Durability of Adhesive Bonds
Long-term performance of bonded joints cannot be predicted from the properties of the adhesive and adherent surface. Complex interfacial and interface chemistry requires the testing of bonded structures. There are close to 50 bond-strength test methods accepted by ASTM International for development of data on adhesive joint properties. Typically tensile, lap-shear, and peel are the main adhesive bond configurations that are used for testing.
Keywords
Epoxies; silicones; polybenzimidazole; water immersion; salt spray
11.1 Introduction
Long-term performance of bonded joints cannot be predicted from the properties of the adhesive and adherent surfaces. Complex interfacial and interface chemistry requires the testing of bonded structures. There are close to 50 bond-strength test methods accepted by ASTM International for development of data on adhesive joint properties. Typically tensile, lap-shear, and peel are the main adhesive bond configurations that are used for testing.
Adhesive bonds must withstand the mechanical forces acting on them but must also resist the service environment. Adhesive strength is affected by many common environments, including temperature, moisture, chemical fluids, and outdoor weathering [1].
In applications where possible degrading elements are present, candidate adhesives should be tested under simulated service conditions. Common ASTM environmental test methods often reported in the literature include the following:
| ASTM D896 | ASTM D2295 |
| ASTM D1151 | ASTM D2557 |
| ASTM D1828 | ASTM D4299 (withdrawn 1990) |
| ASTM D1879 | ASTM D4300 |
The service environments to which adhesive-bonded assemblies are exposed vary from highly protected sealed systems to exterior unprotected systems. The latter may include semiarid sites, such as New Mexico or Arizona, and highly corrosive tropical jungle or marine locations. Durability must always be linked with the service environment. The durability of an adhesive bond in relation to extreme environmental conditions is very much dependent on the surface treatment of the adherends. Another factor that must be considered is the durability of bonds under stress. This is of particular concern in primary structures in aircraft applications. Most other types of bonds are not subject to high loads for prolonged periods. In laboratory ambient storage, no degradation was found in the strength of epoxy/aluminum bonds involving storage for periods as long as 11 years, or in elastomeric adhesive sealant bonds to aluminum and cold-rolled steel stored for up to 5 years [1].
Applied stress will, however, cause an adhesive bond to degrade at a faster rate than an unstressed bond, although stress-relief primers, such as vinyl-phenolic, or stress-relief adhesives, such as nitrile-modified phenolics, will minimize bond degradation due to stress [2].
The ranking of adhesives on the basis of durability is very much dependent on the type of exposure conditions employed. Table 11.1 illustrates a comparison of five common adhesives with regard to (1) typical laboratory accelerated environments and (2) typical weathering environments. Artificial aging (accelerated testing) is considered by some workers to enable ranking of adhesives with respect to their resistance to penetration of water into the adhesive, with corresponding influence on the cohesive strength of the adhesive. The normal outdoor weathering tests, however, rank adhesives with respect to their resistance to penetration of corrosion of the metal along the interface [3].
Table 11.1
Environmental Failure Resistance of Different Adhesive–Aluminum Joints [3]
| Adhesive Type | Laboratory Exposurea (Picatinny Arsenal Tests) | Outdoor Exposureb (Hockney, UK) |
| Nitrile-phenolic (with primer) | Excellent | Excellent |
| Epoxy-phenolic | Good | Excellent |
| Epoxy-polyamide | Good | Very poor |
| Vinyl-phenolic | – | Good |
| Modified epoxy paste | Average | Good |
aChromic acid solution or paste-treated. Lap-shear 1-year storage at 66°C and MIL-STD-304 conditioning. MIL-STD-304 conditioning consists of 30-day cycles under the following conditions: −54°C, 71°C dry heat, 71°C and 95% RH-heat and humidity.
bChromic acid-etched Al adherends. Lap-shear, 90° peel, and honeycomb joint geometries. Two-year exposure in temperate, hot-dry, and hot-wet regions.
Wangsness [4] has made the following general statements about durability of adhesive systems:
• Heat-curing systems possess greater durability than systems that cure at room temperature.
• Surface preparation is a key factor, and it is important in making comparisons between adhesive systems to use the same surface preparation techniques.
• All systems do have an endurance limit. The systems that cure at room temperature have a low endurance limit, 0.7 MPa.
• The chemistry of adhesive systems does have an effect on durability, i.e., highly cross-linked systems such as aromatic amine-cured systems and phenolic systems generally possess superior durability.
• Materials such as nylon that show hydrogen bonding tend to have lower durability.
• Materials such as vinyl polymers can break down to form HCl, which is detrimental to durability.
• The use of chromate-pigmented primer systems greatly enhances durability.
Because of the large number of factors that influence the durability of adhesive-bonded joints, a durability test should be conducted on all systems before they are selected for any particular application. This test should include the adherends, surface preparation, adhesive, and cure parameters needed for each application [4].
11.2 High Temperature
All polymers are degraded to some extent by exposure to high temperatures. Physical properties are lowered after exposure to testing at high temperatures, but they also degrade during thermal aging. Some polymeric adhesives are capable of withstanding temperatures up to 260–316°C continuously. These adhesives are more costly and require facilities for carrying out long, high-temperature cures [1].
For an adhesive to withstand high-temperature exposure, it must have a high melting point or softening point and must be resistant to oxidation. Thermoplastic adhesives may provide excellent bonds at room temperature. As the service temperature approaches the glass-transition temperature of the adhesive, plastic flow results in deformation of the bond and degradation in cohesive strength. Thermosetting adhesives have no melting point and consist of highly cross-linked networks of macromolecules. Many of these materials are suitable for high-temperature applications. The critical factor in thermosets is the rate of strength reduction due to thermal oxidation and pyrolysis [1].
Adhesives that are resistant to high temperatures usually have rigid polymeric structures, high softening temperatures, and stable chemical groups. These same factors make the adhesives very difficult to process. Few thermosetting adhesives can withstand long-term service temperatures above 177°C.
Tape and film adhesives provide high-temperature properties different from those of paste adhesives. The distinguishing feature of tape and film adhesives is that they contain a high proportion of a high-molecular-weight polymer. On the other hand, typical 100% solid paste adhesives or liquid adhesives must, to remain fluid and usable, contain only low-molecular-weight resins. Tape and film adhesives frequently contain polymers with a degree of polymerization of 600 or more and molecular weights of 20,000 or higher. Network polymers made from these high-molecular-weight linear polymers can be very much tougher and more resilient and can provide more recoverable elongation than the highly branched networks formed by curing the low-molecular-weight resins used in paste adhesives. Figures 5.3 and 5.4 illustrate this point by comparing typical tensile-shear data reported by manufacturers of a variety of adhesive types. The best of the tape and film adhesives have higher peak values and broader service-temperature ranges than the best of the 100% solid types [5].
11.2.1 Epoxies
Epoxy adhesives are generally limited to applications below 121°C. Some epoxy adhesives tolerate short-term service at 260°C and long-term service at 149–260°C. These systems were formulated especially for thermal environments by incorporation of stable epoxy co-reactants or high-temperature-curing agents into the adhesive. One of the most successful epoxy co-reactants is an epoxy-phenolic alloy. The excellent thermal stability of the phenolic resins is coupled with the adhesion properties of epoxies to provide an adhesive capable of 371°C short-term operation and continuous use at 177°C [1].
Anhydride-curing agents give unmodified epoxy adhesives greater thermal stability than do most other epoxy-curing agents. Phthalic anhydride, pyromellitic dianhydride, and chlorendic anhydride allow greater cross-linking and result in short-term heat resistance to 232°C. Long-term thermal endurance, however, is limited to 149°C [1].
Epoxy-based adhesive systems offer the advantages of relatively low cure temperatures, no volatiles formed during cure, low cost, and a variety of formulating and application possibilities. The higher-temperature-resistant adhesives lose these advantages in favor of improved thermal-aging characteristics.
11.2.2 Modified Phenolics
11.2.2.1 Nitrile-Phenolic
Of the common modified phenolic adhesives, the nitrile-phenolic blend has the best resistance to elevated temperatures. Nitrile-phenolics have high shear strengths up to 121–177°C, and their strength retention on aging at these temperatures is very good. These materials are available in solvent solutions and unsupported and supported films.
11.2.2.2 Epoxy-Phenolic
These adhesives are mostly used for the military market and are designed for service between 149°C and 260°C. Epoxy-phenolics, however, do not withstand exposure to 177°C as well as nitrile-phenolics. Since cure at 177°C tends to cause outgassing and foaming, 93°C cure for 24 h is recommended.
11.2.3 Polysulfone
This is a high-temperature, hot-melt thermoplastic that has been used as an adhesive. Polysulfone is capable of adhering to hot metals, and has a high softening point (88°C) and outstanding heat stability. It has a 174°C heat-distortion temperature and a 193°C second-order glass-transition temperature. Flexural modulus is retained fairly constant over a wide temperature range. Polysulfone adhesive is supplied as dry pellets. Metal-to-metal joints exhibit high peel and shear strength. Polysulfone maintains its structural integrity up to 193°C. More than 60% of its room-temperature shear strength, as well as excellent creep resistance, is retained at 149°C. Since this is a hot melt, long cure cycles are not needed. The cycle need only be long enough to introduce sufficient heat for adequate wetting of the substrate by the polysulfone. Polysulfone hot melt has been used successfully with clad aluminum alloy, stainless steel, and cold-rolled steel. Adequate surface preparation is important before bonding. Table 11.2 shows bond strength values from 25−55°C to 204°C on aluminum alloy [6].
Table 11.2
Effect of Temperature Variation on Tensile Lap-Shear Strength of Hot-Melt Polysulfone Adhesive (UDEL P-1700) on 2024-T3 Clad Aluminum Alloy (0.05–0.076 mm) Glue Line [6]
| Test Temperature (°C) | Tensile Lap-Shear Strength (MPa) |
| 25 | 24.1 |
| 82 | 18.6 |
| 149 | 15.2 |
| 177 | 13.4 |
| 204 | 3.6 |
For unprimed aluminum, a temperature of 371°C should be used to permit the polysulfone to flow sufficiently to wet the substrate. With this temperature and a pressure of 0.6 MPa, joints with tensile lap-shear strengths above 20.7 MPa are developed in 5 min. Stainless steel has also been bonded with good results at 371°C. Shear strengths above 27.6 MPa are obtained at this temperature. With both carbon steel and aluminum, a bonding temperature of 260°C can be used with satisfactory results if the metal is first primed with a dilute solution (5–10%) of polysulfone applied by spray or flow-coated, and baked for 10 min at 260°C. The primed metal surfaces can then be bonded by pressing for 1 min at 260°C [6].
11.2.4 Silicones
Silicone adhesives have very good thermal stability, but low strength. Their primary application is in nonstructural uses, such as high-temperature pressure-sensitive tapes. Attempts have been made to incorporate silicones in other adhesives, such as epoxies and phenolics, but long cure times and low strength have limited their use [1]. The maximum service temperature for silicone adhesive/sealants is 260°C for continuous operation and up to 316°C for intermittent exposure, depending on the type used.
11.2.5 Polyaromatics
The polyaromatic resins, polyimide and polybenzimidazole (PBI), offer greater thermal resistance than any other commercially available adhesive. The rigidity of their molecular chains decreases the possibility of chain scission caused by thermally agitated chemical bonds. The aromaticity of the structure provides high bond dissociation energy and acts as an “energy sink” to the thermal environments [1].
11.2.5.1 Polyimides
The strength retention of polyimide adhesives for short exposures to 538°C is slightly better than that of an epoxy-phenolic alloy. The thermal endurance of polyimides at temperatures greater than 260°C, however, is unmatched by other commercially available adhesives. Polyimide adhesives are usually supplied as glass-fabric-reinforced film with a limited shelf life. A cure of 90 min at 260–316°C and 0.10–1.4 MPa pressure is necessary for optimum results. High-boiling volatile constituents are released during cure, resulting in a somewhat porous adhesive layer. Because of the inherent rigidity of polyimides, peel strength is low [1].
11.2.5.2 Polybenzimidazoles
These adhesives offer the best short-term performance of any adhesives at elevated temperatures. PBI adhesives, however, oxidize very rapidly and are not recommended for continuous use at temperatures above 232°C. PBI adhesives require a cure at 316°C. As with polyimide adhesives, release of volatiles during cure contributes to a porous adhesive bond. These adhesives are supplied as very stiff, glass-fabric-reinforced films, and are very expensive. Their applications are limited by a long, high-temperature-curing cycle [1].
11.3 Low and Cryogenic Temperatures
Cryogenic adhesives have been defined as those capable of retaining shear strengths above 6.89 MPa at temperatures varying from room temperature to −253°C (20 K). In space vehicles carrying cryogenic fluids and traveling through outer space and reentering the earth’s atmosphere at speeds greater than Mach 3, adhesives encounter temperatures varying from −253°C to 816°C [7–9].
The major use of adhesives for cryogenic applications is for bonding external insulation for both metallic and nonmetallic substrates. Adhesives are also capable of acting as sealants. Many wing structures utilize adhesive-sealed tanks and pressure-type bulkheads. Room-temperature-vulcanizing (RTV) silicones have been evaluated as sealants and adhesives for cryogenic applications. The adhesive strengths obtained with methyl–phenyl RTV silicones were only one-fourth to one-tenth the values for the better structural bonding applications where low tensile and shear forces are anticipated. The RTV silicones may prove useful where high-temperature extremes up to 316°C are encountered for short periods. The better cryogenic adhesives will not tolerate these levels of temperatures [7].
Many problems associated with bonded joints at cryogenic temperatures are the result of stress concentrations and gradients developed within the bond [10,11]. There are a number of causes of stress concentrations in adhesive joints, and a number of these causes are aggravated by cryogenic temperatures. Some of the principal causes are as follows [7]:
• Difference in thermal coefficients of expansion between adhesive and adherends
• Shrinkage of adhesive in curing
• Trapped gases or volatiles evolved during bonding
• Difference in modulus of elasticity and shear strengths of adhesive and adherends
• Residual stresses in adherends as a result of the release of bonding pressure
At room temperature, a low-modulus adhesive may readily relieve stress concentration by deformation. At cryogenic temperatures, however, the modulus of elasticity may increase to a point where the adhesive can no longer effectively release the concentrated stresses. The modulus of elasticity generally increases with decreasing temperature. More constant properties are usually obtained when attempts are made to match the coefficient of expansion of the adhesive to that of the adherends. The thermal conductivity is important in minimizing transient stresses during cooling. These stresses are reduced to minimal by thinner glue lines and higher thermal conductivities.
Polyurethanes are among the better adhesives for cryogenic applications. Room-temperature-curing polyurethane adhesives in current use provide higher ultimate shear and tensile stress and higher peel and shock properties at −253°C than the earlier polyurethanes. This situation is the inverse of what happens to most structural adhesives. The polyurethane adhesives increase in strength at −253°C, but become weaker at ambient and higher temperatures, as shown in Table 11.3 [7].
Table 11.3
Comparison of “Tough” Cryogenic Adhesive Strengths at Liquid Hydrogen and Ambient Temperatures [12]
| Adhesive Type | Tensile-Shear Strength (MPa) | T-Peel Strength (N/m) | ||
| −253°C | Ambient | −253°C | Ambient | |
| Polyurethanea | 51.7 | 10.3 | 12,260 | 3,502–7,004 |
| Nylon-epoxy filmb | 24.8 | 34.5 | 700 | 18,200 |
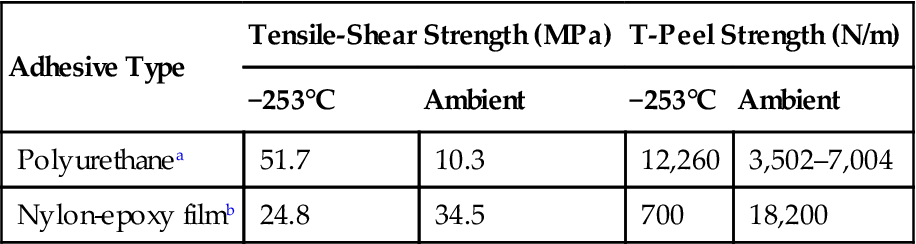
aRoom-temperature-curing paste.
bCure at 149–1,771°C.
The shear-strength properties of several classes of adhesives suitable for cryogenic applications are shown in Figure 11.1. Although bond strengths are reasonably good, the unmodified epoxy resins suffer from brittleness and corresponding low peel and impact strength at cryogenic temperatures. The epoxy-phenolics have excellent cryogenic-temperature strengths, as well as good high-temperature properties. The epoxy-nylons give consistently high strength at cryogenic temperatures. The flexibility of the nylons imparts greater peel strength to the epoxies and produces systems with unusual low-temperature properties. Epoxy-polyamides are readily mixed, easily applied, have good pot life, and can be cured at room temperature to yield a flexible system. Their low-temperature performance, however, is not as good as that of the epoxy-nylons. Vinyl acetal-phenolic adhesives are available as supported and unsupported films, solutions, and solutions with powder, and show reasonably good low-temperature strength. Their strength falls off, however, with decreasing temperature due to the increasing modulus of elasticity characteristic of thermoplastics [13].
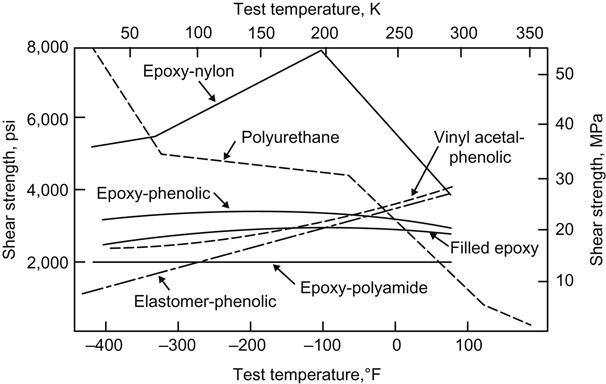
Rubber phenolics (elastomer-phenolics) are of value because of their high peel strengths, but their shear strengths are relatively low. Nitrile-phenolics are examples of this type. Polyurethane adhesives have excellent adhesion to a number of substrates, along with inherently good low-temperature flexibility. Peel strength is excellent at cryogenic temperatures. Epoxy-nylon adhesives mentioned above have higher strength in the low-temperature (−73°C) range, as seen in Figure 11.1, than any other cryogenic adhesive. At liquid-nitrogen temperature (77 K), there is little difference in the shear strengths of the polyurethane and epoxy-nylon types. At liquid hydrogen temperature (20.4 K), however, the same polyurethane adhesives surpass the epoxy-nylons [13].
The National Bureau of Standards at Boulder, Colorado, has prepared an excellent survey for NASA on available reports and publications on cryogenic adhesives and sealants [14].
11.4 Humidity and Water Immersion
Moisture can affect adhesive strength in two significant ways. Some polymeric materials, particularly ester-based polyurethanes, will “revert,” i.e., lose hardness, strength, and in the worst cases, liquefy during exposure to warm, humid air. Water can also permeate the adhesive and preferentially displace the adhesive at the bond interface. This mechanism is the most common cause of adhesive-strength reduction in moist environments [1].
The rate of hydrolytic reversion depends on the chemical structure of the base adhesive, the type and amount of catalyst used, and the flexibility of the adhesive. Certain chemical linkages, such as ester, urethane, amide and urea, can be hydrolyzed. The rate of such attack is fastest for ester-based linkages. Such linkages are present in certain types of polyurethane- and anhydride-cured epoxies. In most cases, amine-cured epoxies provide better hydrolytic stability than anhydride-cured types. The reversion rate of hydrolytic materials is also dependent on the amount of catalyst used in the formulation. The best hydrolytic properties are obtained when the proper stoichiometric ratio of base material to catalyst is used. Reversion is usually much faster in flexible materials because water permeates them more readily [1].
Structural adhesives not susceptible to the reversion phenomenon are also likely to lose strength when exposed to moisture, particularly at high temperatures. The mode of failure in the initial stages of exposure under these conditions is cohesive. After 5–7 days, the failure mode becomes one of adhesion (adhesive failure). Water vapor apparently permeates the adhesive through its exposed edges and concentrates in weak boundary layers at the interface. This effect is very much dependent on the type of adhesive. Nitrile-phenolic adhesives do not fail as a result of the mechanism of preferential displacement at the interface. Failures occur cohesively within the adhesive, even when it is tested after 24 h immersion in water. A nylon-epoxy adhesive degrades rapidly under the same conditions because of permeability and preferential displacement by moisture. Adhesion strength deteriorates more rapidly in an aqueous-vapor environment than in liquid water because of the more rapid permeation of the water vapor. Because of the importance of the interface, primers and surface treatments tend to hinder adhesion strength degradation in moist environments. A fluid primer that easily wets the interface presumably tends to fill in minor discontinuities on the surface. Chemical etching, which removes surface flaws, also improves resistance to high-humidity environments [1].
Environmental effects on adhesive joints are accelerated by stress. Few data are available on this phenomenon, however, because of the time and expense involved with stress-aging tests. It is recognized, however, that the moisture environment significantly decreases the ability of an adhesive to bear prolonged stress [1].
11.4.1 Effects of Surface Preparation on Moisture Exposure
Studies have been conducted [15] on unstressed joint durability after room-temperature water immersion and after 100% RH exposure at 52°C. Table 11.4 gives results on the room-temperature tests in water. Direct comparison of durability for all joints can only be made after 1-year soaking exposure. After 1 year of exposure to this moderately accelerated laboratory weathering, the relative bond retention averages were similar for all but the vapor-degreased surface joints, and ranged between 70% and 80% of initial bond strengths. The vapor-degreased surface joints averaged only 46.2% of their initial bond-strength values. Table 11.5 gives the results after exposure to condensing humidity (100% RH) at 52°C. After 12 months’ exposure, all of the anodized surface joints showed significant retention of bond strength, ranging from a high of 74.9% for the sulfuric acid-anodized surface joints to a low of 54.8% for the phosphoric acid-anodized surface joints. The average bond-strength retention for the acid-etched surface joints in high humidity ranged from 31% for the inhibited alkaline cleaned and chromic sulfuric acid-etched surface joint to 16.1% for the alcohol-phosphoric acid-etched surface joints [15]. Also studied were the effects of stress with the system described above.
Table 11.4
Effects of Surface Treatment on the Durability of 6061-T6 Aluminum Alloy Joints Exposed to Immersion in the Unstressed Condition (Nitrile-Modified Epoxy Paste Adhesive) [15]
| Surface Treatment | Initial Shear Strength (MPa) | Average Percent Retained Bond Strength After Indicated Exposure Time (months) | |||
| 3 | 6 | 12 | 24 | ||
| Vapor degreased | 29.9 | 70.9 | 59.4 | 46.2 | 27.0 |
| Dioxidine 526a (5 min, 25% concentration at RT) | 34.3 | 83.1 | 84.5 | 73.8 | 56.9 |
| Chromic-sulfuric (5 min, 82°C) | 36.8 | 83.9 | 82.6 | 78.2 | 66.2 |
| Chromic acid anodize | 38.0 | 85.6 | 83.6 | 70.9 | NA |
| Phosphoric acid anodize (Boeing procedure, 5–10 V, 20 min) | 44.7 | 89.8 | 80.2 | 74.4 | NA |
| Sulfuric acid anodize (12 asf, 60 min boiling-water seal) | 24.5 | 69.6 | 66.8 | 67.6 | 68.5 |
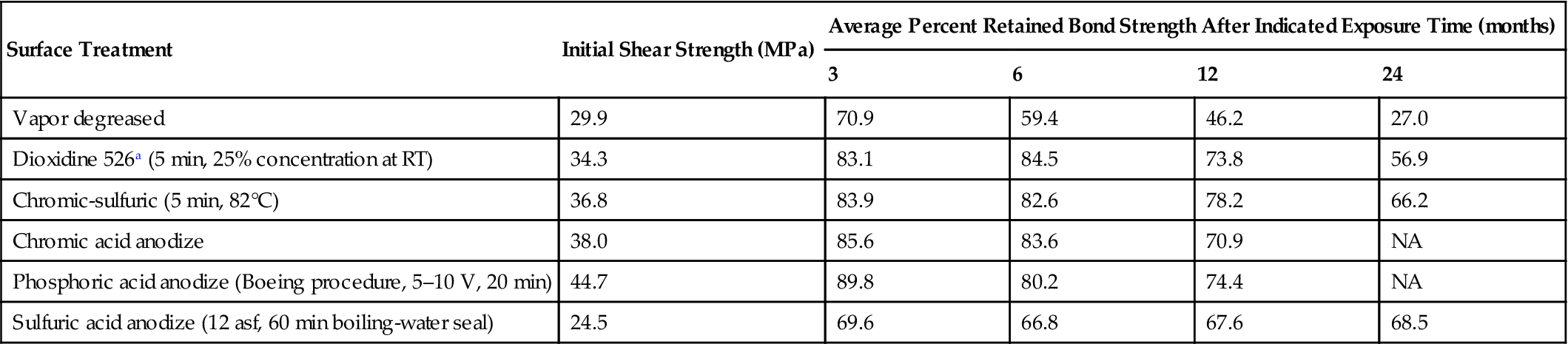
aAmchem Corp.
Table 11.5
Effects of Surface Treatment on the Durability of 6061-T6 Aluminum Alloy Joints Exposed to 100% RH (Condensing Humidity) at 52°C in the Unstressed Condition (Nitrile-Modified Epoxy Paste Adhesive) [15]
| Surface Treatment | Initial Shear Strength (MPa) | Average Percent Retained Bond Strength After Indicated Exposure Time (months) | |||
| 3 | 6 | 12 | 24 | ||
| Dioxidine 526a (5 min, 25% concentration at RT) | 34.3 | 46.1 | 30.2 | 16.1 | 10.9 |
| Chromic sulfuric (5 min, 82°C) | 36.8 | 39.9 | 23.8 | 16.6 | 12.4 |
| Ridoline® 53 (3 min, 82°C) | 37.4 | 51.4 | 36.3 | 31.3 | 26.3 |
| Chromic sulfuric (5 min, 82°C) | 38.0 | 70.7 | 69.8 | 60.9 | NA |
| Phosphoric acid anodize (Boeing procedure, 5–10 V, 20 min) | 44.7 | 70.7 | 62.8 | 54.8 | NA |
| Sulfuric acid anodize (12 asf, 60 min boiling-water seal) | 24.5 | 74.9 | 74.1 | 72.7 | 67.6 |
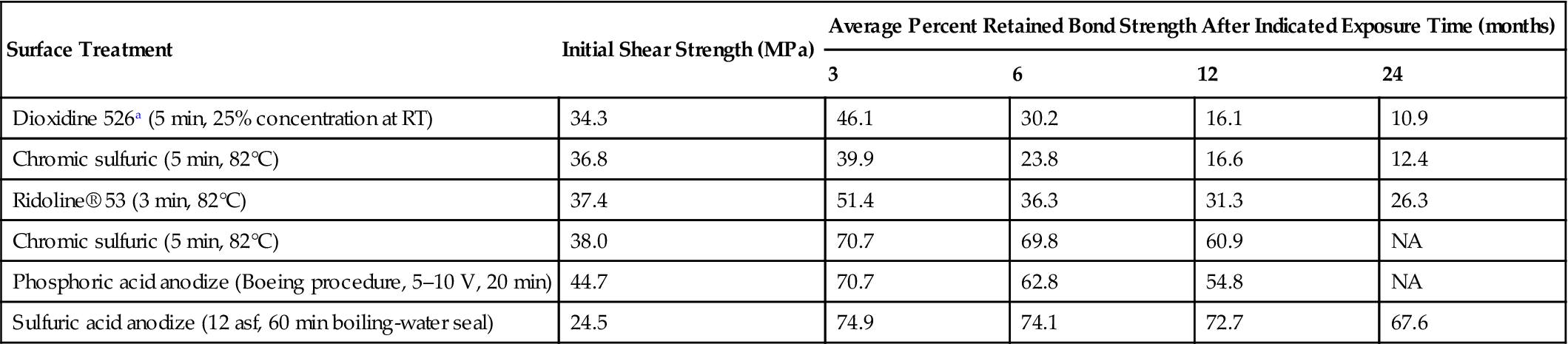
aAmchem Corp.
11.4.2 Stressed Temperature/Humidity Test
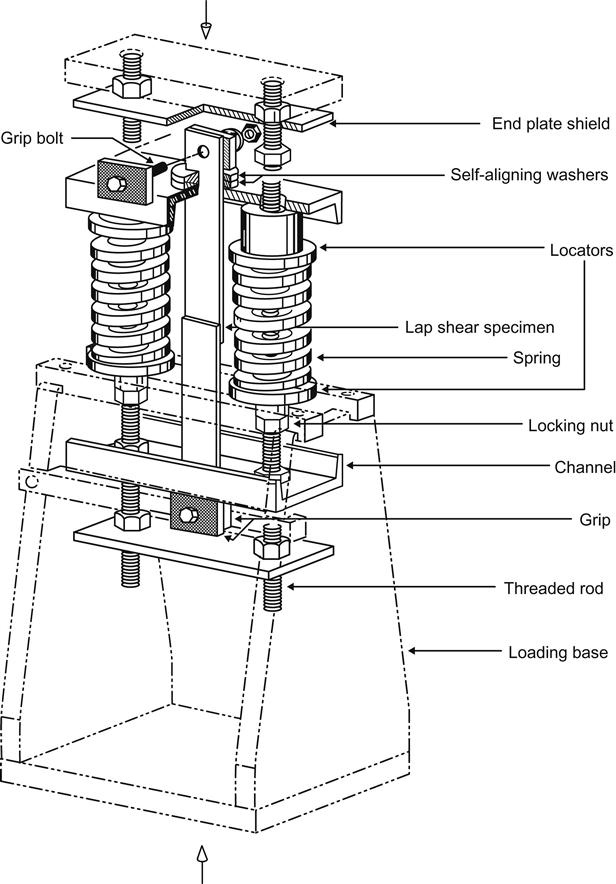
One of the earlier methods used by the US Army for evaluating the durability of adhesive-bonded joints is the stressed temperature/humidity test described in ASTM D2919. Army ARDC workers at Picatinny Arsenal used environmental conditions not listed in the standard test environments of this standard, 60°C and 95–100% RH. These conditions were selected during an evaluation of adhesive-bonding processes used in helicopter manufacture. This stressing method is very time-consuming and often expensive, since it requires the employment of environmental test chambers [16]. Figure 11.2 shows the stressing jig. This jig can be used with multiple units fitted with automatic failure-recording devices.
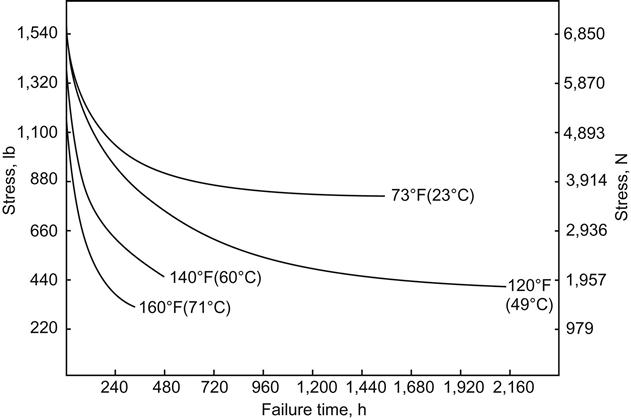
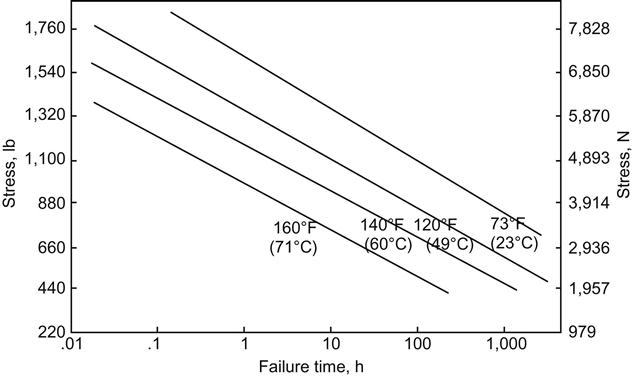
Figures 11.3 and 11.4 show the degradation of the epoxy adhesive bond due to the effect of temperature on the anodic aluminum joint at 95% RH [17]. The plots show that those joints exposed at 23°C and 95% RH degraded rapidly when the stress level exceeded a force of 3914 N, which would be equal to approximately 40% of the room-temperature tensile-shear strength of the joint. At 49°C rapid deterioration occurred at a stress level of 1957 N, or approximately 20% of the room-temperature strength of the joint. The durability or stress versus log time-to-failure curves are shown in Figure 11.4 for the various temperatures at 95% RH. The resultant curves are close to being parallel, indicating that the effect of temperature at a constant RH is constant, and as the temperature increases, the durability decreases as a function of the strength change caused by the change in temperature. These data are for aluminum, but similar results are obtained with titanium. Thus, an increase in % RH will result in a decrease in joint durability and appears to be a significant factor in joint failure [17]. This ASTM method has some drawbacks, such as the large scatter of data and the inability to run very low loads because of the high k-factor of the springs. Springs with lower k-factors could be used, however [18].
11.4.3 Hot-Water-Soak Test
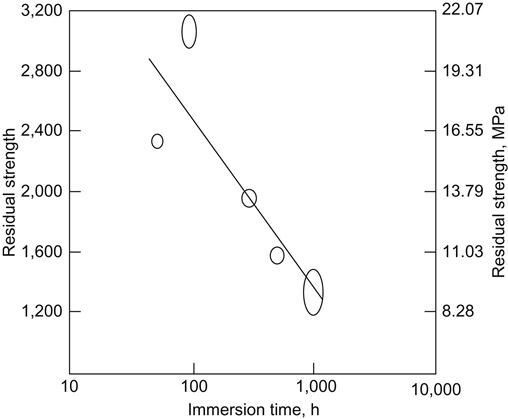
This method involves the soaking of test specimens in a tank of deionized water at 60°C. Specimens are allowed to soak for periods up to 100–1000 h in water at this temperature. At the end of the soak period, the specimens are removed and placed in a container of water also at 60°C. The container is then placed in the test chamber of a universal tensile-testing machine. The chamber is also kept at 60°C. The test specimens are then removed, one at a time, from the water and placed in the grips of the test machine. A thermocouple is attached to the specimen and the temperature monitored. When the temperature of the specimen reaches 60°C, the test is started. The load is applied at a rate of 270–315 N/min until the specimens fail. This test is carried out on at least four specimens and the results are plotted on semilogarithmic paper [14,16]. When the data are plotted as a function of the residual strength versus log of exposure time, the resulting plot is a straight line similar to the stressed durability curves as shown in Figure 11.4 [18].
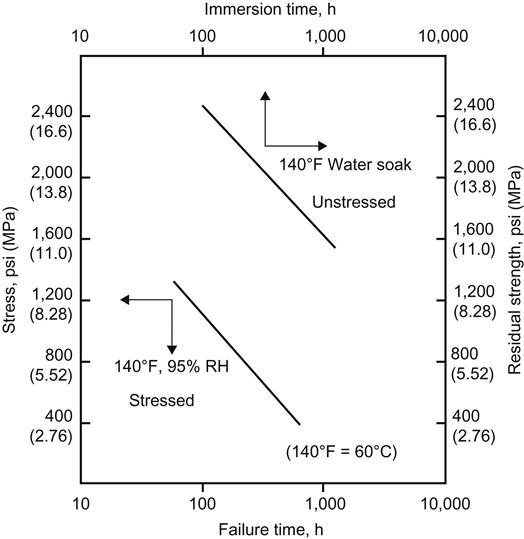
Figure 11.5 shows the data obtained on an epoxy-nitrile film adhesive on 5052-H34 aluminum alloy after immersion in hot water for 50, 100, 300, 500, and 1000 h. This test is very useful because it permits a large number of adhesive-bonded specimens with different adhesives, adherends, and surface pretreatments to be tested at the same time with a relatively small investment in man-hours and equipment. Figure 11.6 shows a comparison of the stressed durability data and unstressed hot-water-soak data on the same epoxy-nitrile film adhesive, using 2024-T3 aluminum alloy. Note the parallelism of the plots. The curve at the lower left was obtained when lap-shear specimens were subjected to various levels of stress and then exposed to an environment of 60°C and 95% RH until failure. The failure time is plotted as a log function. The curve at the upper right is a plot of the data when the same types of lap-shear specimens were subjected to 60°C water for specified periods of time and then tested for their residual strength. In the former case, failure time was recorded. In the latter case, residual strength was determined. The same type of data are obtained from both curves [18].
11.4.4 Fatigue-Life Data
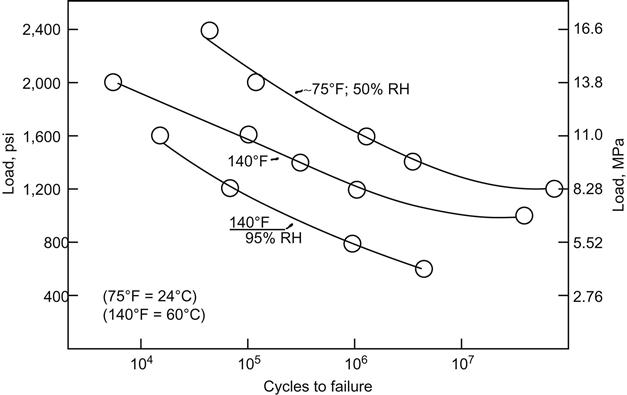
Some bonded joints such as those in helicopters are subjected to constant fatigue, and the operation of a helicopter subjects the aircraft to a constant state of vibration. The operational environment subjects these joints to conditions of elevated temperature and, frequently, high humidity. Environmental exposure is believed to have a strong detrimental effect upon the fatigue endurance of bonded joints in this type of aircraft [19]. A vibration frequency of 1,000 cycles per minute is considered representative of the vibration of aircraft during flight. A military test for the determination of fatigue life has been designed that works in the tension stress cycle from zero to a specific maximum stress of 19.3 MPa. Endurance curves were determined at room temperature, 60°C with 95% RH [19].
Figure 11.7 compares the stress versus log of cycles to failure (S-log N) curves for an epoxy-nitrile film adhesive used for bonding sulfuric-dichromate-etched 2024-T3 aluminum at a frequency of 1,000 cycles per minute at room temperature, 60°C (dry) and at 60°C with 95% RH [15]. In general, the fatigue curves obtained at these environments follow the trend shown in Figures 11.3 and 11.4, where a noticeable drop is experienced when the environment is changed from 23°C to 60°C, and again when the environment becomes more severe with the addition of 95% RH to the 60°C environment [20].
11.5 Saltwater and Salt Spray
Saltwater and especially salt spray are known to have a deleterious effect on adhesive joints. Testing for the effects of salt spray (salt fog) is usually carried out using ASTM B117, “Standard Method of Salt Spray (Fog) Testing.”
Bond durability results on an aluminum alloy are given in Table 11.6 for chromic acid-anodized and four variations of phosphoric acid-anodized surfaces, plus two variations of sulfuric acid-anodized surfaces, i.e., sealed and unsealed. The excellent resistance to surface corrosion in saltwater exposure shown by unbonded anodized surfaces is also shown in the bonded joints. All chromic acid- and phosphoric acid-anodized surface joints were highly resistant to bond failures, which ordinarily occur by undercutting corrosion of the bond line in this environment. The essential absence of such undercutting was noted visually in joints failed deliberately after 12 months’ exposure, and was confirmed by the high percentage of retained bond strength in the range of 79–96%. Although thicker sulfuric acid-anodized coatings would be expected to offer the highest degree of corrosion resistance to salt spray in the sealed and unbonded state, the only bond failure encountered when testing weekly to 50% of initial bond strength was with a sealed sulfuric acid-anodized pretreated joint. This is not surprising, since it is known that an unsealed sulfuric acid-anodized surface can be bonded with higher initial bonding strength and better bond durability. The good bond durability in saltwater exposure of anodized surface pretreated joints is a good reason for selecting this type of pretreatment over the acid-etching procedures for marine applications [15].
Table 11.6
Effect of Surface Treatment and Exposure to 3.5% Saltwater Intermittent Spray on the Durability of 6061-T6 Aluminum Alloy Joints Exposed in the Unstressed Condition (Nitrile-Modified Epoxy Paste Adhesive) [15]
| Surface Treatment | Initial Shear Strength (MPa) | Average Percent Retained Bond Strength After Indicated Exposure Time (months)a | |||
| 3 | 6 | 9 | 12 | ||
| Chromic acid anodize | 38.0 | >50 | >50 | >50 | 82.0 |
| Phosphoric acid anodize (30 V, 30 min) | 39.3 | >50 | >50 | >50 | 89.8 |
| Phosphoric acid anodize (60 V, 18.5 min) | 41.6 | >50 | >50 | >50 | 92.9 |
| Phosphoric acid anodize (110 V, 6 min) | 41.9 | >50 | >50 | >50 | 95.9 |
| Phosphoric acid anodize (Boeing procedure) | 44.7 | >50 | >50 | >50 | 79.3 |
| Sulfuric acid anodize (12 asf, 60 min unsealed) | 27.2 | >50 | >50 | >50 | 75.1 |
| Sulfuric acid anodize (12 asf, 60 min, boil-water seal) | 24.5 | >50 | >50 | >50 | 49.6 |

aIn this procedure, the specimens were stressed weekly to 50% of initial shear strength and then returned to the exposure conditions, provided no bond failure occurred. After 52 weeks of testing, the joints were deliberately failed for quantitative determination of the actual bond strength as shown.
11.5.1 Seacoast Weathering Environment
The results of studies with vapor-degreased, acid-etched, and sulfuric acid-anodized surface joints exposed to a seacoast environment are given in Table 11.7. These tests were carried out for periods as long as 8 years. This type of natural weathering test environment is highly discriminating between the various surface pretreatments. The surprisingly high 62% bond-strength retention for the sealed sulfuric acid-anodized surface joints after 8 years is especially significant when compared to the less-than-1-year survival time for vapor-degreased surface joints and the approximately 700- to 1440-day survival time for alcohol-phosphoric acid and chromic-sulfuric-etched surface joints [11]. Minford [21] showed that the seacoast atmosphere is the most deteriorative to heat-cured epoxies as a group, many failing completely after the end of 4 years’ exposure. Anhydride-cured epoxies give better results and retain about half their initial shear strength after 4 years in this aggressive marine environment. Nitrile-modified epoxies give better results than nonmodified epoxies, as is the case with phenolics and nitrile-phenolics.
Table 11.7
Effect of Surface Treatment and Exposure to Seacoast Environment on the Durability of 6061-T6 Aluminum Alloy Joints Exposed in the Unstressed Condition (Nitrile-Modified Epoxy Paste Adhesives) [15]
| Surface Treatment | Initial Shear Strength (MPa) | Average Percent Retained Bond Strength After Indicated Exposure Time (years)a | |||
| 1 | 2 | 4 | 8 | ||
| Vapor degreased | 29.9 | 0b | – | – | – |
| Deoxidine 526c (5 min, 25% concentration at RT) | 34.3 | 72.4 | 10.9 | 0d | – |
| Chromic sulfuric (5 min, 82°C) | 36.8 | 91.2 | 63.2 | 0e | – |
| Chromic acid anodize | 38.0 | 82.5 | – | – | – |
| Sulfuric acid anodize (12 asf, 60 min boiling-water seal) | 22.6 | 69.4 | 68.2 | 85.4 | 62.3 |
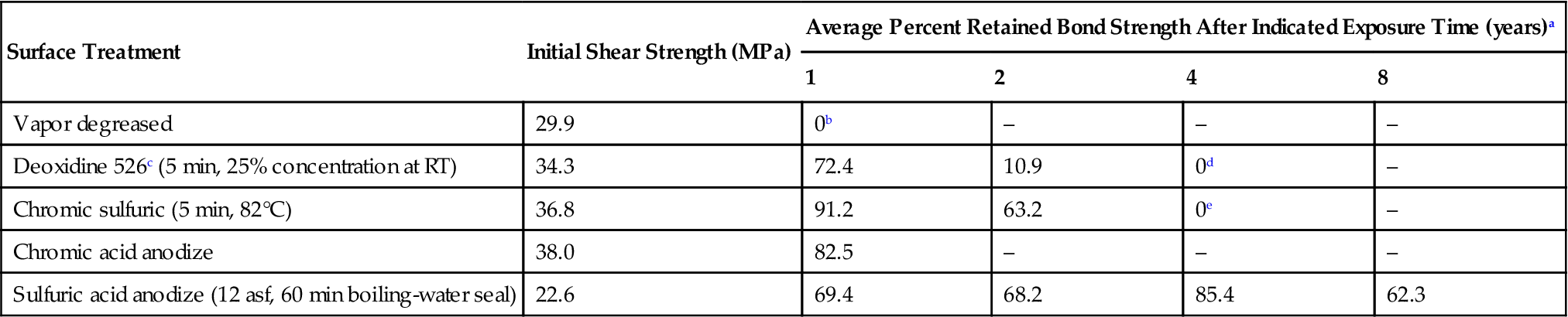
aExposed at Point Judith, Rhode Island.
bTime span of bond failure from 71 to 270 days.
cAmchem Corp.
dTime span of bond failure from 720 to 1,440 days.
eTime span of bond failure from 760 to 1,440 days.
Two-part epoxy adhesives (RT-curing) give poor results in seacoast atmospheres unless a compatible organic sealer is placed over the edge of the bond line. In the case of tape and film adhesives, nylon or nylon-modified epoxy adhesive bonds either failed to survive 4 years’ exposure, or lost 73% of initial strength. Excellent performances were shown by all nitrile-phenolic and phenolic-type adhesives. As a group, all joints fabricated from 10 out of 12 tape and film adhesives tested in a seacoast atmosphere survived for the total test period of 48 months’ exposure. By contrast, no two-part epoxy joints lasted longer than 30 months, and only one heat-cured, one-part-epoxy survived 48 months’ exposure [21].
11.5.2 Saltwater Immersion
Minford also studied the effects of four different phosphoric acid processing conditions under stress and intermittent saltwater immersion testing of 6061-T6 aluminum alloys. None of the joints pretreated by varying phosphoric acid anodizing conditions failed after 480 days’ exposure, even after 10.6 MPa stress. A few of the stressed joints pretreated by chromic acid anodizing failed during the 480 days of exposure, but only at a stress level of approximately 13.8 MPa, or approximately 35% of the initial bond strength. Because of the lower initial bond strength of the sulfuric-acid-acid-anodized surface joints, the highest stress levels imposed were 9.5 MPa (35% of initial bond strength) for the unsealed and 8.6 MPa (35% of initial bond strength) for the sealed joints. After about 100 days, the sealed sulfuric acid-anodized joints failed in exposure, while the corresponding unsealed joints survived after 482 days’ exposure [15].
11.5.2.1 Nitrile-Phenolic Adhesives
Minford has shown the exceptional strength retention of nitrile-phenolics, such as FM-61, on aluminum after extended salt spray, water immersion, and other long-term exposure tests. It is probably true that no other adhesive type exceeds the ability the nitrile-phenolics to maintain good strength on steel or aluminum after extended exposure to water, salts, or other corrosive media, and to prevent undercutting through corrosion of the metal substrate [5].
11.5.3 Boeing/Air Force Studies on Salt-Spray Effects
Studies have been conducted on the effects of corrosive salt-spray environment on bond lines of different bonded systems [22]. The system variations included clad and bare alloys, surface treatments, adhesive primers, and adhesives. Five specimens were fabricated for each of the bonded systems. The specimens were then placed in a salt-spray environment of 5% NaCl at 35°C. The change in wedge-test crack length of each specimen was recorded periodically. At the end of 1 month, one specimen was randomly selected from each bonded system and opened for visual inspection of the bond-line condition, both in the stressed zone (crack-tip zone) and in the unstressed zone. The same procedure was carried out after 2, 3, 6, and 12 months, when the last specimen was removed from test. The conclusions were as follows [22]:
• The phosphoric acid-anodized process provides markedly improved stressed bond joint durability and retards bond-line crevice corrosion (started at an edge) in severely corrosive environments when compared to chromic acid-anodized and FPL etched [22a].
• Stressed-bond joint durability is markedly affected by the adherend prebond surface treatment and the adhesive/primer system in contact with it. This is evidenced by the poor performance of FM 123-L/BR 123 (non-CIAP) adhesive/primer system on FPL-etched and chromic acid-anodized 2024-T3 aluminum alloy, clad and bare, and the superior performance of the same systems when BR 127 (corrosion-inhibiting adhesive primer (CIAP)) is substituted for BR 123 (non-CIAP).
• The wedge-test method is discriminatory and provides a relative ranking for many of the parameters that affect bond-joint durability.
• Clad aluminum in bond lines is undesirable under severely corrosive salt-spray environments.
11.6 Weathering
By far the most detrimental factors influencing adhesives aged outdoors are heat and humidity. Thermal cycling, ultraviolet radiation, and low temperatures are relatively minor factors. When exposed to weather, structural adhesives rapidly lose strength during the first 6 months to 1 year. After 2–3 years, the rate of decline usually levels off at 25–30% of the initial joint strength, depending on the climate zone, adherend, adhesive, and stress level. The following generalizations are important in designing a joint for outdoor service [1]:
• The most severe locations are those with high humidity and warm temperatures.
• Stressed panels deteriorate more rapidly than unstressed.
• Stainless steel panels are more resistant than aluminum panels because of the corrosion in the latter.
• Heat-cured adhesive systems are generally more resistant to severe outdoor weathering than room-temperature-cured systems.
• Using better adhesives, unstressed bonds are relatively resistant to severe outdoor weathering, although all joints will eventually exhibit some strength loss.
11.6.1 Simulated Weathering/Accelerated Testing
Army workers at Picatinny Arsenal carried out a number of experiments in the laboratory (accelerated testing) using MIL-STD-304 conditions, and in actual weathering sites throughout the world [23]. MIL-STD-304 has now been replaced by MIL-STD-331, but the MIL-STD-304 conditions were exposure to alternating cycles of cold (−54°C), dry heat (71°C), and heat and humidity (95% RH) for 30 days. After the exposure period, the aluminum alloy panels used in the studies (2024-T3) were cut into individual specimens and tested at −54°C, 23°C, and 71°C. Eleven types of adhesives were tested. Only one adhesive actually failed. The results are given in Table 11.8. Virtually all the adhesives showed a loss in strength when tested at 71°C, some being more affected than others. One adhesive, #7, the epoxy anhydride room-temperature-cured system, lost approximately 70% of its joint strength at 23°C after cycling. The room-temperature-cured epoxy-polyamide systems (#1) seemed to be the least affected of the adhesive types tested.
Table 11.8
Effect of MIL-STD-304a Conditioning (JAN cycle) on Strength of Bonded Aluminum Alloy 2024-T3 Joints [23]
| Adhesive Type | Test Temperatures (°C) | Average Shear Strength (MIL-STD-304a) (MPa) | |
| Control | Test | ||
| 1. Epoxy-polyamide, room temperature cured | −54 | 11.7 | 15.5 |
| 23 | 12.4 | 15.5 | |
| 71 | 18.6 | 12.4 | |
| 2. Epoxy-polyamide w/mica filler, room temperature cured | −54 | 15.2 | 21.2 |
| 23 | 17.2 | 21.7 | |
| 71 | 15.2 | 7.7 | |
| 3. Resorcinol epoxy-polyamide, room temperature cured | −54 | 17.9 | 16.8 |
| 23 | 24.1 | 21.5 | |
| 71 | 22.8 | 18.8 | |
| 4. Epoxy aromatic amine, room temperature cured | −54 | 11.7 | –b |
| 23 | 13.8 | –b | |
| 71 | 5.0 | –b | |
| 5. Epoxy-polysulfide, room temperature cured | −54 | 12.4 | 13.4 |
| 23 | 13.1 | 11.3 | |
| 71 | 11.7 | 7.4 | |
| 6. Nylon-epoxy, room temperature cured | −54 | 16.6 | 20.0 |
| 23 | 17.9 | 11.9 | |
| 71 | 1.5 | 0.6 | |
| 7. Epoxy-anhydride, room temperature cured | −54 | 16.6 | 13.1 |
| 23 | 20.7 | 6.3 | |
| 71 | 22.8 | 9.2 | |
| 8. Modified epoxy, cured 1 h at 177°C | −54 | 25.5 | 18.6 |
| 23 | 33.8 | 23.4 | |
| 71 | 28.3 | 22.1 | |
| 9. Epoxy-phenolic, cured 45 min at 166°C | −54 | 19.3 | 18.0 |
| 23 | 20.0 | 16.2 | |
| 71 | 20.0 | 15.1 | |
| 10. Nitrile-phenolic, cured 1 h at 177°C | −54 | 32.4 | 36.6 |
| 23 | 31.7 | 26.9 | |
| 71 | 21.2 | 20.0 | |
| 11. Polyurethane, room temperature cured | −54 | 24.1 | 29.0 |
| 23 | 17.9 | 13.6 | |
| 71 | 11.0 | 10.8 | |
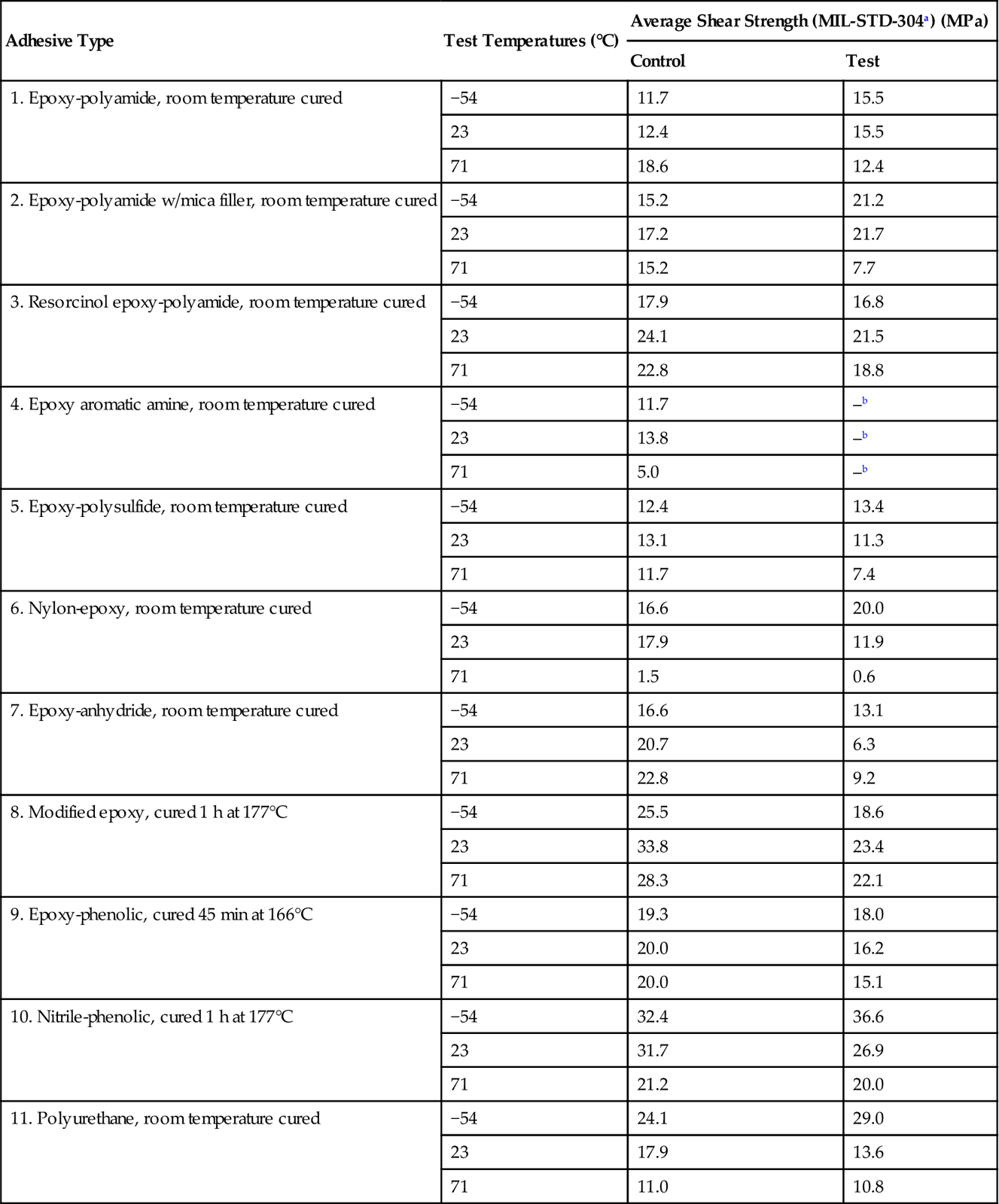
aAlternating cycles of cold (−54°C), dry heat (71°C), and heat and humidity, 71°C (95% RH) for 30 days. MIL-STD-304 has been superseded by MIL-STD-331.
bPanels fell apart.
11.6.2 Outdoor Weathering (Picatinny Arsenal Studies)
Weathering studies after exposures up to 1 year were also made by Picatinny Arsenal on the adhesives covered in Table 11.8, along with several additional adhesives [24]. The results are given in Table 11.9 as percent retention of original joint strength. In addition to controls, the following climates were used:
Table 11.9
Percent Retention of Original Adhesive Joint Strengtha After Weathering 1 Year (2024-T3 Aluminum Alloy) [24]
| Weathering | Adhesive Designation and Type | |||||||||||||
| Epoxy (177° C) | Epoxy (121° C) | Polyamide Epoxy | Epoxy Anhydride | Polyepoxide | Epoxy Powder | Filled Epoxy-Nylon | Epoxy-Resorcinol | Epoxy-Nylon | Epoxy-Phenolic | Nitrile-Phenolic | Silicone (RTV) | Polyurethane | Polyester | |
| Control | 92 | 115 | 115 | 91 | 107 | 82 | 99 | 87 | 100 | 89 | 103 | 200 | 168 | 80 |
| Picatinny | 76 | 84 | 88 | 84 | 76 | 71 | 93 | 80 | 93 | 80 | 90 | 184 | 98 | 95 |
| Panama | 60 | 90 | 90 | 34 | 74 | 104 | 96 | 74 | 98 | 76 | 90 | 208 | 24 | 0 |
| Yuma | 91 | 112 | 137 | 90 | 100 | 117 | 96 | 81 | 102 | 78 | 100 | 300 | 183 | 61 |
| MIL-STD-304 | 58 | 89 | 135 | 32 | 83 | 68 | 105 | 92 | 69 | 72 | 92 | 107 | 135 | 47 |
| Tested at 71°C | ||||||||||||||
| Control | 96 | 124 | 113 | 100 | 139 | 72 | 158 | 98 | 115 | 85 | 98 | 230 | 130 | 138 |
| Picatinny | 88 | 103 | 77 | 71 | 122 | 81 | 82 | 86 | 101 | 82 | 98 | 140 | 57 | 102 |
| Panama | 55 | 83 | 76 | 30 | 90 | 117 | 65 | 65 | 95 | 73 | 88 | 220 | 87 | 0 |
| Yuma | 97 | 127 | 100 | 91 | 174 | 99 | 120 | 89 | 90 | 80 | 103 | 220 | 155 | 162 |
| MIL-STD-304 | 62 | 86 | 84 | 46 | 181 | 69 | 90 | 77 | 22 | 72 | 100 | 170 | 130 | 40 |
| Tested at −54°C | ||||||||||||||
| Control | 95 | 98 | 123 | 83 | 110 | 68 | 112 | 89 | 74 | 94 | 107 | 171 | 102 | 81 |
| Picatinny | 67 | 62 | 119 | 90 | 108 | 68 | 92 | 90 | 78 | 83 | 104 | 172 | 45 | 89 |
| Panama | 67 | 74 | 114 | 85 | 77 | 84 | 107 | 78 | 119 | 78 | 104 | 145 | 27 | 0 |
| Yuma | 67 | 90 | 130 | 98 | 140 | 71 | 97 | 86 | 85 | 85 | 93 | 172 | 64 | 109 |
| MIL-STD-304 | 64 | 83 | 143 | 93 | 145 | 73 | 110 | 76 | 115 | 82 | 119 | 109 | 119 | 121 |
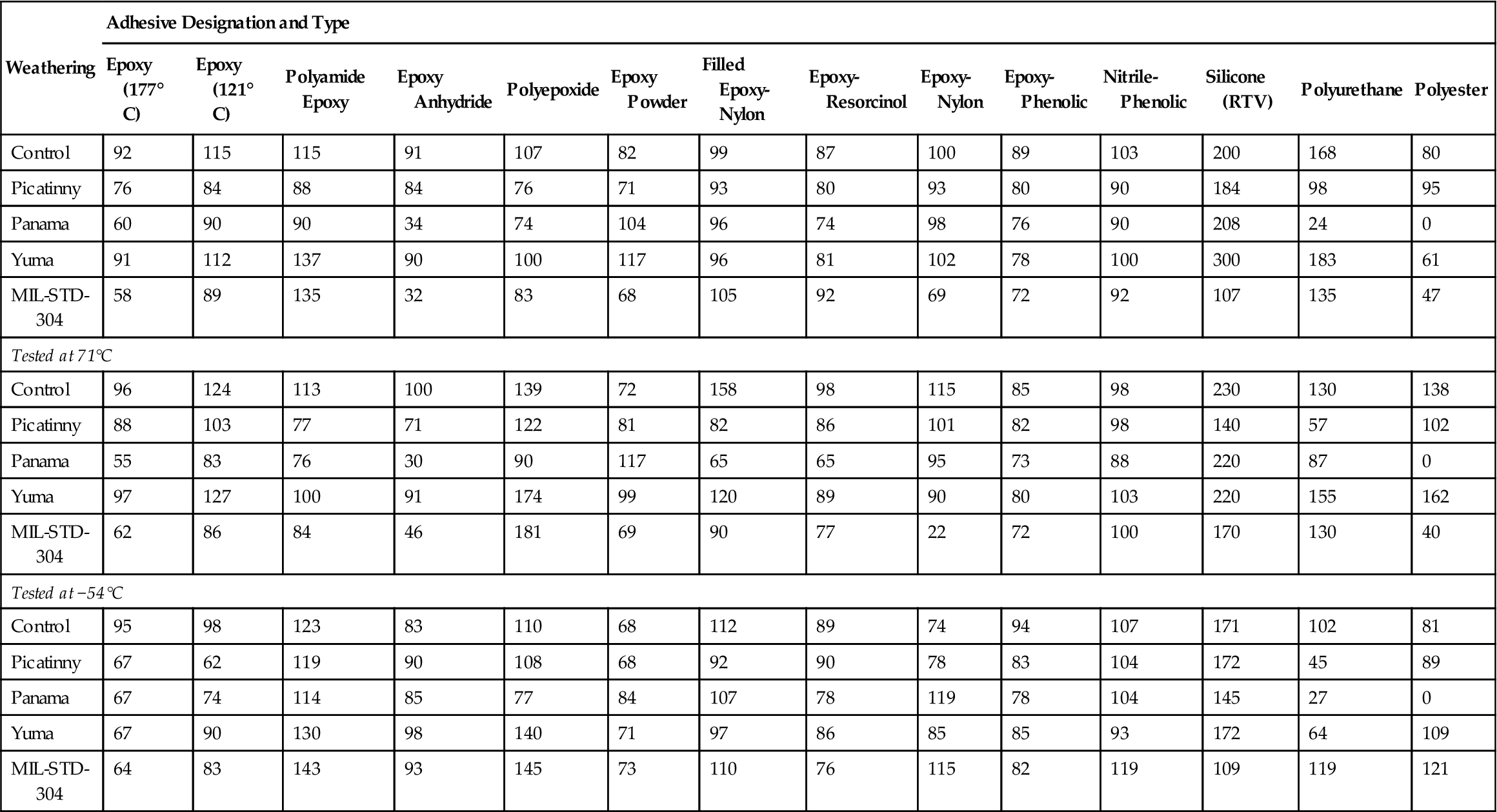
aBased on testing temperature of each.
The results of JAN cycling (MIL-STD-304), as described earlier, were also given. The results in Table 11.10 show that, in general, most of the adhesive joints, when stored in the laboratory (controls), retain most of their original joint strength for 1 year. The joints that were stored in the hot, dry area (Yuma) generally retained most of their original strength. Two adhesives, epoxy-resorcinol and epoxy-phenolic, show a trend toward decreased joint strength. Where climatic conditions subject the bond to humidity and precipitation, i.e., at Picatinny and in Panama, most of the joints show a decrease in joint strength. Four adhesive joints, those with filled epoxy-nylon, unfilled epoxy-nylon, nitrile-phenolic, and silicone respectively, do not appear to be affected to any large extent by weathering, regardless of the site and climatic conditions. The MIL-STD-304 temperature and humidity cycle (now MIL-STD-331) appears to be useful in predicting the changes that occur in panels exposed to high humidity [24].
Table 11.10
Percent Retention of Original Adhesive Joint Strength After 3 Years of Weathering (2024-T3 Aluminum Alloy) [25]
| Weathering | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Tested at 23°C | |||||||||||||||||
| Control | 79 | 94 | 108 | 102 | 109 | 100 | 102 | ND | 92 | 93 | 36 | 110 | 81 | 103 | 250 | 177 | 159 |
| Picatinny | 70 | 68 | 77 | 78 | 58 | 35 | 64 | 63 | 54 | 58 | 65 | 110 | 67 | 95 | 102 | 24 | 22 |
| Panama | 22 | 0 | 53 | 39 | 0 | 0 | 0 | 87 | 90 | 64 | 100 | 0 | 68 | 127 | 250 | 165 | 0 |
| Yuma | 90 | 80 | 103 | 91 | 94 | 103 | 102 | 90 | 95 | 88 | 85 | 77 | 77 | 147 | 183 | 70 | 74 |
| MIL-STD-304 | |||||||||||||||||
| Control | 96 | 90 | 128 | 115 | 105 | 96 | 105 | 80 | 85 | 104 | 103 | – | 89 | 108 | 208 | 180 | 93 |
| Cycled | 58 | 51 | 89 | 135 | 32 | 83 | 0 | 68 | 105 | 92 | 69 | – | 72 | 92 | 167 | 135 | 47 |
| Tested at 71°C | |||||||||||||||||
| Control | 84 | 100 | 107 | 83 | 100 | 256 | 274 | ND | 142 | 94 | 108 | 84 | 80 | 106 | 276 | 195 | 147 |
| Picatinny | 75 | 75 | 89 | 65 | 65 | 95 | 100 | 61 | 70 | 51 | 97 | 109 | 76 | 107 | 115 | 30 | 70 |
| Panama | 39 | 0 | 46 | 43 | 0 | 0 | 0 | 84 | 54 | 59 | 94 | 0 | 70 | 88 | 204 | 71 | 0 |
| Yuma | 103 | 93 | 105 | 85 | 87 | 324 | 154 | 83 | 71 | 84 | 129 | 120 | 85 | 108 | 200 | 88 | 80 |
| MIL-STD-304 | |||||||||||||||||
| Control | 93 | 94 | 129 | 125 | 114 | 289 | 89 | 95 | 176 | 93 | 56 | – | 95 | 107 | 250 | 133 | 140 |
| Cycled | 62 | 56 | 86 | 84 | 46 | 181 | 0 | 69 | 90 | 77 | 22 | – | 72 | 100 | 170 | 130 | 40 |
| Tested at −54°C | |||||||||||||||||
| Control | 79 | 110 | 108 | 118 | 97 | 139 | 97 | ND | 102 | 74 | 81 | 154 | 81 | 128 | 153 | 121 | 92 |
| Picatinny | 73 | 78 | 76 | 111 | 59 | 52 | 59 | 58 | 71 | 64 | 94 | 83 | 68 | 111 | 31 | 44 | 66 |
| Panama | 22 | 0 | 72 | 75 | 0 | 0 | 0 | 69 | 113 | 73 | 98 | 0 | 63 | 121 | 136 | 47 | 0 |
| Yuma | 76 | 73 | 72 | 130 | 111 | 150 | 76 | 70 | 98 | 84 | 95 | 98 | 88 | 137 | 115 | 47 | 86 |
| MIL-STD-304 | |||||||||||||||||
| Control | 97 | 88 | 109 | 115 | 118 | 122 | 119 | 73 | 85 | 81 | 91 | – | 88 | 106 | 208 | 99 | 85 |
| Cycled | 64 | 63 | 83 | 143 | 93 | 145 | 0 | 73 | 119 | 76 | 115 | – | 82 | 119 | 109 | 119 | 120 |
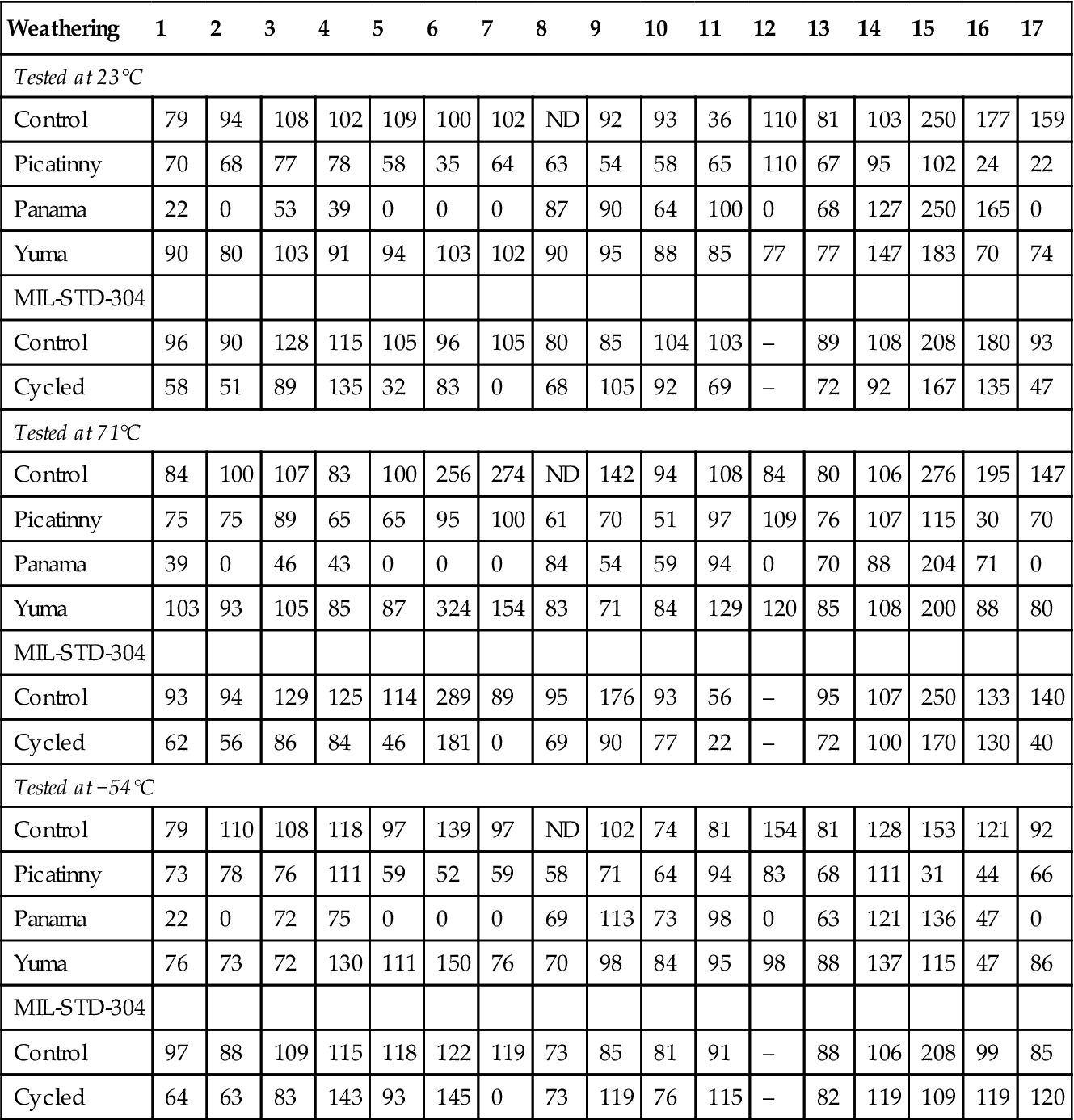
Picatinny Arsenal workers also carried out a 3-year weathering program on aluminum joints, using 17 different adhesives [25]. Of the 17 adhesives, 13 were epoxies or modified epoxies, as epoxy types are most widely used in adhesive-bonding applications. In the study, only 5 of the original 17 adhesives retained a minimum of 50% of their original joint strength and approximately 13.8 MPa at all test temperatures after 2 years of weathering, regardless of the test site. After 3 years, only two adhesives, the phenolic-epoxy film (#13) and the nitrile-phenolic film (#14) met these requirements. Table 11.11 gives the percentage off retention of the original adhesive joint strength for the 17 adhesives after 3 years of weathering at the three test sites and after MIL-STD-304 cycling. It is apparent that the joints stored in the laboratory (controls) retained most of their original strength for 3 years. So did most of the joints weathered at Yuma, Arizona, where humidity and moisture were prevalent, as at Picatinny and Panama, most of the joints showed a decrease in joint strength.
Table 11.11
| Column Heading | Polymer Type | Trade Name | Manufacturer | Cure Temperature (°C) |
| 1 | Epoxy paste, Al-filleda | EC-2086 | 3M Corp. | RT |
| 2 | Epoxy paste, filled | EC-2186 | 3M Corp. | 177 |
| 3 | Epoxy paste, filleda | EC-2214 | 3M Corp. | 177 |
| 4 | Polyamide epoxy | Epon-828/V-140 | Shell Corp. | 121 |
| 5 | Epoxy anhydride | Epon-31-59 | Shell Corp. | RT |
| 6 | Polysulfide epoxy | C-14 | Morton International | RT |
| 7 | Modified epoxy | Epon-913 | Shell Corp. | RT |
| 8 | Epoxy powder | Epon-917 | Shell Corp. | RT |
| 9 | Nylon-epoxy/polyamide | N-159 | 149 | |
| 10 | Resorcinol epoxy/polyamidea | K-159 | RT | |
| 11 | Nylon-epoxy paste | Toresine FS-410 | Teikoku Kagaku Sangyo K.K. | RT |
| 12 | Nylon-epoxy film | FM-1000 | Cytec Engineered Materials | RT |
| 13 | Phenolic-epoxy filma | HT-424 | Bloomingdale Rubber Co | 190 |
| 14 | Nitrile-phenolic filmb | AF-30 | 3M Corp. | 166 |
| 15 | RTV silicone rubber | RTV-102 | General Electric | 177 |
| 16 | Polyether-based polyurethane | PR-1538 | RT | |
| 17 | Styrene-modified polyester | Laminac 4116/4134 | Island Pyrochemical Industries | 82 |
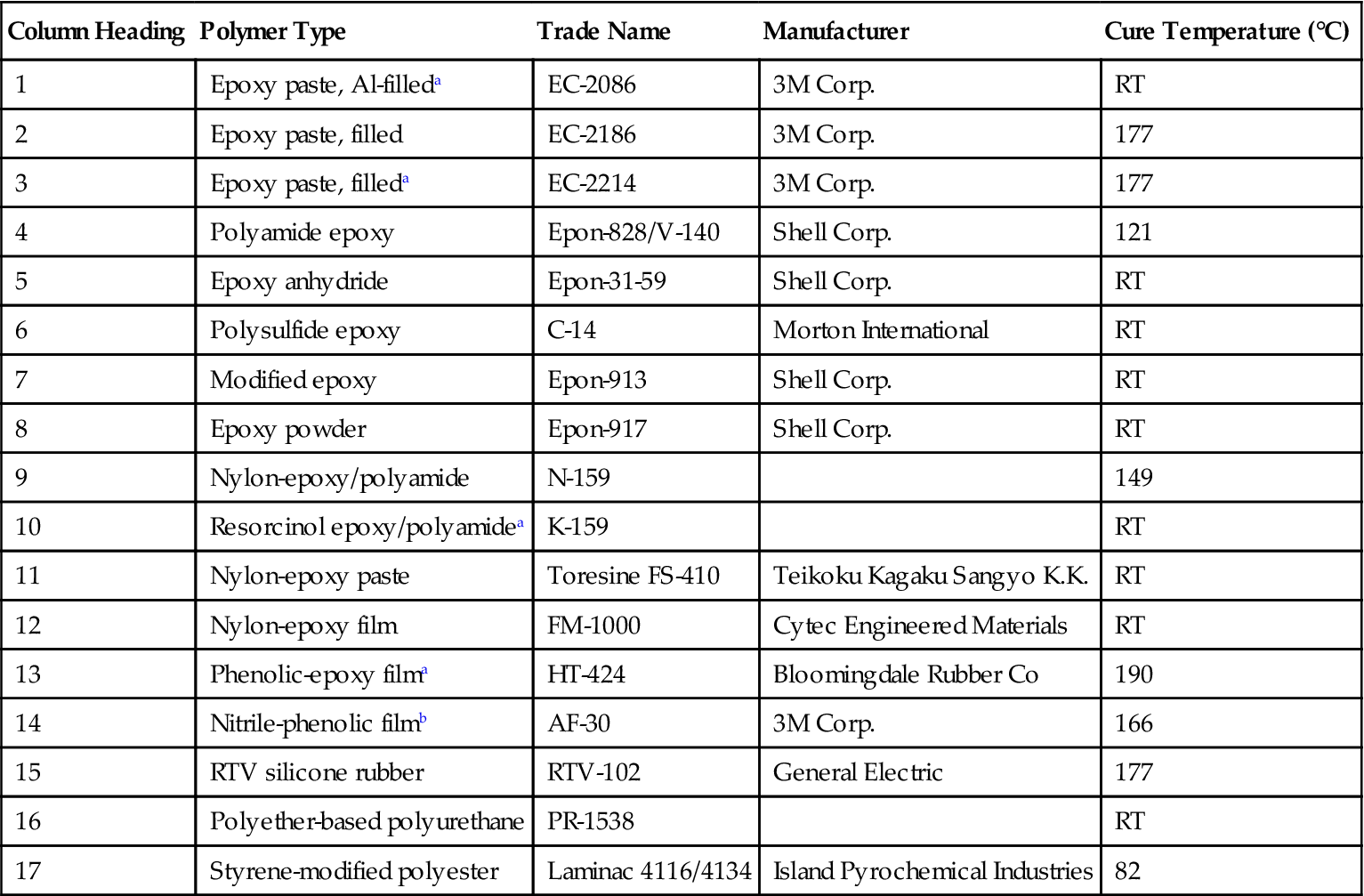
aAdhesive joint retained a minimum of 50% of its original bond strength and approximately 13.8 MPa shear strength at all test temperatures after 2 years of weathering at all test sites.
bAdhesive joint retained a minimum of 50% of its original bond strength and approximately 13.8 MPa shear strength at all test temperatures after 3 years of weathering at all test sites.
The joints made with an aluminum-filled one-component epoxy paste, 177°C curing adhesive (#1), showed very good durability at Yuma. However, these joints showed a marked decrease in joint strength in the high humidity of Panama. At the end of 3 years, there was some evidence of corrosion of the aluminum beneath the bond line. The joints made with another filled, one-component modified epoxy paste, 177°C curing adhesive (#2), showed good retention of joint strength during the 3-year exposure at all sites other than Panama. After 2 years, the Panama joints had lost more than 50% of their original strength due to corrosion of the aluminum. After 3 years, they were so badly corroded that they fell apart in the racks. In general, the joints formed with a 121°C curing, one-component epoxy-paste adhesive (#3) showed a good retention of the original bond strength. However, here again, the higher-humidity sites appeared to have an adverse effect after 3 years. This effect was also noted on the joints made with the polyamide-modified epoxy (#4).
The joints with the two-part epoxy anhydride adhesive (#5), the two-part aliphatic amine-cured polysulfide-modified epoxy (#6), and the two-part mixed amine-cured, filled epoxy (#7), fell apart at the Panama site. Numbers 5 and 6 both fell apart during the second year of the program. The #6 joints fell apart during the third year and also showed a sharp decrease in strength after 3 years at the Picatinny site. Joints #8, #9, #10, and #11 retained better than 50% of their original joint strength after 3 years at all test temperatures and sites.
The joints made using the epoxy-nylon film adhesive (#12) fell apart on the rack at Panama due to crevice and exfoliation corrosion. The epoxy-phenolic film adhesive (#13) and the nitrile-phenolic film adhesive (#14) retained better than 50% of their original joint strength with average joint strengths of approximately 13.8 MPa under all test conditions after exposure to environments of all the test sites. The joints made with the RTV silicone rubber (#15) showed a general increase in bond strength in the early stages of exposure, probably due to further cure. These joints also demonstrated a general retention of the initial bond strength throughout the 3-year period. The joints made with polyurethane (#16) showed signs of degradation at all the outdoor sites. Those made with the polystyrene-modified unsaturated polyester (#17) fell apart in Panama after 2 years exposure. The panels at the other sites generally retained a fair percentage of their original strength [25].
In summary, the joints that retained better than 50% of their original bond strengths at all test temperatures after exposure to 3 years at any of the test sites were #8, #10, #13, and #15. Of these, only #13 and #14 retained approximately 13.8 MPa shear strength at all test temperatures after 3 years at any of the test sites. Five of the joints retained better than 50% of their original bond strength and approximately 13.8 MPa shear strength at all test temperatures after exposure to MIL-STD-304 cycling. These were #1, #2, #3, #13, and #15. Subjection of bonded panels to the temperature and humidity aging of MIL-STD-304 does, in general, tend to show up those adhesive systems which will not form joints that will provide satisfactory performance in highly humid atmospheres [25].
11.7 Chemicals and Solvents
Most organic adhesives tent to be susceptible to chemicals and solvents, especially at elevated temperatures. Among the standard test fluids and immersion conditions (other than water, high humidity, and salt spray) are the following:
• 7 days in JP-4 jet engine fuel
• 7 days in anti-icing fluid (isopropyl alcohol)
Unfortunately, exposure tests lasting less than 30 days are not applicable to many service-life requirements. Practically, all adhesives are resistant to these fluids over a short-time period and at room temperature. Some epoxy adhesives even show an increase in strength during aging in fuel or oil [1]. Hysol Division (Dexter Corporation) reported studies on their Aerospace Adhesive EA 929, a fast-curing, one-part thixotropic epoxy paste adhesive. With gasoline at 24°C and gear oil at 121°C, there was a definite increase in 121°C tensile-shear strength in etched 2024-T3 A1 clad cured 20 min at 204°C. This increase tended to level off after 4–6 months’ immersion [26]. This effect may be due to postcuring or plasticizing of the epoxy by the oil [1].
Epoxy adhesives are generally more resistant to a wide variety of liquid environments than are other structural adhesives. However, the resistance to a specific environment is greatly dependent on the type of epoxy-curing agent used. Aromatic amines, such as m-phenylenediamine, are frequently preferred for long-term chemical resistance [1].
Urethane adhesives generally show good resistance to most chemicals, solvents, oils, and greases.
There is no one adhesive that is optimum for all chemical environments. As an example, maximum resistance to bases almost axiomatically means poor resistance to acids. It is relatively easy to find an adhesive that is resistant to one particular chemical environment. Generally, adhesives which are most resistant to high temperature have the best resistance to chemicals and solvents [1].
The temperature of the immersion medium is a significant factor in the aging properties of adhesives. As the temperature increases, more fluid is generally adsorbed by the adhesive and the degradation rate increases. In summary [1]:
• Chemical resistance tests are not uniform in terms of concentration, temperature, time, or property measured.
• Generally, chlorinated solvents and ketones are severe environments.
• High-boiling solvents, such as dimethylformamide, dimethyl sulfoxide, and Skydrol (Monsanto Corp.) are severe environments.
• Acetic acid is a severe environment.
• Amine-curing agents for epoxies are poor in contact with oxidizing acids.
• Anhydride-curing agents are poor in contact with caustics.
ASTM D896, “Standard Test Method for Resistance of Adhesive Bonds to Chemical Reagents” (covers the testing of all types of adhesives for resistance to chemical reagents. The standard chemical reagents are those listed in ASTM D543 and the standard oils and fuels are given in ASTM D471. Additional supplementary reagents, for which the formulations are given, are Hydrocarbon Mixture No. 1, Standard Test Fuel No. 2, and Silicone Fluid (polydimethylsiloxane).
11.8 Vacuum
The ability of an adhesive to withstand long periods of exposure to a vacuum is of primary importance for materials used in space travel. The degree of adhesive evaporation is a function of its vapor pressure at a given temperature. Loss of low-molecular-weight constituents such as plasticizers or diluents could result in hardening and porosity of adhesives or sealants. Most structural adhesives are relatively high-molecular-weight polymers, and for this reason exposure to pressures as low as 10−9 torr (1.33×10−7 Pa) is not harmful. However, high temperatures, nuclear radiation, or other degrading environments may cause the formation of low-molecular-weight fragments which tend to bleed out of the adhesive in vacuum [1].
The space vacuum is one of the more important components of the space environment. Although volatility of materials at high vacuum is certainly important, volatility of the polymer is usually not high enough to be significant. Polymers, including adhesives, will not volatilize as a result of vacuum alone. Incomplete polymerization, often the result of poor manufacturing processes not detected by quality-control systems, frequently results in the presence of residual lower-molecular-weight species, which, in return, are responsible for observed outgassing of polymeric materials. The vacuum is no real problem in itself when the molecular weight of the polymer is reasonably high and the polymer is reasonably high and the polymer is free of low-molecular-weight components. The effect of vacuum on polymers is not one of evaporation or sublimation, but is a degradation caused by the breaking down of the long-chain polymers into smaller, more volatile fragments. Chain length (molecular weight), extent of branching, and cross-linking have a direct effect upon the rate of decomposition. Polymers which show high decomposition rates in vacuum near room temperature are nylon, polysulfides, and neoprene [27].
Douglas Aircraft reported on a study [24] of outgassing of commercially available (in 1966) structural adhesives, sealants, and seal materials at 10−9 torr (1.33×10−7 Pa). Disks of 1-in diameter were punched from 19 adhesives, sealants, and seal materials. The disks were dried in a desiccator over phosphorus pentoxide, weighed on an automatic balance, and placed in a vacuum under the above-mentioned conditions for 7 days. At the conclusion of the exposure period, each specimen was immediately placed in a desiccator and reweighed for determination of any weight change. After this second weighting, the specimens were exposed to the atmosphere for 1 week and again weighed to determine any additional weight change. Table 11.12 gives a few results of this study and indicates that under ambient conditions, a high vacuum does not cause significant weight loss in the materials [28].
Table 11.12
Effect of 10−7 torr (1.33×10−5 Pa) on Commercial Adhesives/Sealants [28]
| Adhesive | Type | Weight Change (%) | Moisture Change (%) | Manufacturer |
| Lefkoweld 109 | Modified epoxy | −0.03 | +0.60 | Kester Corp. |
| EC2216 B/A | Flexibilized epoxy | −0.06 | +0.61 | 3M Corp. |
| Adiprene L-100+MOCA | Polyurethane | +0.01 | +0.38 | SOLVAY |
| PR 1535 | Polyurethane | +0.01 | +0.44 | PRC-DeSoto International |
| EC 1605 | Polysulfide | −0.23 | +0.39 | 3M Corp. |
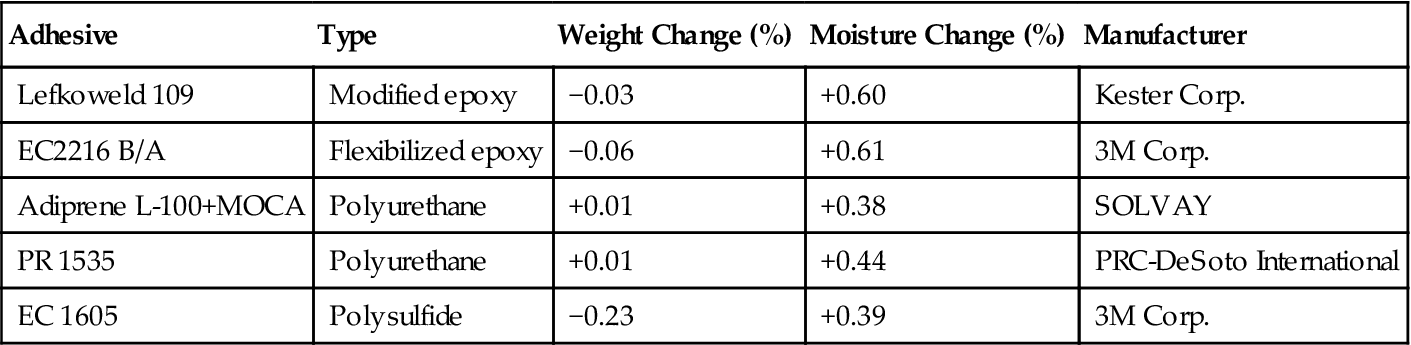
To show the significant effect of temperature on the rate of decomposition and volatilization under vacuum, the same experiment described earlier was conducted at 107°C on the two polyurethane materials. The Adiprene L-100 and MOCA formulation now showed a weight change of −0.75% and the PR 1535 showed a weight change of −1.45%. The 107°C temperature was considerably higher than intended for urethane formulas available at the time of testing [28].
To assure safety and reliability, NASA and other government agencies require all polymeric materials to be qualified and, as a minimum, to pass outgassing tests defined in ASTM-E595. This specification defines two tests: the total mass loss (TML) and the collected volatile condensable materials (CVCMs). The TML is the weight of material lost after exposure for 24 h at 125°C in a vacuum of less than 5×10−5 torr and is specified as 1% or less. The CVCM is the amount of volatiles that condense on a collector plate maintained at 25°C during the same conditions and is required to be 0.1% or less (Table 11.13). Through many years of testing, an extensive databank of materials that pass these requirements is available [29].
Table 11.13
Outgassing Data for Selecting Spacecraft Materials [29]
| Material Information | NASA Outgassing Results (%) | |||
| Adhesive Supplier | Cure Schedule | TML | CVCM | WVR |
| Ablebond® 36-2 (silver-filled epoxy)/Ablestik | 30 min at 150°C | 0.30 | 0.00 | – |
| Ablebond® 71-1 (silver-filled polyimide)/Ablestik | 30 min at 150°C and 30 min at 275°C | 0.17 0.25 |
0.001 0.00 |
0.14 0.17 |
| Ablebond® 84-1 LMI (silver-filled epoxy)/Ablestik | 1 h at 150°C or 2 h at 125°C | 0.12 0.12 |
0.00 0.01 |
0.04 0.09 |
| Ablebond® 84-3 (electrically insulative epoxy)/Ablestik | 1 h at 150°C | 0.23 | 0.00 | 0.16 |
| Ablefilm® 5020K (epoxy film)/Ablestik | 1 h at 150°C | 0.24 | 0.02 | 0.18 |
| Ablefilm® 5025E/Ablestik | 1 h at 150°C or 2 h at 125°C | 0.300.32 | 0.060.08 | 0.08 |
| Ablefilm® 516K/Ablestik | 2 h at 125°C | 0.42 | 0.13 | 0.09 |
| Ablefilm® ECF 550/Ablestik | 30 min at 150°C | 0.49 | 0.10 | 0.18 |
| Epibond® 7275/Vantico | 30 min at 80°C | 1.82 | 0.08 | 0.26 |
| Epo-Tek® H20E (silver-filled epoxy)/Epoxy Technology | 1 h at 150°C | 0.62 | 0.01 | 0.09 |
| Epo-Tek® H35-175MP/Epoxy Technology | 1.5 h at 150°C | 0.30 | 0.02 | 0.18 |
| Epo-Tek® H70 E-4/Epoxy Technology | 12 h at 50°C, 1 h at 80°C, 15 min at 120°C | 1.2 | 0.01 | 0.23 |
| ME 7156/Epoxy Technology | 1.25 h at 175°C | 0.5 | 0.15 | 0.1 |
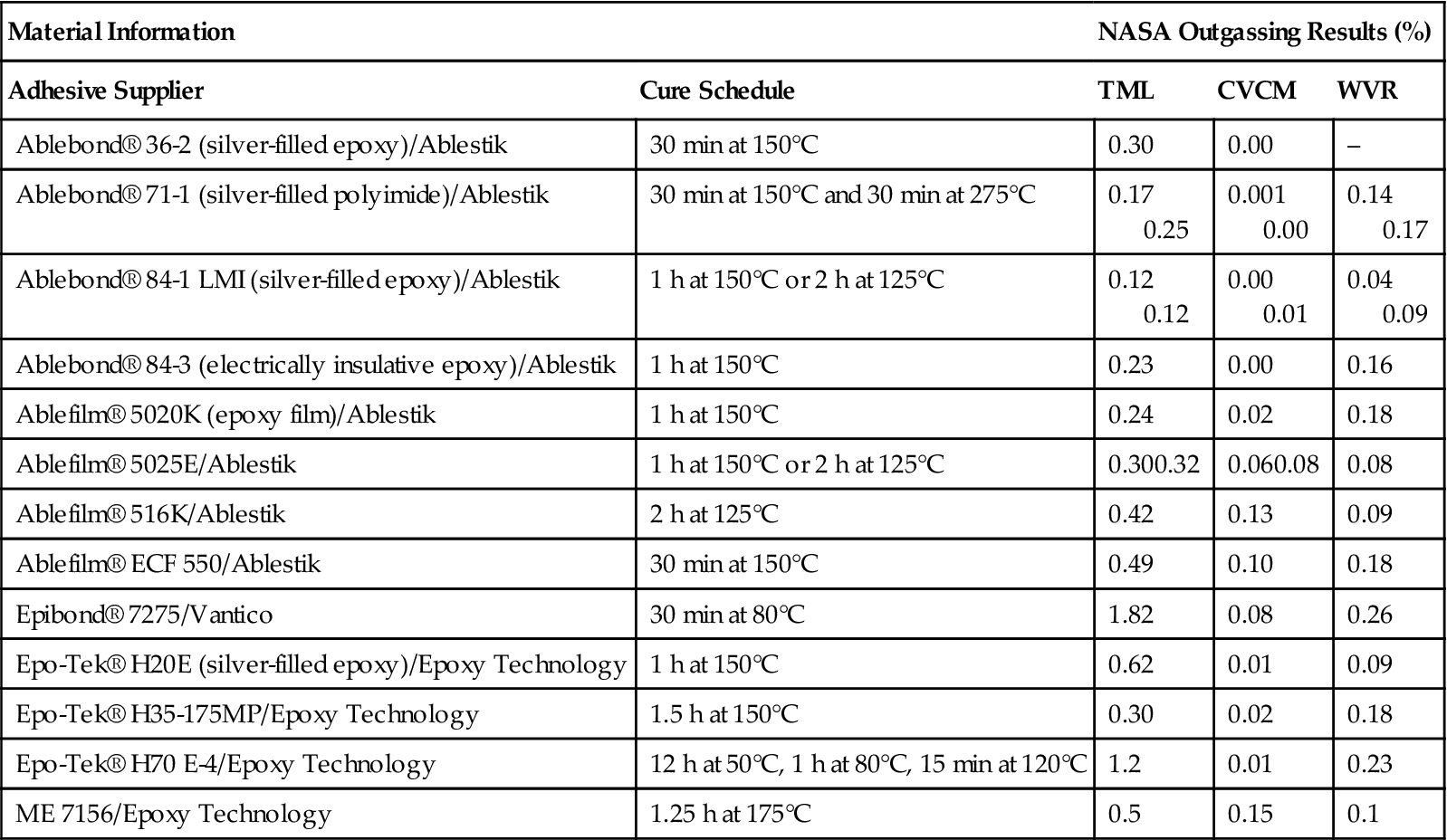
CVCM: collected volatile condensable materials; TML: total mass loss; WVR: weight volatile residue.
Source: Outgassing Data for Selecting Spacecraft Materials, NASA Publication 1124.
11.9 Radiation
High-energy particulate and electromagnetic radiation, including neutron, electron, and gamma radiations, have similar effects on organic adhesives. Radiation causes molecular-chain scission of polymers used in structural adhesives, which results in weakening and embrittlement of the bond. This condition is worsened when the adhesive is simultaneously exposed to elevated temperatures. Figure 11.8 shows the effect of radiation dosage on the tensile-shear strength of structural adhesives. Generally, heat-resistant adhesives have been found to resist radiation better than less thermally stable systems. Fibrous reinforcements, fillers, curing agents, and reactive diluents affect the radiation resistance of adhesive systems. In epoxy-based systems, aromatic-curing agents offer greater radiation resistance than aliphatic types [1,25].
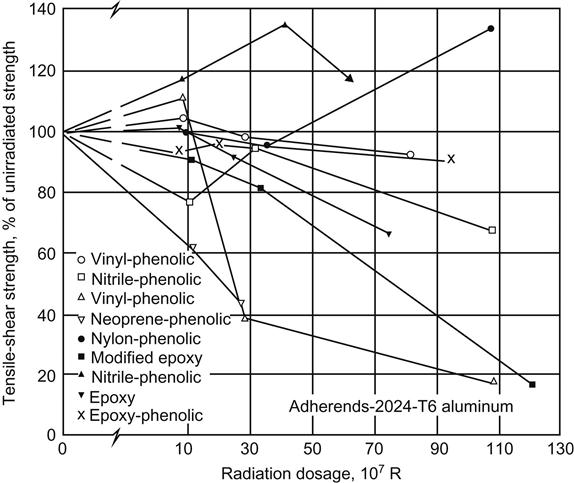
ASTM D1879, “Standard Practice for Exposure of Adhesive Specimens to High-Energy Radiation,” is the test method currently in use. Polyester resins and cured anaerobic products have high radiation resistance based on a radiation spectrum for electrical insulation and materials. Anaerobic resins are classed in a radiation-exposure category with all dose rates based on 100 h and up to 1000 Mrad with a dose rate of 106–107 rad/h, or 1011–1012 neutrons/h. Thread-locking grades of anaerobic adhesives have sustained 2×107 rad without molecular change or loss in locking torque. Anaerobic threaded connections have been exposed to radiation in a reactor for several years with no apparent loss in holding strength [31].
Adhesives generally react to radiation in much the same manner as the plastics or elastomers from which they are derived. Generally, those containing aromatic compounds show good resistance to radiation. Fillers and reinforcing materials improve the radiation stability of these products substantially, while also improving other properties [32].
The following conclusions were reached, in 1979, by Battelle Laboratory investigators on sterilizing radiation effects in polymers that might be used in adhesives [32]:
Polysulfones: These materials can withstand radiation doses greater than 1,000 Mrad without significant effect. (General radiation resistance: excellent.)
Phenol-formaldehyde: These are usually filled or reinforced. The addition of several fillers increases the radiation stability significantly, by as much as 100-fold. Filled resins of this type usually show good radiation resistance up to 500 Mrad or higher. (General radiation resistance: good.)
Epoxies: These materials are above average in radiation resistance of polymers, although this resistance may be varied somewhat, depending on the hardeners used. Resins using aromatic-curing agents generally are more stable than those using aliphatic hardeners. These polymers are stable to radiation doses above 1,000 Mrad. (General radiation resistance: excellent.)
Unsaturated polyesters: These thermoset materials have quite good radiation resistance, especially if they contain mineral fillers or glass fibers. They can be expected to withstand greater than 1,000 Mrads. (General radiation resistance: excellent.)
PIs: These materials are well known for their very high thermal and radiation resistance. They can be expected to withstand radiation doses of about 1,000 Mrad at high temperatures (260°C). (General radiation resistance: excellent.)
Polyurethanes: Properties vary from those of an elastomer to those of hard, rigid cross-linked polymers with mechanical properties showing no reduction after an exposure to 1,000 Mrad. (General radiation resistance: excellent.)
In 1962 a study was carried out on the effects of gamma radiation on the performance of several structural adhesive bonds [28]. The general conclusions were:
• Nitrile-phenolic adhesives are more resistant to radiation damage than epoxy-based adhesives.
• The peel strength of adhesives deteriorates more rapidly than other properties.
• Thick adhesives layers retain useful strength better than thin glue lines. Ten mils (0.01 in or 0.25 mm) is recommended as the minimum glueline thickness where radiation is a factor.
Radiation does not appear to have serious effects on the overlap-shear strength of highly cross-linked adhesives (EC 1469-modified epoxy, AF-31 elastomer-phenolic film, AF-32 elastomer-phenolic film, and EC-1639-modified phenolic). In this study by McCurdy and Rambosek in 1962 [33], the adhesives seemed to benefit slightly from the additional cross-linking caused by the low orders of radiation (to 3,000 Mrad), but eventually began to degrade after 500–600 Mrad. The principal effect noted was embrittlement due to high (800–900 Mrad) amounts of radiation. Very probably there was a loss of cohesive strength due to considerable amount of chain scission, as well as cross-linking. McCurdy and Rambosek also looked at the effects of high temperatures (121°C and 149°C) combined with radiation to 900 Mrad, using the same adhesives listed above, with the exception of the EC-1639-modified phenolic. In these investigations, the high-temperature performance appeared to fall off in a manner parallel to the room-temperature performance. The modified epoxy adhesive maintained its properties up to about 600 Mrad and the modified phenolic was relatively unaffected by very high doses of radiation. The elastomer-phenolic films, as might be expected, were more greatly affected by radiation, since they have a greater flexibility and more sites for cross-linking. These films maintained their performance at room temperature up to 400 Mrad, but fell below the old MIL-A-4090D, type II requirements of 17.2 MPa after about 100 Mrad of radiation [33].
As long as an adhesive maintains its adhesion to the substrate, along with a certain amount of cohesive strength, its performance is relatively unaffected by radiation. The results obtained with a more complex property such as peel strength are given in Table 11.14.
Table 11.14
Effect of Gamma Radiation on T-Peel Strength of Elastomer-Phenolic Film Adhesives [33]
| Adhesive | Radiation Dose (Mrad) | ||||
| 0 N/m | 100 N/m | 300 N/m | 600 N/m | 900 N/m | |
| AF-30 | 8,490 | 5,950 | 2,100 | 876 | 876 |
| FA-31a | 5,250 | 3,500 | 1,400 | 788 | 700 |
| AF-32 | 5,950 | 1,750 | 700 | 525 | 525 |
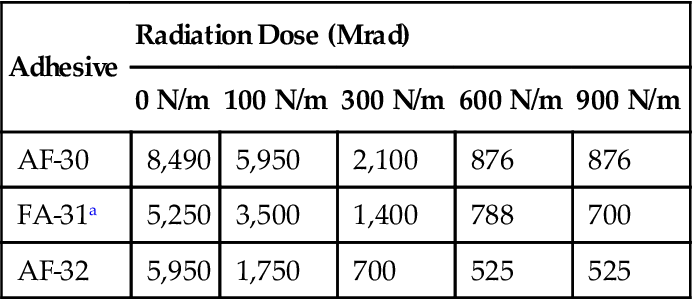
aMost rigid.
An important question is: what is the probable radiation dose during exposure to the space environment? In outer space, unshielded structures may be exposed to as much as 3,500 Mrad/day during a solar storm. Organic structures with minimum shielding are exposed to perhaps 10–15 rad/h. Even at the upper level of 50 rad/h (Van Allen belt), it would take 2,000,000 h (229 years) to reach the 100-Mrad dosage level, and most missions should be completed by that time [33].
Most structural adhesives will perform well when exposed to general radiation encountered in outer space over any reasonable period of time. The rigid metal-to-metal adhesives will perform satisfactorily under fairly high radiation dosages, although it is not recommended that they be exposed directly to the space environment. The other major requirement for radiation resistance of adhesives is for nuclear reactors and related equipment with high radiation flux zones [33].
11.10 Biological Organisms [34,35]
Adhesives in bonded joints may or may not be attacked and degraded by biological organisms (fungi, bacteria, insects, and rodents), depending on how attractive the adhesives are to these organisms. Adhesives based on animal or plant materials (animal and fish glues, starch, dextrins) are much more likely to be affected than synthetic adhesives. Fungi and bacteria are classified as microorganisms, although the former may consist of forms readily visible to the naked eye (mycelia).
Polyurethane resins based on polyether polyols are moderately to highly resistant to fungal attack, while all polyester urethane resins are highly susceptible to such attack. This susceptibility is related to the number of adjacent unbranched methylene groups in the polymer chain. At least two such groups are required for appreciable attack to occur. The presence of side chains on the diol moiety of the polyurethane reduces susceptibility to attack. With the polyethers, attack is dependent on the diol and the diisocyanate used. Adipic acid and diethylene glycol, used in making polyester, are capable of supporting mold growth.
It is not always easy to determine whether deterioration of polyurethane is caused by hydrolytic reversion or by fungal attack. However, a magnifying lens can be used to detect channel-like lesions on the surface of the polyurethane (this is more difficult with adhesives), and below the surface, tubular formations branch out in different directions. This network of tunnels is frequently seen to radiate from a single point. The tunnels contain individual mold hyphae or mold strands of varying thicknesses. Over a long period, and more rapidly when exposed to high temperature, humidity, and light, the damaged material softens increasingly. Eventually, after several months, it becomes a gelatinous mixture of degradation products and fungal hyphae. Of the mold species attacking polyester polyurethanes, Stemphylium is one of the most active. Biodeterioration can be slowed down by the use of hydrolysis inhibitors (stabilizers) and/or certain fungicides, such as 8-hydroxyquinoline.
A report by the Army Natick Research and Development Center describes a program of evaluation of commercial adhesive formulations and bases for microbial susceptibility or resistance. Work will continue on this program on the evaluation of thermoplastic and thermosetting resins and plasticizers used in adhesive formulations [35].
11.11 Test Methods
ASTM Committee D-14 on Adhesives have in the past published five test methods that are applicable to biological attack. Of these methods, four test methods have been withdrawn (without replacement) and are no longer recognized by ASTM. However, the methods can still be applied for comparison purposes.
• ASTM D1382 (withdrawn 1991): Standard Test Method for Susceptibility of Dry Adhesive Films to Attack by Roaches.
• ASTM D1383 (withdrawn 1991): Standard Test Method for Susceptibility of Dry Adhesive Films to Attack by Laboratory Rats.
• ASTM D1877 (withdrawn 1984): Standard Test Method for Permanence of Adhesive-Bonded Joints in Plywood Under Mold Conditions.
• ASTM D4299 (withdrawn 1990): Standard Test Methods for Effects of Bacterial Contamination of Adhesive Preparations and Adhesive Films.
• ASTM D4300: Standard Test Methods for Effects of Mold Contamination on Permanence of Adhesive Preparations and Adhesive Films.
11.12 Adhesive Bond Durability—A Commercial Perspective
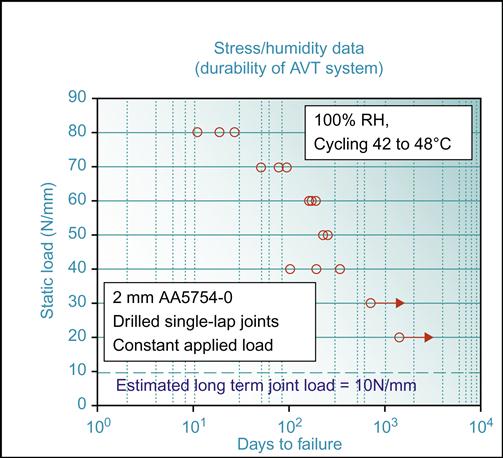
Accelerated test methods are used to estimate the rate at which a bond might be projected to lose its joint strength. The adhesive properties and chemical degradation are most affected by above ambient temperatures and high moisture levels [36]. For example, Alcoa Corp (www.Alcoa.com) has used continuously condensing 100% RH at 52°C, as an accelerated tropical-type laboratory exposure. Rio Tinto Alcan (www.RioTintoAlcan.com) has measured loss of strength of lap and T-peel joints in neutral salt-spray fog at 43°C. The residual strengths of pretreated and etched-only lap-shear joints are shown in Figure 11.9. It is clear that Alcan’s Aluminium Vehicle Technology (AVT) AVT [37] pretreated surfaces have much better strength retention than acid etched material. Clearly the initial joint strengths here give no indication of the durability performance of the surfaces.
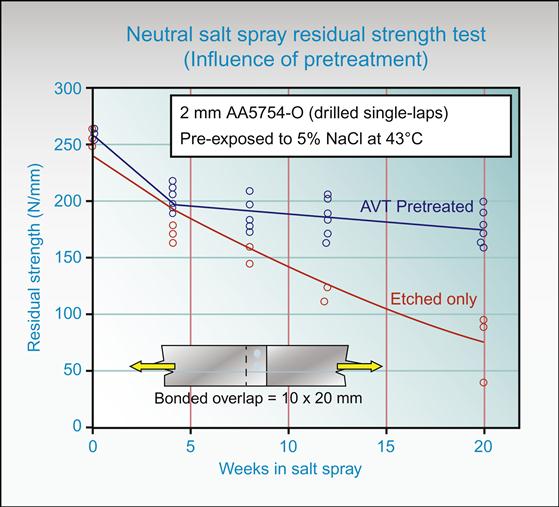
For a structural joint, load and ability to withstand creep under extreme conditions are important. Hence, structural joints are usually exposed to conditions including temperature, moisture, often salt for corrosion, and some form of load.
For example, Ford carries out their “Arizona Proving Ground Equivalent” test where lap joints are subjected to 50oC, 90% RH conditions, with a 15 min immersion in salt solution 5 times a week to add a corrosive step to the cycle [36]. Rio Tinto Alcan has used a similar stress-humidity test (ISO 14615) to discriminate between durability of different adhesives and pretreatment. Here lap joints are exposed under load at 100% RH with temperature cycled hourly between 42°C and 48°C.
Figure 11.10 shows the influence of load on the number of days to failure for joints made with Rio Tinto Alcan’s AVT system. Clearly, load has a major effect on joint life. The results show that it is possible to achieve >3 years’ accelerated exposure life without failure, even with a load which is 2–3 times that normally seen in automotive structures. Although it is difficult to use the results from these tests to predict real life durability, a pretreatment/adhesive system that performs well in accelerated tests provides a good starting point for design and manufacture of bonded joints.