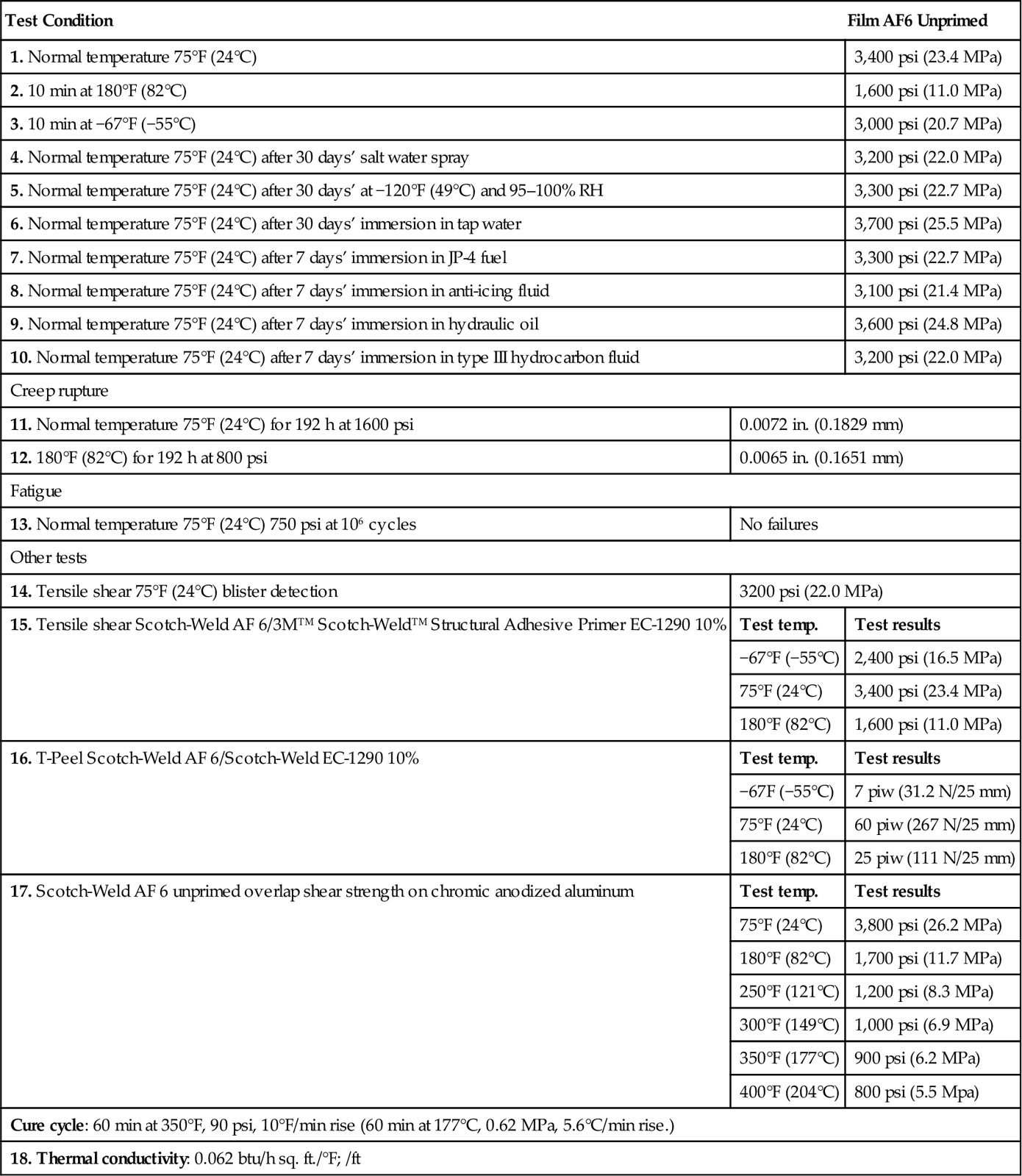
Performance properties of PL 663 on aluminum grade 2024-T3 (anodized with chromic acid) and primed with PL 691 are listed in Table 5.2. Primer was air-dried for 30 min and baked for 80 min at 138°C. Primer was abraded lightly with Scotchbrite, re-primed and air-dried 90 min prior to bonding. Adhesive was 0.05 cm thick, and cured for 135 min at 152°C and 0.69 MPa. Performance test results for Scotch-WeldTM nitrile-phenolic film adhesives can be found in Tables 5.3 and 5.4. Surface preparation, priming and film application techniques have been provided for nitrile-phenolic adhesives [12].
Table 5.4
Scotch-Weld™ AF 13 Bonded Specimens Prepared and Tested According to Military Specification MMM-A-132 Type 1, Class 2 [12]
| Test Condition | ||
| Tensile shear | Test temp. | Test results |
| −67°F (−55°C) | 2,800 psi (19.3 MPa) | |
| 75°F (24°C) | 2,800 psi (19.3 MPa) | |
| 180°F(82°C) | 1,600 psi (8.6 MPa) | |
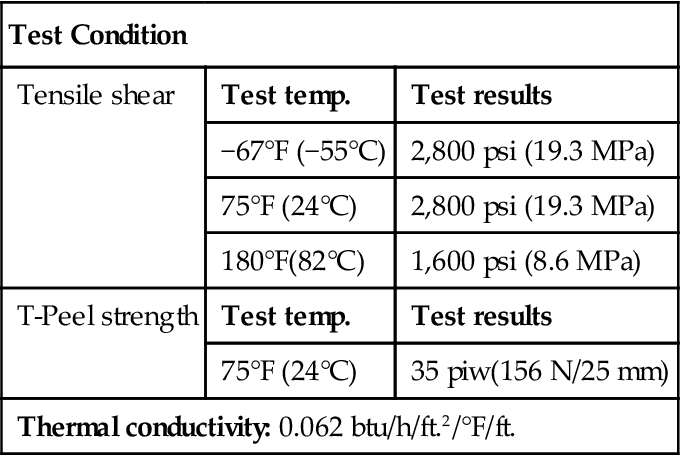
| T-Peel strength | Test temp. | Test results |
| 75°F (24°C) | 35 piw(156 N/25 mm) | |
| Thermal conductivity: 0.062 btu/h/ft.2/°F/ft. | ||
5.4 Anaerobic Adhesives/Sealants
This adhesive group has been promoted for use as a sealant. The adhesives used are acrylate acid diesters (polyester-acrylic). They are essentially monomeric thin liquids that polymerize to form a tough plastic bond when confined between closely fitting metal joints. Contact with air before use keeps the monomeric adhesive liquid. Metal surfaces accelerate the polymerization in the absence of air (anaerobic conditions). These materials will bond all common metals, glass, ceramics, and thermosetting plastics to each other. Phenolic plastics and some plated metals, such as cadmium and zinc, require a primer such as ferric chloride. Polymerization is essentially a free radical-type addition polymerization [13–15].
The most important application of anaerobic adhesives/sealants is as liquid lock washers for screws and bolts. Because of their strong penetrating ability, they can be applied either before or after assembly. The prevailing torque for the strongest grades is many times greater than that of locknuts and lock screws. Cure speed is largely dependent on the parts being joined. There are three basic cure-speed types—fast (5 min to 2 h), medium (2–6 h), and slow (6–24 h)—all at room temperature without primer. Addition of heat will speed up the cure. As a rule, these adhesives will cure outside the connection if the temperature exceeds 93°C, despite the presence of inhibiting air. Heat cures up to 149°C are practical. Anaerobic adhesives can be cured faster with accelerators or primers, especially on inactive surfaces (nonmetals). The recommended primers are degreasing solvents, which, on evaporation, leave a light deposit of a catalyst to speed up curing.
The operating conditions of the end use environment dictate the threadlocking formulation selected. The newest threadlocking technologies offer many advantages formerly unavailable, including surface insensitivity, high temperature resistance, and chemically resistance, as well as formulations engineered to withstand extreme vibration. Recent advances in the stability and reactivity of threadlocking materials have allowed the development of semisolid “stick” formulations that complement their liquid counterparts. Semisolid threadlocking products work well in overhead or hard to see applications where liquids might be too messy or potential migration might be a problem [16].
These anaerobic adhesives fill all surface irregularities and tolerance gaps and effectively seal clearances up to 0.76 mm. They can be applied by high-speed applications in moving production lines. The cured film has excellent chemical resistance to most liquids and gases within an operating temperature range of −54°C to 232°C.
Anaerobic structural adhesives combining urethane-modified acrylic technology have been developed for more exacting applications. These adhesives can be formulated to meet the requirements of Federal Specification MMM-A-132. Anaerobic sealants and threadlocking products are designed to withstand normal tensile and shear loading. The applications of these products often subject them to shear loading. Anaerobic adhesives can now withstand continuous aging at 232°C. Resistance to salt spray is also excellent [13–15]. An extremely good, although perhaps outdated source of information is a chapter on anaerobic adhesives by Burnham [17] and Adams [18].
The use of anaerobic adhesives has become increasingly popular in appliance assembly applications, as they provide a number of manufacturing benefits. Typically used to augment the seal or holding force of a mechanically joined appliance assembly, anaerobic adhesives serve as threadlockers, thread sealants, retaining materials, and flange sealants [19].
The greatest challenges faced with traditional anaerobic adhesives have been: (i) promoting cure on a wide range of metallic and nonmetallic substrates that may be contaminated with grease or oil, (ii) long-term exposure to elevated temperatures >150°C, and (iii) long-term exposure to high levels of mechanical stress.
The use of anaerobic adhesives has become increasingly popular in appliance assembly applications, as they provide a number of manufacturing benefits. Typically used to augment the seal or holding force of a mechanically joined appliance assembly, anaerobic adhesives serve as threadlockers, thread sealants, retaining materials, and flange sealants.
5.5 Aromatic Polymer Adhesives (Polyaromatics)
Considerable progress has been made in improving the thermal and oxidative stabilities of organic resins at high temperatures. Heat-resistant resins and polymers have been developed as adhesives to meet the needs of the aircraft industry (supersonic aircraft) and space vehicles (missiles, satellites, rockets), where resistance to temperatures approaching 316°C is required throughout the life of bonded assemblies based on metals and reinforced plastic composites. The oxidative stability of organic polymers is improved by the incorporation of aromatic and heterocyclic rings (such as imide, imidazole, and thiazole) into the molecules of the polymer.
Aromatic polymers have many desirable properties such as good lap shear strength, thermal stability, and tensile strength, which make them useful for a wide variety of applications. The term aromatic polymer is used herein to mean a polymer, which has aromatic groups incorporated in the repeat unit of their backbone chain. Such polymers include polyimides (PIs), polyetherimides, polysulfones, polyether sulfones, polyaryl ether ketones, polycarbonates, polyarylates, and the like [20].
The most important resins available for use as adhesives in high temperature structural applications are PIs and polybenzimidazoles (PBIs), both of which are described later (see Sections 5.35 and 5.33). These resins are supplied as prepolymers containing open heterocyclic rings, which are soluble and fusible. At elevated temperatures, the prepolymers undergo condensation reactions leading to ring closure and the formation of insoluble and infusible cured resins [8]. Table 5.5 provides examples of performance properties of a polyetherimide adhesive (Ultem® is trademark of SABIC Corp). IP-600 stands for Thermid IP-600 for which the chemical formula is shown in Figure 5.1.
Table 5.5
Performance Properties of a PI Adhesive (Ultem®) [20]
| Material* | Aging Time (Days) | Tensile Strength MPa (PSI) | % Ultimate Elongation |
| ULTEM | 0 | 28.36 (4,110) | 83 |
| ULTEM/IP-600 | 0 | 22.34 (3,238) | 58 |
| ULTEM | 7 | 31.28 (4,533) | 3 |
| ULTEM/IP-600 | 7 | 44.28 (6,418) | 10 |
| ULTEM/IP-60 0 | 14 | 41.88 (6,070) | 5 |
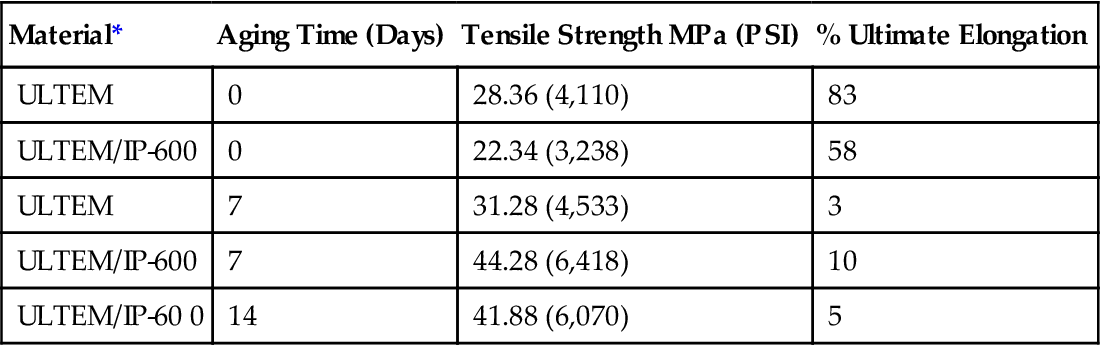
*All samples pressed at 315°C (600°F).
The high temperature adhesives are available in film and tape form. They show better bond strengths above 260°C in air than do epoxy-phenolic, although the latter gives better strength retention after exposure to water or other polar liquids at lower temperatures. The major disadvantages are their high cost, generally 10 or more times that of epoxy-based adhesives, the difficulty in handling or curing, and the problems involved in the elimination of volatiles during cure in order to obtain a void-free bond. A long and careful series of cure and post-cure steps at progressively increasing temperatures up to the 316–371°C range, coupled with intermittent application and release of high-clamping pressure, is required to obtain optimum results. Currently, only PIs can be used in the 260–427°C service temperature range [22–24].
5.6 Asphalt
Asphalt is a low-cost thermoplastic material, which is highly temperature dependent. The addition of a thermoplastic rubber at 1–5% by weight greatly reduces the dependence of viscosity on temperature. Useful operating temperature ranges can often be doubled in this manner. The addition of a thermoplastic rubber, such as butyl rubber or polyisobutylene, at 10–30% by weight produces a truly thermoplastic product with elasticity, resilience, and high cohesive strength. Such mixtures are useful as sealants. Asphalt emulsions are used to increase solid content, improve water resistance, and lower the cost of laminating adhesives. Such adhesives are used in laminating paper and other packing materials where a water-barrier layer is required. Another important application is in roofing and flooring adhesives [3,25,26].
A commercial example, FiberTite® FTR 390 (Table 5.6), is a waterborne rubberized asphalt adhesive designed specifically for adhering FiberTite®-FB “Fleece Back” membranes used in roofing structures to a variety of compatible substrates [27]. Examples of compatible substrates include approved polyisocyanurate insulation, gypsum-based cover boards, approved base sheets and “dry” and sealed cellular lightweight insulating concrete. FiberTite® FTR 390 is characterized by a high degree of workability unique for adhesives of the waterborne type. The adhesive exhibits a degree of pressure sensitivity uncommon in rubber asphalt-based adhesives. There are no fire or toxicity hazards. Temperature and water resistance factors of the cured adhesive are excellent.
Table 5.6
Physical Properties of FiberTite FTR 390 Rubberized Asphalt Adhesive [27]
| Color | Black |
| Viscosity | 18,000 cps |
| Solid weight | Approx. 72.0% |
| Coverage | 60 ft.2/gal. (1.5 m2/l) |
| V.O.C. | 3 g/l |
| Application | Brush or heavy roller |
| Open/cure time | 10–30 min depending on weather conditions |
| Shelf life | 6 months |
| Storage | Closed container/between 50° and 80°F |
| Wt. Gal. | 9 lb. (1.1 kg/l) shipped in 5 gal. pails |
| Working temp. range | 50°F and rising/up to 90°F |
5.7 Butyl Rubber Adhesives
Butyl rubber is an elastomeric polymer used widely in adhesives and sealants, both as primary binders and as tackifiers and modifiers. Butyl rubber is poly(methylpropene-co-2-methyl-1,3-butadiene) or poly(isobutylene-co-isoprene). The latter is a copolymer of isobutylene with a small amount of isoprene [28]. These materials have relatively low strength and tend to exhibit creep under load. They are useful in packaging applications where their low permeability to gases, vapors, and moisture can be exploited. Butyl rubber is also used as an adhesive sealant. It is generally applied from a solvent-based solution [3,7]. Table 5.7 summarizes some of the important properties of butyl rubber adhesives.
Table 5.7
Properties of Elastomeric Polymers in Nonstructural Applications [7]
| Adhesives | ||||||||||
| Natural Rubber (Polyisoprene) | Reclaimed Rubber | Butyl Rubber | Polyisobutylene | Nitrile Rubber | Styrene-Butadiene Rubber (SBR) | Polyurethane | Polysulfide (Thiokols) | Silicone | Neoprene (Chloroprene) | |
| Description | Solvent solutions, latexes, and vulcanizing type | Solvent solutions, some water dispersions; most are black, some gray and red | Solvent system, latex | Solvent solution | Latexes, solvent solutions compounded with resins, metallic oxides, fillers, etc. | Solvent solutions and latexes; because tack is low, rubber resin is compounded with tackifiers and plasticizing oils | Two-part liquid or paste | Two-part liquid or paste | Solvent solution: heat or RT-curing and pressure sensitive; RT-vulcanizing solvent-free pastes | Latexes and solvent solutions, often compounded with resins, metallic oxides, fillers, etc. |
| Curing method | Solvent evaporation, vulcanizing type by heat press RT (two-part) | Evaporation of solvent | Solvent evaporation, chemical cross-linking with curing agents and heat | Evaporation of solvent | Evaporation of solvents and/or heat pressure | Evaporation of solvent | RT or higher | RT or higher | Solvent evaporation, RT or elevated temperature | Evaporation of solvent |
| Usual adherends | Natural rubber, masonite, wood, felt, fabric, paper, metal | Rubber, sponge rubber, fabric, leather, wood, metal, painted metal, building materials | Rubber, metals | Plastic film, rubber, metal foil, paper | Rubber (particularly nitrile), metal, vinyl plastics | Fabrics, foils, plastic film laminate, rubber and sponge rubber, wood | Plastics, metals, rubber | Metals, wood, plastics | Metals, glass, paper, plastics, rubber (including silicone and butyl rubber), and fluorocarbons | Metals, leather, fabric, plastics, rubber (particularly neoprene), wood-building materials |
| Advantages | Excellent resilience, moisture, and water resistance | Low cost, applied very easily with roller coating, spraying, dipping, or brushing; gains strength rapidly after joining; excellent moisture and water resistance | Excellent aging characteristics; chemically cross-linked materials have good thermal properties | Good aging characteristics; used as tackifiers in other adhesives; also provide softness and flexibility and improve adhesion by “wetting out” substrates | Most stable synthetic rubber adhesive, excellent oil resistance, easily modified by addition of thermosetting resins | Good heat aging and water resistance; uniform appearance, nonstaining light color, disperses easily in hydrocarbon solvents; low cost | Excellent adhesion at cryogenic temperatures and excellent retention of elasticity and shock resistance at these temperatures | Resistance to water, organic solvents, greases, oils, salt water; excellent aging and weathering resistance; superior low-temperature flexibility (flexible down to −62°C) | Retain flexibility (peel) over a wide temperature range; resistant to moisture, hot water, oxidation, corona discharge, and weathering | Good resistance to water, salt spray, biodeterioration, aliphatic hydrocarbons, acetone and ethyl alcohol, lubricants, weak acids and alkalies; shows good shear and peel strengths |
| Limitations | Becomes quite brittle with age; poor resistance to organic solvents; does not bond well to metals | Becomes quite brittle with age; poor resistance to organic solvents | Metals should be treated with an appropriate primer before bonding; attacked by hydrocarbons | Attacked by hydrocarbons; poor thermal resistance | Does not bond well to natural rubber or butyl rubber | Strength characteristics poor; tendency to creep, lack of tack requires a tackifier for use in adhesives | Poor resistance to hydrolytic degradation (reversion), even in the polyether type | Poor high-temperature resistance; usually softens at 70–94°C, with little strength retention above 120°C | Some forms (acid-curing) may corrode electrical equipment | Unsuitable for contact with aromatic and chlorinated hydrocarbons, certain ketones, and strong oxidizing agents; cold flow at shear strengths >2.9 MPa |
| Special characteristics | Excellent tack, good strength, shear strength 0.21–1.23 MPa; peel strength 98.1 N/m; surface can be tack-free to touch and yet bonds to similarly coated surface | Low cost, widely used; peel strength higher than natural rubber; failure occurs under relatively low constant loads | Low permeability to gases, good resistance to water and chemicals, poor resistance to oils, low strength | Sticky, low-strength bonds; copolymers can be cured to improve adhesion, environmental resistance, and elasticity; good aging resistance; poor thermal resistance; attacked by solvents | Most versatile rubber adhesive; superior resistance to oil and hydrocarbon solvents; inferior in tack range, but most dry tack-free, an advantage in precoated assemblies; shear strengths of 1.03–13.8 MPa, higher than neoprene, if cured | Usually better aging properties than natural or reclaimed rubber; low dead-load strength similar to reclaimed rubber; useful temperature range from −40°C to 71°C | Excellent tensile shear strength from −240°C to 93°C; poor resistance to moisture before and after cure; good adhesion to plastics | Resistant to a wide range of solvents, oils, and greases, good gas impermeability; resistant to water, sunlight, ozone; retains flexibility over a wide temperature range; not suitable for permanent load-bearing applications | Of primary interest in pressure-sensitive type used for tape; high strengths for other forms are reported from −73°C to 260°C; limited service to 371°C; excellent dielectric properties | Superior to other rubber adhesives in most respects—strength, quick-setting; maximum temperature to 93°C, sometimes 177°C; good aging resistance; resistant to light, weather, mild acids, and oils |
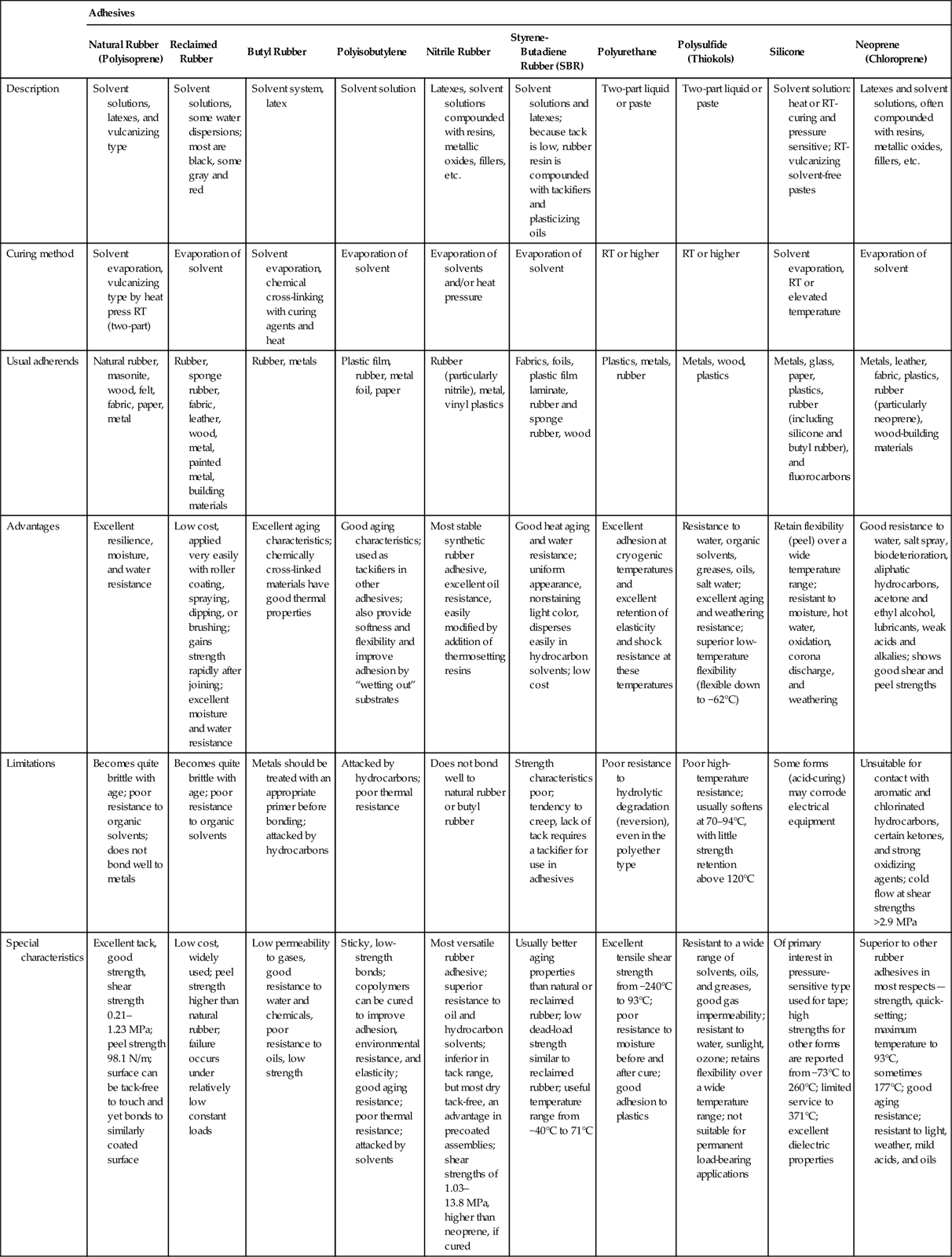
Butyl rubber and asphalt adhesives compete in applications such as flashing applications. Table 5.8 shows a side-by-side comparison of those two adhesives indicating advantages for the butyl rubber over rubberized asphalt adhesive.
Table 5.8
Comparison of Properties of Butyl Rubber and Rubberized Asphalt Adhesives [29]
| Butyl Rubber | Rubberized Asphalt | |
| Cold temperatures (30°F) | Maintains flexibility | Becomes hard and brittle |
| Hot temperatures (180°F) | Maintains stability | Becomes soft and flowable |
| Sealant compatibility | Compatible with all sealant types | May react with petroleum-based sealants |
| Aging | Zero offgassing, retains adhesive characteristics | Offgassing of VOCs changes composition, reducing effectiveness |
| VOCs | Low to zero VOCs can contribute to LEED credits | Higher VOCs which will offgas. Should not be used indoors |
5.8 Cellulose Ester Adhesives
These include cellulose acetate, cellulose acetate butyrate, cellulose caprate, and cellulose nitrate (nitrocellulose or pyroxylin). Cellulose esters are used for bonding leather, paper, and wood. While not generally used with metals, specific nonporous substrates such as cellophane (regenerated cellulose) and glass are sometimes bonded with cellulose nitrate or other cellulose esters applied from solution [3,8].
Cellulose acetate is the most important ester produced from cellulose; however, its use in adhesives is limited. Both the triacetate with the degree of substitution >2.75 and secondary acetate (degree of substitution of 2.4–2.6) are used industrially in plastics and textiles. The triacetate is soluble in mixtures of organic solvents, and the secondary acetate is soluble in acetone. Cellulose acetate is more heat resistant than cellulose nitrate but is less water resistant and tends to become brittle with age [30].
Mixing in ether cellulose acetate butyrate improves the performance of cellulose acetate as an adhesive by overcoming some of its efficiencies. Cellulose acetate butyrate is soluble in a greater range of organic solvents than is the pure acetate, and it is more compatible with common plasticizers. It can be applied either as a hot-melt adhesive or in a solvent solution. Grease resistance of cellulose acetate butyrate allows its use in paper sizing and coatings to make the paper more resistant to staining [30].
Cellulose acetate and cellulose acetate butyrate are water clear and more heat resistant, but less water resistant, than cellulose nitrate. Cellulose acetate butyrate has better heat and water resistance than cellulose acetate and is compatible with a wide range of plasticizers. Cellulose nitrate is tough, develops strength rapidly, is water resistant, bonds to many surfaces, and discolors in sunlight. The dried adhesive (nitrocellulose) is highly flammable [7,31].
5.9 Cellulose Ether Adhesives
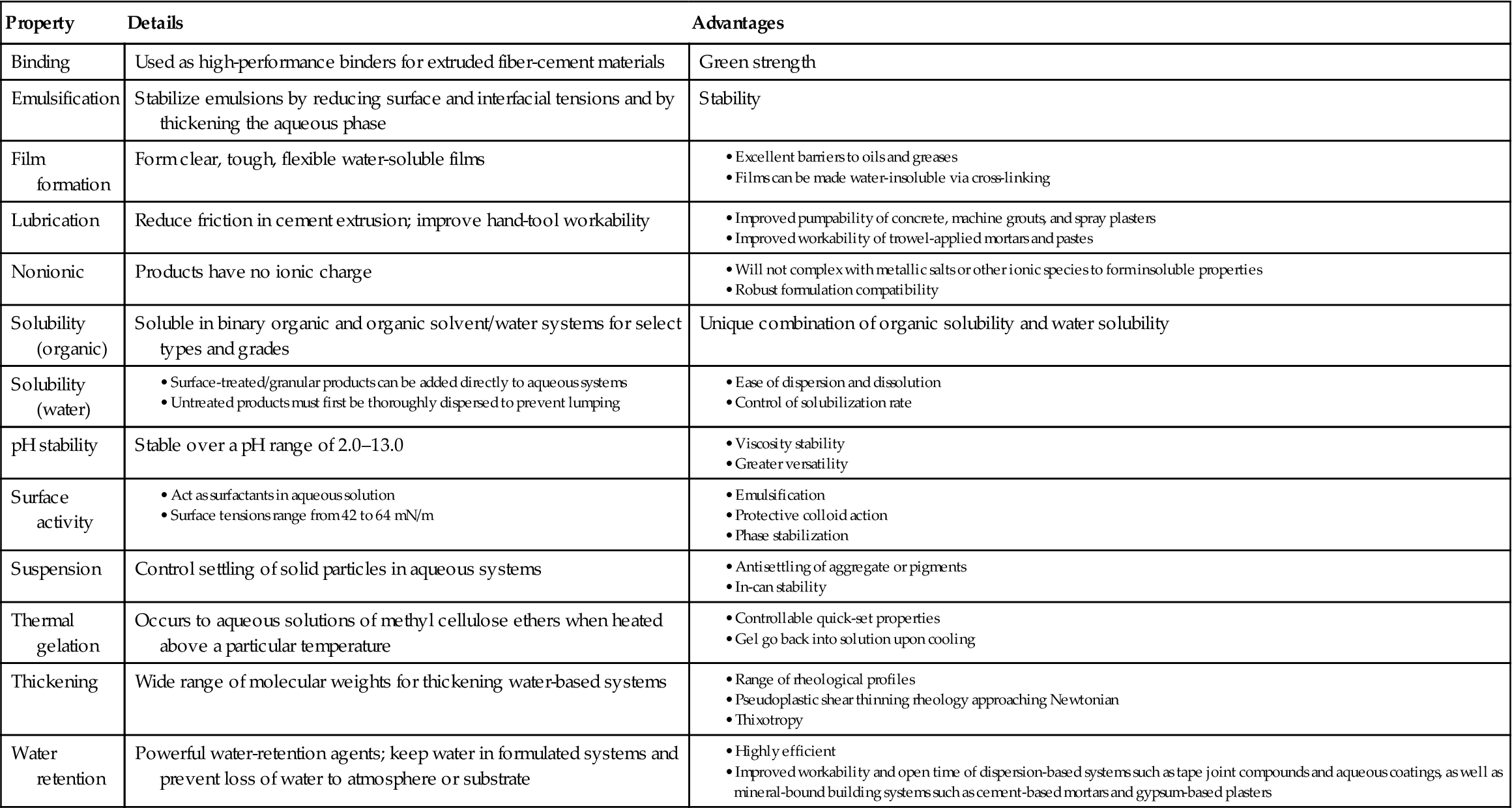
Cellulose ethers are water-soluble polymers derived from cellulose that is the most abundant natural polymer. For more than 60 years, these products have played a significant role in a host of applications, from construction products, ceramics, and paints to foods, cosmetics, and pharmaceuticals [32].
For construction products, cellulose ethers act as thickeners, binders, film formers, and water-retention agents. They also function as suspension aids, surfactants, lubricants, protective colloids, and emulsifiers. In addition, aqueous solutions of certain cellulose ethers thermally gel, a unique property that plays a key role in a variety of applications.
These include ethyl cellulose, hydroxy ethyl cellulose, methyl cellulose, sodium carboxy methyl cellulose, and benzyl cellulose. Ethyl and benzyl cellulose can be used as hot-melt adhesives. Methyl cellulose is a tough material, completely nontoxic, tasteless, and odorless, which makes it a suitable adhesive for food packages. It is capable of forming high-viscosity solutions at very low concentrations, so it is useful as a thickening agent in water-soluble adhesives. Hydroxy ethyl cellulose and sodium carboxy methyl cellulose can also be used as thickeners. The cellulose ethers have fair to good resistance to dry heat. Water resistance varies from excellent for benzyl cellulose to poor for methyl cellulose [7,8,31]. Properties and advantages of cellulose ether adhesives are summarized in Table 5.9.
Table 5.9
Properties and Advantages of Cellulose Ether Adhesives [32]
5.10 Conductive Adhesives
Appropriate fillers have been used to produce adhesives with high thermal or electrical conductivity for specialized applications. The basic resins used include epoxies, urethanes, silicones, and polysulfones. Epoxies, however, are the most widely used resins [33–35].
5.10.1 Electrically Conductive Adhesives (Chip-Bonding Adhesives)
Synthetic resins are made electrically conductive by the addition of either metallic fillers or conductive carbons. The carbon can be either an amorphous carbon, such as acetylene black, or finely divided graphite. Usually finely divided silver flake is used in conductive epoxies and conductive coatings. Silver has the advantage of having moderately conductive salts and oxides so that slight oxidation or tarnishing can be tolerated. The resistivity techniques give much lower values than methods involving thin glue lines, such as ASTM D2739 that measures volume resistivity, where interfacial resistance plays an important role [33].
Silver is preferable to gold as filler because it is less costly and has lower resistivity. Under conditions of high humidity and DC voltage, however, silver is reported to undergo electrolytic migration to the surface of the adhesive. Microspheres of silver-coated copper do not migrate; nor does gold. The highest silver loading possible is about 85% by weight. Silver loadings lower than about 65% by weight cause sharp drops in conductivity, but offer higher adhesive strengths. Carbon (graphite) gives fairly low conductivities.
Aside from silver and gold, other common metallic fillers include nickel, aluminum, and copper. Each of these metals presents particular compounding problems. Silver is often used in flake form, therefore making it more difficult to achieve particle-to-particle contact than with spherical metal particles. A stearate coating is applied to the silver flake to improve its dispersibility. The stearate tends to outgas at elevated temperatures. The outgassing may contaminate critical parts, such as those in microelectronic applications. Some silver products are uncoated and do not evolve outgassing products. Copper and aluminum form oxide films, which reduce electrical conductivity by hampering particle-to-particle contact [33].
Electrically conductive adhesives are used in microelectronic assemblies [36]. These applications include attachment of fine lead wires to printed circuits, electroplating bases, metallization on ceramic substrates, grounding metal chassis, bonding wire leads to header pins, bonding components to plated-through holes on printed circuits, wave-guide tuning, and hole-patching. Conductive adhesives are applied as substitutes for spot welding when welding temperatures build up excess resistance at the weld because of oxide formation.
Another application is in ferroelectric devices used to bond electrode terminals to the crystals in stacks. These adhesives replace solders and welds where crystals tend to be deposited by soldering and welding temperatures. Bonding of battery terminals is another application when soldering temperatures may be harmful. Conductive adhesives form joints with sufficient strength, so they can be used as structural adhesives where electrical continuity, in addition to bond strength, is required, as in shielded assemblies [37]. Sharpe [38] has published an excellent comprehensive review of electrically conductive adhesives.
Commercial conductives adhesives come in a variety of cure mechanisms, some of which are described here [39].
5.10.1.1 Snap Cure Conductive Adhesives
The snap curable conductive adhesives provide excellent adhesion and reliability. For applications with large coefficient of thermal expansion (CTE) mismatches between substrates, or fine pitch flip chip interconnections where electrical conductivity is desired in only one direction, we have an electrically conductive adhesive product to meet the challenge.
5.10.1.2 Heat Cure Conductive Adhesives
Electrically conductive heat cure adhesives are required for a number of manufacturing challenges. They include products with varying cure speeds, viscosity, and pot life. For example, there are silver-filled adhesives that rapidly cure at 150°C and 210°C while bonding to a wide variety of surfaces including silicon, ceramic, plastics, and metals. The adhesives survive operations in the temperature range of −55°C to 150°C [40].
5.10.1.3 Room Temperature Cure Adhesives
These electrically conductive adhesive products cure at room temperature and can be used for bonding and sealing applications that require superior electrical and mechanical properties.
5.10.1.4 Two-Component Conductive Adhesives
Two-component electrically conductive adhesives include products that provide high peel and tensile lap shear strength over a broad temperature range, and silver-filled epoxies recommended for electronic bonding and sealing applications.
5.10.2 Thermally Conductive Adhesives
With increased miniaturization of systems and increased circuit density, today’s electronics generate large amounts of heat. These trends in electronics will continue to make removal of this excess energy even more critical for future applications. If the heat is not carried off and dissipated, the operational lifetime and reliability of the electronics can be reduced. This is a problem that needs to be addressed for everything from individual devices to electronic modules and systems [41].
The use of thermally conductive adhesives in electrical/electronic assemblies has been described [36,38]. In these applications, temperature rises due to evolution of heat from components including resistors, transformers, etc. in high-density circuits is often critical and a cause for concern. Heat sinks and fans are mechanical means that are used to keep the temperature of the electronics at a minimum, but materials also play a critical role. Materials are used to couple the electronics and heat sinks or fan sinks, as well as to couple interfaces with lids, baseplates, and heat spreaders.
Design considerations for these applications must include thermally conductive parts (heat sinks) for removing heat from the circuitry involved. The circuitry may or may not be encapsulated. In confined circuitry, as on a printed-circuit board, nonencapsulated heat sinks bonded in place are one solution. In this case, aluminum is usually the preferred heat-sink material because of its lightweight and high thermal conductivity. If good dielectric properties are required, a high concentration of inorganic or mineral fillers can be used.
A typical thermally conductive epoxy system used as an adhesive, as well as for other purposes, has a thermal conductivity of 0.0026 cal/cm/s/°C and a volume resistivity of 1.5×1015 ohm cm. Fillers include alumina (aluminum oxide), beryllia (beryllium oxide), other unspecified inorganic oxides, boron nitride, and silica. Boron nitride is an excellent choice as a thermally conductive filler except that its content reaches a maximum at about 40% by weight in epoxy resins. The resultant products are always thixotropic pastes. Beryllia powder has excellent thermal conductivity by itself, but when mixed with a resin binder its conductivity drops drastically. It is also highly toxic and high in cost. Alumina is a commonly used filler to impart thermal conductivity in resins [33].
A variety of noncorrosive, thermally conductive silicone adhesives are available that are suitable for use in bonding hybrid circuit substrates, connecting power semiconductor components and devices to heat sinks as well as for use in other bonding applications requiring thermal conductivity. The flowable versions are used as thermally conductive potting materials for transformers, power supplies, coils, and other electronic devices that require improved thermal dissipation [41].
Tape adhesives can be made thermally conductive by the dispersion of small articles of a conductive filler such as Saint-Gobain boron nitride (BN) PCTH3MHF and spherical aluminum oxide (Al2O3) available from Denka Corp. [42]. For example, 3M Corp offers pressure-sensitive adhesive (PSA) tapes filled with thermally conductive ceramic particles and flame retardant fillers. This product is designed with a thin polyester (PET) film and a soft acrylic polymer. It conforms to surfaces to which it adheres thus providing contact surface area for heat transfer [43].
5.11 Cyanoacrylate Adhesives
These so-called “wonder” adhesives (Superglue) are marginally thermosetting materials and were first introduced commercially by Eastman Chemicals in 1958. Loctite Corp (now part of Henkel Corp) acquired the cyanoacrylate (CA) business in the 1960s and later developed its own technology. CAs have found application in many different industries even in medicine. Cyanoacrylate features and limitations include [44]:
Excellent adhesion to a wide variety of substrates
High strength possible on polyolefins and fluorocarbons using primers
Blooming/frosting, except Chenso
Difficult to cure fillet or exposed liquid adhesive without activator or UV light
Stress cracking could occur to some plastics
Thermal and chemical stability not as good as with certain other structural adhesives
Cyanoacrylates form strong thermosetting bonds between many materials without heat or an added catalyst. They are particularly useful in bonding metal to nonmetal. Lap-shear strengths of 13.7 MPa have been reported. However, the resistance of these adhesives to moisture is still somewhat low [3]. These materials set very quickly when squeezed out to thin films between many types of adherends.
As with other acrylics, the monomers are liquids of low viscosity that polymerize very easily in the presence of a slightly basic surface containing adsorbed water. Polymerization is ionic. The resulting polymers have different properties, depending on the alkyl group. The methyl ester (methyl-2-cyanoacrylate) is the most commonly used compound. This material is formulated with a thickener (to prevent starved joints from being formed) and a plasticizer to make it more resistant to shock loading. The thickener can be a polymer of the same monomer. An essential feature is a stabilizer to prevent polymerization in the adhesive container, which is usually made of polyethylene [45].
The polymerization of cyanoacrylates is inhibited by low pH (high acidity), thus it does not proceed satisfactorily on acid surfaces such as wood. The suggested incorporation of poly-N-vinyl pyridine or polyethyleneamine, or even simple amines, presumably serves the dual purpose of thickening the liquid and increasing the pH.
Adhesives based on higher homologs than the methyl form have been in use for a number of years. These include the ethyl, propyl, and butyl esters of cyanoacrylic acid. Moisture resistance of the methyl-2-cyanoacrylate is only fair. Ethyl cyanoacrylate has been shown to form stronger bonds than the methyl form between several different types of plastic surfaces. The higher homologs, however, generally do not form bonds as strongly as the methyl form [46].
The most important step in the successful application of a cyanoacrylate adhesive is the application of a thin adhesive film between two well-mated surfaces. The thinner the film is, the faster the rate of bond formation, and the higher the bond strength. Bond strength is dependent on proper surface preparation.
In general, aging properties of the cyanoacrylates are good. Rubber-to-rubber and rubber-to-metal bonds typically have endured outdoor weathering for over 7 years. These bonds have also passed stringent water-immersion and salt-spray tests. Plastic-to-plastic and plastic-to-rubber bonds have aged satisfactorily for 3–5 years. Metal-to-metal bonds generally age rather poorly, except under special conditions where the minimal glue line is exposed to moisture. Solvent resistance is also generally satisfactory. Dilute alkaline solutions weaken the bond considerably, while dilute acid solutions weaken it to a lesser degree. Impact resistance is generally poor, because of the thin, inflexible bond.
This is especially true with two rigid substrates such as metals. The methyl cyanoacrylate bond melts at approximately 165°C. Prolonged exposure to temperatures in this range results in a gradual but permanent breakdown of the bond. Generally, the upper temperature limit for continuous exposure is about 77°C. At low temperatures bonds remain intact at least down to −54°C. Grades of cyanoacrylates with specialized improved properties are available. For example, one grade has improved heat resistance to 246°C, high viscosity, and very fast setting ability [46].
Among the advantages of the cyanoacrylates are the following:
• High bond strength with thin glue line
• No added catalyst or mixing needed
• No solvent to evaporate during bond formation
• Contact pressure is usually sufficient
• Economical because of minute quantities needed, although relatively expensive.
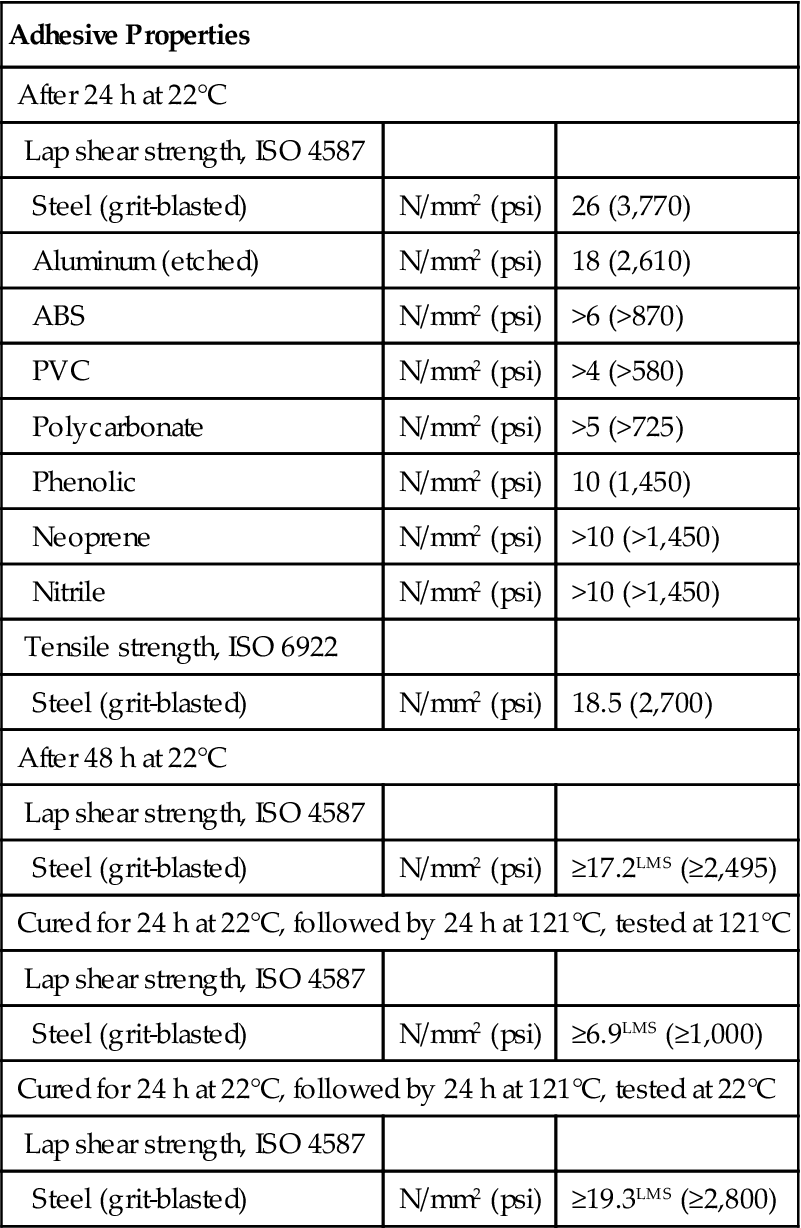
The Loctite Corporation offers a rubber-toughened cyanoacrylate adhesive such as 380 Black Max® which is reported to achieve improved strength, resiliency, and fast fixturing at the expense of a rather limited shelf life (about 4 months) [47]. This adhesive cures to fixturing strength in 2 min in the case of most substrates. It reaches 80% strength in 24 h and full strength in 72 h (Table 5.10). On aluminum, its average strength is 16.6 MPa after full room temperature cure, versus 6.2 MPa for a typical epoxy adhesive and 3.8 MPa for “instant” adhesives. After 240 h of tensile-shear thermal cycling tests, this adhesive improved its strength to 21.3 MPa for “instant” adhesive. Loctite claims that this adhesive is consistently 20 times stronger than epoxies on aluminum, 10 times stronger on neoprene, 4 times stronger on steel, and 2 times stronger on epoxy/glass after the tests. This adhesive is designed for assembly-line cure [48].
Table 5.10
Typical Performance of Cured Loctite 380 Cyanoacrylate Adhesive [47]
| Adhesive Properties | ||
| After 24 h at 22°C | ||
| Lap shear strength, ISO 4587 | ||
| Steel (grit-blasted) | N/mm2 (psi) | 26 (3,770) |
| Aluminum (etched) | N/mm2 (psi) | 18 (2,610) |
| ABS | N/mm2 (psi) | >6 (>870) |
| PVC | N/mm2 (psi) | >4 (>580) |
| Polycarbonate | N/mm2 (psi) | >5 (>725) |
| Phenolic | N/mm2 (psi) | 10 (1,450) |
| Neoprene | N/mm2 (psi) | >10 (>1,450) |
| Nitrile | N/mm2 (psi) | >10 (>1,450) |
| Tensile strength, ISO 6922 | ||
| Steel (grit-blasted) | N/mm2 (psi) | 18.5 (2,700) |
| After 48 h at 22°C | ||
| Lap shear strength, ISO 4587 | ||
| Steel (grit-blasted) | N/mm2 (psi) | ≥17.2LMS (≥2,495) |
| Cured for 24 h at 22°C, followed by 24 h at 121°C, tested at 121°C | ||
| Lap shear strength, ISO 4587 | ||
| Steel (grit-blasted) | N/mm2 (psi) | ≥6.9LMS (≥1,000) |
| Cured for 24 h at 22°C, followed by 24 h at 121°C, tested at 22°C | ||
| Lap shear strength, ISO 4587 | ||
| Steel (grit-blasted) | N/mm2 (psi) | ≥19.3LMS (≥2,800) |
Light cure cyanoacrylates are a new and revolutionary adhesive technology that was developed in response to demand for an adhesive that offered all the advantages of cyanoacrylates and light cure adhesives, yet none of the limitations. This highly versatile new adhesive technology emits minimal vapors; surface cures immediately when exposed to light, there is easy adaptation into production lines, and no second-step accelerators or activators are required. Light cure cyanoacrylates undergo fixture tack-free in seconds upon exposure to low intensity ultraviolet and/or visible light sources. Any adhesive located in shadowed areas or behind opaque substrates cures naturally and quickly at room temperature due to a secondary moisture cure mechanism [49].
5.12 Delayed-Tack Adhesives
Delayed-tack adhesives require activation often by moisture, heat/pressure, or contact. Stamps and envelopes are two examples of moisture activation. A common example of heat-activated adhesive is the one applied to wood edge tapes (veneers). These tapes are used to cover the raw edges of plywood in applications such as shelving. A heated iron is used to press the wood tape against the edge to which it is being applied. The heat activates the adhesive, which becomes tacky and bonds to the wood edge.
In the packaging field, acrylics are often used for delayed-tack adhesive coatings for labels. Copolymer dispersions of acrylic ester with vinyl acetate, vinyl chloride, or styrene are usually employed for these applications. The backing material, usually paper, is coated after the dispersion has been modified accordingly. The coated papers are tack-free under normal conditions, so the sheets and cuttings can be rolled up or stacked. These adhesives consist primarily of one or a mixture of polymer film formers in dispersion form and one or several crystalline plasticizers. The plasticizer is usually employed in dispersion form with small particle size. Resin solutions or dispersions are added as additives for obtaining certain adhesive effects. The adhesive coating must be dried at a temperature below the melting point of the plasticizer in order to obtain a tack-free product [50].
Labels produced in this manner are applied according to the following procedure. The adhesive coat is heated directly by infrared radiation or hot air, or by hot plates from the reverse side, to a temperature above the melting point of the plasticizer. The polymer is plasticized (i.e., the coating is tackified by the molten plasticizer, which is present in excess). Under this condition, the label can be bonded to the substrate by applying a slight pressure. Adhesion to glass, metals, polyvinyl chloride (PVC), wood, etc., is durable even after the plasticizer has recrystallized [50].
Other polymers that can provide delayed-tack adhesives include styrene-butadiene copolymers, polyvinyl acetate, polystyrene, and polyamides. Solid (crystalline) plasticizers for these adhesives include dicyclohexyl phthalate, diphenyl phthalate, N-cyclohexyl-p-toluene sulfonamide, and o/p-toluene sulfonamide. Adhesives with different heat-activation temperatures could be obtained because of the range of melting points available. Delayed-tack adhesives have a large number of uses, such as coating paper for labels on bread packages, cans, etc.
5.13 Elastomeric Adhesives
Elastomeric adhesives are used in applications where the joint [51]:
Elastomeric adhesive compositions including a high softening point tackifier resin in combination with a base polymer can be used to create laminates having effective adhesion and elastic properties [52]. These adhesives are both natural and synthetic rubber-based materials, usually with excellent peel strength, but low shear strength. Their resiliency provides good fatigue and impact properties. Except for silicone, which has high temperature resistance, their uses are generally restricted to temperatures in the range of 66–93°C. A significant amount of creep (flow-under-load) occurs at room temperature. The basic types of elastomeric adhesives used for nonstructural applications are shown in Table 5.7. These systems are generally supplied as solvent-based solutions, latex, cements, and pressure-sensitive tapes.
Solvent solutions and latex cements require the removal of the solvent from the adhesive before bonding can take place. This is accomplished by simple or heat-assisted evaporation. Some of the stronger or more environmentally resistant rubber-based adhesives require an elevated-temperature cure. Only slight pressure is usually required with PSAs to obtain a satisfactory bond. These adhesives are permanently tacky and flow under pressure, and thus they provide intimate contact with the adherend surface.
In addition to PSAs, elastomers are used in the construction industry for mastic compounds. Neoprene and reclaimed rubber mastics are used to bond gypsum board and plywood flooring to wood framing members. The mastic systems cure by evaporation of solvent through the porous substrates. Elastomer-adhesive formulation is particularly complex because of the need for antioxidants and tackifiers [7].
Table 5.7 summarizes the properties and characteristics of elastomeric adhesives for nonstructural applications. Individual elastomeric adhesive types are discussed in this chapter under separate headings.
One of the most widely used elastomeric adhesives in the industry is one-part polyurethane. It can be used as general-purpose adhesive and sealant with applications in nearly all markets because of its favorable performance/cost balance.
5.14 Epoxy Adhesives
This class of compounds is one of the most important adhesive groups with applications ranging from consumer to aerospace markets. Epoxies are thermosets and are cross-linked during the cure cycle. The chemical structure for a simple epoxy (ethylene oxide) in its unhardened state is shown in Figure 5.2. All epoxy compounds contain two or more of these groups. Epoxy resins form adducts with vinyl, acrylic, and polyester resins producing compounds such as phenol novolac, cresol novolac, bis-[4(2,3-epoxy propyoxy) phenyl] methane, and phenol hydrocarbon novalac [53].
Epoxy resins may vary from low-viscosity liquids to high melting point solids. More than two-dozen types are known. Tens of curing agents, including commonly available compounds such as amines, primary and secondary amines, and anhydrides, are used. Only a few of these are used widely in adhesive formulations [37].
Of all the thermosetting plastics, epoxies are more widely used than any other plastic, in a variety of applications. There are resin/hardener systems (two-part) that cure at room temperature, as well as one-part systems that require extreme heat cures to develop optimum properties (e.g., 121°C and 177°C). Proper selection of various hardeners, resins, modifiers, and fillers allows the development of desired properties for a particular application. Because of the wide versatility and basic adhesive qualities, epoxies make excellent structural adhesives that can be engineered to widely different specifications. Essentially no shrinkage occurs during polymerization because epoxies are completely reactive producing no volatiles during cure. Epoxy adhesives can be formulated to meet a wide variety of bonding requirements. Systems can be designed to perform satisfactorily at a temperature of −157°C or at 204°C [54].
Epoxy adhesives form strong bonds to most materials, in addition to excellent cohesive strength (good attraction to itself). Epoxy adhesives also have excellent chemical resistance and good elevated-temperature capabilities. As with many other structural adhesives, to obtain maximum strength, particularly under adverse conditions, substrate surfaces must be prepared carefully. Epoxies yield good to excellent bonds to steel, aluminum, brass, copper, and most other metals. Similar results are obtained with thermosetting and thermoplastic plastics and with glass, wood, concrete, paper, cloth, and ceramics. The adherends to which epoxy is being bonded usually determine the adhesive formulation. Epoxy adhesives have relatively low peel strengths [54].
One-part epoxy adhesives include solvent-free liquid resins, solutions in solvent, liquid resin pastes, fusible powders, sticks, pellets and paste, supported and unsupported films, and preformed shapes to fit a particular joint. Two-part epoxy adhesives are usually comprised of the resin and the curing agent, which are mixed just prior to use. The components may be liquids, putties, or liquid and hardener powder. They may also contain plasticizers, reactive diluents, fillers, and resinous modifiers. The processing conditions are determined by the curing agent employed. In general, two-part systems are mixed, applied within the recommended pot life (a few minutes to several hours), and cured at room temperature for up to 24 h, or at elevated temperatures to reduce the cure time. Typical cure conditions range from 3 h at 60°C to 20 min at 100°C [8].
With an aliphatic amine (e.g., diethylenetriamine) curing agent at room temperature, the resin is cured in 4–12 h to an extent sufficient to permit handling of the bonded assembly. Full strength develops over several days. A compromise between cure rate and pot life must be made. Too rapid a cure at room temperature results in the formation of an unspreadable mixture in the mixing pot. Heat buildup (exothermic reaction) can be restricted by lowering the temperature of the mixture, limiting the size of the batch, or using shallow mixing containers. Actions such as these will extend the pot life of the adhesive. Contact bonding pressures usually suffice, but small pressures from 0.016 to 0.02 MPa result in more uniform joints with maximum strength. One-part systems incorporate a hardening agent which requires heat to activate curing. A period of 30 min at 100°C is typical [8].
5.14.1 Hardening Agents for Epoxy Adhesives
Hardeners used in curing bisphenol-A epoxy resins, the type most commonly used in adhesives, include the following [31]:
• Aliphatic polyamine hardeners: These are used in adhesive systems capable of curing at normal or slightly elevated temperatures. The most important examples are diethylenetriamine, triethylenetetramine, and diethylenepropylamine.
• Fatty polyamides: These are condensation products of polyamines and unsaturated fatty acids. They are high-melting linear polyamides of the nylon type, containing carboxyl end groups and amide groups along the chain. The amount of hardener required for curing is large and the proportion is not critical. These materials are used to impart flexibility, as well as for curing. Fatty polyamides are probably the most widely used epoxy curing agents.
• Aromatic polyamine hardeners: These mostly solid hardeners include metaphenylenediamine, diaminodiphenylmethane, and diaminodiphenyl sulfone. In general, these hardeners provide poorer bond strengths and are more sensitive to temperature cycling than the aliphatic amines. Shrinkage is also high.
• Anhydride hardeners: These materials are organic polycarboxylic anhydrides. Most require severe curing cycles. They provide thermal stability superior to that of the amines. Anhydride-cured epoxies are often brittle and require a flexibilizer, which will result in reduced heat and chemical resistance.
• Boron trifluoride hardeners: Boron trifluoride monoethylamine melts at 95°C and is used in one-part adhesives.
• Miscellaneous curing agents: The most important is dicyandiamide, used frequently in metal bonding. This material melts at about 200°C and is nonreactive at room temperature, so it is convenient for use in a one-package adhesive in the form of a powder or rod.
5.15 Epoxy-Phenolic Adhesives
These relatively expensive adhesives account for only a small fraction of the current usage of structural adhesives. They are used primarily for military applications designed for service between 149°C and 260°C. Epoxy-phenolics are blends of thermosetting phenolic and epoxy resins. They are supplied as viscous liquids, which may contain solvents, or as glass-cloth or fabric-supported films or tapes. They are often modified with fillers and thermal stabilizers [22–24].
Solvent blends are usually force-dried at 80–90°C for 20 min before assembly of adherends. Curing generally lasts for 30 min at 95°C at contact pressure, followed by 30 min to 2 h at 165°C and 0.07–0.4 MPa pressure. Postcuring is used to obtain optimum curing at elevated temperatures [8].
Applications are for high-temperature structural bonding of metals including copper and its alloys, titanium, galvanized iron and magnesium, glass and ceramics, and phenolic composites. Epoxy-phenolics are also applied in bonding honeycomb sandwich composites. Liquid forms are often used as primers for tapes. These materials display excellent shear and tensile strength over a wide temperature range. Films give better strengths than liquid systems. Peel and impact strengths are usually poor.
Epoxy-phenolic film and tape adhesives have good resistance to weathering, aging, water, weak acids, aromatic fuels, glycols, and hydrocarbon solvents. The service-temperature range is −60°C to 200°C, but special formulations are suitable for end uses at cryogenic temperatures down to −260°C [8].
5.16 Epoxy-Polysulfide Adhesives
These adhesives are products of reaction between an epoxy resin and liquid polysulfide polymer, usually catalyzed by an additional tertiary amine [9]. They are available as two-part liquids or pastes that are usually cured at room or higher temperatures to rubbery solids that provide bonds with excellent flexibility and chemical resistance. Epoxy-polysulfide adhesive forms satisfactory bonds to different substrates. Shear strength and elevated-temperature properties are low, but resistance to peel and low temperature is acceptable [7,8].
Curing is usually for 24 h at 20°C or up to 20 min at 100°C. Bonding pressures are low, in the range of 0.07–0.16 MPa. A disagreeable sulfur odor forms during processing, rendering ventilation necessary. Resistance to water, salt spray, hydrocarbon fuels, alcohols, and ketones is acceptable. Resistance to weathering properties is excellent.
Epoxy-polysulfide adhesives are suitable for use down to −100°C and lower temperatures. Some blends have been used down to liquid nitrogen temperatures of −198.5°C. The maximum service temperature is about 50°C to 71–82°C [55,56]. The resistance of bonds to moisture is quite high, but may deteriorate if the bonds are stressed. Some formulations will corrode copper adherends.
Applications of epoxy-polysulfide adhesives primarily include structural assemblies requiring some degree of resilience. Epoxy-polysulfides are used in bonding concrete for floors, airport runways, bridges and other concrete structures, metals, glass and ceramics, wood, rubber, and some plastics. They are particularly durable in outdoor applications where temperature extremes (freeze-thaw cycles) will be encountered [8]. Epoxy-polysulfides can be heavily filled without adversely affecting their properties [55,56].
5.17 Film and Tape Adhesives (See also Section 5.3)
A number of high-strength structural adhesives are currently supplied in film and tape form. Although the bond strengths provided by both film and tape and one-component pastes are generally similar, there are several advantages of using film and tape [3]:
• Provide uniform, controlled glue line thickness
• Speed and ease of application (a clean, solvent-free operation is facilitated)
• Two-sided films can be prepared for use in lightweight sandwich constructions. The honeycomb side will provide good filleting, while the skin side will provide high peel strength. If one side of the film is tacky, it is easier to align the assembly to be bonded.
In some film adhesives, a cover or knitted fabric is used to support the polymer film. It will also carry a part of the load and will provide improved bond strength by more efficient distribution of the applied forces. Film adhesives are produced in two forms: unsupported, or alternatively, supported on a flexible carrier such as glass, cloth, nylon, or paper. The carrier will usually have little effect on adhesive properties [3]. The adhesive polymer is usually elastomeric, blended with curing agents, fillers, and other ingredients and is usually extruded, calendered, or cast into 0.1–0.4-mm-thick unsupported films. This type is called film adhesive. When the mixture is cast, or calendered onto a mesh support, such as woven or nonwoven mesh of glass or other fibers, the resulting product is called tape adhesive.
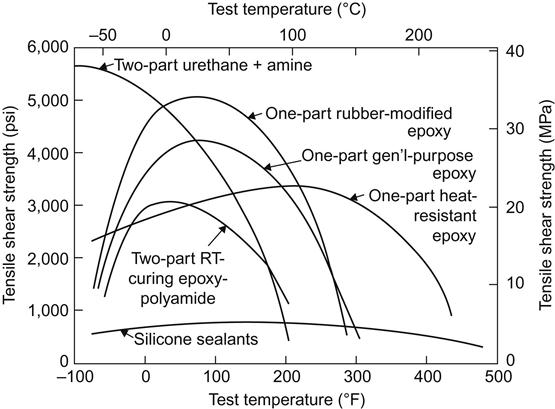
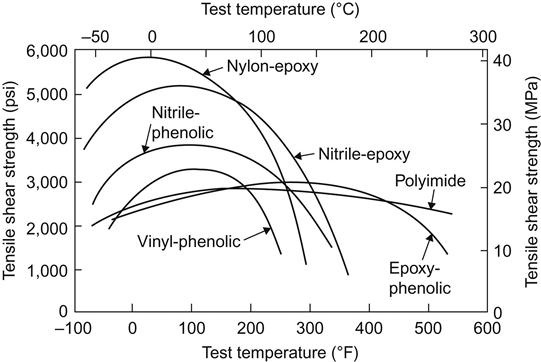
Films and tapes may be either soft and tacky or stiff and dry. They may be room temperature storable, or may require refrigeration between manufacture and the time of use. Most film and tape adhesives are cured at elevated temperatures and pressures. Film and tape adhesives differ from paste and liquid adhesives in that the former contains a high proportion of high-molecular-weight polymer. The 100% solid paste and liquid adhesives contain only low-molecular-weight resins to permit them to remain fluid and usable. The film and tape adhesives contain components that permit them to be much tougher and more resilient than paste adhesives. Figures 5.3 and 5.4 compare typical tensile shear data for a number of adhesive types. It should be noted that the best film and tape types have higher peak values and broader service temperatures than the best 100% solid adhesives [57].
The handling and reliability advantages of tape and film adhesive include ready to use, no need for mixing, no degassing, and no possibility for error in adding catalyst. Tapes permit a variety of lay-up techniques, which facilitate the production of virtually defect-free structures. The use of a mesh support helps to control the bond-line thickness with tape adhesives, avoiding thin, adhesive-starved areas where curvature or external pressure is the greatest.
Tape and film adhesives are generally composed of three components [22–24]:
• A high-molecular-weight backbone polymer providing the elongation, toughness, and peel. This is the thermoplastic or elastomeric component
• A low-molecular-weight cross-linking resin, invariably either an epoxy or a phenolic (thermosetting types)
Exceptions to this generalization are the epoxy-phenolic adhesives, which are composed of two thermosetting adhesives.
Film and tape adhesives are also frequently called “two-polymer” or “alloyed adhesives.” With few exceptions, all successful film and tape adhesive are, or have been, one of the types shown in Tables 5.11 and 5.12. The adhesive types based on phenolic cross-linking resins liberate volatiles during cure, while the types based on epoxies only need sufficient pressure to maintain alignment and compensate for cure shrinkage [22–24].
Table 5.11
Most Important Tape and Film Adhesives [22–24]
| Adhesive Type | Backbone Polymer | Cross-Linking Resin | Catalyst | High-Pressure Cure |
| Nylon-epoxy | Soluble nylon | Liquid epoxy | DICY-type | No |
| Elastomer-epoxy | Nitrile rubber | Liquid epoxy | DICY-type | No |
| Nitrile-phenolic | Nitrile rubber | Phenolic novalac | Hexa, sulfur | Yes |
| Vinyl-phenolic | PVB or PVF | Resol phenolic | Acid | Yes |
| Epoxy-phenolic | Solid epoxy | Resol phenolic | Acid | Yes |
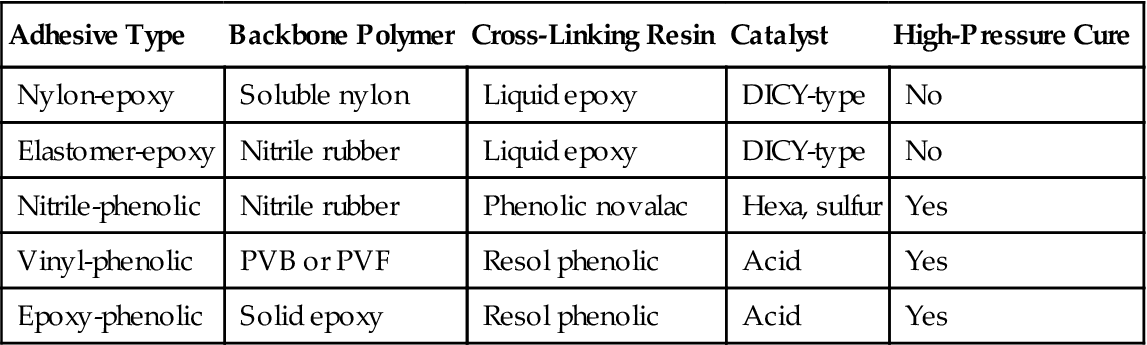
DICY, dicyandiamide; PVB, polyvinyl butyral; PVF, polyvinyl fluoride.
Table 5.12
Range of Bond Strengths of Tape and Film Adhesives at Room Temperature [22–24]
| Adhesive Type | Tensile-Shear Strength (MPa) | T-Peel Strength (N/m) |
| Nylon-epoxy | 34–49 | 14,000–22,750 |
| Elastomer-epoxy | 26–41 | 3,850–15,750 |
| Nitrile-phenolic | 21–31 | 2,625–10,500 |
| Vinyl-phenolic | 21–31 | 2,625–6,065 |
| Epoxy-phenolic | 14–22 | 1,050–2,100 |
5.18 Furane Adhesives
These are dark-colored synthetic thermosetting resins containing the chemical group known as the furane ring (Figure 5.5).
These compounds include the condensation polymers of furfuraldehyde (furfural) and furfuryl alcohol. On addition of an acid, these furane compounds polymerize, passing through a liquid resinous state, and have adhesive properties. Volatile loss during cure is low, thus bonding pressure need not be high. Resistance to boiling water, organic solvents, oils, and weak acids and alkalis is good. However, strong oxidizing agents attack these materials. High-temperature resistance depends on the type and quantity of catalyst. For continuous exposure, service temperatures up to 150°C are acceptable.
Furane resin adhesives are used as bonding agents or modifiers of other adhesive materials. Applications include surfacing and bonding agents for flooring compositions and acid-resistant tiles, chemically resistant cements for tank linings, phenolic laminates (shear strengths up to 40 MPa), binder resins for explosives and ablative materials used in rockets and missiles at 1,250°C service temperatures, foundry core boxes, and binder resins for carbon and graphite products [8,31].
Furane adhesives are suitable for gap-filling applications because their strength is maintained with thick glue lines. For this reason, the resins are used as modifiers for urea-formaldehyde adhesive to improve gap-filling and craze resistance. As furanes are compatible with a variety of other resins, they are used in mixtures with silicates and carbonaceous materials for chemically resistant grouting compositions [8].
5.19 Hot-Melt Adhesives
Hot-melt adhesives are thermoplastic bonding materials applied as melts that achieve a solid state and resultant strength on cooling. These thermoplastic 100% solid materials melt in the temperature range from 65°C to 180°C. Theoretically, any thermoplastic can be a hot-melt adhesive, but the 10 or so preferred materials are usually solid up to 79.4°C or higher, then melt sharply to give a low-viscosity fluid that is easily applied and is capable of wetting the substrate to be bonded, followed by rapid setting upon cooling. When hot-melt adhesives are used, factors such as softening point, melt viscosity, melt index, crystallinity, tack, heat capacity, and heat stability must be considered, in addition to the usual physical and strength properties [3,8].
The plastics used in hot-melt applications are generally not newly developed materials. However, the combination of properly formulated resins and application equipment to handle these resins has contributed much to the success of hot-melt technology [3]. While most hot-melt adhesives melt at about 79.4°C, they are usually applied at much higher temperatures, from 149°C to 288°C. In addition to the thermoplastic polymers, other ingredients are incorporated to improve processing characteristics, bonding characteristics, or service properties. Stabilizers retard oxidation, tackifiers improve bond strength, waxes reduce viscosity and alter surface characteristics, and various fillers increase viscosity, melting point, and bond strength. Hot melts are sold only by a manufacturer’s number or name designation, with no generic identification, as is common for most other adhesives. This is why comparison of competing brands of similar hot-melt adhesives is not easy [58].
One of the most important characteristics of hot-melt adhesives is service temperature. Service temperatures of hot melts are low because of their low melting temperatures, which is a disadvantage. These materials also flow under load over extended time. Thermoplastics have some of the characteristics of viscous liquids and, with a few exceptions, are not dimensionally stable under load. This is why hot melts are recommended primarily for hold-in-place operations with negligible load requirements. The main disadvantages of hot melts are limited strength and heat resistance. Unlike other adhesives, the set-up process is reversible and, at about 77°C, most hot melts begin to lose strength. The maximum shear load capacity is usually about 3.4 MPa [59]. Lap-shear strengths up to 4.3 MPa have been reached with hot-melt adhesives used to bond untreated high-density polyethylene to untreated high-density polyethylene [60].
5.19.1 Foamable Hot-Melt Adhesives
These materials were introduced in 1981. The process involves introducing a gas, normally N2 or O2, into the hot-melt adhesive in a volumetrically metered fashion using a two-stage gear pump. Typically, the volume of the adhesive is increased by 20–70%. Although all adhesives foam under these conditions, the quality of the foam depends on the individual adhesives. Foamed hot-melt adhesives can be used on the same substrates on which standard hot melts are used. A superior bond can often be obtained on metal, plastics, and paper products, as well as on heat-sensitive and porous substrates. This is because of the characteristics resulting from foaming including increased spreading ability, larger open time, shorter set time, increased penetration, and reduced thermal distortion over traditional hot melts. Polyethylene, in particular, gives excellent results. Typical applications include gasketing and sealants [61].
5.19.2 Ethylene-Vinyl Acetate (EVA) and Polyolefin Resins
These are the least costly resin materials used in hot melts. Their applications include bonding paper, cardboard, wood, fabric, etc., for use at −34°C to 49°C. Compounded versions can be used for nonload-bearing applications up to about 71°C. EVAs and polyethylenes represent the highest volume of hot-melt adhesives used, primarily in packaging and wood-assembly applications [58].
5.19.3 Polyamide (Nylon) and Polyester Resins
These compounds are the next stop up in strength and general service in hot-melt adhesives. These so-called “high-performance” hot melts are used to assemble products made from glass, hardboard, wood, fabric, foam, leather, hard rubber, and some metals. Service temperatures range from −40°C to about 82°C. A number of formulations are available that can be used at >93°C. Some are capable of being used in nonload-bearing applications at >149°C [58].
Polyester-based hot melts are generally stronger and more rigid than the nylon compounds. Polyesters have sharp melting points because of their high crystallinity, a decided advantage in high-speed hot-melt bonding. Frequently, they have a combination of high tensile strength and elongation. Both nylon and polyester adhesives are sensitive to moisture during application. Nylons combine good strength with flexibility. If nylon compounds are not stored in a dry area, they may pick up moisture, which may cause foaming in the heated adhesive. This problem, in turn, may produce voids in the applied adhesive layer, reducing bond strength. Moisture affects polyesters in a somewhat different manner by hydrolysis of the molecular structure of the resin, thereby lowering the molecular weight and viscosity. This is precisely why polyester hot melts should not be used in reservoir-type application systems [48].
5.19.4 Other Hot-Melt Adhesives
Other materials include polyester-amides and those formulated from thermoplastic elastomers. The former are said to have the desirable properties of polyesters, but with improved application characteristics. The principal base polymers in thermoplastic elastomers are used mostly in pressure-sensitive applications, replacing other adhesives, such as contact cements, to eliminate solvent emission problems. These materials are used for applications such as tape products and labels, which require relatively low strength [58]. One particular thermoplastic rubber formulation provides paper tear in the range from −23°C to 60°C. This adhesive may also be applied by a gun for attaching items such as plastic molding to wooden cabinet doors [62].
Thermoplastic elastomer hot melts are not as strong as the polyesters, but are stronger than conventional thermosetting rubbers. They provide good flexibility and toughness for applications requiring endurance and vibration resistance, and have good wetting properties. These compounds are quite viscous, even at 232°C, because of their high molecular weights. This characteristic renders them more difficult to apply than the nonelastomeric materials, unless they are formulated with other ingredients [62].
An example of a 100% solid, nonflammable, heat-activated hot-melt adhesive recommended for structural bonding of aluminum, steel, copper, brass, titanium, fabric, and some plastics is 3M Company’s Scotch-Weld™ Thermoplastic Adhesive Film 4060. Strength data are shown in Table 5.13 [64]. Bonding using this clear, amber, unsupported film adhesive takes place rapidly. The speed of bonding is limited only by the heat-up time required to reach the optimum bonding temperature of 149°C at a pressure sufficient to maintain contact between the surfaces to be bonded. The adhesive can also be preapplied to parts days or months in advance of the actual bonding operation. When parts are ready to be bonded, heat is applied to the previously applied adhesive to quickly activate the material for bonding. Typical applications include nonload-bearing honeycomb panels, application of decorative trim, and installation of electronic parts [63,64].
Table 5.13
Strength Characteristics of Thermo-Bond Thermoplastic Adhesive Film Used to Bond to Etched Aluminuma [63]
| Temperature (°C) | Overlap Shear Strength (psi) | T-Peel Strength (per inch width) |
| 23 | 1,160 | 16.5 |
| 38 | 1,090 | 17.5 |
| 52 | 990 | 19.2 |
| 66 | 910 | 18.0 |
| 79 | 590 | 15.0 |
a(1) Overlap shear (OLS) made by bonding 20 mil etched aluminum to 63 mil etched aluminum using 160°C bond-line temperature, 2 secs dwell, 14 lb gauge pressure. (2) Peel bonds made by bonding 4.5 mil aluminum foil to test substrates using 320°F (160°C) bond-line temperature, 2 s dwell, 14 lb gauge pressure. (3) Adhesion tests done at 2 in/min for peel, 2 in/min for OLS.
The newest generation of structural acrylic adhesives has made significant inroads towards resolving some of the objectionable issues of this group [65]:
Some of the advantages of the new structural acrylics are listed in Table 5.14.
5.20 Inorganic Adhesives (Cements)
These materials are widely used because they are durable, fire resistant, and inexpensive when compared with organic materials. Inorganic adhesives are based on compounds such as sodium silicate, magnesium oxychloride, lead oxide (litharge), sulfur, and various metallic phosphates. The characteristics of some of the more important commercial materials are summarized.
5.20.1 Soluble Silicates (Potassium and Sodium Silicate)
Sodium silicate is the most important of the soluble silicates. This material is often called “water glass” and is ordinarily supplied as a colorless, viscous water solution displaying little tack. Positive pressure must be used to hold the substrates together. This material will withstand temperatures up to 1,100°C. The main applications of sodium silicate adhesives are in bonding paper and making corrugated boxboard, boxes, and cartons. They are also used in wood bonding and in bonding metal sheets to various substrates; in bonding glass to glass, porcelain, leather, textiles, stoneware, etc.; bonding glass-fiber assemblies; optical glass applications; manufacture of shatter-proof glass; bonding insulation materials; refractory cements for tanks, boilers, ovens, furnaces; acid-proof cements; fabrication of foundry molds; briquettes; and abrasive polishing wheel cements. Soluble silicates may also be reacted with silicon fluorides or silica to produce acid-resistant cements with low shrinkage and a thermal expansion approaching that of steel [8,66].
5.20.2 Phosphate Cements
These cements are based on the reaction product of phosphoric acid with other materials, such as sodium silicate, metal oxides and hydroxides, and the salts of the basic elements. Zinc phosphate is the most important phosphate cement and is widely used as “permanent” dental cement. It is also modified with silicones to produce dental filling materials. Compressive strengths up to 200 MPa are typical of these materials, which are formulated to have good resistance to water. Copper phosphates are used for similar applications, but they have a shorter useful life and are used primarily for their antiseptic qualities. Magnesium, aluminum, chromium, and zirconium phosphates are also used [8].
5.20.3 Basic Salts (Sorel Cements)
These are basic salts of heavy metals, usually manganese or magnesium cement or magnesium oxychloride cement. They are suitable for dry locations where 2–8 h of hardening will permit their immediate use for bonding many refractory materials, ceramics, and glass. The final strength will be in the range of 48–69 MPa. Magnesium oxychloride is an inorganic adhesive notable for its heat and chemical resistance. It is usually supplied as a two-part product (magnesium oxide and magnesium chloride) which is mixed at the time of use. Copper is added to overcome the tendency to dissolve in water. These cements resist damage by cooking fats and greases, repel vermin, and prevent the growth of molds and bacteria. They also conduct static electricity from flooring and similar materials [8,66].
5.20.4 Litharge Cements
Mixtures of glycerin and litharge (lead oxide or PbO) are used as adhesives in the repair of tubs and sinks, pipe valves, glass, stoneware, and common gas conduits. A mixture of one part slightly diluted glycerin with two to three parts of lead oxide requires approximately 1 day to form a crystalline compound. The resulting cement resists weak acids and nitric acid, but reacts with sulfuric acid. These materials have also been used as ceramic seals in potting electronic equipment [8,66].
5.20.5 Sulfur Cements
Liquid sulfur (melting point 388°C) can really be considered an inorganic hot-melt adhesive. This material should not be exposed to temperatures higher than 93°C because of a marked change in the coefficient of expansion at 96°C as a result of a phase change. The addition of carbon black and polysulfides improves its physical properties. Tensile strength values of about 4.0 MPa have been reported, which decrease to 3.0 MPa after 2 years of exposure to water at 70°C. The principal use of sulfur cements is for acid tank construction, where high resistance to oxidizing acids, such as nitric and hydrofluoric acid mixtures at 70°C, is required. Resistance to oleic acid, oxidizing agents, and strong bases or lime is poor. Adhesion to metals, particularly copper, is satisfactory [8].
5.20.6 Sauereisen’s Adhesives
Sauereisen’s adhesives [67] and potting compounds are inorganic, ceramic-based materials. These specialty cements are composed of high-purity, inert fillers such as silica, alumina, or zircon. The nature of these materials, when formulated in a dense matrix with an appropriate binder, is to exhibit high thermal conductivity and electrical insulation. When dispensed, Sauereisen cements exhibit the consistency of a thick cream until they harden and fully cure. Sauereisen products bond to ceramics, metals, and glass, which make them ideal for many electrical instruments that operate at high temperature. Some end-use components that require technical cements include heating elements, resistors, halogen lamps, igniters, and thermocouples. The original Sauereisen adhesive, formulated in 1899, is still in demand today.
5.21 Melamine-Formaldehyde Adhesives (Melamines)
These synthetic thermosetting resins are condensation products of unsubstituted melamines and formaldehyde. They are equivalent in durability and water resistance to phenolics and resorcinols. Melamines are often combined with ureas to reduce cost. Melamines have higher service temperatures than those of ureas [7,8,31].
Amino resins, including melamines, have been discussed in considerable detail [68]. Another comprehensive discussion is by Pizzi [69,70].
5.22 Microencapsulated Adhesives
Microencapsulation is a method for separating an activating solvent or a reactive catalyst from the adhesive base. The materials, whether solid or liquid, are packaged in very small “microscopic” capsules. When adhesion is desired, the encapsulated solvent is released by breaking the capsules by heat or pressure, and a tacky adhesive with instant “grab” is produced. In addition to solvents, small quantities of plasticizers or tackifiers may be contained in the capsules. The capsules are made of gelatin and are insoluble in water and neutral to the solvents. Heat-activated adhesives are another form of microencapsulation. A blowing agent is mixed with the solvent in the capsule. Upon application of heat, the blowing agent vaporizes and ruptures the capsule, releasing either the entire adhesive or the solvent needed to make the adhesive tacky. A third form of encapsulation involves two-part adhesives, such as epoxy or polyurethanes. In this type, both the base resin and the catalyst are stored in the same container. The catalyst can be released by pressure, or by other means, to cure the adhesive [57].
5.23 Natural Glues
These adhesives include vegetable- and animal-based materials and have been replaced by synthetic resin adhesives. Their occasional uses are usually limited to paper, paperboard, wood, and metal foil. Hide glue forms a strong and long-lasting bond and was the most common woodworking glue for thousands of years until its replacement with man-made adhesives. Hoof glue is still used today in cabinetry and other fine woodworking projects where the joints must be extremely fine if not invisible.
Shear strengths range from 0.034 to 6.9 MPa. Few natural glues retain their strengths at temperatures above 100°C. Most of these materials have poor resistance to moisture, vermin, and fungus, but they do have good resistance to organic solvents. Common natural glues are discussed below.
5.23.1 Vegetable Glues
These adhesives are soluble or dispersible in water and are produced or extracted from natural sources. Other adhesives, such as rubber cements, nitrocellulose, and ethyl cellulose lacquer cements, are also produced from plant sources, but are not water soluble or water dispersible and are therefore not classified as vegetable glues [71].
Starch adhesives are derived primarily from the cassava plant, although other starch sources may be used. Starch is usually heated in alkaline solutions, such as NaOH, followed by cooling to room temperature to prepare the dispersions. After cooling, they are applied as cold-press adhesives. They develop their strength by loss of water into a wood substrate. Tack is developed rapidly; normal wood processing takes 1–2 days at room temperature and 0.5–0.7 MPa. Starch adhesives are also used for paper cartons, bottle labeling, and stationery applications. Joint strengths are low compared to other vegetable adhesive types. These adhesives are resistant to water and biodeterioration, and their resistance to these environments is improved by adding preservatives [8]. An example of starch adhesive application is found in Military Specification MIL-A-17682E, “Adhesive, Starch,” for use in mounting paper targets to target cloth. In this specification, the starch source must be wheat. Readers may recall using “flour and water” to make a simple paste for school and home use. It should be noted that this source of starch is not subjected to heating in alkaline solution, and, therefore, does not have the strength of the commercial material.
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch. They have the same general formula as carbohydrates but are of shorter chain length. Industrial production is generally performed by acidic hydrolysis of potato starch. Other catalytic agents used include enzymes, alkali, and oxidizing agents. Dextrins are water soluble, white to slightly yellow solids that are optically active. These adhesives can be used in formulations for many different substrates. Their applications are primarily for paper and paperboard. Laminating adhesives are usually made from highly soluble white dextrins and contain fillers such as clay, as well as alkalis or borax. Blends of white dextrins and gums are common. Urea-formaldehyde is often added to produce water resistance [71]. Military Specification MIL-A-13374D, “Adhesive, Dextrin, for Use in Ammunition Containers,” covers four classes of dextrin adhesives for use in making spirally wound containers and chipboard spacers.
Soybean glue (nitrogenous protein soybean) is the most common plant protein adhesive derived from seeds and nuts, hemp, and Zein. These adhesives are inexpensive and can be applied in making semiwater-resistant plywood and for coating some types of paper. The protein from the soybean is separated out mechanically and used much like casein protein, with the addition of calcium salts to improve water resistance. The soybean glues are used as room temperature-setting glues to produce interior-type softwood plywood, where only limited moisture resistance is needed. Soybean glues have been largely replaced by protein-blend glues, such as combinations of soybean and blood proteins, and by phenolics for plywood bonding. Cold-press bonding of plywood with soybean adhesives requires 4–12 h at 0.70–1.0 MPa. Hot-press bonding requires 3–10 min at 100–140°C and 1.0–1.5 MPa pressure. Water resistance of soybean glues is limited, although they become stronger on drying similar to casein glues. These materials will biodeteriorate under humid conditions unless inhibitors are used. Their resistance to heat and weathering is poor; therefore, they are restricted to indoor applications. Soybean glues are filled for paper and paperboard lamination, cardboard and box fabrication, and particle binders [8].
The Rosin family’s most common form of adhesive is colophony, a hard amorphous substance derived from the oleoresin of the pine tree. This material is applied in solvent solution form as a hot-melt mastic. It has poor resistance to water, is subject to oxidation, and has poor aging properties. Plasticizers are usually added to reduce its brittleness. Bond strengths are moderate and develop rapidly. These materials are used as temporary adhesives in bonding paper and as label varnishes. They are also used as components of PSAs based on styrene-butadiene copolymers and in hot-melt adhesives and tackifiers. These materials have been largely replaced by synthetic-resin adhesives [13]. One specialized form of rosin adhesive is Canada Balsam, covered by the obsolete Military Specification MIL-C-3469C, titled “Canada Balsam.” This material was intended for cementing optical elements.
5.23.2 Glues of Animal Origin
The term animal glue usually is confined to glues prepared from mammalian collagen, the principal protein constituent of skin, bone, and muscle. When treated with acids, alkalis, or hot water, the normally insoluble collagen slowly becomes soluble. These glues are divided into those derived directly and indirectly from animals, including mammals, insects, and fish, as well as milk products. The category should not be called “animal glues,” as that term is a specialized form.
Casein glue is the protein of (skim) milk, from which it is obtained by precipitation. Dry-mix casein glues are simply mixed with water before use. Casein glues are used at room temperature and set or hardened by loss of water (to the wood substrate) and by a degree of chemical conversion of the protein to the more insoluble calcium derivative. Applications include packaging, where the adhesives are used to apply paper labels to glass bottles. In woodworking, they are applied in laminating large structural timbers for interior applications. They are also used for general interior woodworking applications, including furniture. They cannot be used outdoors, although they are more resistant to temperature changes and moisture than other water-based adhesives. Casein adhesives will tolerate dry heat up to 70°C, but, under damp conditions, the adhesives lose strength and are prone to biodeterioration. Chlorinated phenols can be used to reduce this tendency to deteriorate. These glues are often compounded with materials such as latex and dialdehyde starch to improve durability. They have generally good resistance to organic solvents [7,72].
Blood albumen (blood glues) are used in much the same manner as casein glues. The proteins from animal blood in slaughtering are precipitated out, dried, and sold as powders, which are then mixed with water, hydrated lime, or sodium hydroxide. The blood proteins undergo some heat coagulation so that they can be hardened by hot-pressing and by loss of water. Processing usually takes 10–30 min at 70–230°C for plywood, with bonding pressures of 0.5–0.7 MPa. Porous materials require only several minutes at 80°C. Cold-press applications are also possible. Blood glues are used to a limited degree in making softwood plywood, sometimes in a combination with casein or soybean proteins. They have also been used as extenders for phenolic-resin glues for interior-type softwood plywood. Another application is for bonding porous materials, such as cork, leather, textiles, and paper, and for packaging, such as in bonding cork to metal in bottle caps.
Animal glues (bone and hide glues): The term “animal glues” is normally reserved for glues prepared from mammalian collagen, the principal constituent of skin, bone, and sinew. Other types of glues obtained from animal sources are usually referred to according to the material from which they are derived (casein, blood, fish, and shellac). Bone glues are made from animal bones, while hide glues are made from tannery waste. These glues are supplied as liquid, jelly, or solid in the form of flake, cube, granule, powder, cake, slab, etc., for reconstitution with water. They are used primarily for furniture woodworking, but also for leather, paper, and textiles, and as adhesive binders for abrasive paper and wheels. Liquid hide glues are normally supplied with a gel depressant added to the molten-glue mixture. This is done to assure that the dispersion remains liquid when cooled to room temperature so that it can be bottled. These glues harden only by the loss of water to the adherend, which must be relatively porous [73–75].
The processing conditions for animal glues are dependent on the type of glue. These glues set at temperatures in the range of 80–90°C, or they may set at room temperature. Bonding pressures range from contact pressure to 1–1.4 MPa for hardwoods and 0.35–0.70 MPa for softwoods. Application periods range from 5 min to several hours. Hide glues are stronger than bone glues. The bond strengths of these glues usually exceed the strengths of wood and fibrous adherends. High-strength joints are obtained where the bonds are kept under dry conditions. Structural applications are limited to interior uses. These glues are gap filling, which makes them useful where close-fit joints are not feasible and filler products are not required [8].
Animal glue is the primary adhesive component in gummed tapes used in sealing commercial solid fiber and corrugated shipping uses, as well as the more common lightweight types used in retail packaging [76].
Fish glues are by-products of desalted fish skins, usually cod, and have properties similar to animal skin and hide glues, which have largely replaced them in woodworking applications. Fish glues were the forerunners of all household glues. Many of the original industrial applications were developed because fish glue was liquid and had an advantage over animal glues, which required a heated glue pot. Fish glue has been in use for more than 100 years. Even with the many synthetic adhesives available today, there are applications that require the unique properties of fish glue [8,77].
Fish glues are available in cold-setting liquid form that does not gel at room temperature. Solvents such as ethanol, acetone, or dimethyl formamide may be added to facilitate the penetration of the glue into substrates that may be coated or finished (e.g., paper, leather, and fabrics). These glues may be exposed to repeated freezing and thawing cycles without adverse effects. Initial tack is excellent on remoistening dry fish-glue films with cold water. The water resistance of dried glue films can be improved by exposure to formaldehyde vapors, which renders the fish gelatin component insoluble. Fish glues bond well to glass, ceramics, metals, wood, cork, paper, and leather. The main uses are in the preparation of gummed tapes with animal/fish glue compositions, and in the bonding of stationery materials. Latex, animal glues, dextrins, and polyvinyl acetate adhesives are sometimes modified with fish glues to improve wet-tack properties. High-purity fish glues are important photoengraving reagents. Service temperature ranges from −1°C to 260°C and shear strength (ASTM D905) is 22 MPa with 50% wood failure [8,77].
Shellacs are thermoplastic resins derived from insects. They are used in alcoholic solutions or as hot-melt mastics. They have good electrical insulating properties, but are brittle unless compounded with other materials. Shellacs are resistant to water, oils, and grease. Bond strengths are moderate. Shellacs are used to bond porous materials, metals, ceramics, cork, and mica. They are also used as adhesive primers for metal and mica, for insulating sealing waxes, and as components of hot-melt adhesives. Shellacs are the basic components of de Khotinsky cement. Their application has declined over time because of their high cost [8].
Shellac is used as an alternative to alkyd resins in binding mica splitting to produce mica board. This is pressed into shapes used as insulation in electric motors, generators, and transformers. Mica tape, used as insulators in motors and generator coil slots, is fabricated by bonding mica flakes to glass cloth and tissue paper with shellac or silicones [78].
5.24 Neoprene (Polychloroprene) Adhesives
This type of synthetic rubber has been used extensively to bond aluminum. Its characteristics are summarized in Table 5.7. Neoprene is ordinarily used in organic solvents for convenient application. Although properties of neoprene and natural rubber are similar, neoprene generally forms stronger bonds and has better resistance to aging and heat. Solvent-based neoprene cements are also used extensively as shoe adhesives. For structural applications, neoprene is usually combined with a phenolic resin plus a number of other additives for curing and stabilizing the mixture (neoprene-phenolic). Both cold-setting and heat-curing formulations can be prepared [3].
Neoprene is a general-purpose adhesive used for bonding a wide range of materials. Gap-filling properties are satisfactory. Neoprene joints may require several weeks of conditioning to yield maximum strength. The unalloyed adhesives should not be used for structural applications requiring shear strengths of >2 MPa because they are likely to creep under relatively light loads. Tack retention is generally inferior to that of natural rubber. Loads of 0.2–0.7 MPa can be sustained for extended periods soon after bonding [8,26].
5.25 Neoprene-Phenolic Adhesives
These alloy adhesives are thermosetting phenolic resins blended with neoprene (polychloroprene) rubber. They are available in solvent solutions in toluene, ketones, or solvent mixtures, or as unsupported or supported films. The supporting medium may be glass or nylon cloth. Neoprene-phenolic adhesive may be used to bond a variety of substrates such as aluminum, magnesium, stainless steel, metal honeycombs and facings, plastic laminates, glass, and ceramics. Wood-to-metal bonds are often primed with neoprene-phenolic adhesives.
Compounding with neoprene rubber increases flexibility and peel strength of phenolic resins and extends the high-temperature resistance. The film form is preferred for applications where solvent removal is problematic. The higher curing temperatures provide the highest strengths. Curing takes place under heat and pressure. The film is ordinarily cured at 150–260°C for 15–30 min at 0.35–1.8 MPa bonding pressure. The liquid adhesives are ordinarily dried at 80°C and then cured for 15–30 min at 90°C and contact pressure of 0.7 MPa. The bond may be removed from the hot press while still hot. The liquid adhesive may be used as a metal primer for film adhesives [7,8].
The normal service temperatures of these adhesives range from −57°C to 93°C. Because of their high resistance to creep and most severe environments, neoprene-phenolic joints can withstand prolonged stress. Fatigue and impact strengths are excellent. However, shear strength is lower than that of other modified phenolic adhesives [7].
5.26 Nitrile-Epoxy (Elastomer-Epoxy) Adhesives
The term nitrile-epoxy is frequently used to signify elastomer-epoxy, even though this is not the only elastomer-epoxy available. Table 5.7 summarizes some of the important properties of this important adhesive. The maximum bond strength of these adhesives is generally below the maximum attainable with nylon-epoxies at room temperature. A major advantage of these adhesives, however, is that their peel strength does not decrease as abruptly at subzero temperatures as do the peel values of the nylon-epoxies. Bond durability of these high-peel elastomer-epoxies is satisfactory, as measured by most long-term moisture tests, but it does not match the durability of the vinyl-phenolic or nitrile-phenolic systems [22–24]. Nitrile-epoxies should not be used in applications involving exposure to marine environments or under continuous immersion in water [79].
5.27 Nitrile-Phenolic Adhesives
These adhesives are usually made by blending a nitrile rubber with a phenolic novalac resin, along with other compounding ingredients. Usage in tape, film, or solution form is very high. The major uses include bonding brake shoes and clutch disks in the automotive industry. They are also used in aircraft assembly and in many other smaller applications thanks to their low cost, high bond strengths at temperatures up to 121°C, and exceptional bond durability on steel and aluminum. Nitrile-phenolics exhibit exceptionally high durability after extended exposure to salt spray, water immersion, and other corrosive environments. They constitute the most important tape adhesives (Tables 5.11 and 5.12). Their important disadvantages include the need for cure under high pressure (1.38 MPa), while the trend is toward reduced cure pressures; and the need for high temperature (149°C), long dwell cures, while the trend is toward adhesives that cure rapidly at or below 121°C [7].
The liquid nitrile-phenolic adhesives are dried at 80°C and cured for 15–30 min at 90°C at contact to 0.70 MPa pressure [8].
5.28 Nitrile Rubber Adhesive
This is one of the most important synthetic thermoplastic elastomers. Nitrile rubber is a copolymer of butadiene and acrylonitrile. The copolymer usually contains enough acrylonitrile (>25%) so that good resistance to oil and grease can be obtained. Adhesive properties also increase with increasing nitrile content. These adhesives are used to bond vinyls, other elastomers, and fabrics where good wear, oil, and water resistance are important. Compatibility with additives, fillers, and other resins is another advantage of this material [3,80]. Table 5.7 summarizes the properties of nitrile rubber.
5.29 Nylon Adhesives
Nylons are synthetic thermoplastic polyamides of relatively high molecular weight that have been used as the basis for several types of adhesive systems. They are used as solution adhesives, as hot-melt adhesives, and as components of other adhesive-alloy types (nylon-epoxy and phenolic-nylon). The high-molecular-weight products are generally referred to as modified nylons. Low- and intermediate-molecular-weight materials are also available. The latter two are more commonly used in hot-melt formulations and the modified nylons are often blended with small amounts of a phenolic resin to improve surface wetting (hence nylon-phenolic).
Solution systems of low- and intermediate-molecular-weight nylon resins can be coated on paper, metal foil, or plastics, and when heat-activated will act as adhesives for these substrates. Modified nylons have fair adhesion to metals, good low- and high-temperature properties, and good resistance to oils and greases, but poor resistance to solvents [3,8].
Certain specialty nylon resins with low melting temperatures have been used quite successfully with extrusion techniques. Both nylon and high-molecular-weight polyamide resins that are chemically related to dimer acid-based polyamides are used in high-strength metal-to-metal adhesives; they are applied by extrusion [3].
Nylon (polyamide) use in hot-melt adhesives has been discussed briefly in Section 5.19.
5.30 Nylon-Epoxy Adhesives
These are possibly the best film-and-tape structural adhesives available. Their tensile strength of >48 MPa and climbing-drum peel strengths of >26,265 N/m are the highest available in structural adhesives. These adhesives also have exceptional fatigue and impact strengths. Low-temperature performance is good down to the cryogenic range, except that brittleness occurs at cryogenic temperatures (−240°C). Other disadvantages include poor creep resistance and extreme sensitivity to moisture [3,22–24]. Property data on these adhesives are shown in Tables 5.11 and 5.12.
Nylon-epoxy film adhesives have the tendency of picking up substantial amounts of water before use. They also tend to lose bond strength rapidly after use on exposure to water or moist air. After 18 months of exposure to 95% RH, conventional nitrile-phenolic adhesive loses only a fraction of its initial strength, going from 21 to 18 MPa in tensile shear. On the other hand, one of the best nylon-epoxy adhesives available degraded from about 34 to 6.8 MPa in just 2 months under the same test conditions [81]. A considerable effort has been made to solve this moisture problem, but nitrile-epoxy or acetal-toughened epoxy film adhesives are still superior in durability [22–24].
5.31 Phenolic Adhesives
These adhesives, more properly called phenol-formaldehyde adhesives, are condensation products of formaldehyde and a monohydric phenol [31]. They dominate the field of wood adhesives and represent one of the largest volumes of any synthetic adhesive. Phenolics are also among the lowest cost adhesives and may be formulated as water dispersions, to allow penetration into the cell structure of wood which is important for the formation of permanent bonds. Beyond the wood and wood products area, unmodified phenolics are used mainly as primers, to prepare metal surfaces for bonding, and as binders, for such varied products as glass wool insulation mats, foundry sand, abrasive wheels, and brake lining composites. Phenolics are supplied either as one-component, heat-curable liquid solution, as powder, or as liquid solution to which catalysts must be added. The curing mechanisms are different for these two groups [3].
5.31.1 Acid-Catalyzed Phenolics
The acid-catalyzed phenolics form wood joints requiring from 1 to 7 days conditioning, depending on the end use. Metals bonded with these adhesives require priming with a vinyl-phenolic or rubber-resin adhesive before bonding. These adhesives have good gap-filling properties, but they are not recommended as structural adhesives unless their glue line pH is higher than 2.5. Glass or plastic mixing vessels are required because of the acidic nature of these adhesives. The mixed adhesive is exothermic (gives off heat) and temperature sensitive. These adhesives are cured under the conditions listed in Table 5.15.
Table 5.15
Cure Conditions of Acid-Catalyzed Phenolics [8]
| General-purpose | 3–6 h at 20°C |
| Timber (hardwood) | 15 h at 15°C and 1.2 MPa |
| Timber (softwood) | 15 h at 15°C and 0.7 MPa |
Curing time is reduced by increasing the curing temperature. Resistance to weather, boiling water, and biodeterioration is satisfactory. Resistance to elevated temperatures is also satisfactory, but inferior to that of heat-cured phenolic and resorcinol adhesives. Excess acidity due to poor control of the acid catalyst content often leads to wood damage on exposure to warm humid air. The durability of joints at high and low temperatures for extended periods is usually acceptable. These adhesives are used for woodwork assemblies, where the service temperature does not exceed 40°C. Applications include furniture construction and, to a minor extent, plywood fabrication. This adhesive is also used to join metal to wood for exterior use [8].
5.31.2 Hot-Setting Phenolics
The hot-setting form of phenol-formaldehyde adhesive is supplied in spray-dried powder to be mixed with water, as alcohol, acetone as water-solvent solutions, or as glue films [70,82,83]. It may be compounded with fillers and extenders. The gap-filling properties of this type of phenolic adhesive are poor and inferior to the acid-catalyzed phenolic adhesives. Joints require conditioning up to 2 days. Although durable and resistant to many solvents, the bonds are brittle and prone to fracture under vibration and sudden impact. These adhesives are used as additives to other materials to form adhesives for glass and metals, or modifying agents for thermoplastic elastomer adhesives, or as components of thermoplastic resin-elastomer adhesives for metal bonding [8].
Hot-setting phenolic adhesives are processed for up to 15 min at 100–150°C and at 0.7–1.7 MPa bonding pressure. The film form is processed for up to 15 min at 120–150°C and at 0.7–1.4 MPa. This type of phenolic is resistant to weather, boiling water, and biodeterioration. It has superior temperature stability to that of the acid-catalyzed form. Applications of this adhesive include fabrication of exterior-grade weather- and boil-proof plywood and for bonding glass to metal for electric light bulbs [8].
5.32 Phenoxy Adhesives
These materials are synthetic thermoplastics in the form of polyhydroxy ethers. Phenoxy adhesives are supplied as one-component systems in powder, pellet, or film forms. They may be dissolved in solvents or supplied as special shapes. Phenoxies act as hot melts and set upon cooling. The liquid forms require removal of the solvent by drying before bonding. Time and temperature are important factors in obtaining maximum strength; bonding pressure is not critical. Typical conditions include bonding for 30 min at 192°C, 2–3 min at 260°C, or 10 s at 300–350°C, and pressure from contact to 0.17 MPa. Phenoxies are used as structural adhesives for rapid assembly of metals and rigid materials, for continuous lamination of metal to metal (cladding) or wood and flexible substrates, paper, cloth, metal foil, and plastic laminations. Other applications include pipe jointing (with fiber type), assembly of automotive components, and bonding polymeric materials such as polyester film, polyurethane foam, acrylics, and phenolic composites. They are also used as components of hot-melt adhesives for conventional applications [8].
Phenoxy adhesives withstand weathering and resist biodeterioration. They have excellent resistance to inorganic acids, alkalies, alcohols, salt spray, cold water, and aliphatic hydrocarbons but swell in aromatic solvents and ketones. Thermal stability is adequate, with a service temperature range of −62°C to 82°C. Resistance to cold flow and creep is high, even at 80°C. These adhesives provide rigid, tough glue lines with high adhesive strength. Shear strengths are similar to epoxies, and for metals generally exceed 17 MPa, possibly approaching 27.5 MPa. Film thickness is not critical and can be as little as 0.012 mm.
Liquid adhesives do not usually provide maximum bond strengths, as complete solvent removal may be difficult. Hot-melt adhesive systems may also present difficulties. Thermal degradation can occur before the resin is completely melted, unless plasticizers are used [9]. Plasticizers used are diphenyl phthalate, tricresyl phosphate, and dicyclohexyl phosphate (DCHP), which are used in hot-melt formulations. Unplasticized phenoxies give peel strengths of 3,152–5,253 N/m in bonding Neolite to Neolite. Formulations with 60% DCHP raise the peel strength to 5,078–5,213 N/m [57,84,85]. Good adhesion has been obtained with substrates such as copper, brass, steel, aluminum, wood, and many other nonmetallic substances [3].
5.33 PBI Adhesives
These adhesives are supplied in film form on glass cloth. Normally, filler (usually aluminum) and antioxidants are among the components. PBIs are thermoplastics, although their thermoplastic nature is not evident below 371°C. These materials were developed specifically for use in high-temperature applications. They are relatively stable in air up to 288°C in short-term exposures. PIs are superior for long-term strength retention. PBIs are expensive and are limited to the bonding of high-temperature metals (stainless steel, beryllium, and titanium). PBIs are of greatest interest to aerospace engineers for use in the adhesive assembly of lightweight honeycomb structures for supersonic aircrafts, missiles, and other space systems. They are somewhat sensitive to moisture at room temperature; lap-shear strength drops gradually on heating to 316°C, then more rapidly at higher temperatures. Figure 5.6 shows the effect of heat aging at 371°C, compared with PIs [3].
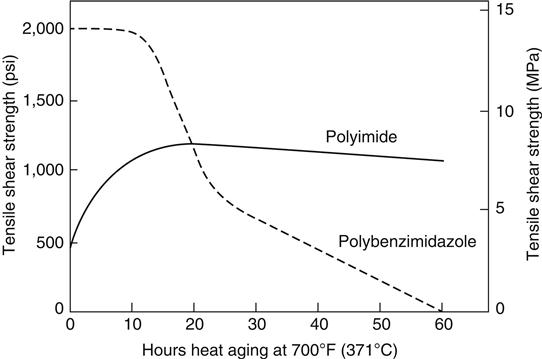
Processing is normally carried out in a preheated press at 370°C with pressure maintained at 0.03 MPa for 30 s. The pressure is then increased to 0.6–1.4 MPa and the glue line temperature maintained at 370°C for 3 h. The temperature is then reduced to 260°C or less and the assembly is removed from the press. Autoclave techniques can also be employed. For improved mechanical properties, postcuring in an inert atmosphere (nitrogen, helium, or vacuum oven) is recommended. The desirable conditions are 24 h each at 316, 345, 370, and 400°C followed by 8 h at 427°C in air to achieve maximum properties [8]. Obviously, these “literature” recommendations should be checked against manufacturers’ recommendations, but they provide a starting point for PBI processing.
PBI adhesives have good resistance to salt spray, 100% humidity, aromatic fuels, hydrocarbons, and hydraulic oils. About 30% loss of strength occurs after exposure to boiling water for 2 h. Electrical properties are fairly constant throughout the temperature range up to 200°C. Thermal stability at high temperatures for short periods is satisfactory, such as exposure at 540°C for 10 min or at 260°C for 1,000 h. The useful service-temperature range as adhesives is −250°C to 300°C [9]. Note that this includes the cryogenic temperature range.
5.34 Polyester Adhesives
Polyester adhesives may be divided into two distinct groups: saturated (thermoplastic) and unsaturated (thermosetting). The saturated polyesters are reaction products of difunctional acids and difunctional alcohols or glycols. Their adhesive applications are minor, except in hot melts (high performance). The unsaturated (thermosetting) polyesters, which require a catalytic cure, have a few uses as adhesives. These usually involve bonding of polyester substrates. Polyester adhesives are also used in patching kits for repair of fiberglass boats, automobile bodies, and concrete flooring.
Other minor uses include bonding polyester laminates to polyester or to metal, and as adhesives for optical equipment. CR-39 allyl diglycol carbonate is an example of the latter. This material, in the cured condition, exhibits improved abrasion and chemical resistance over other transparent adhesive resins and displays the good heat resistance and dimensional stability associated with thermosetting systems. These properties are retained on prolonged exposure to severe environmental conditions. CR-39, which stands for Columbia Resin 39, is an allyl resin, a special type of unsaturated polyester [3,8,58].
5.35 PI Adhesives
For a general discussion of these high-temperature adhesives, compared with PBI, see Section 5.5. These adhesives are synthetic thermosetting resins formed by the reaction of a diamine and a dianhydride. As with PBIs, they were developed specifically for high-temperature aerospace applications. PI adhesives are superior to PBIs for long-term strength retention, as shown in Figure 5.6 [8].
PI adhesives are supplied as solutions of the PI prepolymer in solvent, or in film form, usually containing fillers, such as aluminum powder, on a glass-cloth interliner. Processing is as follows: liquid form—removal of solvent by heat or under reduced pressure and by precuring the resin to the desired degree (B-staging), usually at 100–150°C. The volatile content may range from 8% to 18% (w/w) after B-staging. Final cure (C-staging) is carried out in stages over the range 150–300°C or higher. The film form may require B-staging. Their typical cure schedule involves heating to 250°C over a 90-min period and maintaining at this temperature for 90 min. Postcuring at higher temperatures up to 300°C and beyond is recommended when maximum mechanical properties are required. Bonding pressures should be in the range of 0.26–0.65 MPa. Like PBIs, the adhesives require tedious processing, compared to other adhesives.
PI adhesives have good hydrolytic stability and salt-spray resistance and excellent resistance to organic solvents, fuels, and oils. They are resistant to strong acids, but are attacked slowly by weak alkalies. Ozone causes the deterioration of the adhesive bond. Service temperature range is from −196°C to 260°C for long-term exposure, but these materials will withstand short exposures (200 h) up to 250°C and 10 min at 377°C. PIs are exceptionally good high-temperature electrical insulation materials. They also have exceptional resistance to atomic radiation (electrons and neutrons). PI materials are used as structural adhesives for high- and low-temperature applications, down to the cryogenic range, for bonding metals such as stainless steel, titanium, and aluminum, and generally in aircraft applications. They are also used in preparing glass-cloth-reinforced composites for electrical insulation, and in bonding ceramics [8].
PI adhesives require higher cure temperatures than epoxy-phenolic adhesives. Curing at 250°C is usually adequate when service temperatures do not exceed the cure temperature. Volatiles are released during the cure of PI adhesives. The best results are therefore obtained when the volatiles can freely escape (e.g., honeycomb or perforated-core structures). For long-term aging at temperatures in the range of 204–316°C, PIs are superior to PBI and epoxy-phenolic adhesives.
According to Edson [86], PI adhesives are capable of withstanding temperatures up to 316°C for hundreds of hours, and up to 204°C for thousands of hours. Thermal “spikes” of 538–816°C can be accommodated. PIs are several times more expensive than epoxies. According to Alvarez [87], PI adhesives can be processed at 177°C and postcured at 232°C to produce bonds capable of 316°C service. The “exchange” grade of PI polymers has a processing range of 177–288°C at a pressure of 0.10 MPa. These materials will withstand 316°C with normal 232°C postcuring. PIs are useful for bonding high-temperature metals like titanium and graphite/PI composite for use at 260–316°C [36,88].
5.36 Polyisobutylene Adhesives
These thermoplastic elastomers are covered briefly in Table 5.7. Polyisobutylene is a homopolymer [28].
5.37 Polystyrene Adhesives
Polystyrene is a transparent, colorless thermoplastic resin available in solvent solution or aqueous emulsion form. In both forms, applications are limited to conditions where at least one of the adherends is porous. An example is sticking polystyrene tiles onto a plaster wall. Polystyrene adheres well to wood, but not to plastics, except itself. For bonding polystyrene, a low-molecular-weight styrene polymer with a peroxide catalyst is used. This adhesive polymerizes in the glue line [31].
With some woods, shear strengths up to 13 MPa can be obtained. Polystyrene is used as a modifier for other adhesives such as unsaturated polyesters, hot-melt materials, and in optical cements. Resistance to high temperatures is limited. The heat-distortion temperature is about 77°C. Electrical insulating properties are excellent. Polyester adhesives have good resistance to water, nuclear radiation, and biodeterioration. However, they generally have poor resistance to chemicals. Other undesirable properties include high flammability and a tendency to brittleness and crazing. Copolymers of styrene and butadiene (SBR), also described in Table 5.7, are much less brittle and more valuable as adhesives. These materials are commonly used in footwear for bonding leather and rubber soles [8,31].
5.38 Polysulfides (Thiokols)
Polysulfides are flexible materials belonging to the synthetic rubber family. Some of the more important characteristics of polysulfide adhesive/sealants are tabulated in Table 5.7. Although polysulfides are primarily used as sealants for automotive, construction, and marine uses, they are used to some extent as flexibilizing hardeners for epoxy adhesives. Their sulfur linkages combine good strength with the ability to rotate freely, resulting in a strong, flexible polymer. Polysulfides utilize atmospheric moisture to accelerate cure. A two-component system is usually used, consisting of formulated polysulfide and formulated lead dioxide catalyst. Moisture converts a portion of the lead dioxide catalyst to a faster reacting form [3].
Polysulfides cure at room temperature and reach maximum strength in 3–7 days. Polysulfides and epoxies are mutually soluble in all proportions. Polysulfides are also alloyed with phenolics [8].
Curing agents may be furnished in powder, paste, or liquid form. The activity of the metallic curing agents is a function of surface area, thus increasing the importance of particle size. As it is necessary to obtain a fairly complete dispersion throughout the polymer in order to achieve complete cure, it is generally more effective to combine the lead oxide with a plasticizing agent to form a paste. A finished polysulfide adhesive/sealant will generally contain the following ingredients as a minimum:
2. Reinforcing filler to increase strength and reduce cost
3. Plasticizer to modify modulus and hardness
Heat, humidity, and sulfur will accelerate the cure [89,90].
5.39 Polysulfone Adhesives
These are temperature-resistant thermoplastic adhesives which require fairly high temperatures for heat activation after solvents have been removed [5]. Polysulfones are a family of tough, high-strength thermoplastics which maintain their properties over a temperature range from −101°C to >149°C. Bakelite’s UDEL Polysulfone P-1700 has the following properties: tensile strength 70 MPa; flexural strength 106 MPa; heat-distortion temperature 174°C; second-order glass transition temperature 191°C. The flexural modulus is maintained over a wide temperature range. At 149°C, more than 80% of the room-temperature stiffness is retained. Resistance to creep is excellent. Polysulfone adhesives are resistant to strong acids and alkalis, but attacked and/or dissolved by polar organic solvents and aromatic hydrocarbons [91].
These adhesives maintain their structural integrity up to 191°C. More than 60% of their room-temperature shear strength as well as excellent creep resistance are retained at 149°C. Cure cycles need only be long enough to introduce enough heat to wet the substrate with the P-1700 polysulfone. For unprimed aluminum, a temperature of 371°C should be used after drying the adhesive film for 2–4 h at 121°C to remove the equilibrium moisture. With a platen temperature of 371°C and a pressure of 0.55 MPa, joints with tensile lap-shear strengths of >21 MPa are developed in 5 min. Higher temperatures at shorter dwell times may be used whenever the metal will tolerate such temperatures. Tensile-shear strengths of >27.5 MPa have been obtained with stainless steel after pressing at 371°C [91].
Polysulfone adhesives have good gap-filling properties. In general, polysulfone adhesives combine the high strength, heat resistance, and creep resistance of a thermosetting-type adhesive with the processing characteristics and toughness of a high-molecular-weight thermoplastic [91].
5.40 Polyurethane Adhesives
Urethane polymers [92] have been used in flexible and rigid foams, cryogenic sealants, and abrasion-resistant coatings. Their application as adhesive has been expanding.
The principal use of polyurethanes is in bonding plastics that are difficult to bond, usually to a dissimilar material or to metals. Cured urethanes are lightly cross-linked thermoset resins, almost thermoplastic. This gives them a flexible rubbery characteristic. A brief description of their characteristics is given in Table 5.7. Their flexibility combined with good adhesion insures good bonding to flexible plastics, where peel strength is important. The outstanding feature of urethanes is strength at cryogenic temperatures. Table 5.16 compares the strength of urethane, epoxy-nylons, and epoxy-polyamides at −240°C [3].
Table 5.16
Comparison of Typical Urethane Adhesive with Other Adhesives on Aluminum at –240°C [3]
| Adhesive | Lap Shear Strength (MPa) | Peel Strength (N/m) |
| Urethane | 55.2 | 4,550 |
| Epoxy-nylon | 31.7 | Brittle |
| Epoxy-polyamide | 11.0 | Brittle |
Polyurethanes are one-component thermoplastic systems in solvents (ketones, hydrocarbons) often containing catalysts in small amounts to introduce a degree of thermosetting properties. They are also available as two-part thermosetting products in liquid form, with or without solvents. The second part is a catalyst. The one-part solvent type is used for contact bonding of tacky adherends following solvent release or heat-solvent reactivation of dried adhesive coating. The two-part thermosetting products are mixed and fully cured at 20°C in 6 days. They may also be heat-cured in 3 h at 90°C or in 1 h at 180°C. Bonding pressures range from contact to 0.35 MPa [8].
A one-component urethane prepolymer adhesive (available from H.B. Fuller Co., www.HBFuller.com) is designed for bonding various substrates, including plastic to plastic, plastic to metal, and metal to metal [93]. This adhesive can be used to bond imperfectly matched substrates and can be used for tack welding. No priming of the substrate surface is required, except for a solvent wipe. Average tensile strength and elongation according to ASTM D638, after 30 min cure at 127°C have been listed in Table 5.17.
Table 5.17
Average Tensile Properties of Cured One-Component Polyurethane Adhesive According to ASTM D638
| Temperature (°C) | Tensile Strength (MPa) | Elongation (%) |
| −40 | 54 | 8.7 |
| 22 | 16.6 | 32 |
| 82 | 4 | 22 |
| 127 | 2.2 | 16 |
Another urethane one-part adhesive (Urethane Bond) developed by Dow Corning is cured by moisture in the air at room temperature. This material requires a thin glue line and clamping to produce the strongest joints. The resultant bonds are moisture resistant and are claimed to work well on polystyrene, PVC, and acrylics, and fairly well on polyethylene [94]. There are excellent references for additional study of this adhesives family [70,95,96].
5.41 Polyvinyl Acetal Adhesives
Polyvinyl acetal [97] is the generic name for a group of polymers that are products of the reaction of polyvinyl alcohol and an aldehyde. In preparing these acetals, polyvinyl acetate is partially hydrolyzed to an alcohol. As adhesives, the most common acetals are those from formaldehyde, namely the formal, and from butyraldehyde, the butyral. The properties of these polymers are largely dependent on the molecular weight and on the degree of hydrolysis of the acetate. As an adhesive, the butyral (polyvinyl butyral) is much more important than the formal (polyvinyl formal). This is because of its more ready solubility and lower melt viscosity, and because it is softer and more flexible, thus yielding better peel strength and higher apparent adhesion with thin adhesives. In the two-polymer adhesive system, the formal is at least as important as the butyral [31].
Polyvinyl butyral is commonly used in safety-glass laminates. Polyvinyl acetals are used in making thermoset resins more flexible to obtain structural adhesives for metals.
5.42 Polyvinyl Acetate Adhesives
The most widely used resin in water-dispersion form is polyvinyl acetate in homopolymer and copolymer variety. Polyvinyl acetate latex is the basis for the common household “white glue,” of which Elmer’s® is probably the most well known. Products of this type are good adhesives for paper, plastics, metal foil, leather, and cloth. Their major use is in packaging for flexible substrates. This material is also used as a lagging adhesive to bond insulating fabric to pipe and duct work in steam plants and ships. It is also used in frozen-food packaging where low-temperature flexibility is important. Polyvinyl acetates are used in hot-melt adhesive formulations. Other uses include bookbinding and the lamination of foils. Organic solvent solution and water dispersion are two common forms of polyvinyl acetate adhesives.
For wood bonding, 10 min to 3 h at 20°C and contact to 1 MPa pressure is recommended. These adhesives have low resistance to weather and moisture. Resistance to most solvents is poor, although they withstand contact with grease, oils, and petroleum fluids and are not subject to biodeterioration. The cured films are light stable, but tend to soften at temperatures approaching 45°C. Polyvinyl acetates are low-cost adhesives with high initial-tack properties. They set quickly to provide almost invisible glue lines. Curing for 1–7 days is recommended before handling the bonded assemblies. Maximum bond strength up to 14 MPa can be reached by baking the adhesive films, followed by solvent reactivation and assembly. Polyvinyl acetates tend to creep under substantial load. They have satisfactory gap-filling properties [8].
Polyvinyl acetate adhesives are used in the construction of mobile homes. The purpose is to provide temporary bonds during construction until the units are supported on foundations. They provide strong initial bonds that develop strength quickly. Immediate strength and stiffness are needed to resist stress induced by flexing and racking of long mobile homes as they are moved within the factory and during hauling and lifting at the construction site [98].
Polyvinyl acetate glues should be applied at 16–32°C working temperatures. They soften when sanded [99,100].
5.43 Polyvinyl Alcohol Adhesives
This is a water-soluble thermoplastic synthetic resin with limited application as an adhesive [101]. The chief uses are in bonding porous materials such as leather, cork, and paper in food packaging, and as a remoistenable adhesive. It is available as a water solution with good wet-tack properties. It sets by losing water to give a flexible transparent bond with good resistance to oils, solvents, and mold growth, but poor resistance to water. It is nontoxic and odorless. Cured films are impermeable to most gases. The maximum service temperature is about 66°C. Polyvinyl alcohol is also used as a modifier for other aqueous adhesive systems to improve film-forming properties, or to promote adhesion. These materials are used with dextrins and starches to provide low-cost laminating adhesives. They are also used for envelopes and stamps [8].
5.44 Polyvinyl Butyral Adhesives
See the discussion of these adhesives under polyvinyl acetal adhesives (see Section 5.41).
5.45 Premixed Frozen Adhesives
Ablestik Laboratories in Gardena, CA [102], has available frozen reactive adhesives, such as epoxies, in disposable tubes or syringes ranging upward in size from 1 cm3. These adhesives are packed in dry ice and shipped in insulated cartoons. Included in each carton is a safety indicator that is formulated to melt and lose shape when exposed to temperatures unsafe for adhesive storage. Storage life at −40°C before use is usually from 2 to 6 months. In use the frozen adhesive is thawed to room temperature and applied within 2 h after thawing. These adhesives eliminate production-line delays caused by on-the-job mixing of messy two-part adhesives, saving valuable assembly time. They also guarantee accurate formulation of components. Another advantage is the reduction in the possibility of workers contracting dermatitis from handling irritating amine curing agents [103].
5.46 Pressure-Sensitive Adhesives
The most common application of PSAs is in tape form. In the dry state, PSAs are aggressively and permanently tacky at room temperature and firmly adhere to a variety of dissimilar surfaces without the need for more than finger or hand pressure [104,105]. They require no activation by water, solvent, or heat in order to exert a strong adhesive holding force.
Most PSAs are based on natural rubber. Rubber by itself has very low tack, and adhesion to surfaces thus requires addition of tackifying resins based on rosins, petroleum, or terpenes. Hydrogenated resins are added to enhance PSAs’ long-term aging. Adhesives based on acrylic polymers and natural rubbers are the leading PSAs. These acrylics have good ultraviolet (UV) stability, are resistant to hydrolysis, and are water white with good resistance to yellowing or aging. Acrylic-based PSAs have poor creep properties, compared to natural rubber. Blends of natural rubber and SBR also produce excellent PSAs. Other less desirable adhesives include polyisobutylene and butyl.
The adhesives discussed above are all applied in the solution and hot-melt forms. These are pressure sensitive at room temperature. These materials may be based on EVA copolymers tackified with various resins and softeners. They produce rather soft adhesives with poor cohesive strength. Their use is small, mostly on label stock. Of wider interest are hot-melt adhesives based on the block copolymers of styrene with butadiene or isoprene. Vinyl ether polymers are also used, particularly in medicinal self-adhering plasters or dressings [106].
Silicone adhesives are used to a small extent in PSAs. These products are based on silicone rubber and synthetic silicone resins. They have excellent chemical and solvent resistance, excellent elevated-temperature resistance, excellent cold-temperature performance, and high resistance to thermal and oxidative degradation. Their disadvantages include lack of aggressive tack and high cost (three to five times as much as acrylic systems) [107].
PSAs are often supplied to the final consumer coated onto a substrate such as cellophane tape or insulating tapes based on plasticized PVC film. These consist of the backing film, a primer or key coat, and the adhesive. If the product is to be rolled up in tape form, a release coat may be applied to the back of the film to reduce unwind tension when the tape is applied; otherwise it is omitted. The adhesive, generally of the types discussed here, is usually applied from an organic solvent. Aqueous dispersions and hot-melt forms, however, could be used. The coating weights range from 10 g/m2 upward, but are generally around 20–50 g/m2. The primer is applied at a coating weight of 2–5 g/m2 from solvent or aqueous dispersion. Nitrite rubber, chlorinated rubbers, and acrylates are common primers.
A graft copolymer of MMA and natural rubber can be used as a primer coat for plasticized PVC. The release coat is also applied to a lightweight coating at 1–5 g/m2. Acrylic acid esters of long-chain fatty alcohols, polyurethanes incorporating long aliphatic chains, and cellulose esters have also been used as release coats. Almost any material that can be put through a coating process can be used as adhesive backing [106].
5.47 Resorcinol-Formaldehyde Adhesives
These adhesives cure by the addition of formaldehyde, compared to phenolics, which cure on addition of strong acids [8,70,83]. Commercially, these adhesives are supplied as two-part systems. A liquid portion, the “A” part, is the resinous constituent. It is generally a solution of the preformed formaldehyde-deficient resin in a mixture of alcohol and water, with solid content of about 0%. This resin is stable if kept in closed containers at or below room temperature. The pH at which the liquid is buffered controls the reactivity of the glue. The solid portion, or “B” part, is a solid, powdered mixture of paraformaldehyde, or “para,” and fillers. The para is selected for control of glue-mix working life and curing efficiency. Once the “A” and “B” portions are mixed, the pot life of the mixture is limited. Many of these glue mixtures are exothermic (upon mixing), increasing the mix temperature and thus speeding the cross-linking reaction. Consequently, the pot life is reduced considerably. In these cases, it is important to remove the heat by stirring and cooling as rapidly as the heat is generated. Actual gluing may take place anywhere in the range of 21–43°C, with clamping at moderate pressures [108].
These adhesives are suitable for exterior use and are unaffected by water (even boiling water), molds, grease, oil, and most solvents. Their applications primarily include wood, plywood, plastics, paper, and fiberboard [7]. Resorcinol-formaldehydes are excellent marine-plywood adhesives. Curing at room temperature normally takes 8–12 h, while phenolic wood adhesives require a high-temperature cure. The adhesives are also used for indoor applications because of their high reliability [3,108].
5.48 Rubber-Based Adhesives
See Elastomeric Adhesives (see Section 5.13).
5.48.1 Silicone Adhesives
Silicones are semi-inorganic polymers (polyorganosiloxanes) that may be fluid, elastomeric, or resinous, depending on the types or organic groups on the silicone atoms and the extent of cross-linkage between polymer chains [8,109,110]. An example of silicone resin structure is seen in Figure 5.7.
The silicone resins owe their high heat stability to the strong silicon–oxygen–silicon bonds. The resin systems vary significantly in their physical properties as a result of the degree of cross-linkage and the type of radical (R) within the monomer molecule. In this regard, the chief radicals are methyl, phenyl, or vinyl groups [111].
These polymers have unusual properties and are used both to promote and to prevent adhesion. Silicones have good heat stability, chemical inertness, and surface-active properties. Applications of silicone adhesive fall into four types [3]:
Silicones have not found broad use as adhesives, relative to the total consumption volume, because of their high cost. Their applications are numerous and varied. Silicones are applied where organic materials (based on carbon) cannot withstand exposure to the environmental conditions, superior reliability is required, or their durability gives them economic advantages. As coupling agents, silicones are widely used for surface treatment of fiberglass fabric for glass-reinforced laminates. The adhesion of epoxy or polyester to glass cloth is improved both in strength and in moisture resistance of the cured bond by the use of silicone-coupling agents. The retention of flexibility and a fraction of strength at a temperature range from cryogenic to >260°C is an advantage of silicones. Generally, the room temperature mechanical properties of silicone adhesives are quite low compared to typical polymers [3].
The excellent peel strength properties of silicones are more important in joint designs than the tensile or lap-shear properties. Some examples of peel and lap-shear strengths with silicones are presented in Table 5.18.
Table 5.18
Examples of Peel and Lap-Shear Strength of Silicone Adhesives [3]
| Adherends | Peel Strength |
| Rubber to aluminum | 2,975–3,500 N/m |
| Urethane sealant to aluminum | |
| Without primer | 612 N/m |
| With silicone-coupling agent | 2,450 N/m |
| Lap-shear strength | |
| Metal-to-metal | 1.7–3.4 MPa |
Silicone applications in adhesives include:
• Two-part adhesive for bonding insulating tapes to magnet wire (Class M performance).
• One- or two-part adhesives for pressure-sensitive tapes, used in the temperature range of −62°C to 260°C. End-uses of these tapes include some in the electronics and aerospace industries.
Silicone use in primers includes:
• Bond promoters with phenolic binders for foundry sand on abrasive wheels
• Filler treatment in filled polyester or epoxy coatings (epoxy concrete patching formulations)
• Improved bonding of polysulfide or urethane sealants to metal substrates or glass.
In some cases, silicone is as effective when blended into an adhesive formulation as when it is applied separately as a primer. For silicone-coupling agents, moisture adsorbed on the substrate plays an important role in attaching the silicone molecule through hydrolysis. The opposite end of the molecule contains a chemical group such as a vinyl or amine, which is reactive with the epoxy, polyester, or other resin that is to be adhered to the substrate. In this manner, a single layer of silicone molecules “couples” the resin to the substrate. In addition to bond strength, moisture resistance also improves [3].
Silicone adhesives cure without the application of heat or pressure to form permanently flexible silicone rubber. The rubber remains flexible despite the exposure to high or low temperatures, weather, moisture, oxygen, ozone, or UV radiation. This makes them useful for joining and sealing joints in which considerable movement can be expected, such as intermediate layers between plastics and other materials of construction (e.g., acrylic glazing). Several types of silicone adhesives/sealants are available, including one-part and two-part systems.
One-part silicone systems are ready to use, require no mixing, present no pot-life problem, and are generally the least expensive. Conventional one-part adhesive/sealants are available with two different types of cure systems: acid and nonacid cure. Both require moisture from the atmosphere to cure. The acid-curing type has the greatest unprimed adhesion and the longest shelf life. The nonacid-curing type is used when the acetic acid released by the cure reaction may cause corrosion, or be otherwise objectionable [112].
The two-part silicone adhesive/sealants do not require moisture to cure and produce a superior deep-section cure. Two types are available: addition-cure and condensation-cure. Addition of curing produces no by-products, can be heat accelerated, produces negligible shrinkage, and provides the best high-temperature resistance of all silicone adhesives. Condensation-cure silicones are not easily inhibited and can be used on a greater variety of materials [112].
Dow Corning Corp. offers an improved silicone adhesive/sealant for high-temperature use. This is a one-part, nonslumping paste that cures to a tough, rubber solid at room temperature on exposure to water vapor in the air. This material is said to perform at temperatures ranging from −65°C to 260°C for continuous operation, and to 316°C for intermittent exposure. This material will meet the requirements of MIL-A-46106A (2), Type 1 (see http://mil-spec-industries.com). The adhesive/sealant is acid cured and acetic acid is evolved during cure [113].
Table 5.19 summarizes some of the characteristics of silicone adhesives.
Table 5.19
Principal Polymers Used for Solvent-Based Adhesives and Solvent- or Water-Based Adhesives [26]
| Solvent-based | |
| Nitrocellulose | Cyclized rubber |
| Cellulose acetate butyrate | Polyisobutylene |
| Solvent- or water-based | |
| Natural rubber | Polyvinyl ether |
| SBR | Polyvinylidene chloride and copolymers |
| Butyl rubbers | Polyacrylate and polymethacrylate |
| Neoprene rubbers | Polyamide |
| Nitrile rubbers | Asphalt |
| Reclaim rubbers | Urea-formaldehyde |
| Polyvinyl acetate and copolymer | Phenol-formaldehyde |
| Polyvinyl chloride copolymer | Resorcinol-formaldehyde |
| Resin esters | |
5.49 Solvent-Based Systems
Natural and synthetic rubber and synthetic resins are soluble in organic solvents resulting in cements, resin solutions, or lacquers. In addition, there are many cellulose derivatives, such as nitrocellulose, ethyl cellulose, and cellulose acetate butyrate, used in preparing solvent-based adhesives. Solvent-based adhesives are also prepared from cyclized rubber, polyamide, and polyisobutylene. Low-molecular-weight polyurethane and epoxy compounds can be used with or without solvent. On the other hand, high-molecular-weight types or prepolymers require solvent to make application possible [26].
Solvents, or solvents containing small amounts of bodying resin, are used for bonding thermoplastic resins and film adhesives. An example is toluol, which can be used to soften and dissolve polystyrene molded articles to allow joining the softened pieces. Ketones can be used to bond PVC films in a similar manner. A small amount of resin can be used to thicken the solvent so that a sufficient amount would stay in place to dissolve the substrate. It should be noted, however, that solvent welding of molded plastics can cause stress cracking and weakening of the structure as the parts age [26].
Another class of solvent-based dispersion is the organosols. In this case, vinyl chloride copolymer resins are dispersed in suitable nonvolatile plasticizers and solvent. The solvent is evaporated and the remaining film is heated to approximately 177°C. The heat helps dissolve the resin in the plasticizer, and a tough, flexible film is obtained on cooling to room temperature.
The major polymers used for solvent-based adhesives are listed in Table 5.19.
Solvent-based adhesives are more expensive than water-based products. They usually make bonds that are more water-resistant and have higher tack and early strength than water-based adhesives. Solvent-based adhesives also wet oily surfaces and some plastics considerably better than water-based adhesives. Organic solvents must be handled in explosion-proof equipment and precautions need to be taken during application. Ventilation to remove toxic hazards must also be provided to avoid exposure of personnel to solvent vapors [26].
5.50 Thermoplastic Resin Adhesives
A thermoplastic resin adhesive is one that melts or softens on heating and rehardens on cooling without (within certain temperature limits) undergoing chemical change. At temperatures above the melting point, an irreversible chemical change such as depolymerization or oxidative degradation could take place. When used as adhesives, thermoplastic resins are applied in the form of solutions, dispersions in water, or solids. They are usually set by solidification, which is a purely physical means. When applied as solution or dispersion, adhesion follows evaporation or absorption of the liquid phase, as in solvent activation. When applied by melting and cooling the solids, the terms “hot-melt” or “melt-freeze” are used to describe the method of application. Although the terms “setting” and “curing” are frequently used synonymously for both thermoplastic and thermosetting adhesives, the term “setting” is more common with thermoplastic adhesives. When a chemical reaction such as polymerization occurs, the term “curing” is more appropriate.
Although thermoplastic adhesives fall into many different chemical classes, they are all composed predominantly of linear macromolecules. Most thermoplastic resins are capable of bonding a wide variety of substrates such as paper, wood, and leather. Some are capable of bonding rubbers, metals, and some plastics, without special surface treatment. The most notable exceptions are the silicone and fluorocarbon plastics [31].
5.51 Thermoplastic Rubber (For Use in Adhesives)
Thermoplastic rubber is a relatively new class of polymer. It has the solubility and thermoplasticity of polystyrene, while at ambient temperatures it has the toughness and resilience of vulcanized natural rubber or polybutadiene. These rubbers are actually block copolymers. The simplest form consists of a rubbery mid-block with two plastic end blocks (A-B-A), as shown in Figure 5.8. Examples of commercial products are Kraton® and Solprene® [114,115]. These materials are often compounded with plasticizers to decrease hardness and modulus, eliminate drawing, enhance pressure-sensitive tack, improve low-temperature flexibility, reduce melt and solution viscosity, decrease cohesive strength or increase plasticity if desired, and substantially lower material costs. Low levels of thermoplastic rubbers are sometimes added to other rubber adhesives. These materials are used as components in the following applications: PSAs, hot-melt adhesives, heat-activated assembly adhesives, contact adhesives, reactive contact adhesives, building construction adhesives, sealants, and binders. Two common varieties of thermoplastic rubber adhesives are styrene-butadiene-styrene (S-B-S) and styrene-isoprene-styrene (S-I-S) [25].
5.52 Thermosetting Resin Adhesives
A thermosetting synthetic resin is one that undergoes an irreversible chemical and physical change during curing to become substantially infusible and insoluble. The term thermosetting is applied to the resin both before and after curing. Some thermosetting adhesives are condensation polymers and some are addition polymers. The important thermosetting resin adhesives are urea-formaldehydes, melamine-formaldehydes, phenol-formaldehydes, resorcinol-formaldehydes, epoxies, polyisocyanates, and polyesters [31].
5.53 UV-Curing Adhesives
UV-curable adhesives and in general radiation-curable adhesives use UV light or other radiation sources to initiate curing. A permanent bond forms without application of heat by means of free-radical chemistry. The advantages of UV curing include lower application temperature (120–140°C), solvent-free, improved shear resistance at higher temperature, improved chemical resistance, and lower equipment installation costs. A disadvantage of UV-curing adhesives is that one substrate is usually required to be transparent to UV light. Some UV resin systems utilize a secondary cure mechanism to complete the curing of the adhesive regions that are shielded from UV rays. Electron beam, in contrast, does not have this advantage and penetrates through most materials.
UV-curing adhesives are available in a number of chemical systems, most of which are polymer based. These systems include acrylics and acrylates, epoxies, polyurethanes, polyesters, silicones, and vinyl and vinyl esters. The most common UV-curable adhesives are the acrylics. Specially modified acrylic and epoxy adhesives can be cured rapidly by UV radiation. In the case of epoxy adhesives, the adhesives can be preirradiated after application to the substrate before closing the bond line. These adhesive systems are offered by most major suppliers [116].
The cure time of different UV-curable adhesives vary, ranging from instant to several hours. Typically, UV exposure starts the process, which begins with tackiness of the adhesive and requires a given length of time to set fully. Longer cure times are required at lower curing temperatures.
There are a wide variety of UV-curing materials available for a broad range of applications. UV-curing resins are used to protect laminated flooring or to coat the “peel and stick” labels you use. We will look at two types of high performance, engineering adhesive typically used in product assembly [117].
The first type of adhesive to become familiar with is an epoxy-based material. While some people use the term epoxy as a generic reference to all high-performance engineering adhesives, it has a specific meaning within the adhesives world. It is also different from other adhesive types, particularly the acrylic-based adhesives.
Epoxy adhesives use a catalytic cure mechanism. The catalyst is a by-product from the reaction of the photoinitiator to UV light. By definition, a catalyst is something that promotes a chemical reaction, but is not consumed in the reaction. One consequence of this is that UV-curing epoxy adhesives can exhibit a shadow curing capability—material that is not directly exposed to UV light will cure, sooner or later.
Epoxy adhesives are also easy to modify for special purposes. For example, they can be filled with carbon, silver, or gold to provide electrical conductivity. Other additives can enhance thermal conductivity, while maintaining electrical insulation. Additional performance properties of epoxy-based adhesives that can be modified include impact resistance, shrinkage, glass transition temperature, high-temperature strength, surface specific adhesion characteristics, and chemical or moisture resistance.
Acrylic adhesives result from an entirely different chemistry and a different type of photoinitiator. Curing of acrylic adhesives results from a free radical mechanism. The free radicals are produced by the photoinitiator when it is exposed to UV light. However, the free radicals are consumed in the adhesive cure process, so acrylic adhesives can cure only where UV light is delivered. At least one of the components being bonded must be UV transparent to some degree. Another consequence of this cure mechanism is that no shadow cure capability is evident.
Modification of properties in acrylic adhesives is more often conducted at the chemical level, through changes in formulation or combination with other base resins. Wide ranging properties can include impact resistance, surface insensitivity, environmental resistance, etc. The emergence of urethane acrylate adhesives, as well as acrylated epoxies, begins to make simplistic adhesive classifications more challenging.
5.54 Urea-Formaldehyde Adhesives (Ureas)
These adhesives are commonly called urea glues [68–70]. They are the condensation products of unsubstituted urea and formaldehyde. They are usually two-part systems, consisting of the resin and the hardening agent (liquid or powder). They are also available as spray-dried powders with incorporated hardener. The latter is activated by mixing with water. Fillers are also added. Curing is normally accomplished under pressure without heat. For general purposes, curing is carried out for 2–4 h at 20°C and 0.35–0.70 MPa bonding pressure. In manufacturing of plywood, adhesion is accelerated by heat assist. Typical conditions include a 5–10-min dwell at 120°C and up to 1.6 MPa pressure. Timber (hardwood) is cured for 15–24 h at 20°C and 0.14 MPa. Softwood curing conditions are a dwell of 15–24 h at 20°C and 0.70 MPa. Bonding pressure depends on the type of wood, shape of parts, and similar factors.
The most common application of urea-formaldehyde adhesives is in plywood. Urea glues are not as durable as other types, but are suitable for a wide range of service applications. When glue line thickness ranges from 0.05 to 0.10 mm, the bond strength usually exceeds the strength of the wood. However, when glue line thickness exceeds 0.37 mm, the gap-filling properties are poor. Thick glue lines craze and weaken the joints unless special modifiers, such as furfural alcohol resin, are incorporated. These adhesives are not suitable for outdoor applications or extreme temperatures [7,8,31].
5.55 Vinyl-Epoxy Adhesives
These structural adhesive alloys are polyvinyl acetals.
5.56 Vinyl-Phenolic Adhesives
These structural adhesive alloys are also polyvinyl acetals. They may be phenolic-vinyl butyral or phenolic-vinyl formal [8]. “Vinyl” in vinyl-phenolic adhesive is a somewhat misleading term referring either to polyvinyl formal or to polyvinyl butyral. Vinyl phenolics generally have excellent durability, both in water and in other adverse environments. Cure takes place at 177°C for the polyvinyl formal-phenolic and at 150°C for the polyvinyl butyral-phenolic. These adhesives provide excellent performance, primarily as film adhesives. Grades that cure at lower temperatures and pressures yield higher hot strength, higher peel strength, and have other performance advantages [8,22–24]. Tables 5.11 and 5.12 and Section 5.3 provide useful information on these adhesives and their strength properties.
Cure conditions for the polyvinyl formal-phenolic film consist of 177°C for 5 min or 150°C for 30 min at 0.35–3.5 MPa bonding pressure. Curing of polyvinyl butyral-phenolic film requires a temperature of 150°C at 0.10–0.20 MPa pressure. Polyvinyl formal-phenolic film, the most common form, retains adequate strength when exposed to weather, mold growth, salt spray, humidity, and chemical agents such as water, oils, and aromatic fuels. These adhesives generally have good resistance to creep, although temperatures up to 90°C produce creep and softening of some formulations. Fatigue resistance is excellent, with failure generally occurring in the adherends rather than in the adhesive, which has a service temperature range of −60°C to 100°C [8].
5.57 Polyvinyl Formal-Phenolics
These structural adhesives are used in bonding metal to metal in aircraft assemblies, metal honeycomb panels, and wood-to-metal sandwich construction. Other applications include bonding cyclized rubber and, in some cases, vulcanized and unvulcanized rubbers and copper foil to plastic laminates for printed circuits. They are also applied as a primer for metal-to-wood bonding with resorcinol or phenolic adhesives. Polyvinyl formal-phenolics are among the best thermosetting adhesives for metal-honeycomb and wood–metal structures. These adhesives are generally equivalent to nitrile-phenolics for strength, but have slightly better self-filleting properties for honeycomb assembly. They are superior to epoxy types where strength in sandwich construction is desirable [8].
5.58 Polyvinyl Butyral-Phenolics
These are used in bonding metal or reinforced plastic facings to paper (resin impregnated) honeycomb structures, cork and rubber compositions, cyclized and unvulcanized rubbers, steel to vulcanized rubber, and electrical applications. They are also used as primer for metals to be bonded to wood with phenolics. Polyvinyl butyral-phenolics lack the shear strength and toughness of the polyvinyl formal-phenolic type [8].
5.59 Vinyl-Resin Adhesives
Several vinyl monomers are used to prepare thermoplastics that are useful in certain adhesive applications. The most important vinyl resins for adhesives are polyvinyl acetate, polyvinyl acetals (butyral and formal), and polyvinyl alkyl ethers. PVC and copolymers of both vinyl chloride and vinyl acetate with other monomers, such as maleic acid esters, alkyl acrylates, maleic anhydride, and ethylene, are also used to produce solvent-based adhesives [3].
5.60 Water-Based Adhesives
These adhesives are made from materials that can be dispersed or dissolved only in water. Some of these materials are the basis of solvent-based adhesives and are the principal materials used for liquid-adhesive formulations given in Table 5.20.
Table 5.20
Principal Polymers Used Exclusively for Water-Based Adhesives [26]
| Starch and dextrin | Casein |
| Gums | Sodium carboxymethylcellulose |
| Glue (animal) | Lignin |
| Albumen | Polyvinyl alcohol |
| Sodium silicate |
Table 5.19 lists polymers used for both water- and solvent-based adhesives. Water-based adhesives cost less than the equivalent solvent-based compounds. Even inexpensive organic solvents are costly when compared to water. The use of water eliminates problems of flammability, emission, and toxicity associated with organic solvents. However, in most cases, water-based adhesives must be kept from freezing during shipment and storage because of possible permanent damage to both the container and the contents [26].
There are two general types of water-based adhesives: solutions and latexes [118]. Solutions are made from materials that are soluble only in water or in alkaline water. Examples of materials that are soluble only in water include animal glue, starch, dextrin, blood albumen, methyl cellulose, and polyvinyl alcohol. Examples of materials that are soluble in alkaline water include casein, rosin, shellac, copolymers of vinyl acetate or acrylates containing carboxyl groups, and carboxymethyl cellulose.
Latex is a stable dispersion of a polymeric material in an essentially aqueous medium. An emulsion is a stable dispersion of two or more immiscible liquids held in suspension by small percentages of substances called emulsifiers. In the adhesives industry, the terms latex and emulsion are sometimes used interchangeably. There are three types of latex: natural, synthetic, and artificial. Natural latex refers to the material obtained primarily from the rubber tree. Synthetic latexes are aqueous dispersions of polymers obtained by emulsion polymerization. These include polymers of chloroprene, butadiene-styrene, butadiene-acrylonitrile, vinyl acetate, acrylate, methacrylate, vinyl chloride, styrene, and vinylidene chloride. Artificial latexes are made by dispersing solid polymers. These include dispersions of reclaimed rubber, butyl rubber, rosin, rosin derivatives, asphalt, coal tar, and a large number of synthetic resins derived from coal tar and petroleum [119].
Latex adhesives replace solvent-based adhesives more easily than solution adhesives. Most latex adhesives are produced from polymers that were not designed for use as adhesives. This is why they require extensive formulation in order to obtain the proper application and performance properties. Application methods for latex adhesives include brush, spray, roll coat, curtain coat, flow, and knife coat. The bonding techniques used for latex adhesives are similar to those used for solvent adhesives. The following techniques are commonly used [113,118]:
• Wet bonding: Used when at least one of the bonded materials is porous. The adhesive is usually applied to one surface only. Bonding takes place while the adhesive is still wet or tacky.
• Open-time bonding: In this method, the adhesive is applied to both surfaces and allowed to stand “open” until suitable tack is achieved. At least one of the adherends should be porous.
• Contact bonding: In this method, both surfaces are coated and the adhesive is permitted to become dry to the touch. Within a given time, these surfaces are pressed together and near ultimate bond strength is immediately achieved. In this method, both surfaces may be nonporous. Neoprene latex adhesives are commonly utilized in contact bonding.
• Solvent reactivation: In this method, the adhesive is applied to the surface of the part and allowed to dry. To prepare for bonding, the adhesive is reactivated by wiping with solvent or placing the part in a solvent-impregnated pad. The surface of the adhesive tackifies and the parts to be bonded are pressed together. This method is suitable only for relatively small parts.
• Heat reactivation: In this method, a thermoplastic adhesive is applied to one or both surfaces and allowed to dry. To bond, the part is heated until the adhesive becomes soft and tacky. The bond is made under pressure while hot. After cooling a strong bond is obtained. This method is common for nonporous heat-resistant materials. It can also be used in a continuous in-line operation. The adhesive is applied in liquid form to a film or sheet, force-dried with heat to remove the water, and then laminated to a second surface while still hot. Temperatures are usually in the range of 121–177°C.
Solid contents of latex adhesives are in the 40–50% range compared to about 20–30% for solvent-based adhesives. The main disadvantage of latex adhesives is the longer drying time required before tack or strength develops. On the other hand, latex adhesives have good brushability and usually require less pressure to pump or spray than solvent-based adhesives. Prior to drying, they can be cleaned up with water.