Chapter 1
Introduction
1.1 Introduction
An aerosol is defined as a suspension of liquid or solid in a gas. Aerosols are often discussed as being either ‘desirable’ or ‘undesirable’. The former include those specifically generated for medicinal purposes and those intentionally generated for their useful properties (e.g. nanotechnology, ceramic powders); the latter are often associated with potential harmful effects on human health (e.g. pollution). For centuries, people thought that there were only bad aerosols. Early writers indicated a general connection between lung diseases and aerosol inhalation. In 1700, Bernardo Ramazzini, an Italian physician, described the effect of dust on the respiratory organs, including descriptions of numerous cases of fatal dust diseases (Franco and Franco, 2001).
Aerosols are at the core of environmental problems, such as global warming, photochemical smog, stratospheric ozone depletion and poor air quality. Recognition of the effects of aerosols on climate can be traced back to 44 BC, when an eruption from Mount Etna was linked to cool summers and poor harvests. People have been aware of the occupational health hazard of exposure to aerosols for many centuries. It is only relatively recently that there has been increased awareness of the possible health effects of vehicular pollution, and in particular submicron particles.
The existence of particles in the atmosphere is referred to in the very early literature (see Husar, 2000; Calvo et al., 2012). In the 1800s, geologists studied atmospheric dust in connection with soil formation, and later that century meteorologists recognised the ability of atmospheric particles to influence rain formation, as well as their impact on both visible and thermal radiation (Husar, 2000).
The environmental impact of the long-range transport of atmospheric particles has also been widely discussed (Stohl and Akimoto, 2004). Around 1600, Sir Francis Bacon reported that the Gasgogners of southern France had filed a complaint to the King of England claiming that smoke from seaweed burning had affected the wine flowers and ruined the harvest. During the eighteenth century, forest fires in Russia and Finland resulted in a regional haze over Central Europe. Even then, Wargentin (1767) and Gadolin (1767) (quoted in Husar, 2000) indicated that it would be possible to map the path of the smoke based on the locations of the fires and its appearance at different locations. Danckelman (1884) mentions that hazes and smoke from burnings in the African savannah have been observed in various regions of Europe since Roman times.
The possibility of atmospheric particles forming from gaseous chemical reactions was pointed out by Rafinesque (1819). In his paper entitled ‘Thoughts on Atmospheric Dust’, he makes a number of pertinent observations: ‘Whenever the sun shines in a dark room, its beams display a crowd of lucid dusty molecules of various shapes, which were before invisible as the air in which they swim, but did exist nevertheless. These form the atmospheric dust; existing every where in the lower strata of our atmosphere’; ‘The size of the particles is very unequal, and their shape dissimilar’.
In spite of the widespread occurrence of aerosols in nature and their day-to-day creation in many spheres of human activity, it is only in comparatively recent times that a scientific study has been made of their properties and behaviour. During the late nineteenth and early twentieth centuries, many scientists working in various fields became interested in problems that would now be considered aerosol-related. The results were fairly often either byproducts of basic research, related to other fields or just plain observations that roused curiosity. Several of the great classical physicists and mathematicians were attracted by the peculiar properties of particulate clouds and undertook research on various aspects of aerosol science, which have since become associated with their names, for example Stokes, Aitken and Rayleigh.
Whatever the usage, the fundamental rules governing the behaviour of aerosols remain the same. Rightly or wrongly, the terms ‘aerosols’ and ‘particles’ are often freely interchanged in the literature. Aerosols range in size range from 0.001 µm (0.001 µm =10−9 m = 1 nm = 10 Å) to 100 µm (10−4 m), so the particle sizes span several orders of magnitude, ranging from almost macroscopic down to near molecular sizes. All aerosol properties depend on particle size, some very strongly. The smallest aerosols approach the size of large gas molecules and have many of the same properties; the largest are visible grains that have properties described by Newtonian physics.
Figure 1.1 shows the relative size of an aerosol particle (diameter 0.1 µm) compared with a molecule (diameter 0.3 nm, average spacing 3 nm, mean free path 70 nm (defined as the average distance travelled by a molecule between successive collisions)).
Figure 1.1 Relative size of an aerosol particle (diameter 0.1 µm) compared with a molecule (diameter 0.3 nm).

There are various types of aerosol, which are classified according to physical form and method of generation. The commonly used terms are ‘dust’, ‘fume’, ‘smoke’, ‘fog’ and ‘mist’. Virtually all the major texts on aerosol science contain definitions of the various categories. For example, for Whytlaw-Gray and Patterson (1932):
Dust: ‘Dusts result from natural and mechanical processes of disintegration and dispersion.’
Smoke: ‘If suspended material is the result of combustion or of destructive distillation it is commonly called smoke.’
while more recently, for Kulkarni, Baron and Willeke (2011):
Dust: ‘Solid particles formed by crushing or other mechanical action resulting in physical disintegration of a parent material. These particles have irregular shapes and are larger than about 0.5 µm.’
Smoke: ‘A solid or liquid aerosol, the result of incomplete combustion or condensation of supersaturated vapour. Most smoke particles are submicrometer in size.’
It is clear right from the early literature that dust and smoke are not defined in terms of particle size but in terms of their formation mechanism.
The actual meanings of ‘smoke’ and ‘dust’ have recently been the subject of an appeal at the New South Wales Court of Appeal (East West Airlines Ltd v. Turner, 2010). The New South Wales Dust Diseases Tribunal had previously found in favour of a flight attendant who inhaled smoke in an aircraft. The initial trial judge concluded that ‘In ordinary common parlance, dust encompasses smoke or ash. Dust may need to be distinguished from gas, fume or vapour. The distinction would be that dust comprises particulate matter. Smoke comprises particulate matter and, accordingly, is more comfortably described as dust rather than gas, fume or vapour. I do not consider that there is a distinction between smoke and dust such that smoke cannot be dust. When the particulate matter settled, it would, to most people, be recognised as dust. If, through the microscope or other aid, one could see the particulate matter without the smoky haze, most people would recognise the particulate matter as dust. The dictionary definitions would encompass smoke as dust’.
The Court of Appeal stated:
… His Honour did not find that, as a matter of general principle, ‘smoke’ was a ‘dust’ … This was not a decision as to a point of law but a factual determination. There was ample evidence before his Honour to justify that conclusion.
Various governments worldwide have instigated standards to protect workers from toxic substances in workplaces. For example, the American Conference of Governmental Industrial Hygienists (ACGIH) publishes a list of over 600 chemicals for which ‘threshold limit values’ have been established. Approximately 300 of these are found in workplaces in the form of aerosols. Aerosol science is thus central to the study, characterisation and monitoring of atmospheric environments. Aerosols can cause health problems when deposited on the skin, but generally the most sensitive route of entry into the body is through the respiratory system. Knowledge of the deposition of particulate matter in the human respiratory system is important for dose assessment and the risk analysis of airborne pollutants. The deposition process is controlled by physical characteristics of the inhaled particles and by the physiological factors of the individuals involved. Of the physical factors, particle size and size distribution are among the most important. The same physical properties that govern aerosols in the atmosphere apply within the lungs.
Aerosols in the atmosphere are either primary or secondary in nature. Primary aerosols are atmospheric particles that are emitted or injected directly into the atmosphere, whereas secondary aerosols are atmospheric particles formed by in situ aggregation or nucleation from gas-phase molecules (gas to particle conversion). Particles in the atmosphere consist of a mixture of solid particles, liquid droplets and liquid components contained within the solid particles. Particles are variable in relation to their concentration and their physicochemical and morphological characteristics. Particles can be products of combustion, suspensions of soil materials, suspensions of sea spray or secondary formations from chemical reactions in the atmosphere (Figure 1.2).
Figure 1.2 Schematic representation of the chemical reactions and processes associated with the chemical composition of particulate matter.

Aerosols have diverse effects ranging from those on human health to those on visibility and the climate. They are also very important in public health and understanding of their dynamics is essential to the quantification of their effects. Human exposure to aerosols occurs both outdoors and indoors. They are also important in numerous technological applications, such as the delivery of drugs to the lungs, delivery of fuels for combustion and the production of nanomaterials.
The World Health Organization's Global Burden of Disease WHO GBD project concluded that that 3.2 million people die prematurely every year from cancer, heart disease and other illnesses that are attributable to particulate air pollution; 65% of these deaths occur in Asia. Brauer et al. (2012) have reported that 99% of the population in South and East Asia lives in areas where the WHO Air Quality Guideline (annual average of 10 µg/m3) for PM2.5 is exceeded. Particulate matter pollution was also ranked ninth of all the risk factors in terms of years lost due to disability by Brauer et al. (2012).
Aerosols have the potential to change the global radiation balance. A 2007 report by the Intergovernmental Panel on Climate Change (IPCC) estimated the effect of aerosols on the climate since the start of the industrial era to be around 20% of that of greenhouse gases. Aerosols are thought to be responsible for a negative forcing and therefore to have mitigated some of the expected global warming over this period (Kulmala, Riipinen and Kerminen, 2012).
1.2 Size and Shape
Particle size is the most important descriptor for the prediction of aerosol behaviour. When its particles are all the same in size, an aerosol is termed ‘monodisperse’. This is extremely rare in nature. Generally, particles vary in size, and this is called ‘polydisperse’. When its particles are chemically identical, an aerosol is called ‘homogeneous’. Particle shapes can be divided into three general classes:
- Isometric: The particle's three dimensions are roughly equal, for example spherical particles.
- Platelets: The particle has two long dimensions and a third small one, for example leaves and discs.
- Fibres: The particle has one long dimension and two much smaller ones, for example needles and asbestos.
Most of our knowledge regarding aerosol behaviour relates to isometric particles. Concern over the health hazards of fibres has prompted their study.
When particles are spherical, their radius or diameter can be used to describe their size. Since most particles are not spherical, however, other parameters must be used. Often the diameter is defined in terms of particle setting velocity. All particles with similar settling velocities are considered to be the same size, regardless of their actual size, composition or shape. The two most common definitions are:
- Aerodynamic diameter (see Chapter 2): The diameter of a unit-density sphere with the same aerodynamic properties as the particle in question. This means that particles of any shape or density will have the same aerodynamic diameter if their settling velocity is the same.
- Stokes diameter: The diameter of a sphere of the same density as the particle in question that has the same settling velocity as that particle.
Stokes diameter and aerodynamic diameter differ only in that Stokes diameter includes the particle density whereas aerodynamic diameter does not. Equivalent diameter is also commonly used. When particle size is measured by a specific technique, the measurement usually corresponds to a specific physical property; if electrically induced motion is used then a mobility equivalent diameter is reported.
1.3 Size Distribution
Determination of the aerosol size distribution is one of the most important aspects in the measurement and modelling of aerosol dynamics. The diameter of an ambient particle can be determined by various means, including light-scattering measurements, characterisation of the aerodynamic resistance of the particle and measurement of its electrical mobility or settling velocity. It is necessary to refer to an equivalent diameter independent of the measurement method and therefore the Stokes and aerodynamic equivalent diameter have been introduced. The aerodynamic diameter is defined as the diameter of a spherical particle with equal settling velocity as the particle under consideration but with material density of 1 g/cm3 (Hinds, 1999).
Particles can be categorised according to their size based on (i) their observed modal distribution (Hinds, 1999), (ii) the 50% cut-off diameter or (iii) dosimetric variables related to human exposure. In the latter case, the most common divisions are PM2.5 and PM10. PM10 is defined as airborne particulate matter passing through a sampling inlet with a 50% efficiency cut-off at 10 µm aerodynamic diameter that transmits particles below this size (European Commission, 2008); PM2.5 is similarly defined. The division in particle size is related to the possibility of PM2.5 particles penetrating to the lower parts of the human respiratory tract. In the modal distribution, several subcategories can be observed: nucleation mode, Aitken mode, accumulation mode, ultrafine particles and fine and coarse particles. These terms are discussed in Chapter 2.
An important region of the size distribution is the ultrafine part of the nuclei mode. Understanding of the physics and chemistry of very small clusters containing a few hundreds of molecules represents a theoretical and experimental challenge. Figure 1.3 depicts some typical aerosol size ranges and their related properties.
Figure 1.3 Particle size range for aerosols.

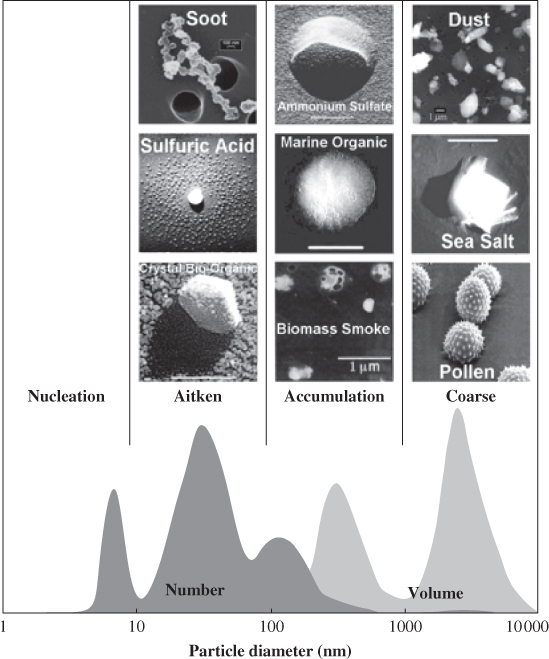
The various aerosol modes are associated with different sources and mechanisms of formation and with different chemical characteristics, as depicted in Figure 1.1 for the number and volume distributions. Examples of aerosols in the Aitken mode include soot, sulfuric acid and crystal bio-organic particles; in the accumulation mode, ammonium sulfate, marine organics and biomass smoke; and in the coarse mode, dust, sea salt and pollen.
Figure 1.4 shows an example of the aerosol size distribution and morphology obtained from electron microscopy. Number and volume size distribution are depicted together with the chemical composition by size for a number of aerosol types.
Figure 1.4 Aerosol size distribution and morphology for various aerosol types. Reproduced with permission from Heintzenberg et al. (2003). Copyright © 2003, Springer Science + Business Media.
The logarithmic canonical distribution of particle mass is used to describe aerosol dynamics. The multilognormal model is widely used to describe aerosol size distribution (Seinfeld and Pandis, 2006; Lazaridis, 2011). The multilognormal distribution is mathematically expressed as:
1.1 
where n is the number of modes, Ni the number concentration in each mode, Dp the aerosol diameter, Dpg,i the geometric mean diameter in each mode and σg,i the geometric standard deviation.
Aerosol behaviour in the atmosphere is controlled by internal and external processes. Internal processes act within the system boundaries, while external processes processes act across boundaries (Whitby and McMurry, 1997). Internal processes include coagulation, condensation, evaporation, adsorption/desorption, heterogeneous chemistry and nucleation mechanisms (Figure 1.5). External processes involve convection, diffusion and the effect of external forces such as thermophoresis (Hinds, 1999).
Figure 1.5 Internal and external processes that control aerosol behaviour.

Figure 1.6 presents some typical atmospheric aerosol distributions by number and volume. The volume distribution has different features to the number distributions; it is usually bimodal, with a minimum ∼1 µm (the dividing limit between coarse and fine particles). The arithmetic distribution has a maximum at the ultrafine mode (nucleation mode), whereas the volume distribution presents two logarithmic distributions, one at the accumulation mode and one at the coarse mode. It should be remembered that 1 million 1 µm particles have the same volume as a single 100 µm.
Figure 1.6 Typical ambient aerosol distributions by number and volume. (Adapted from Seinfeld and Pandis, 2006.)

The separation of fine and coarse particles is a determined factor, since particles in these two regions are different with respect to their source, chemical composition, processes for removal from the atmosphere, optical properties and effects on human health (Hinds, 1999; Lazaridis, 2011).
1.4 Chemical Composition
The composition of an atmospheric aerosol is determined from its source, which can include the emission of primary and secondary particles produced in the atmosphere. The main components of an aerosol include sulfate, nitrate, ammonium, chloride, elemental carbon, organic carbon, water, chloride and crustal material (Seinfeld and Pandis, 2006).
Crustal material, biogenic matter and sea salt make up the majority of natural aerosols. Anthropogenic aerosols comprise primary emitted soot (elemental carbon) and secondary formed carbonaceous material (organic carbon) and inorganic matter (nitrates, sulfates, ammonium and water).
Figure 1.7 shows the distribution of particles and the physicochemical processes associated with different particle sizes.
Figure 1.7 Physicochemical processes related to aerosol particle size.

An important part of secondary aerosol particles in the atmosphere is composed of secondary formed organic matter (Turpin and Huntzicker, 1991) produced from the oxidation of organic compounds. Partitioning of gas-particle organic compounds in the atmosphere is important in determining their association with fine particulate matter (Seinfeld and Pandis, 2006; Lazaridis, 2011). The number of different chemical forms of organic matter and the absence of direct chemical analysis mean that fractional aerosol yields, fractional aerosol coefficients and adsorption/absorption methodologies for describing the incorporation of organic matter in the aerosol phase are mainly experimentally determined. An important pathway for secondary organic particle formation arises from biogenic hydrocarbons. There are very large quantities of globally emitted biogenic hydrocarbons that are highly reactive (Hoffmann et al., 1997).
Bioaerosols include all airborne particles of biological origin; that is, bacteria, fungi, fungal spores, viruses and pollen, as well as their fragments, including various antigens. Aerodynamic diameters can range from about 0.5 to 100 µm (Nevalainen et al., 1991; Cox and Wathes, 1995). Airborne microorganisms become nonviable and fragmented over time, due to desiccation. Indoor air contains a complex mixture of (i) bioaerosols such as fungi, bacteria and allergens and (ii) nonbiological particles such as dust, tobacco smoke, cooking-generated particles, motor vehicle exhaust particles and particles from thermal power plants. Exposure to several of these biological entities, as well as to microbial fragments such as cell-wall fragments and flagella and microbial metabolites such as endotoxin, mycotoxins and volatile organic compounds (VOCs), can result in adverse health effects. In particular, an increase in asthma attacks and bronchial hyper-reactivity has been correlated to increased bioaerosol levels. Bioaerosols are usually measured in standard colony forming units per volume (CFU/cubic metre counts). An in-depth consideration of bioaerosols can be found in Chapter 16 and in a recent review by Després et al. (2012).
1.5 Measurements and Sampling
According to Kerker (1997), the first recorded use of laboratory-generated aerosols was by Leonardo da Vinci (1452–1519), who wanted to account for the blue colour of the sky. Centuries later, Tyndall (1869) noted that if a beam of light was passed through a suspension and viewed at an angle against a dark background, the presence of particles was revealed by the scattered light. Tyndall's legacy to aerosol science was great (Gentry and Lin, 1996), including in particular his proposal of a connection between the light scattered by an aerosol during the early stages of its formation, when the particles were small, and the colour of the sky and the polarisation of light. Tyndall assumed that all small particles behaved in this manner and considered the light of the sky a specific instance of a general physical phenomenon. This work and a theoretical treatment by Rayleigh (1871) gave the scattering of blue light by very small particles and the preferential transmission of red light, so strikingly exemplified by the vivid colours of sunset, a ready explanation. At first Rayleigh believed the blue sky was caused by the presence of fine particles such as those Tyndall had experimented with, but sometime later he revised this notion, noting that particles as such were not necessary and that the blue sky ‘can be explained by diffraction from the molecules of air themselves’.
In the past, exploding wires were used to generate aerosols. Although scientific interest in the phenomenon didn't truly begin until the 1920s, the first paper on exploding wires was read before the Royal Society in December 1773 by Nairne (1774). He used an exploding wire to prove that the current in all parts of a series circuit is the same. Some 40 years later, Singer (1815) and Singer and Crosse (1815) reported on more experiments involving exploding wires. Faraday (1857) demonstrated how exploding wires could be uses to produce a metal film or mirror. He was the first scientist to systematically use the exploding wire technique to generate aerosols. He was also first to characterise the aerosols and to develop techniques that allowed certain of their optical properties to be examined (Gentry, 1995).
The requirement to measure aerosols in a range of fields has increased dramatically over the last 2 decades. As a result, there are now a large number of instruments on the market, ranging from small portable devices for personnel exposure monitoring to research-laboratory-based instrumentation. Selection of an instrument depends upon the aims of your research and on determining compliance with standards, quantifying trends and identifying hotspots. In other words, you must decide on (i) what you want to measure (which metric: number, mass, volume and size distribution or concentration), (ii) whether measurement response time critical, (iii) how long you will sample for and (iv) whether you need to collect a sample.
Any sample should be representative of its environment, taking into account timing, location and particle size distribution. As will be discussed in Chapter 3, the sampling system can influence the transmitted sample. Particles do not behave in the same way as gas molecules when dispersed in air. They deposit under gravity, impact on bends due to particle inertia, are deposited on internal surfaces by molecular and turbulent diffusion and are affected by thermal, electrostatic and acoustic forces.
Generally, particulate sampling devices are divided into two types: those that collect a sample on a substrate and those that conduct in situ real-time measurements. With the former, one most ensure that the substrate is compatible with subsequent analysis: for gravimetric analysis, the substrate should be weight-stable; for microscopy, the filter should be transparent to radiation (optical or electron); for biological aerosol, recovery of organisms from the filter should be possible; and for chemical analysis, the substrates should have low levels of the compound under analysis or be capable of incineration. With the latter, either extractive or external sensing techniques can be used. Extractive methods require the aerosol to be brought into the instrument (e.g. optical particle counters), whereas external sensing methods are noninvasive.
In summary, a wide variety of techniques and instruments are available by which to measure and characterise aerosols. Each has advantages and disadvantages in terms of size range, concentration range, measurement resolution, speed of response and so on. New instruments are always being developed.
References
Brauer, M., Amann, M., Burnett, R.T. et al. (2012) Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environmental Science and Technology, 45, 652–660.
Calvo, A.I., Alves, C., Castro, A. et al. (2012) Research on aerosol sources and chemical composition: past, current and emerging issues. Atmospheric Research, 120–121, 1–28.
Cox, C.S. and Wathes, C.M. (1995) Bioaerosols in the environment, in Bioaerosols Handbook (eds C.S. Cox and C.M. Wathes), Lewis Publishers, Boca Raton, FL, pp. 11–14.
Danckelman, V. (1884) Die Bevoelkungsverhaeltnisse des suedwstlichen Africas. Meteorologische Zeitschrift, 8, 301–311.
Després, V.R., Huffman, J.A., Burrows, S.M. et al. (2012) Primary biological aerosol particles in the atmosphere: a review. Tellus Series B, 64, 15598. doi: 10.3402/tellusb.v64i0.15598
East West Airlines Ltd v. Turner (2010). NSWCA 53; BC201001873—01 Apr 2010—Supreme Court of New South Wales, Court of Appeal.
European Commission (2008) Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Official Journal of the European Union, L 152, 1–44.
Faraday, M. (1857) Experimental relations of gold (and other metals) to light. Philosophical Transactions of the Royal Society of London, 147, 145–181.
Franco, G. and Franco, F. (2001) Bernardino Ramazzini: the father of occupational medicine. American Journal of Public Health, 91, 1382.
Gadolin, J. (1767). Bedenken von Sonnenrauch. Abhandlung Der Königlichen Schwedischen Akademie der Wissenschaften, Abhandlungen für die Monate April, Mai, Juni, 1767.
Gentry, J.W. (1995) The aerosol science contributions of Michael Faraday. Journal of Aerosol Science, 26, 341–349.
Gentry, J.W. and Lin, J.C. (1996) The legacy of John Tyndall in aerosol science. Journal of Aerosol Science, 27, S503–S504.
Heintzenberg, J., Raes, F., Schwartz, S. et al. (2003) Tropospheric aerosols, in Atmospheric Chemistry in a Changing World (eds G. Brasseur, R. Prinn and A.P. Pszenny), Springer, Berlin, Heidelberg, pp. 125–156.
Hinds, W.C. (1999) Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd edn, John Wiley & Sons, Inc., New York.
Hoffmann, T., Odum, J.R., Bowman, F. et al. (1997) Formation of organic aerosols from the oxidation of biogenic hydrocarbons. Journal of Atmospheric Chemistry, 26, 189–222.
Husar, R.B. (2000). Atmospheric aerosol science before 1900, in History of Aerosol Science, (eds Preining, O. and Davis E.J.), Verlag der Oesterreichischen Akademie der Wissenschaften, Wien, pp. 25–36.
Kerker, M. (1997) Light scattering instrumentation for aerosol studies: an historical overview. Aerosol Science and Technology, 27, 522–540.
Kulkarni, P., Baron, P.A. and Willeke, K. (2011) Aerosol Measurement: Principles, Techniques, and Applications, 3rd edn, John Wiley & Sons, Inc., Hoboken, NJ.
Kulmala, M., Riipinen, I. and Kerminen, V.M. (2012) Aerosols and climate change, in From the Earth's Core to Outer Space, (ed Haapala I.), Springer, Berlin, Heidelberg, pp. 219–226.
Lazaridis, M. (2011) First Principles of Meteorology and Air Pollution, Springer Science + Business Media, Dordrecht.
Nairne, E. (1774) Electrical experiments. Philosophical Transactions of the Royal Society of London, 64, 79–89.
Nevalainen, A., Pasanen, A.L., Niininen, M. et al. (1991) The indoor air quality in Finnish homes with mold problems. Environment International, 17, 299–302.
Rafinesque, C. (1819) Thoughts on atmospheric dust. American Journal of Science, 1, 397–400.
Rayleigh, L. (1871) On the scattering of light by small particles. Philosophical Magazine, 41, 446–454.
Singer, G.J. (1815) Some account of the electrical experiments of M. De Nelis, of Malines in the Netherlands. Philosophical Magazine, 46, 259–264.
Seinfeld, J.H. and Pandis, S.N. (2006) Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 2nd edn, John Wiley & Sons, Inc., New York.
Singer, G.J. and Crosse, A. (1815) Account of some electrical experiments of M. De Nelis, of Malines in the Netherlands: with an extension of them. Philosophical Magazine, 46, 161–166.
Stohl, A. and Akimoto, H. (2004) Intercontinental Transport of Air Pollution, Springer, Berlin.
Turpin, B.J. and Huntzicker, J.J. (1991) Secondary formation of organic aerosol in the Los Angeles Basin: a descriptive analysis of organic and elemental carbon concentrations. Atmospheric Environment. Part A. General Topics, 25, 207–215.
Tyndall, J. (1869) On the blue colour of the sky, the polarisation of skylight, and the polarisation of light by cloudy matter generally. Proceedings of the Royal Society of London, 17, 223–233.
Wargentin, P. (1767). Anmerkungen ueber Sonnenrauch. Abhandlung Der Königlichen Schwedischen Akademie der Wissenschaften, Abhandlungen für die Monate April, Mai, Juni, 1767.
Whitby, E.R. and McMurry, P.H. (1997) Modal aerosol dynamics modeling. Aerosol Science and Technology, 27, 673–688.
Whytlaw-Gray, R. and Patterson, H.S. (1932) Smoke: A Study of Aerial Disperse Systems, Edward Arnold and Company, London.
