3
Hydrothermal Carbon Materials for the Oxygen Reduction Reaction
Kathrin Preuss*, Mo Qiao*, and Maria‐Magdalena Titirici
Queen Mary University of London, School of Engineering and Materials Science and Materials Research Institute, Mile End Road, London, E1 4NS, UK
3.1 Introduction
Nowadays, climate change seems almost unstoppable, unless governments manage to drastically reduce their greenhouse gas emissions through legislation and encourage alternative energy technologies. Although renewable energy resources are a promising alternative to burning fossil fuels, their widespread commercialization is still limited, mostly due to high cost and implementation. A good example for this are fuel cells, where the use of platinum (Pt) as a catalyst significantly increases the cost, due to Pt being a noble and scarce metal with resources limited to very few regions of the globe. The hydrogen oxidation reaction (HOR) at the anode proceeds fast with platinum as a catalyst, allowing very low concentrations of Pt to be used. Contrary to this, reaction kinetics for the oxygen reduction reaction (ORR) at the cathode side are sluggish on Pt, requiring a multiple of Pt loading than at the anode side as well as having a low tolerance to poisoning and poor stability [1]. Considering these issues, research efforts are primarily focused on replacing platinum at the cathode side for the ORR in order to reduce the cost of fuel cells. Ideally, novel catalysts are aimed at working without any metal, noble or non‐noble, to prevent any future conflicts regarding shortage of resources and price fluctuations.
These metal‐free ORR catalysts are typically based on carbons doped with different heteroatoms such as nitrogen, sulfur, boron, or phosphorus. Although a deep understanding for the active sites in these metal‐free materials is still missing, assumptions on the enhanced catalytic activity are mostly based on the differences in electronegativity and atomic size between the carbon atoms and the heteroatom dopants. With nitrogen being the most common dopant among metal‐free carbons, the different species of nitrogen can add beneficial properties to the carbon material. Pyridinic‐N can offer a lone electron pair, whereas graphitic‐N can introduce an extra electron in the delocalized π system, both of which enhance the basicity and electron‐donor capacity of the material and thus its ORR activity [2]. A lot of research has been carried out in order to understand which of the nitrogen species are truly involved in the enhanced catalytic activity; although some suggest that pyridinic‐N is the active species [3], others claim it is graphitic‐N [4, 5]. More recent studies indicate that a synergistic effect between both of these species causes the improved ORR activity, where Kim et al. [6] proposed an interconversion of the two N species during the ORR process, whereas Lai et al. [7] observed an influence of pyridinic‐N on the onset potential of the catalyst and of graphitic‐N on its limiting current. Likewise, the effect of nitrogen species on the selectivity of the catalyst toward the two‐ or four‐electron pathway also still remains unclear with adverse findings being reported [3, 8, 9]. Similar to nitrogen, doping boron or phosphorus can cause a disruption in the charge uniformity of the carbon framework due to their difference in electronegativity compared with the carbon atoms [2]. Although nitrogen has a higher electronegativity than carbon, creating a positive charge on the neighboring carbon atom, boron and phosphorus have a lower electronegativity, creating a positive charge on the dopant itself. When co‐doping nitrogen with boron or phosphorus, this charge density profile can change the chemisorption of oxygen by acting as a bridge to transfer electrons directly from the positively charged atom to O2, thereby weakening the OO bonds and increasing the ORR activity [2]. Due to its large atomic size compared with carbon or nitrogen, doping sulfur can cause structural defects and increased polarizability additional to enhancing the spin density of neighboring carbon atoms, which can facilitate easier interaction between molecules in the electrolyte [2, 10]. Density functional theory (DFT) calculations on N‐doped graphene (NG) suggested that catalytically active sites were more likely to have either a high positive charge or spin density [11, 12], where a similar trend could be assumed for other heteroatom‐doped carbons.
There are a number of procedures to synthesize these metal‐free ORR materials, ranging from chemical vapor deposition (CVD) [13–15], various thermal treatments [16–18], templating methods [19–21], to hydrothermal carbonization [22–24]. These materials include graphite/graphene [25–27], carbon nanotubes (CNTs) [14, 28, 29], carbon aerogels [10, 22, 24, 30–32], carbon nanodots [33, 34], carbon spheres [35, 36], carbon nanosheets [23, 37], and many more. Among these synthesis strategies, hydrothermal carbonization presents an environmental friendly and inexpensive approach to convert earth‐abundant biomass, biowaste, or their precursors into useful carbon materials [38]. This process is based on the concept of coal formation, which occurred over thousands of years on earth, converting biomass with water as a solvent in a lab autoclave into carbon materials under self‐generated pressure and mild temperatures (180–200 °C) in a matter of hours. Surface area, pore properties, morphology, and chemical composition of the resulting carbon material are highly dependent on the type of starting material as well as the reaction conditions. Although soft plant tissue and biomass precursors such as glucose, without a crystalline cellulose scaffold, usually form small carbon spheres with interstitial porosity, hard plant biomass containing structural crystalline cellulose results in a replica similar to its original structure [39]. The hydrothermal carbonization of pure glucose (Scheme 3.1) proceeds via different steps starting with an isomerization to fructose, which is then dehydrated to hydroxymethylfurfural (HMF). HMF can further be hydrolyzed to levulinic acid and formic acid, but it can also undergo further complex reactions such as self‐condensation, Diels–Adler reactions, and cross‐condensation with aldehydes or ketones. These newly formed compounds can then polymerize/nucleate and lastly carbonize via additional intermolecular dehydration [40].

Scheme 3.1 Chemical reactions involved during the hydrothermal carbonization of glucose: (a) dehydration of glucose to HMF and potentially to levulinic and formic acid; (b) self‐condensation of HMF; (c) Diels–Alder HMF condensations; and (d) reaction of acetone with HMF.
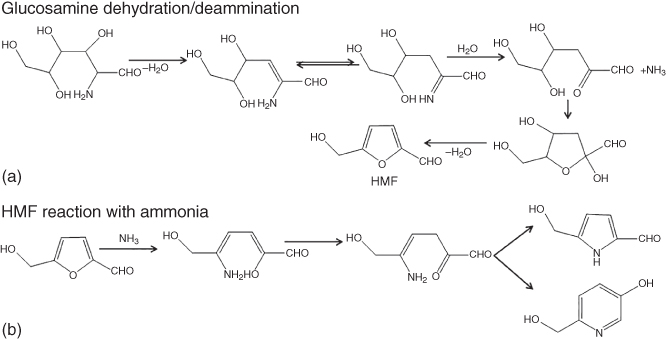
Scheme 3.2 Chemical reactions involved in (a) glucose dehydration–deamination to HMF and ammonia and (b) reaction of HMF with ammonia.
In the presence of additional nitrogen‐containing precursors, for example proteins, additional reactions occur between the reducing sugar and the amines, which are classified as Maillard reactions, changing the typical HTC formation by creating nitrogen‐doped carbons [41]. Scheme 3.2 shows some model reactions during the hydrothermal carbonization of glucosamine as a carbon‐ and nitrogen‐containing precursor, which undergoes dehydration and deamination to form HMF and ammonia. HMF can further react with ammonia or other amines to form pyrrole‐ and pyridine‐like compounds via various steps. The reactions occurring during the hydrothermal carbonization of any biomass is a complex process. Schemes 3.1 and 3.2 give a simplified overview of some of the reactions, more detailed information of general HTC mechanism, and Maillard reactions can be found elsewhere [42, 43].
Furthermore, HTC can also be used to promote the assembly process of chemical reactants with other preformed carbon nanostructures. For example, graphene or CNTs can be combined with nitrogen‐containing biomass precursors such as chitosan or urea to create functionalized hybrid materials [44–46]. The scalability and easy processability of the HTC technique is hereby remarkably favored over other existing methods to produce carbon–carbon composites such as CVD and chemical or thermal exfoliation.
In the following, HTC will be discussed in relation to sustainable biomass precursors, ranging from food to plant‐based and waste biomass toward synthetical biomass precursors and their performance as ORR catalysts. Further, HTC will be presented as an assembly process of chemical reactants with other preformed carbon nanostructures, resulting in multifunctionalized carbon–carbon composites and their electrochemical performance.
3.2 Sustainable HTC Catalysts for the Oxygen Reduction Reaction
Although reducing the cost of a fuel cell is one matter, making this renewable energy resource overall more sustainable is crucial for their future application as well. A well‐known definition of sustainability was defined in 1987 by the Brundtland Commission as “… development that meets the needs of the present without compromising the ability of future generations to meet their own needs” [47]. To achieve this concept of sustainability and cost reduction, biomass and biomass precursors can be a promising starting material for metal‐free ORR catalyst. Synthesis of ORR materials from biomass or biomass precursors usually follows two heat treatments: first, the starting material is converted into doped carbons via hydrothermal carbonization, which is then followed by a high‐temperature carbonization under inert atmosphere, sometimes in the presence of activation agents, to increase the material's conductivity/graphitization, surface area, and stability [2]. The origin of this biomass can be classified into food‐based, plant/food waste and synthetic biomass precursor like glucose and will be discussed separately as follows.
3.2.1 Catalysts from Food‐Based Biomass
In 2012, Zhu et al. [33] produced bifunctional fluorescent carbon nanodots from soy milk. As their research focused on photoluminescent properties, the authors used the liquid phase retained after the HTC without any further heat treatment/activation. Due to that, a high nitrogen content of 10.4 at.% and additional phosphorus doping of 0.7 at.% were obtained. Their material showed catalytic activity toward the ORR in alkaline media, although considerably lower than a commercial platinum standard, with a calculated electron transfer number of 3.15, suggesting a mixed two‐ and four‐electron pathway. The rather poor performance of the material could be explained by the missing pyrolysis step resulting in presumably the absence of graphitic nitrogen species [48], which was shown to influence ORR activity besides pyridinic‐N (as discussed earlier) as well as the amorphous nature of the material. Although not outstanding in their ORR activity, the fluorescent carbon nanodots showed surprisingly good stability with a current loss of only 9% after 7000 s of testing.
Gao et al. [35] used fermented rice, which was a pre‐steamed mix of rice and yeast fermented over 2–4 days, to synthesize N‐doped carbon spheres via hydrothermal carbonization followed by high‐temperature activation with ZnCl2 and subsequent washing with HCl to remove the metal salt. Their most active material was pyrolyzed at 700 °C and exhibited a high Brunauer‐Emmet‐Teller (BET) surface area of 2106 m2 g−1 as well as high nitrogen doping of 6.2 at.%. Samples pyrolyzed at lower temperatures (500 °C) resulted in drastically larger surface areas (3998 m2 g−1) and nitrogen doping (10 at.%) but less favorable ORR performance and electron transfer numbers. This trend is presumably due to the less graphitic nature of the carbon pyrolyzed at lower temperatures as well as the differently distributed nitrogen species. A control sample pyrolyzed without previous HTC treatment showed very poor catalytic performance, suggesting that the HTC process is a crucial step in fixing the nitrogen content as well as creating a large surface area and favorable porous structure. A clear difference could be seen between their sample made from fermented and unfermented rice, where the fermentation process had a positive influence on the catalytic activity, which suggests that the added yeast could play a role, most likely due to traces of iron being incorporated from yeast, as has been mentioned elsewhere [36].
The same authors also published a similar synthesis route starting with the hydrothermal carbonization of bamboo fungus followed by high‐temperature activation with and without ZnCl2 and acid washing [49]. They also investigated the influence of treatment technique and conditions on the resulting materials. The highest nitrogen content of 2.42 at.% was found in the sample produced via HTC of bamboo fungus and activated in the presence of ZnCl2 at 800 °C, due to in situ incorporation of N during the HTC process. Large surface areas were achieved in the presence of ZnCl2, regardless of its addition during (1821 m2 g−1) or after (1895 m2 g−1) the HTC process, compared with activation without ZnCl2 (516 m2 g−1) or direct activation without HTC (131 m2 g−1). The highest surface area of 2376 m2 g−1 was reported for the sample activated at 700 °C in the presence of ZnCl2. Electrochemical testing in alkaline media revealed the best performance for the sample synthesized via HTC and activated with ZnCl2 at 800 °C when compared with the different treatment techniques and temperatures. They concluded that the high nitrogen content as well as a favorable mixture of nitrogen species is responsible for the sample's superior performance.
An interesting approach was made by Liu et al. [37] in 2014, who hydrothermally carbonized nori algae with and without melamine to synthesize N,S‐doped carbon catalysts with subsequent carbonization at 1000 °C and acid washing. The presence of melamine not only increased the nitrogen content from 1.42 to 2.63 at.%, but also increased the surface area from 494 to 538 m2 g−1, whereas sulfur doping decreased from 0.89 to 0.23 at.%. Their sample containing melamine outperformed the platinum standard in alkaline media in terms of onset potential and limiting current with an electron transfer number of 3.97 and hydrogen peroxide yield below 5% and showed a considerably better catalytic activity compared with the sample without melamine. They attributed this drastic improvement in performance to the higher nitrogen content. Compared with platinum, their sample also showed outstanding stability with a current loss of only 5% after 8 h and excellent methanol tolerance. Although the authors mention that it is well known that traces of iron typically occur in nori biomass, no further analysis was carried out to determine if any traces of iron were left in the sample after acid washing. Although traces of Fe might have an influence on their sample's beneficial onset potential, the difference between their two samples (with and without melamine) clearly shows that the good limiting current can be ascribed to the higher nitrogen content/surface area. Similar to Gao et al. [35], a control sample without previous hydrothermal carbonization and no additional melamine showed a significantly lower surface area of 383 m2 g−1 and poor catalytic activity, despite having a lower ID:IG ratio compared with the other samples, which the authors attributed to less defect sites due to the absence of heteroatom doping.
Although far from competing with a commercial platinum standard, Liu et al. [22] produced nitrogen‐doped carbon aerogels by hydrothermal carbonization of cantaloupes followed by KOH activation at different temperatures and acid washing (Figure 3.1). The authors were able to show that activation with KOH favorably increased the material's surface area and pore properties (for example from 53 to 530 m2 g−1 at 600 °C). An increased temperature during the KOH activation also increased the surface area and provided a beneficial mix of micro‐ and mesopores as well as nitrogen species. They found their best sample to be pyrolyzed at 800 °C consisting of micropores around 1.6 and 1.8 nm as well as mesopores of 2.7, 3.7, and 20 nm with a surface area of 1778 m2 g−1, compared with 530 and 787 m2 g−1 when pyrolyzed at 600 and 700 °C, respectively. X‐ray photoelectron spectroscopy (XPS) studies showed that the increased temperature yielded a higher carbon content and a lower oxygen content compared with the samples treated at 600 and 700 °C. With a nitrogen content of 2.01 at.%, made up of around 40% of pyridinic‐N and 20% of graphitic‐N, the sample pyrolyzed at 800 °C had the second highest overall N content, with 2.14 at.% being the highest for the 700 °C sample and 1.46 at.% the lowest for the one at 600 °C. Unsurprisingly, the sample pyrolyzed at 800 °C showed the best electrocatalytic performance toward ORR, which the authors attributed to the hierarchical pore structure, the high surface area, and the beneficial mix of pyridinic‐N to graphitic‐N. Although falling short behind a commercial platinum standard in terms of onset potential and limiting current, their sample exhibited an electron transfer number of 3.9 and showed excellent stability with 88% of current remaining after 20 000 s compared with only 69% for the Pt standard.
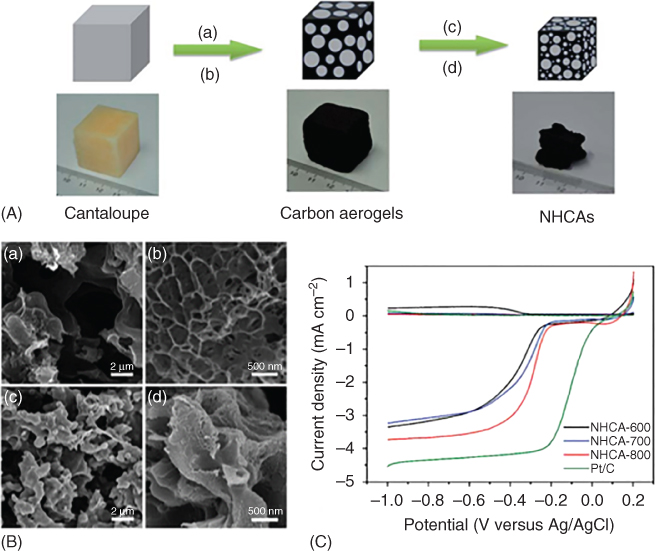
Figure 3.1 (A) schematic illustration of the synthesis process of nitrogen‐doped carbon aerogels from cantaloupe, by hydrothermal carbonization (a), freeze‐drying (b), infiltration with KOH (c), and high‐temperature treatment (d). (B) scanning electron microscopy (SEM) images for carbon aerogels from cantaloupe pyrolyzed at 700 °C (a, b) and 800 °C (c, d). (C) linear sweep voltammogram for carbon aerogels from cantaloupe treated at different temperatures compared with a commercial platinum standard in 0.1 M KOH.
Source: Liu et al. (2016) [22]. Copyright 2016. Reproduced with permission from Royal Society of Chemistry.
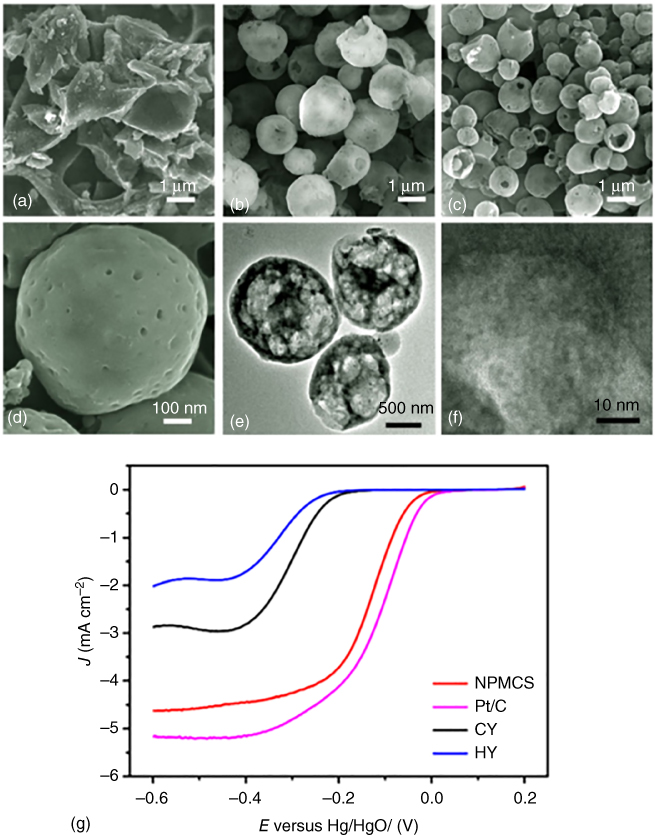
Figure 3.2 SEM of (a) directly pyrolyzed dry yeast (CY); (b) hydrothermally carbonized dry yeast (HY); (c, d) hydrothermally carbonized and subsequently pyrolyzed dry yeast (NPMCS); (e, f) TEM of NPMCS; and (g) linear sweep voltammogram of differently treated dry yeast samples and a commercial platinum standard (Pt/C) in 0.1 M KOH.
Source: Yu et al. 2017 [36]. Copyright 2017. Reproduced with permission from Springer.
Nitrogen‐ and phosphorus‐co‐doped mesoporous hollow carbon spheres were reported by Yu et al. [36] in 2016, who used commercial baking yeast as a starting material. The authors observed a clear benefit when the dry yeast was treated via hydrothermal carbonization before the pyrolysis step due to the presence of water during the HTC, which helped restore the yeast's original spherical shape as can be seen in Figure 3.2. Compared with direct pyrolysis of dry yeast, which resulted in a small surface area and pore volume (221 m2 g−1 and 0.29 cm3 g−1) as well as an irregular carbon block morphology, the rehydrated HTC sample exhibited a high surface area and pore volume of 1223 m2 g−1 and 1.56 cm3 g−1. XPS analysis revealed a nitrogen and phosphorus content of 3.35 and 1.69 at.%, respectively. Electrochemical testing of the hollow carbon spheres showed promising results compared with a platinum standard with an average electron transfer number of 3.7 and excellent stability, retaining 84% of its current after 9 h, as well as a good methanol tolerance. Although the authors claim their material to be metal‐free, they mention that small amounts of iron can be present in commercially available dry yeast. As XPS did not reveal Fe, it is likely to be below the standard detection limit, which does not mean that any Fe present would not have an influence on the material's electrocatalytic activity. Studies have shown that amounts as low as 18 ppm of metal residues can drastically boost a material's activity toward the ORR [50]. Considering these findings, the good performance of Yu et al.'s mesoporous hollow carbons spheres should be considered with care.
As always with food‐based biomass, a conflict over the use of food can be caused when utilizing food resources for new energy applications such as biofuels or biocatalysts. Although promising metal‐free catalysts have been presented in this subchapter, feasibility in terms of upscaling any of these synthesis approaches should be considered with care due to the arising contradiction with food shortage and rising food prices.
3.2.2 Catalysts from Food Waste and Plant Biomass food waste"?>
A more viable alternative for the synthesis of metal‐free catalyst could be plant and food waste, which unarguably cannot interfere with food supply and would even offer a useful solution to deal with food and plant waste. A very promising and user‐friendly approach was presented by Chen et al. [23] in 2014, where they used parts of the plant Typha orientalis, also known as cattail, which can be won by low cost and high volume production due to its ability to adsorb nutrients easily leading to vast amounts of biomass. They carried out hydrothermal carbonization of the flower spikes followed by washing with water, freeze‐drying, and annealing in NH3 atmosphere at temperatures between 750 and 850 °C, which resulted in a 3D interpenetrated network structure made of nanosheets (Figure 3.3). The sample treated at 800 °C (NCS‐800) showed the highest nitrogen content of 9.1 at.%, followed by NCS‐750 with 7.5 at.% and NCS‐850 with 5.8 at.%. A control sample was also annealed under pure N2 atmosphere at 800 °C, which resulted in low nitrogen doping of only 1.8 at.% and a low surface area of 307 m2 g−1. NCS‐800 had the lowest surface area of 646 m2 g−1, compared with 692 m2 g−1 for NCS‐750 and 898 m2 g−1 for NCS‐850. Electrochemical testing revealed clear superiority of NCS‐800 over the other samples both in alkaline and in acidic media, even outperforming the platinum standard in alkaline media in terms of onset potential and limiting current with an electron transfer number between 3.85 and 3.96 and hydrogen peroxide yield below 3.5% as well as showing a better stability and methanol tolerance than platinum both under alkaline and acidic conditions. The authors concluded that multiple factors contributed to their catalyst's outstanding performance, one being freeze‐drying compared with drying in air or at 100 °C, which helped maintaining the porous network structure, another being the high nitrogen content, where mostly pyridinic and pyrrolic nitrogen are present. Furthermore, they believe that the nanosheet morphology offers a large surface area for easily accessible active sites for the ORR as well as the three‐dimensional network structure providing accelerated reactant and electron transport. Overall, Chen et al. proved that it is possible to synthesize a green metal‐free catalyst from plant waste with outstanding ORR performance without using any harsh chemicals or treatment steps.
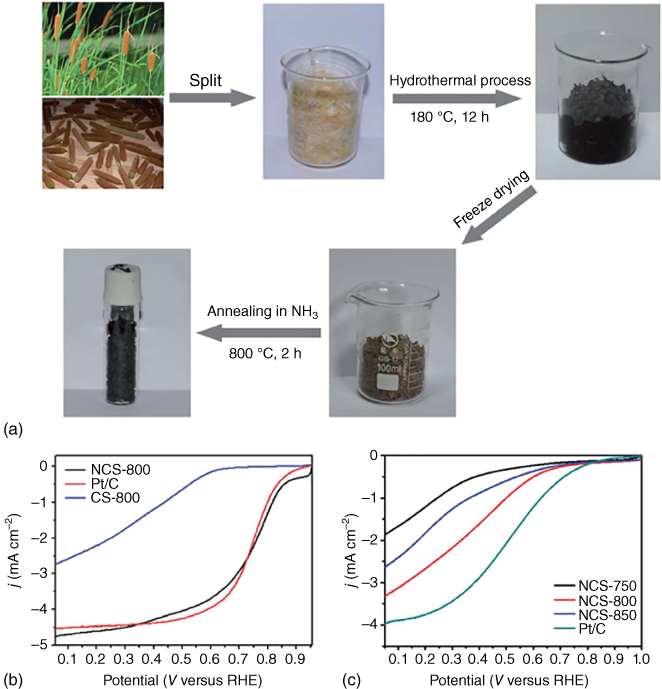
Figure 3.3 (a) Schematic for the preparation of nitrogen‐doped nanoporous carbon nanosheets made from flower spikes of Typha orientalis. Linear sweep voltammograms for samples pyrolyzed at different temperatures (750, 800, and 850 °C) under NH3 atmosphere (NCS‐) or under N2 atmosphere (CS‐) compared with a commercial platinum standard in 0.1 M KOH (b) and 0.5 M H2SO4 (c).
Source: Chen et al. 2014 [23]. Copyright 2014. Reproduced with permission from Royal Society of Chemistry.
Similar to Zhu et al. [33] , Zhang et al. [34] reported the synthesis of nitrogen‐doped carbon nanodot/nanosheet aggregates in the liquid phase after the HTC of dried and ground monkey grass. Their material resulted in carbon nanosheets with incorporated graphitic nanodots with a N:C atomic ratio of 3.41%. Their material showed photoluminescent properties as well as good catalytic activity in alkaline media when compared with a commercial platinum standard showing similar onset potential and limiting current as well as a high electron transfer number close to 4. Their samples also proved superior tolerance to methanol and outstanding stability with a decrease in current of only 5.5% after 5 h. The authors attributed the good catalytic performance to the high pyridinic nitrogen content in their sample, which they supported via DFT calculations. With these, they showed that pyridinic nitrogen has a higher positive charge and stronger oxygen adsorption compared with pyrrolic‐N or graphitic‐N. Their calculations also highlighted a stronger dissociative adsorption of pyridinic‐N and pyrrolic‐N compared with undoped carbon or graphitic‐N, with which they explained the high electron transfer number of their material. It should be mentioned that the authors considered only one N species at a time for their calculations, whereas a synergistic effect between all nitrogen species present might be a more realistic view on the species' influence on the ORR.
Liu et al. [51] converted rice straw into nitrogen‐doped carbon for microbial fuel cells by hydrothermal carbonization, followed by freeze‐drying and annealing under NH3 atmosphere at different temperatures. As expected, the nitrogen content and surface area decreased with an increasing treatment temperature, resulting in a nitrogen content of 7.65, 5.57, and 3.32 at.% and a surface area of 494, 350, and 270 m2 g−1 for the samples treated at 800, 900, and 1000 °C, respectively. The control sample treated under argon atmosphere resulted in a low nitrogen content of 1.12 at.% and a surface area of 99 m2 g−1. The authors attributed these changes to an activation effect of NH3 by etching of carbon atoms. Regardless of surface area and nitrogen content, their best electrochemically performing sample (in 50 mM phosphate buffer solution (PBS)) was the one treated at 900 °C under NH3 atmosphere with an electron transfer number of 3.86 and a H2O2 yield of below 7%, although the onset potential and limiting current were falling short of a commercial platinum standard. Their sample's nitrogen consisted mostly of pyridinic‐N and pyrrolic‐N, which the authors attributed to the good ORR performance. Further, they concluded that their sample treated at 900 °C had the most beneficial balance of nitrogen content, surface area, and graphitization compared with the samples treated at 800 and 1000 °C.
Another type of plant waste was used by Zhou et al. [52], who collected plant moss (Weisiopsis anomala) from concrete walls to synthesize nanostructured N‐doped carbons by hydrothermal carbonization with subsequent pyrolysis under nitrogen atmosphere followed by acid washing. Their materials exhibited a highly developed porous network with graphene‐like features and nanodots on the surface, as can be seen in Figure 3.4. By a comparative one‐step pyrolysis of moss, they were able to show that these nanodots were formed during the hydrothermal carbonization and attached to the material via self‐assembly. The two‐step process also resulted in a considerably higher surface area of 409 m2 g−1 compared with 255 m2 g−1 when pyrolyzed at 900 °C. The highest surface area of 420 m2 g−1 was achieved when using the two‐step process and a pyrolysis temperature of 1000 °C, although the higher temperature resulted in a lower nitrogen content of 1.1 at.% compared with 1.6 at.% at 900 °C. For comparison, the authors also prepared a sample by ultrasonication after the HTC to remove the nanodots from the materials surface, which resulted in a slightly lower surface area of 325 m2 g−1 after the pyrolysis at 900 °C but a similar nitrogen content of 1.5 at.%. The dominant N species in all samples was found to be graphitic nitrogen. Electrochemical testing revealed the superior performance of the sample prepared via the two‐step process pyrolyzed at 900 °C, which showed a slightly less positive onset potential when compared with a platinum standard but a better limiting current in alkaline media with outstanding stability, methanol tolerance, and an electron transfer number close to 4. Their testing clearly demonstrated the crucial role of nanodots on the material's surface in terms of its catalytic activity. The authors also compared the material's N species before and after the ORR testing, which revealed a drastic increase in oxidized N while the content of graphitic N remained unchanged, leading to the assumption that pyrrolic‐N gets oxidized and pyridinic‐N ends with OH attachments after the catalytic testing, which indicates its important role during ORR. Overall, the authors concluded that their catalyst's outstanding performance without any harsh chemicals or treatments can be attributed to its high surface area and porous structure, offering a high active site density combined with a favorable N species composition.
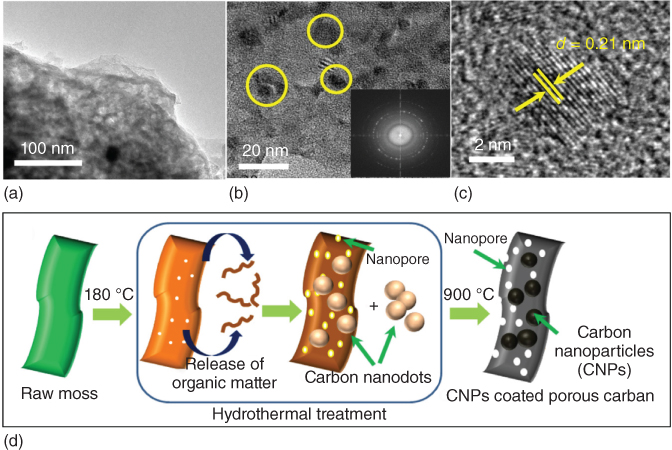
Figure 3.4 TEM images of hydrothermal carbon from plant moss pyrolyzed at 900 °C (a–c) and proposed mechanism for the formation of the porous carbon material with carbon nanoparticles (d).
Source: Zhou et al. 2016 [52]. Copyright 2016. Reproduced with permission from Elsevier.
An interesting finding was made by Zhang et al. [53], who made fluorescent N‐doped carbon nanoparticles by H2O2‐assisted HTC of cocoon silk. They found a relationship between the concentration of H2O2 and the resulting particle size, where a higher H2O2 concentration resulted in smaller nanoparticles. Their sample synthesized in 5 M H2O2 resulted in nitrogen doping of 15.19 at.% with an additional sulfur content of 0.09 at.%, all samples showed an amorphous character. As the authors focused on their material's photoluminescent properties, reporting of electrochemical testing was poor, although they showed improvement of the catalytic activity toward the ORR with increased H2O2 concentration, which they attributed to an enhanced electron conduction ability.
Starting from actual waste biomass, Yuan et al. [54] used waste pomelo peel to produce a nitrogen‐doped carbon with three‐dimensional interconnected framework structure based on the original structure of the precursor material. After shredding the peel, soluble impurities were removed with boiling water and hydrothermal carbonization was carried out, followed by annealing at different temperatures under NH3 atmosphere. The authors investigated the influence of annealing temperature and presence of NH3, where they achieved the highest surface area of 1445 m2 g−1 for the sample treated at 1000 °C under NH3 atmosphere, followed by the sample annealed at 1000 °C under argon atmosphere with a surface are of 967 m2 g−1. Decreasing the annealing temperature resulted in significantly lower surface areas of 714 and 518 m2 g−1 for 900 and 800 °C, respectively. All samples consisted of pores smaller than 5 nm; X‐ray diffraction (XRD) and Raman spectra showed unordered, amorphous structures. Electrochemical testing clearly revealed superior performance for the sample treated at 1000 °C under NH3 atmosphere in alkaline and acidic media, although with a less‐favorable onset potential and limiting current compared with a platinum standard. Their sample proved outstanding methanol tolerance and stability with an electron transfer number of 3.7 and a H2O2 yield of 11.9% in alkaline media. The authors attributed their catalysts good catalytic performance to its structure and composition, where they claimed that the high surface area and porous structure can facilitate the active sites and offer a better exposure as well as improve mass transport. They also suggested that the high graphitic nitrogen content can act as active sites and that the interconnected structure of their material can accelerate electron and reactant transport. Although exhibiting the second highest surface area, the sample pyrolyzed under argon atmosphere showed very poor catalytic activity in alkaline and acidic media, which, although not further specified by the authors, will likely be due to the absence of nitrogen dopants.
3.2.3 Catalysts from Biomass Precursors
As discussed earlier, real biomass, may it be a food source or waste product, proves to be a promising starting material for ORR catalysts, although the issue of compositional variability based on the species, location, harvest, and storage of the biomass should not be neglected. Studies have shown that there can be significant differences in the elemental composition of the same plant species/food waste between various habitats as well as between different parts of the same plant (e.g. leaves, husk, and pith) or food waste (e.g. lobster shell from claw or carapace) [55, 56]. Based on these concerns, many researchers have fallen back on using biomass precursors, which, for example can be won from real biomass, such as glucose, cellulose, chitosan, and many more to ensure repeatability of the process.
One of the first hydrothermal carbon ORR catalysts from sustainable biomass precursors was reported by Wohlgemuth et al. [10] in 2012 who synthesized nitrogen‐ and sulfur‐co‐doped carbon aerogels from glucose and ovalbumin, a protein from chicken egg white. Their synthesis was based on the earlier reports by the same research group [41, 57] who established the beneficial role of ovalbumin during the hydrothermal carbonization of glucose to create a nitrogen‐doped porous network structure, where the protein acts as a structure directing/surface stabilizing agent as well as a nitrogen source based on the aforementioned Maillard reactions during the HTC process. The authors investigated co‐doping nitrogen and sulfur on the material's properties and catalytic performance by simple addition of a sulfur precursor (2‐thienyl‐carboxaldehyde) to the reaction mixture before hydrothermal carbonization, followed by supercritical CO2 drying and pyrolysis at 900 °C under an inert atmosphere. Their control sample without the addition of any sulfur resulted in a surface area of 267 m2 g−1 and 3.6 at.% of nitrogen, whereas the addition of sulfur slightly decreased the surface area to 224 m2 g−1 and nitrogen content to 3.3 at.% with a sulfur doping of 0.5 at.%. Compared with other sulfur precursors, they found that the addition of 2‐thienyl‐carboxaldehyde does not interfere with the formation of the aerogel structure and the cross‐linking process can proceed similar to that in the sulfur‐free version. Electrochemical testing in alkaline and acidic media clearly revealed superior performance for the N/S‐co‐doped version over the purely nitrogen‐doped sample, although less favorable than a platinum standard. Surprisingly, stability testing showed a better stability over platinum of the N/S‐doped sample in acidic but not in alkaline media. The authors explained this trend due to sulfur sites being oxidized or decomposed in alkaline media, while they are being protonated and thus chemically more stable under acidic conditions.
Shortly after, the same research group reported nitrogen‐doped carbon aerogels prepared under the same conditions but based on the hydrothermal carbonization of glucose catalyzed by borax [24]. For this, they either mixed glucose with borax to obtain a nitrogen‐free aerogel or they added an additional nitrogen source (2‐pyrrol‐carboxaldehyde) to obtain N‐doped materials. With this, the authors presented an approach to easily tune their materials' surface area and nitrogen content by simply varying the concentration of borax to the nitrogen source. They found that with an increasing borax concentration, the particle size decreased and thus the surface area increased, while the nitrogen content remained similar (3.0–3.5 wt%). An increase in the concentration of nitrogen source resulted in an increased nitrogen content (4.6–6.3 wt%) with a slight decrease in surface area. The sample without additional N doping resulted in the highest surface area of 373 m2 g−1 after pyrolysis. Electrochemical testing revealed a clear influence of surface area and nitrogen doping on the catalytic activity, where a trend could be found between high surface area, high nitrogen content, and favorable catalytic performance. They found their most catalytically active material in alkaline media to be made with the highest borax and nitrogen source concentration, resulting in a surface area of 265 m2 g−1 and a nitrogen content of 6.3 wt%. Their sample had a less positive onset potential but a more negative limiting current when compared with a platinum standard with an electron transfer number of 3.3 and an outstanding stability and methanol tolerance. It should be mentioned that the authors found 35 ppm of iron via inductively coupled plasma (ICP) analysis in their sample, which they did not correlate with their good performance but nevertheless should be considered with care.
In the same year, Brun et al. [30] synthesized nitrogen‐doped aerogels based on a phenolic–saccharide reaction by combining carbohydrate derivatives with phenolic compounds via hydrothermal carbonization followed by supercritical CO2 drying and pyrolysis under N2 atmosphere at 950 °C. The authors suggested two possible mechanisms in which the phenolic compound can act as a cross‐linker, either by intermolecular condensation with dehydrated sugars like HMF or by electrophilic aromatic substitutions to create the highly porous aerogel network. Their sample with the best onset potential and limiting current was made from glucose and N‐acetylglucosamine with a surface area of 450 m2 g−1 and a nitrogen content of 3.3 at.% (22% pyridinic, 18% pyrrolic, 35% graphitic), as can be seen in Figure 3.5a,b, although the highest electron transfer number (around 3.7) was found for the sample made with only N‐acetylglucosamine with a lower surface area of 224 m2 g−1 and a nitrogen content of 4.5 at.% (13% pyridinic, 26% pyrrolic, and 36% graphitic), which the authors attributed to the more favorable combination of pyridinic‐N to pyrrolic‐N. A comparison with pure hydrothermal carbon microspheres, a nitrogen‐free and a nitrogen‐containing aerogel, revealed a positive influence of the aerogel's porous network structure and surface area on the catalytic activity regardless of nitrogen doping (Figure 3.5c).

Figure 3.5 (a) Aerogel obtained after the hydrothermal carbonization of glucose and N‐acetylglucosamine and (b) TEM of aerogel after pyrolysis at 950 °C; (c) Linear sweep voltammogram in 0.1 M KOH for a commercial platinum standard (dotted gray line), the N‐doped aerogel from glucose and N‐acetylglucosamine after pyrolysis (green circles), a nitrogen‐free carbon aerogel (blue triangles), and nitrogen‐free HTC microspheres after pyrolysis (black squares).
Source: Brun et al. 2013 [30]. Copyright 2013. Reproduced with permission from Royal Society of Chemistry.
Another type of nitrogen‐doped carbon aerogels was reported by Alatalo et al. [31] who combined glucose or cellulose with soy bean flour via hydrothermal carbonization followed by freeze‐drying and high‐temperature pyrolysis under inert N2 atmosphere at 1000 °C. The mix of cellulose and soy bean flour resulted in a higher surface area of 697 m2 g−1 compared with 449 m2 g−1 for the glucose/soy bean flour mix, whereas the nitrogen content was reverse with 1.9 and 0.5 wt%, respectively. The sample made from glucose and soy bean flour exhibited a more favorable onset potential and limiting current with an electron transfer number of 3.3–3.7 and a H2O2 yield below 30%, which the authors attributed to the higher nitrogen content.
Based on the work of Wohlgemuth et al. [10], Preuss et al. [32] further developed the glucose/ovalbumin system to synthesize dual‐ and triple‐doped carbon aerogels with nitrogen, sulfur, and boron. They also investigated the influence of O2 activation during the pyrolysis at 1000 °C using a mixed atmosphere of N2 and different air concentrations. Although the sample pyrolyzed under pure N2 atmosphere only resulted in a surface area of 110 m2 g−1, an increase in the air concentration in the atmosphere generated considerably higher surface area of up to 1150 m2 g−1 for 4% of O2, although at the expense of yield due to burning of material in the presence of oxygen. This drastic increase in surface area was explained by oxidation of the carbon surface, basically etching away carbon atoms and creating porosity. The authors concluded that an O2 concentration of 2% during the pyrolysis is most favorable to obtain materials with a good surface area (around 890 m2 g−1), nitrogen content (4 at.%), and acceptable degree of yield loss (Figure 3.6a). By adding different heteroatom precursors to the reaction mixture before the hydrothermal carbonization, they obtained various doped materials, where they found that the addition of boric acid as a boron source resulted in differently structured aerogels with a lower surface area of around 670 m2 g−1, as can be seen in Figure 3.6b. They suggested that, similar to the borax‐mediated aerogels [24], boric acid had a catalytic effect and determined the structure of the aerogel leaving the protein as a sole nitrogen source, which was supported by a higher nitrogen content of 5.1 at.%. Electrochemical testing in alkaline media revealed a similar onset potential for the N‐doped and the N/B‐doped samples, whereas the best limiting currents were achieved by samples containing boron (N/B and N/S/B), which was attributed to the higher nitrogen content (Figure 3.6c). Their best performing sample overall with an electron transfer number of 3.5 and a H2O2 yield below 23%, with 85% of current remaining after 3 h of stability testing, was the co‐doped sample with nitrogen and boron, which the authors attributed to a synergistic effect between N and B.

Figure 3.6 TEM images of doped carbon aerogels made from glucose and ovalbumin (a) and additional boric acid (b). Linear sweep voltammogram in 0.1 M KOH for doped carbon aerogels (N – nitrogen, S – sulfur, B – boron) compared with a commercial platinum standard (c).
Source: Preuss et al. 2017 [32]. Copyright 2017. Reproduced with permission from Royal Society of Chemistry.
Starting with chitosan as a biomass precursor, Wu et al. [58] produced nitrogen‐ and nitrogen/sulfur‐doped carbon nanoparticles by hydrothermal carbonization of chitosan without and with sulfur powder followed by pyrolysis under inert N2 atmosphere at 900 °C. For comparison, they also synthesized a nitrogen‐doped sample by a one‐step process without HTC, which resulted in a low surface area of 198 m2 g−1 with unfavorable catalytic activity. Both samples synthesized via the two‐step process resulted in a nitrogen doping of 3.4 at.% as well as in a similar surface area of 533 and 579 m2 g−1 for the N‐ and the N/S‐doped version, respectively. The sulfur content was determined to be 1.4 at.% in the N/S‐doped version. Both samples showed promising catalytic activity in alkaline media, with a similar onset potential to platinum for the N/S‐doped version and a better limiting current for both as well as electron transfer numbers of 3.9 and 4 for the N‐ and N/S‐doped versions, respectively.
3.2.4 Discussion
It became clear that sustainable biomass resources no matter if food, plant, waste, or biomass precursors present a promising alternative as starting materials for metal‐free ORR catalysts. Interestingly, various authors [35, 37, 52, 58] report how hydrothermal carbonization of the biomass under mild conditions (usually around 180 °C), before the high temperature pyrolysis, results in a more favorable catalytic performance, often rendering the use of harsh chemicals/metal salts for activation or post‐treatment doping unnecessary by creating a beneficial porous structure as well as utilizing any heteroatom precursor more efficiently.
Table 3.1 Summary of best performing sample from each presented reference in Section 3.2, all potentials mentioned are versus Ag/AgCl.
| References | Icon in Figure 3.7 | Main precursor | HTC conditions | Pyrolysis temperature/activation | Onset potential of catalyst (V) | Onset potential of Pt standard (V) | Limiting current of catalyst at −0.6 V | Limiting current of Pt at −0.6 V | Electron transfer number | Stability (current remaining) | Dopants | Name |
| 3.2.1 | ||||||||||||
| [33] | — | Soy milk | 180 °C, 3 h | — | −0.15 | 0.06 | / | / | 3.15 | 91% after 7000 s | N/P | FCN |
| [35] | Fermented rice | 180 °C, 24 h | 700 °C/ZnCl2 | −0.06 | 0.07 | −4.46 | −5.56 | ∼4.0 | / | N | N‐CSs | |
| [36] | Baking yeast | 180 °C, 12 h | 800 °C/— | −0.04 | −0.02 | −4.61 | −5.17 | 3.73 | 84% after 32 400 s | N/P | NPMCS | |
| [49] | Bamboo fungus | 180 °C, 24 h | 800 °C/ZnCl2 | 0.01 | 0.18 | −3.40 | −4.00 | 3.55 | / | N | HAZ‐800 | |
| [37] | Nori biomass | 190 °C, 8 h | 1000 °C/ melamine | 0.01 | 0.01 | −5.86 | −5.61 | 3.97 | 97% after 30 000 s | N/S | HNORI‐M‐AT | |
| [22] | Cantaloupe | 180 °C, 12 h | 800 °C/KOH | −0.2 | −0.03 | −3.57 | −4.29 | 3.90 | 88% after 20 000 s | N | NHCA‐800 | |
| 3.2.2 | ||||||||||||
| [23] | Typha orientalis | 180 °C, 12 h | 800 °C/NH3 | −0.08 | −0.05 | −4.42 | −4.42 | 3.90 | 84% after 10 000 | N | NCS‐800 | |
| [34] | Ophiopogon japonicus | 180 °C, 10 h | — | −0.08 | −0.07 | −4.13 (1000 rpm) | −4.0 (1000 rpm) | 3.94 | 94.5% after 20 000 s | N | N‐CNAs | |
| [51] | Rice straw | 180 °C, 12 h | 900 °C/NH3 | −0.15 | −0.01 | −4.20 | −5.60 | 3.87 | / | N | H‐NC‐900 | |
| [52] | Plant moss | 180 °C, 24 h | 900 °C/— | −0.03 | 0.01 | −4.48 (1100 rpm) | −4.08 (1100 rpm) | 3.94 | 92% after 10 000 s | N | TMC900 | |
| [54] | Pomelo peel | 180 °C, 16 h | 1000 °C/NH3 | 0.01 | 0.01 | −4.82 | −5.50 | 3.7 | / | N | N‐PC‐1000 | |
| 3.2.3 | ||||||||||||
| [10] | Glucose/Ovalbumin | 180 °C, 5.5 h | 900 °C/— | −0.13 | 0.08 | −5.21 | −5.80 | 3.32 | 62% after 12 000 s | N/S | CA‐TCA_900 | |
| [24] | Glucose/borax | 180 °C, 18 h | 900 °C/— | −0.17 | 0.03 | −6.23 | −4.78 | 3.3 | 85% after 12 000 s | N | N3B3_900 | |
| [30] | Glucose/phloroglucinol | 180 °C, 20 h | 950 °C/— | −0.13 | 0.03 | −4.40 | −4.78 | 2.8 | 80% after 12 000 s | N | 950‐G1AG1 | |
| [31] | — | Glucose/soy bean flour | 180 °C, 5.5 h | 1000 °C/— | −0.05 | 0.01 | / | / | 3.5 | / | N | GluSo_1000 |
| [32] | Glucose/ovalbumin | 180 °C, 5.5 h | 1000 °C/N2/O2 | −0.14 | 0.01 | −3.8 | −5.0 | 3.5 | 85% after 10 000 s | N/B | CNB | |
| [58] | Chitosan/sulfur | 180 °C, 12 h | 900 °C/— | −0.04 | −0.04 | −5.62 | −4.39 | 4 | / | N/S | S,N‐GCNP | |
aValues estimated from linear scan voltammogram (LSV) graphs (unless otherwise noted at 1600 rpm).
/ – denotes no information given.
Potentials were converted as follows:
ERHE = EAg/AgCl + E0Ag/AgCl + 0.0591 × pH
ERHE = EHg/HgO + E0Hg/HgO + 0.0591 × pH
For a better understanding, precursors, synthesis conditions, and electrochemical values for the best performing sample of each reference presented above are shown in Table 3.1. Additionally, Figure 3.7 illustrates limiting current density versus onset potential of each sample. If judging the catalysts purely by their onset potential and limiting current, major variations among themselves become obvious. Here, one of the best performing materials reported was synthesized from nori biomass [37], whereas the lowest performance was reached using cantaloupe [22], although the catalyst loading of the nori‐derived material on the electrode surface was considerably higher with around 510 μg cm−2 compared with only 200 μg cm−2 for the cantaloupe‐derived material. Likewise, two catalysts synthesized from the same starting material, glucose and ovalbumin, one co‐doped with nitrogen/sulfur [10] and the other with nitrogen/boron [32], exhibited a similar onset potential, but very different limiting current densities (−5.21 mA cm−2 for N/S and −3.8 mA cm−2 for N/B), where again the catalyst loaded on the electrode was more than double for the N/S‐doped material. Considering these discrepancies, Figure 3.7 can only give a broad overview and its values should not be taken as definitive. Due to lack of standardized procedures for evaluating a material's catalytic activity toward the ORR, a true comparison between samples synthesized by different research groups is hard to carry out. Another issue regarding the performance gap of so‐called metal‐free ORR catalysts can be the unknown presence of metal traces in some samples. Most of the time, absence of sufficient analysis techniques (X‐ray fluorescence, inductively coupled plasma spectroscopy), which would be able to detect these metal traces, poses the problem, as characterization via XPS or EDX is not able to detect below a certain limit. Studies have shown that metal traces even as low as 18 ppm can boost a material's catalytic ORR activity [50]. Statements by authors about the possible presence of iron in biomass like yeast [36] or nori [37] or possible metal residues left behind after using ZnCl2 as an activation agent, without further proving their actual absence should be considered with great care. Similarly, authors claiming that the presence of 35 ppm of iron does not affect their “non‐metal” catalyst's performance [24] without showing proof (e.g. cyanide poisoning) is worrying. In the end, discussions about the possible presence of metal traces in catalysts, particularly when starting from food waste or plant biomass without the addition of metal salts for activation or doping, should be balanced against the fact of easily converting a readily available waste product into a cheap and promising ORR catalyst.
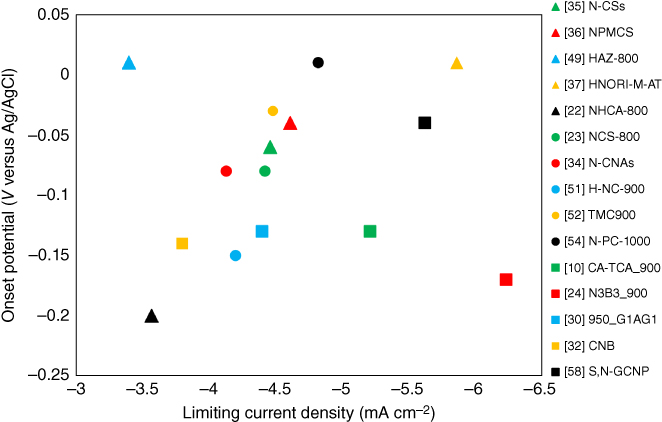
Figure 3.7 Graphical overview of onset potential and limiting current density for the best performing sample of each reference presented in Section 3.2, taken from Table 3.1.
3.3 Carbon–Carbon Composites Based Electrocatalysts
Graphene is a one‐atom‐thick layer carbon material consisting of sp2 hybrid carbon atoms that are densely packed into a two‐dimensional (2D) honey comb crystal lattice. This unique structure containing the delocalized π‐system provides graphene and its allotropes remarkable chemical and physical properties such as high electrical and thermal conductivity, high stiffness, mechanical strength, and high specific surface area. These distinctive properties make graphene a very promising candidate to overcome the limitations of other forms of carbon materials used in energy conversion and storage. There are different formats of graphene materials including graphene sheets, platelets, ribbons, oxidized graphene, chemically/thermally reduced graphene, CNTs, and oxidized unzipped graphene ribbons originating from CNTs, etc. Each of these forms is different in terms of edge/defects and functional groups on the surface and hence their properties are slightly different. However, the overall basic structure and properties are very similar. In the following, different types of graphene will not be further differentiated and graphene will be used as a generic term for any of the above‐mentioned materials.
3.3.1 Carbon Nanostructures/Biomass‐Derived Hydrothermal Carbon Composites
Wu and coworkers in 2013 produced NG materials using urea as a nitrogen source. They have found that increasing the amount of urea resulted in a higher N content and promoted reduction of graphene oxide (GO) in the as‐prepared NG. The explanation was that during the HTC process, ammonia was slowly released from urea, which reacted with oxygen‐containing functional groups leading to N doping; meanwhile, ammonia also increased the pH of the solution, leading to a more effective deoxygenation of GO [44].
Zheng and coworkers also developed NG using the HTC synthesis of graphene with urea as a nitrogen precursor, followed by annealing at 600 °C under Ar atmosphere. The annealing process led to an increased ratio of graphitic‐N and a significant decrease in pyrrolic‐N. The annealing treatment resulted in a more positive half‐wave potential, more obvious diffusion‐limited region, and showed a preference for a four‐electron transfer process [59]. Bao and coworkers produced a sandwich‐like structure comprising N‐doped porous graphene/carbon (NPGC) composites, using glucose as a carbon source and ethylenediamine (EDA) as a nitrogen source. The as‐obtained NPGC‐950 showed excellent ORR activity, which is comparable with that of commercial Pt on carbon. The remarkable catalytic activity was attributed to its high surface area (1512 m2 g−1) and the high ratio of effective nitrogen‐active sites (0.26 at.% of pyridinic‐N and 0.69% of graphitic‐N) [27].
Our group has recently produced a series of nitrogen‐doped carbon–graphene hybrids. The composites were synthesized via hydrothermal process using chitosan as a nitrogen precursor and GO to provide an electrical conductive substrate. The obtained composites were then further carbonized at high temperatures (1000 °C). The hydrothermal process promotes the self‐assembly of chitosan‐derived HTC carbon on the graphene substrate, whereas the annealing treatment further promotes the production of N‐derived active sites. The graphene ratio in the starting dispersion varied from 0 to 5 wt% and 25 wt%, resulting in HTC‐graphene composites with different amounts of graphene (Figure 3.8). This has helped in elucidating the different roles of both the active sites and the conductivity of the carbon matrix toward ORR performance. Our conclusion is that low electrical conductivity limits access to the active sites; however, the amount and type of active sites play a key role in ORR activity when conductivity is improved and is no longer a limitation [45].

Figure 3.8 (a) Scheme illustration of the preparation of NC/rGO composites: (i) hydrothermal carbonization of the mixture of GO and chitosan at 200 °C for 12 h. (ii) after washing and freeze‐drying, the as‐obtained material was annealed at 1000 ° C for 2 h. (b–d) SEM images of (b) NC, (c) NCG0.05, and (d) NCG0.25 obtained after the HTC process with different chitosan/GO ratios. SEM images of (e) NC‐1000, (f) NCG0.05–1000, and (g) NCG0.25–1000 obtained after high‐temperature treatment. (h) Detailed comparison between ORR performance and material properties.
Source: Qiao et al. 2016 [45]. Copyright 2016. Reproduced with permission from Royal Society of Chemistry.
Niu and coworkers in 2016 developed a rationally designed strategy for one‐pot, N‐doped‐carbon‐dot‐decorated GO hybrid (N‐C dots/GO). Urea was applied not only as a nitrogen source but also as an anchor for citric acid (which was the precursor for the carbon dots) on GO via the amidation reaction. The GO/urea/citric acid intermediate was carbonized to form in situ N‐doped C‐dots on GO. The onset potential of the N‐doped C‐dots/GO is slightly more negative than that of commercial Pt–C (∼100 mV), whereas the current density is comparable with that of Pt–C [46].
3.3.2 Assembly of Carbon Nanostructures and Biomass‐Derived Carbon Materials Using Hydrothermal Processes
Apart from acting as a catalytic component itself, graphene and CNTs can also be used as an effective carrier for other active components. Hydrothermal process hereby plays a role in promoting the assembly of the carbon nanostructures instead of hydrothermal carbonization process. Fei and coworkers in 2014 synthesized graphene quantum dot (GQD)/graphene hybrid nanoplatelets with B,N co‐dopant. The GQD was prepared by oxidizing anthracite coal in acid. The hydrothermal process promotes the self‐assembly of GQD on graphene substrate, whereas B was doped by a subsequent annealing process. The doping amount can be controlled by adjusting the annealing duration. The optimized hybrids demonstrated a remarkable ORR activity with ∼15 mV more positive onset potential and similar limiting current density compared with commercial Pt–C [25]. Hu and coworkers produced N‐doped carbon dots decorated on graphene (N‐CDs/G) via hydrothermal treatment in the year 2014. The N‐CDs were obtained by electrochemical etching of coal‐based rods in an electrolyte, which contained ammonia, then the N‐CDs were combined with GO via hydrothermal process to produce the N‐CDs/G hybrid. The as‐prepared N‐CDs/G hybrids showed comparable activity and better stability in comparison to the commercial Pt‐C catalyst in alkaline electrolyte [60]. The hydrothermal process promoted the self‐assembly of GQD on the surface of GO via interactions between the hydroxyl and the carbonyl functional groups of GO and GQDs, leading to the formation or GQD/G hybrid nanoplatelets. The graphene sheets not only serve as 2D platforms to facilitate the uniform distribution of GQDs on the 2D substrate but also improve the electron transfer to interconnect the GQDs via the high electrical conductivity. Meanwhile, the porous scaffold of the as‐prepared GQD/G nanosheets enhanced the facile mass transfer such as electrolyte and electro‐reactants/products. The GQD with rich exposed edges and oxygen‐containing functional groups, on the other hand, allows for easy incorporation of dopants as potential active sites for the ORR reaction.
3.3.3 Hydrothermal Assembly of Other Carbon Nanostructures
Chen and coworkers in 2013 reported a NG/CNT composite prepared by HTC process of GO, oxidized CNTs, and ammonia as a nitrogen precursor at 180 °C for 12 h [61]. The N content of graphene sheets and CNTs were 3.2 and 1.3 at.%, respectively, according to the EDX analysis of transmission electron microscopy (TEM). The as‐obtained nitrogen‐doped graphene/carbon nanotube nanocomposite (NG‐NCNT) exhibited a higher catalytic activity than both NG and nitrogen‐doped carbon nanotube (NCNT) nanocomposite, which demonstrated a synergistic effect of graphene and CNTs. The authors attributed this synergistic effect to (i) the insertion of 1‐dimensional CNTs between graphene sheets, thus preventing the graphene sheets from restacking and increasing the exposed active sites, and (ii) the 3D interpenetrated network structure promoted electron and mass transfer.
Jiang and coworkers in 2014 further developed amine‐functionalized hole graphene (AFHG) by a subsequent etching in the presence of KOH to produce holes on graphene flakes. The large number of holes in the graphene sheet gave more active edge N atoms and enhanced the ORR catalytic activity [62]. In fact, chemical etching using KOH, H2O2, etc., has been widely employed to produce holy graphene nanosheets either during or after the HTC process, as an effective method to promote the content of pyridinic‐N and other edge‐derived active sites [62–64].
Ensafi and coworkers in 2016 produced pyridine‐functionalized N graphene via HTC treatment of NH4OH aqueous with GO suspension [65].
Amino‐functionalized graphene (AG) was synthesized by Yanglong's group via a HTC process of graphite oxide in the presence of ammonia solution. They have demonstrated the important contribution of the amino group by eliminating the amino group by diazotization and hydrolysis and demonstrated the superiority of solution‐phase synthesis in the design and synthesis of N‐containing electrocatalyst. They have also studied the role of various nitrogen species via adjusting the annealing temperature following the HTC process. The results showed that the onset potential and electron transfer number of the ORR process were determined by graphitic‐N and amino‐N, whereas the improved ORR‐saturated current density resulted from the total amount of graphitic‐N and pyridinic‐N [66].
Chen and coworkers in 2014 produced sulfurized graphene nanosheets via a low‐temperature process. The S‐rGO‐180 (rGO, reduced graphene oxide) showed a close onset potential and an even higher saturated current density than commercial Pt‐C, demonstrating more positive onset potential and larger current density compared with the samples without S doping. The Na2S used in this work not only acts as a S source but also as a reductant to reduce GO into rGO. The authors believed that the abundant open edges and defects in graphitic frame works introduced by S doping were the main reasons for the improved ORR performance [67].
Heteroatom‐co‐doping strategies are also widely applied into graphene HTC assembly of carbon nanostructures/hydrothermal carbon composites. For example, Su and coworkers in 2013 produced N,S‐co‐doped 3D graphene frameworks via a one‐pot HTC treatment using NH4SCN as N,S dual‐containing precursor. The NH4SCN decomposited into highly reactive N/S‐rich species such as NH3, H2S, and CS2 to react with the defective sites and oxygen functionalities on GO substrate. GO at the same time was reduced to rGO and assembled into 3D graphene frames during the HTC process. The as‐obtained sample contains 12.3 and 18.4 wt% of N,S, respectively. It showed a four‐electron transfer pathway, comparable kinetic‐limiting current density, better stability, and tolerance to methanol compared with commercial Pt–C [68]. Moreover, B,N‐co‐doped graphene has also been developed as an effective ORR catalyst. The synergistic effect between B and N resulted in comparable saturated current density, better durability, and methanol tolerance compared with commercial carbon. Those B,N‐co‐doped graphene are normally prepared combining HTC treatment to dope N, followed by an annealing process to further dope B into the graphene matrix [69–71].
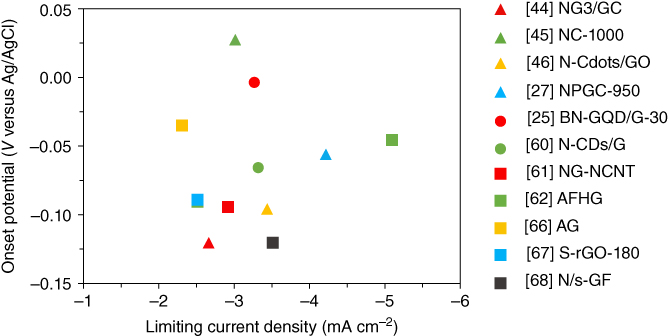
Figure 3.9 Graphical overview of onset potential and limiting current density for the best performing sample of each reference presented in Section 3.3, taken from Table 3.2.
Table 3.2 Summary of best performing sample from each presented reference in Section 3.3, all potentials mentioned are versus Ag/AgCl.
| References | Icon in Figure 3.9 | Main precursor | HTC conditions | Pyrolysis temperature/activation | Onset potential of catalyst (V) | Onset potential of Pt standard (V) | Limiting current of catalyst at −0.6 V | Limiting current of Pt at −0.6 V | Electron transfer number | Stability (current remaining) | Dopants | Name |
| 3.3.1 | ||||||||||||
| [44] | Urea+GO | 170 °C, 12 h | / |
−0.12 (800 rpm) |
0.08 |
−2.65 (800 rpm) |
−3.3 (800 rpm) | ∼3 | / | N | NG3/GC | |
| [45] | Chitosan + GO | 200 °C, 12 h | 1000 °C for 2 h under N2 |
0.028 (1600 rpm) |
0.065 | −3 | −5 | / | 80% after 12 000 s | N | NC‐1000 | |
| [46] | Citric acid, urea, GO | 200 °C, 12 h | / | −0.095 | 0.135 | −3.42 | −4.08 | 3.2 | / | N | N‐Cdots/GO | |
| [27] | Glucose, GO, EDA | 180 °C, 12 h | 950 °C |
−0.055 (1600 rpm) |
−0.035 | −4.2 | −4.8 | ∼3.75 | 82% after 21 600 s | N | NPGC‐950 | |
| 3.3.2 | ||||||||||||
| [25] | Coal, GO | 180 °C, 14 h | 1000 °C for 0.5 h under Ar/NH3, boric acid | ∼ 0 (1600 rpm) |
∼‐0.015 (1600 rpm) |
−3.25 | −3.3 | 3.93 | 73% after 20 000 s | B,N | BN‐GQD/G‐30 | |
| [60] | Coal, GO, ammonia | 180 °C, 12 h | / |
∼‐0.0650 (1600 rpm) |
∼‐0.035 (1600 rpm) |
−3.3 | −4.2 | 3.6–4.0 | 90.6% after 20 000 s | N | N‐CDs/G | |
| 3.3.3 | ||||||||||||
| [61] | Ammonia, GO, CNT | 180 °C for 12 h | / | −0.095 (1600 rpm) | −0.025 (1600 rpm) | −2.9 | −3.05 | 3.7 |
88% After 8000 cycle |
N | NG‐NCNT | |
| [62] | Ammonium hydroxide, GO | 180 °C for 12 h | / | −0.045 (1600 rpm) | −0.005 (1600 rpm) | −5.08 (at‐0.8 V) | −4.76 (at‐0.8 V) | 3.6–4.0 |
> 93% After 36 000 s |
N | AFHG | |
| [66] | Ammonia, GO | 200 °C for 12 h | / | −0.035 (1600 rpm) | −0.005 (1600 rpm) | −2.3 | −3.6 | 3.4–4.0 | 82% after 20 000 s | N | AG | |
| [67] | Na2S, GO | 180 °C for 6 h | / | −0.09 (1600 rpm) | −0.07 (1600 rpm) | −2.5 | −2.8 | 3.8 | 96.3% after 10 000 s | S | S‐rGO‐180 | |
| [68] | NH4SCN, GO | 180 °C for 6 h | / | −0.12 (1600 rpm) | −0.05 (1600 rpm) | −3.5 (at −0.8 V) | −4 (at −0.8 V) | ∼3 | 75.2% after 20 000 s | N, S | N/S‐GF | |
Values estimated from LSV graphs (unless otherwise noted at 1600 rpm).
/ – denotes no information given.
Potentials were converted as follows:
ERHE = EAg/AgCl + E0Ag/AgCl + 0.0591 × pH
ERHE = EHg/HgO + E0Hg/HgO + 0.0591 × pH
3.3.4 General Discussion and Comparison
Detailed information of the typical samples discussed above are shown/listed in Figure 3.9/Table 3.2. For the low‐temperature HTC‐synthesized samples, the addition of graphene leads to a more positive onset potential, higher limiting current density, and improves the electron transfer number and decreases H2O2 yield especially at high overpotentials [45, 46, 60], because the graphene structure promotes the electron transfer during the ORR reaction. The performance of the as‐prepared carbon–carbon composites distinctly varies with different ratio of precursors, which leads to a different assembly of carbon matrix and distribution of active sites [45]. The addition of small amounts of graphene could lead to remarkable changes/improvements in morphology, physical and chemical properties, and electrochemical performance.
Among different formats of graphene materials, GO is the main option as the graphene precursor for HTC assembled carbon–carbon nanocomposites (Figure 3.9). This is because the rich edges, topological defects, and oxygen‐containing functional groups on the panel structure of GO possess abundant free electrons, which favor the hybridization of graphene with HTC precursors [72, 73]. During the hydrothermal process, the GO is converted into rGO showing a higher electrical conductivity, whereas biomass and other precursors with heteroatom sources are hydrothermally carbonized into heteroatom doped carbon. However, because of the high O:C ratio, the electrical conductivity of HTC‐derived rGO is not as high as other formats of graphene materials (e.g. CNTs and thermally reduced graphene), which limits the further improvement of the catalytic activity of the composites. On this consideration, addition of reducing agents (citric acid, etc.) would be expected to promote the removal of the oxygen‐containing functional groups, thus further promoting the electrical conductivity of the composites. High‐temperature annealing has been applied to further improve the electrical conductivity of carbon matrix; this treatment improves the catalytic activity to some extent but is still controversial because this method causes dramatic loss of heteroatom‐derived active sites under high temperatures [25, 27, 45].
The assembly of as‐prepared nanostructures, for example, carbon quantum dots (CQDs), on graphene and CNTs represents another promising technique for electrocatalysis. The CQDs derived from biomass precursors usually possess a high N content and a stable carbonaceous structure; meanwhile, the superior conductivity of graphene in the composites compensates for the poor electrical conductivity of quantum dots and thus further facilitates the electron transfer [72, 73]. Overall, hybridization of NG/CNT composites is regarded as an effective strategy to produce electrocatalysts with 3D structure; this structure results in improved mass and electron transfer, thus leading to superior catalytic activity [29, 61, 74, 75].
Another limitation is that almost all the catalysts discussed were performed in alkaline electrolyte. An efficient HTC catalyst for ORR in acid media, where a proton‐exchange‐membrane fuel cell (PEMFC) works, has been seldom explored.
3.4 Summary and Conclusions
Overall, it has been demonstrated that hydrothermal carbonization is a powerful process to convert biomass into useful carbon materials as well as to create promising carbon–carbon hybrid materials that can be used as electrocatalysts for the ORR in fuel cells. Metal‐free catalysts synthesized from sustainable resources, such as food or plant waste, demonstrated beneficial catalytic activity toward the ORR, sometimes even comparable with commercial platinum standards in terms of onset potential and limiting current density and almost always outperforming platinum in regard to stability and methanol tolerance. Although presenting a promising starting material, concerns about repeatability and conflicts over food‐based materials are limiting the true possibility of replacing platinum with these kinds of materials. Thus, synthetically produced biomass precursors such as glucose create a similarly sustainable and inexpensive way of creating novel metal‐free electrocatalyst with favorable ORR performance.
Hydrothermal carbonization can also be effectively used to achieve the hybridization of graphene‐based carbon–carbon nanocomposites. The graphene and CNTs in the composites usually act as a conductive substrate and sometimes also as a template to direct the pyrolysis process during the HTC. Moreover, due to easy aggregation of graphene nanosheets, the introduction of biomass‐derived HTC nanocarbon structures between the layers is an effective strategy to avoid the nanosheets from aggregating, which helps maintain its superior electrochemical properties. Metal‐free carbon–carbon nanocomposites synthesized via HTC demonstrated comparable onset potential (more positive than approximately −0.125 V versus AgAgCl‐sat.) and limiting current density (∼3–5 mA cm−2) with commercial platinum standards and exhibited better long‐term stability (current retention higher than 70% after 10 000 s of test) and methanol tolerance, which proves their promising potential to become an alternative electrocatalyst to commercial Pt–C.
References
- 1. Katsounaros, I., Schneider, W.B., Meier, J.C. et al. (2012). Hydrogen peroxide electrochemistry on platinum: towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys. 14 (20): 7384.
- 2. Daems, N., Sheng, X., Vankelecom, I.F.J., and Pescarmona, P.P. (2014). Metal‐free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2 (12): 4085.
- 3. Lee, K.R., Lee, K.U., Lee, J.W. et al. (2010). Electrochemical oxygen reduction on nitrogen doped graphene sheets in acid media. Electrochem. Commun. 12 (8): 1052–1055.
- 4. Luo, Z., Lim, S., Tian, Z. et al. (2011). Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property. J. Mater. Chem. 21 (22): 8038.
- 5. Niwa, H., Horiba, K., Harada, Y. et al. (2009). X‐ray absorption analysis of nitrogen contribution to oxygen reduction reaction in carbon alloy cathode catalysts for polymer electrolyte fuel cells. J. Power Sources 187 (1): 93–97.
- 6. Kim, H., Lee, K., Woo, S.I., and Jung, Y. (2011). On the mechanism of enhanced oxygen reduction reaction in nitrogen‐doped graphene nanoribbons. Phys. Chem. Chem. Phys. 13 (39): 17505.
- 7. Lai, L., Potts, J.R., Zhan, D. et al. (2012). Exploration of the active center structure of nitrogen‐doped graphene‐based catalysts for oxygen reduction reaction. Energy Environ. Sci. 5 (7): 7936.
- 8. Qu, L., Liu, Y., Baek, J.‐B., and Dai, L. (2010). Nitrogen‐doped graphene as efficient metal‐free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4 (3): 1321–1326.
- 9. Niwa, H., Kobayashi, M., Horiba, K. et al. (2011). X‐ray photoemission spectroscopy analysis of N‐containing carbon‐based cathode catalysts for polymer electrolyte fuel cells. J. Power Sources 196 (3): 1006–1011.
- 10. Wohlgemuth, S.‐A., White, R.J., Willinger, M.‐G. et al. (2012). A one‐pot hydrothermal synthesis of sulfur and nitrogen doped carbon aerogels with enhanced electrocatalytic activity in the oxygen reduction reaction. Green Chem. 14 (5): 1515.
- 11. Zhang, L. and Xia, Z. (2011). Mechanisms of oxygen reduction reaction on nitrogen‐doped graphene for fuel cells. J. Phys. Chem. C 115: 11170–11176.
- 12. Zhang, L., Niu, J., Dai, L., and Xia, Z. (2012). Effect of microstructure of nitrogen‐doped graphene on oxygen reduction activity in fuel cells. Langmuir 28 (19): 7542–7550.
- 13. Kawaguchi, M., Kawashima, T., and Nakajima, T. (1996). Syntheses and structures of new graphite‐like materials of composition BCN(H) and BC3N(H). Chem. Mater. 8 (6): 1197–1201.
- 14. Wang, S., Iyyamperumal, E., Roy, A. et al. (2011). Vertically aligned BCN nanotubes as efficient metal‐free electrocatalysts for the oxygen reduction reaction: a synergetic effect by co‐doping with boron and nitrogen. Angew. Chem. Int. Ed. 50 (49): 11756–11760.
- 15. Liu, Z.‐W., Peng, F., Wang, H.‐J. et al. (2011). Phosphorus‐doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew. Chem. Int. Ed. 50 (14): 3257–3261.
- 16. Wang, X., Li, X., Zhang, L. et al. (2009). N‐doping of graphene through electrothermal reactions with ammonia. Science 324 (5928): 768–771.
- 17. Sheng, Z.‐H., Shao, L., Chen, J.‐J. et al. (2011). Catalyst‐free synthesis of nitrogen‐doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 5 (6): 4350–4358.
- 18. Nagaiah, T.C., Kundu, S., Bron, M. et al. (2010). Nitrogen‐doped carbon nanotubes as a cathode catalyst for the oxygen reduction reaction in alkaline medium. Electrochem. Commun. 12 (3): 338–341.
- 19. Liu, R., Wu, D., Feng, X., and Müllen, K. (2010). Nitrogen‐doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 49 (14): 2565–2569.
- 20. Yu, D., Zhang, Q., and Dai, L. (2010). Highly efficient metal‐free growth of nitrogen‐doped single‐walled carbon nanotubes on plasma‐etched substrates for oxygen reduction. J. Am. Chem. Soc. 132 (43): 15127–15129.
- 21. Rao, C.V., Cabrera, C.R., and Ishikawa, Y. (2010). In search of the active site in nitrogen‐doped carbon nanotube electrodes for the oxygen reduction reaction. J. Phys. Chem. Lett. 1 (18): 2622–2627.
- 22. Liu, R., Xi, X., Xing, X., and Wu, D. (2016). A facile biomass based approach towards hierarchically porous nitrogen‐doped carbon aerogels. RSC Adv. 6: 83613–83618.
- 23. Chen, P., Wang, L.‐K., Wang, G. et al. (2014). Nitrogen‐doped nanoporous carbon nanosheets derived from plant biomass: an efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 7 (12): 4095–4103.
- 24. Wohlgemuth, S.‐A., Fellinger, T.‐P., Jäker, P., and Antonietti, M. (2013). Tunable nitrogen‐doped carbon aerogels as sustainable electrocatalysts in the oxygen reduction reaction. J. Mater. Chem. A 1 (12): 4002–4009.
- 25. Fei, H., Ye, R., Ye, G. et al. (2014). Boron‐ and nitrogen‐doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 8 (10): 10837–10843.
- 26. Jiang, Z., Zhao, X., Tian, X. et al. (2015). Hydrothermal synthesis of boron and nitrogen codoped hollow graphene microspheres with enhanced electrocatalytic activity for oxygen reduction reaction. ACS Appl. Mater. Interfaces 7 (34): 19398–19407.
- 27. Men, B., Sun, Y., Li, M. et al. (2016). Hierarchical metal‐free nitrogen‐doped porous graphene/carbon composites as an efficient oxygen reduction reaction catalyst. ACS Appl. Mater. Interfaces 8 (2): 1415–1423.
- 28. Suryanto, B.H., Chen, S., Duan, J., and Zhao, C. (2016). Hydrothermally driven transformation of oxygen functional groups at multiwall carbon nanotubes for improved electrocatalytic applications. ACS Appl. Mater. Interfaces 8 (51): 35513–35522.
- 29. Zhang, Y., Jiang, W.J., Zhang, X. et al. (2014). Engineering self‐assembled N‐doped graphene–carbon nanotube composites towards efficient oxygen reduction electrocatalysts. Phys. Chem. Chem. Phys. 16 (27): 13605–13609.
- 30. Brun, N., Wohlgemuth, S.A., Osiceanu, P., and Titirici, M.M. (2013). Original design of nitrogen‐doped carbon aerogels from sustainable precursors: application as metal‐free oxygen reduction catalysts. Green Chem. 15 (9): 2514.
- 31. Alatalo, S.‐M., Qiu, K., Preuss, K. et al. (2016). Soy protein directed hydrothermal synthesis of porous carbon aerogels for electrocatalytic oxygen reduction. Carbon 96: 622–630.
- 32. Preuss, K., Tănase, L.C., Teodorescu, C.M. et al. (2017). Sustainable metal‐free carbogels as oxygen reduction electrocatalysts. J. Mater. Chem. A 5 (31): 16336–16343.
- 33. Zhu, C., Zhai, J., and Dong, S. (2012). Bifunctional fluorescent carbon nanodots: green synthesis via soy milk and application as metal‐free electrocatalysts for oxygen reduction. Chem. Commun. (Camb.) 48 (75): 9367–9369.
- 34. Zhang, H., Wang, Y., Wang, D. et al. (2014). Hydrothermal transformation of dried grass into graphitic carbon‐based high performance electrocatalyst for oxygen reduction reaction. Small 10 (16): 3371–3378.
- 35. Gao, S., Chen, Y., Fan, H. et al. (2014). Large scale production of biomass‐derived N‐doped porous carbon spheres for oxygen reduction and supercapacitors. J. Mater. Chem. A 2 (10): 3317.
- 36. Yu, Y.‐N., Wang, M.‐Q., and Bao, S.‐J. (2017). Biomass‐derived synthesis of nitrogen and phosphorus co‐doped mesoporous carbon spheres as catalysts for oxygen reduction reaction. J. Solid State Electrochem. 21 (1): 103–110.
- 37. Liu, F., Peng, H., You, C. et al. (2014). High‐performance doped carbon catalyst derived from nori biomass with melamine promoter. Electrochim. Acta 138: 353–359.
- 38. Titirici, M.‐M., White, R.J., Brun, N. et al. (2015). Sustainable carbon materials. Chem. Soc. Rev. 44: 250–290.
- 39. Titirici, M.M., Thomas, A., Yu, S.H. et al. (2007). A direct synthesis of mesoporous carbons with bicontinuous pore morphology from crude plant material by hydrothermal carbonization. Chem. Mater. 19 (17): 4205–4212.
- 40. Titirici, M.‐M. and Antonietti, M. (2010). Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 39 (1): 103–116.
- 41. White, R.J., Yoshizawa, N., Antonietti, M., and Titirici, M.‐M. (2011). A sustainable synthesis of nitrogen‐doped carbon aerogels. Green Chem. 13 (9): 2428.
- 42. Titirici, M.‐M. (2013). Sustainable Carbon Materials from Hydrothermal Processes. Oxford, UK: Wiley.
- 43. Nursten, H. (2005). The Maillard Reaction: Chemistry, Biochemistry and Implications. Cambridge, UK: RSC Publishing.
- 44. Wu, J., Zhang, D., Wang, Y., and Hou, B. (2013). Electrocatalytic activity of nitrogen‐doped graphene synthesized via a one‐pot hydrothermal process towards oxygen reduction reaction. J. Power Sources 227: 185–190.
- 45. Qiao, M., Tang, C., He, G. et al. (2016). Graphene/nitrogen‐doped porous carbon sandwiches for the metal‐free oxygen reduction reaction: conductivity versus active sites. J. Mater. Chem. A 4 (32): 12658–12666.
- 46. Niu, W.‐J., Zhu, R.‐H., Yan, H. et al. (2016). One‐pot synthesis of nitrogen‐rich carbon dots decorated graphene oxide as metal‐free electrocatalyst for oxygen reduction reaction. Carbon 109: 402–410.
- 47. Basiago, A.D. (1995). Methods of defining ‘sustainability’. Sustain. Dev. 3: 109–119.
- 48. Briscoe, J., Marinovic, A., Sevilla, M. et al. (2015). Biomass‐derived carbon quantum dot sensitizers for solid‐state nanostructured solar cells. Angew. Chem. Int. Ed. 54 (15): 4463–4468.
- 49. Gao, S., Fan, H., and Zhang, S. (2014). Nitrogen‐enriched carbon from bamboo fungus with superior oxygen reduction reaction activity. J. Mater. Chem. A 2: 18263–18270.
- 50. Wang, L., Ambrosi, A., and Pumera, M. (2013). “Metal‐free” catalytic oxygen reduction reaction on heteroatom‐doped graphene is caused by trace metal impurities. Angew. Chem. Int. Ed. 52 (51): 13818–13821.
- 51. Liu, L., Xiong, Q., Li, C. et al. (2015). Conversion of straw to nitrogen doped carbon for efficient oxygen reduction catalysts in microbial fuel cells. RSC Adv. 5 (109): 89771–89776.
- 52. Zhou, L., Fu, P., Wen, D. et al. (2016). Self‐constructed carbon nanoparticles‐coated porous biocarbon from plant moss as advanced oxygen reduction catalysts. Appl. Catal., B 181: 635–643.
- 53. Zhang, Z., Xu, S., and Wu, P. (2015). H2O2‐assisted hydrothermal process: a green, versatile route to synthesize size‐controllable nitrogen‐doped fluorescent carbon nanoparticles from natural macromolecules. Part. Part. Syst. Charact. 32 (2): 176–181.
- 54. Yuan, W.‐J., Feng, Y., Xie, A. et al. (2016). Nitrogen‐doped nanoporous carbon derived from waste pomelo peel as metal‐free electrocatalyst for the oxygen reduction reaction. Nanoscale 8704–8711.
- 55. Liu, L., Ye, X.P., Womac, A.R., and Sokhansanj, S. (2010). Variability of biomass chemical composition and rapid analysis using FT‐NIR techniques. Carbohydr. Polym. 81 (4): 820–829.
- 56. Boßelmann, F., Romano, P., Fabritius, H. et al. (2007). The composition of the exoskeleton of two crustacea: the American lobster Homarus americanus and the edible crab Cancer pagurus. Thermochim. Acta 463 (1–2): 65–68.
- 57. Baccile, N., Antonietti, M., and Titirici, M.‐M. (2010). One‐step hydrothermal synthesis of nitrogen‐doped nanocarbons: albumin directing the carbonization of glucose. ChemSusChem 3 (2): 246–253.
- 58. Wu, T.X., Wang, G.Z., Zhang, X. et al. (2015). Transforming chitosan into N‐doped graphitic carbon electrocatalysts. Chem. Commun. 51 (7): 1334–1337.
- 59. Zheng, B., Wang, J., Wang, F.‐B., and Xia, X.‐H. (2013). Synthesis of nitrogen doped graphene with high electrocatalytic activity toward oxygen reduction reaction. Electrochem. Commun. 28: 24–26.
- 60. Hu, C., Yu, C., Li, M. et al. (2015). Nitrogen‐doped carbon dots decorated on graphene: a novel all‐carbon hybrid electrocatalyst for enhanced oxygen reduction reaction. Chem. Commun. 51 (16): 3419–3422.
- 61. Chen, P., Xiao, T.‐Y., Qian, Y.‐H. et al. (2013). A nitrogen‐doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 25 (23): 3192–3196.
- 62. Jiang, Z., Jiang, Z., Tian, X., and Chen, W. (2014). Amine‐functionalized holey graphene as a highly active metal‐free catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2 (2): 441–450.
- 63. Jiang, Z., Jiang, Z., and Chen, W. (2014). The role of holes in improving the performance of nitrogen‐doped holey graphene as an active electrode material for supercapacitor and oxygen reduction reaction. J. Power Sources 251: 55–65.
- 64. Sun, J., Wang, L., Song, R., and Yanga, S. (2016). Enhancing pyridinic nitrogen level in graphene to promote electrocatalytic activity for oxygen reduction reaction. Nanotechnology 27 (5): 55404.
- 65. Ensafi, A.A., Jafari‐Asl, M., and Rezaei, B. (2016). Pyridine‐functionalized graphene oxide, an efficient metal free electrocatalyst for oxygen reduction reaction. Electrochim. Acta 194: 95–103.
- 66. Zhang, C., Hao, R., Liao, H., and Hou, Y. (2013). Synthesis of amino‐functionalized graphene as metal‐free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2 (1): 88–97.
- 67. Chen, Y., Li, J., Mei, T. et al. (2014). Low‐temperature and one‐pot synthesis of sulfurized graphene nanosheets via in situ doping and their superior electrocatalytic activity for oxygen reduction reaction. J. Mater. Chem. A 2 (48): 20714–20722.
- 68. Su, Y., Zhang, Y., Zhuang, X. et al. (2013). Low‐temperature synthesis of nitrogen/sulfur co‐doped three‐dimensional graphene frameworks as efficient metal‐free electrocatalyst for oxygen reduction reaction. Carbon 62: 296–301.
- 69. Xu, C., Su, Y., Liu, D., and He, X. (2015). Three‐dimensional N,B‐doped graphene aerogel as a synergistically enhanced metal‐free catalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 17 (38): 25440–25448.
- 70. Tai, J., Hu, J., Chen, Z., and Lu, H. (2014). Two‐step synthesis of boron and nitrogen co‐doped graphene as a synergistically enhanced catalyst for the oxygen reduction reaction. RSC Adv. 4 (106): 61437–61443.
- 71. Patil, I.M., Lokanathan, M., and Kakade, B. (2016). Three dimensional nanocomposite of reduced graphene oxide and hexagonal boron nitride as an efficient metal‐free catalyst for oxygen electroreduction. J. Mater. Chem. A 4 (12): 4506–4515.
- 72. Zheng, Y., Jiao, Y., Chen, J. et al. (2011). Nanoporous graphitic‐C3N4@carbon metal‐free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 133 (50): 20116–20119.
- 73. Yu, D., Nagelli, E., Du, F., and Dai, L. (2010). Metal‐free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 1 (14): 2165–2173.
- 74. Chen, L., Du, R., Zhu, J. et al. (2015). Three‐dimensional nitrogen‐doped graphene nanoribbons aerogel as a highly efficient catalyst for the oxygen reduction reaction. Small 11 (12): 1423–1429.
- 75. Zhang, Z. and Wu, P. (2014). A facile one‐pot route towards three‐dimensional graphene‐based microporous N‐doped carbon composites. RSC Adv. 4 (85): 45619–45624.
