Chapter 18: Schizophrenia
Since the discovery of chlorpromazine in 1953, the number of antipsychotic medications has increased tremendously. Most of these drugs affect the dopaminergic neurotransmission (primarily blocking dopamine D2 receptors). Antipsychotics registered until 2008 could be classified into 3 groups based on receptor occupancy profile rather than using their chemical structure. Conventional (or first-generation antipsychotics, like haloperidol and fluphenazine) with a predominant D2 receptor affinity have benefits in the treatment of delusions and hallucinations (positive symptoms of schizophrenia), which are parallel with a characteristic side-effect profile of extrapyramidal symptoms (EPS) and prolactin elevation. Atypical antipsychotics—which are characterized by a higher antagonist affinity to serotonin (5-hydroxytryptamine, 5HT) 5HT2 receptors than to dopamine D2 receptors—are generally thought to be effective in alleviation of negative symptoms (such as anhedonia, and lack of motivation) beyond the positive symptoms domain of schizophrenia, with a reduced EPS burden (such as clozapine, olanzapine, risperidone). A third group of novel antipsychotics has a partial dopamine agonist profile (aripiprazole), with a lack of or minimal EPS. The nondopaminergic N-methyl-D-aspartate (NMDA) antagonist model of schizophrenia raised new gulatamatergic targets for treatment. NMDA antagonists ketamine and phencyclidine produce wide range of symptoms characteristic for schizophrenia, such as positive and negative symptoms as well as cognitive impairment. Compounds that blunt gulatamatergic activity (for example lamotrigine) or NMDA receptor glycine site allosteric agonists have both showed improvement of schizophrenic symptoms in combination with antipscychotics. The first compound that produced improvement in monotherapy for the treatment of schizophrenia is LY214023, which is a metabotropic Glutamate 2/3 (mGlu2/3) receptor agonist. This chapter reviews neurobiological background of treatments and clinical experiences proven in controlled clinical trials.
Schizophrenia is the most common psychotic disorder, which affects approximately 1% of the population across various cultures. It is a chronic and debilitating illness, which typically manifests during the adolescent and early adulthood stages and causes significant social and economic burden. Schizophrenia is accompanied by a mixture of symptoms such as delusions, hallucinations, and excitement, but essentially it is characterized by “weakening of the mainsprings of volition,” “lowered mental efficiency,” “unsteadiness of attention,” and “inability to shift, arrange and correct ideas, and to accomplish mental grouping of ideas,” as described for the first time by Emil Kraepelin [1]. Meta-analyses of schizophrenic populations have shown the clustering of symptoms into at least three distinct major symptoms domains, which include positive symptoms (such as hallucinations, delusions thought disorder, and paranoidity), negative symptoms (anhedonia, social withdrawal, and thought poverty), and cognitive dysfunctions (inattention as well as disturbances in executive functions and working memory). Genetic, brain imaging, nosological, clinical, and pharmacological studies show the evidence that schizophrenia is a heterogeneous group of disorders.
Since 1953 when chlorpromazine was discovered, hundreds of compounds have been developed for the treatment of schizophrenia. With the development of the first generation antipsychotics, it has for the first time become possible to treat psychotic (positive) symptoms of schizophrenia, such as hallucinations and delusions. These first-generation antipsychotics are generally not considered an efficacious treatment for negative symptoms (such as anhedonia and lack of motivation) or cognitive deficits associated with schizophrenia, and they also cause significant extrapyramidal side effects (like parkinsonism and tardive dyskinesia), as well endocrine disturbances (hyperprolactinemia). After the discovery of clozapine, followed by many other second-generation antipsychotics, the treatment opportunities of schizophrenia improved by partial curability of negative symptoms and by significant decrease of extrapyramidal symptoms. However, only 10–20% of patients with schizophrenia recover fully to the premorbid level of functioning; 60–75% of patients suffer continuously from mental impairment (psychotic, affective, and cognitive impairment), and the remaining 15–20% of patients are treatment resistant (with highly troublesome outcome) [2].
18.1 Neurobiology (Neurochemistry) of Schizophrenia
The pathophysiology of schizophrenia is complex, with multiple factors that contribute to the disturbance of brain functions. Schizophrenia does not result in the dysfunction of one single neurotransmitter system, but in fact it originates from dysregulation of several interacting systems, which include the dopaminergic system, serotonergic (5-hydroxitryptamine, 5HT) system, and gulatamatergic system. More detailed information about neurobiology of schizophrenia see in the following handbooks: D'Haenen and den Boer, 2002, and Kenneth et al., 2002, in the Further Readings.
18.1.1 Dopaminergic Dysregulation
The dopaminerg model of schizophrenia has been supported by three major strains of evidence as follows: 1) all efficacious antipsychotics were effective D2 receptor antagonists, 2) drugs that increase brain dopaminergic activity (DA) induce psychosis in healthy subjects or exacerbate psychotic symptoms in schizophrenics, and 3) more dopaminergic neurochemical alterations are detected in the brain of schizophrenics as compared with healthy controls.
Positive symptoms of schizophrenia have long been considered the result of increased activity of mesolimbic dopaminergic neurons, whereas negative symptoms are the consequences of the decreased activity of the mesocortical dopaminergic pathways. The mesolimbic and striatal dopaminergic system is controlled by the prefrontal cortex (PFC). The lesion of DA neurons in the PFC increased the levels of DA. Its metabolites, such as homovanillic acid (HVA) and dihydroxyphenylacetic acid (DOPAC), as well DA D2 binding site in the striatum and conversely the injection of apomorphine (a DA agonist) into PFC, decreased the striatal dopaminergic activity. Results of studies that examined correlation between plasma HVA concentration and schizophrenic symptoms are ambiguous, but a positive correlation was found between plasma HVA concentration and clinical severity of symptoms in several studies that employed multiple sampling of plasma HVA (and reduced intraindividual variance). Assuming that the mesolimbic and/or the mesocortical dopamine activity is etiologically significant in schizophrenia but that most HVA in the cerebrospinal fluid (CSF) is produced by the nigrostriatal dopamine system, the predictive value of HVA measures in CSF is poor.
The dopamine overactivity model of schizophrenia was not unanimously supported by measurements of dopamine receptor binding at brain regions: the positive findings might be confounded by the effect of previous antipsychotic medication, and the increased receptor density may be a consequence of antipsychotic drug treatment maintained until death. Postmortem studies show increased D2 and D4 but not D1 receptor affinity in various subcortical regions, such as the caudate, nucleus accumbens, and amygdala. However, most in vivo [positron emission tomography (PET)] studies of drug-naive patients have not supported the hypothesis of elevated D2 receptor densities in the striatum. DA D1 receptors did not differ significantly between subjects with schizophrenia and healthy subjects—using [(11)C]SCH 23390 binding—in any of the brain regions or for any of the binding measures studied; however, significant correlation was observed between Brief Psychiatric Rating Scale scores on the negative symptoms with the binding B(max) in the right frontal cortex.
18.1.2 Serotonergic Dysregutaion
The following data support the involvement of the serotonergic (5HT) system in the pathogenesis of schizophrenia: 1) 5HT2A (partial) agonists induced psychosis and hallucinations, 2) the neuroendocrine-challenged paradigms, and 3) atypical antipsychotics show prominent 5HT2 antagonist profile.
The 5HT theory emerged from human observation that the lysergic acid diethylamide (LSD), or 4-iodo-2,5-dimethoxyphenylisopropylamine and mescaline, produced hallucinations predominantly through a common route of action of 5HT2A receptor agonism. The LSD psychosis model failed to show a good face validity; visual hallucinations produced by it are highly unusual in schizophrenia. However, acoustic hallucinations, paranoid delusions, conceptual disorganization, and cognitive impairments that characterize schizophrenia are grossly absent during LSD intoxication. Most studies found no significant differences in the CSF concentration of 5HT metabolite 5-hydroxy-indol-acetilic acid (5-HIAA); however, a few papers reported decrease of 5-HIAA CSF concentration in schizophrenia [3]. Neurochemical evidence suggests that 5HT2A receptor stimulation contributes to facilitation of synthesis and release of DA, which influences either the firing rates of DA neurons and/ or the 5HT2A heterorecptors situated on DA nerve terminals. Activation of 5HT2A receptors in the medial PFC caused a calcium-dependent increase in the frequency of excitatory postsynaptic potentials and currents. These effects can be blocked by the application of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonists or the activation of group mGlu2 receptors. These results suggest that glutamate release plays a key role: It represents a common pathway for the actions of serotonergic hallucinogens that, the 5HT2A agonists, increase 5HT release in the medial prefrontal cortex secondary to stimulation of glutamate release [4].
Neuroendocrine challenge paradigms are widely used in psychiatric research to probe the functioning of a given neurotransmitter system. Because serum prolactin and cortisol are regulated by central 5HT pathways, serotonergic agonists such as D-fenfluramine can be used to study the functional status of central serotonergic synapses in schizophrenia. Several studies showed a significantly enhanced prolactin response to D-fenfluramine in schizophrenic patients, and the prolactin response significantly correlated with negative symptoms. This finding suggests either sensitization or upregulation of 5HT2A/5HT2C postsynaptic receptors. In addition, substantial evidence indicates a direct role of the prefrontal 5HT2A receptors in the cognitive process. Working memory dysfunction is a core symptom of schizophrenia [5].
Clozapine, olanzapine, and risperidone, which are atypical antipsychotics with dominant 5HT2 antagonist profile, blunted felnfluramine-induced prolactin response in schizophrenics. Clozapine, but not haloperidol, blocked the effect of 5HT (partially 5HT2A) agonist, m-chlorophenylpiperazine on adrenocorticotropic hormone, and prolactin release, which demonstrated that clozapine, in contrast to haloperidol, is a functional potent 5HT1c antagonist. The postmortem literature reports increased 5HT1A binding in the prefrontal cortex in schizophrenia; however, a PET study using [[11]C]WAY 100635 as radiotracer for 5HT1A receptor did not detect differences in 5HT1A binding in medication-free schizophrenics compared with normal controls [6].
18.1.3 Gulatamatergic Dysregulation
The anesthetics phencyclidine (PCP) and ketamine are known to produce dissociative and psychotic symptoms with considerable similarity to schizophrenic psychotic symptoms such as the following: positive and negative symptoms, as well as cognitive impairment (deficits of memory, attention, and abstract reasoning), disruptions in smooth-pursuit eye movements, and prepulse inhibition of startle. A direct comparison of healthy volunteers who received subanesthetic doses of ketamine and individuals with schizophrenia shows similar disruptions in working memory and thought disorder between the two groups. A consequence of blocking N-methyl D-aspartate (NMDA) receptors is the excessive release of glutamate in the cerebral cortex; the consequent overstimulation of postsynaptic neurons might explain the cognitive and behavioral disturbances associated with the NMDA receptor hypofunction state.
So far, clozapine and lamotrigine have been investigated in this human experimental psychosis paradigm and have been shown to be effective in suppressing the expression of psychotomimetic symptoms. Haloperidol, which is a DA D2 antagonist, did not suppress ketamine-induced psychosis [7]. However, drugs that facilitate GABAA neurotransmission (e.g., lorazepam) attenuate partially ketamine-induced reactions. These data support the hypothesis that the gulatamatergic neurotransmission has a key role in promoting psychotic symptoms that interact with numerous other neurotransmitter systems, like 5HT and GABA.
Brain imaging studies also provide new solid evidence of gulatamatergic dysregulation in schizophrenia. Reduced NMDA receptor binding have reported in the hippocampus of medication-free schizophrenic patients with (128I)CNS-1261 SPECT [8]. Gray matter changes, such as volume reduction in mediofrontal cortex, prior to manifestation of psychosis could be related to abnormal neuronal plasticity of excitotoxitcity caused by elevated glutamate level [9]. Typical and atypical antipsychotics seem to modulate this system toward normality.
Glutamate neurons have played a key role in the orchestration of the other brain neurons that have been suggested in the pathophysiology of schizophrenia. These neurons include GABA interneurons because reduced GABA synthesis was detected in the inhibitory GABA neurons in the dorsolateral PFC in schizophrenia, dopamine neurons, and serotonergic neurons. The bursting of dopamine-containing neurons—a key response to environmental stimuli—is dependent on NMDA receptor activation.
18.1.4 Cholinergic Dysregulation
A shift of dynamic balance of dopaminergic/cholinergic neurotransmission (increased dopaminergic and/or reduced cholinergic activity) contributes to the development of psychotic symptoms. Neuroimaging and postmortem brain studies (using M1 and M4 selective antagonist [3H]pirenzepine binding) show the role of muscarinic acetylcholine receptors in the pathology of schizophrenia. The numbers of both muscarinic and nicotinic receptors are decreased in schizophrenia. Functional polymorphism of 267A/C in the muscarinic 1 (M1) receptors has been associated with cognitive deficits of the disorder, and the α7 nicotinic receptor has been linked genetically to schizophrenia. The muscarinic/acetylcholinergic system interacts in several other transmitter pathways. M4 receptors are most highly expressed in brain regions rich in dopamine and dopamine receptors. The basal forebrain cholinergic complex—which projects throughout the cerebral cortex—is regulated by GABA-mediated output from the nucleus accumbens. An abnormal dopaminergic tone in the nucleus accumbens might alter the activity of cholinergic projections to the cortex, which thereby links an alteration in cortical function to subcortical dopamine dysregulation. However, M1/M4 agonists such as oxotremorine preferentially increase cortical DA release and inhibit amphetamine-induced DA release in the nucleus accumbens.
Additional, indirect data suggests a key role of muscarinic abnormalities in schizophrenia: Clozapine (and obviously N-desmethylclozapine), with its unique efficacy profile, shows the highest affinity to the muscarinic M1 and M4 receptors.
18.2 Antipsychotics
18.2.1 Receptor-binding Profile Antipsychotics
Small molecules in the treatment of schizophrenia are characterized by clinical and/or pharmacodynamic properties rather than their chemical structures. The first-generation antipsychotics are labeled as “conventional” compared with later developed, “atypical” antipsychotics. However, a considerable level of imprecision exists in the use of this terminology for the second-generation drugs versus atypical antipsychotics. Conventional antipsychotics vary widely in the chemical structures (see Table 18.1), but these drugs share a common route of action of blocking DA D2 receptors with the same or similar affinity in the mesolimbic and nigrostriatal areas of the brain. These drugs are considered conventional based on the long-lasting experience that antipsychotic effect cannot be observed without a certain level of extrapyramidal (neuroleptic) symptoms. For establishing the neuroleptic threshold, several methods have been developed, for example the handwriting test, which can be used to detect the effect of D2 receptor blockade. The appearance of the motoric symptoms such as the handwriting becomes slowed (bradykinesia) and letters decreased in size (micrigraphia), indicate the Parkinsonian (neuroleptic) threshold. Conventional antipsychotics can be classified on a potential scale of low-medium-high, which is based on the affinity to DA D2 receptors and the average therapeutic dose compared with chlorpromazine [10].
Table 18.1 Classification of conventional and atypical antipsychotics by chemical structure
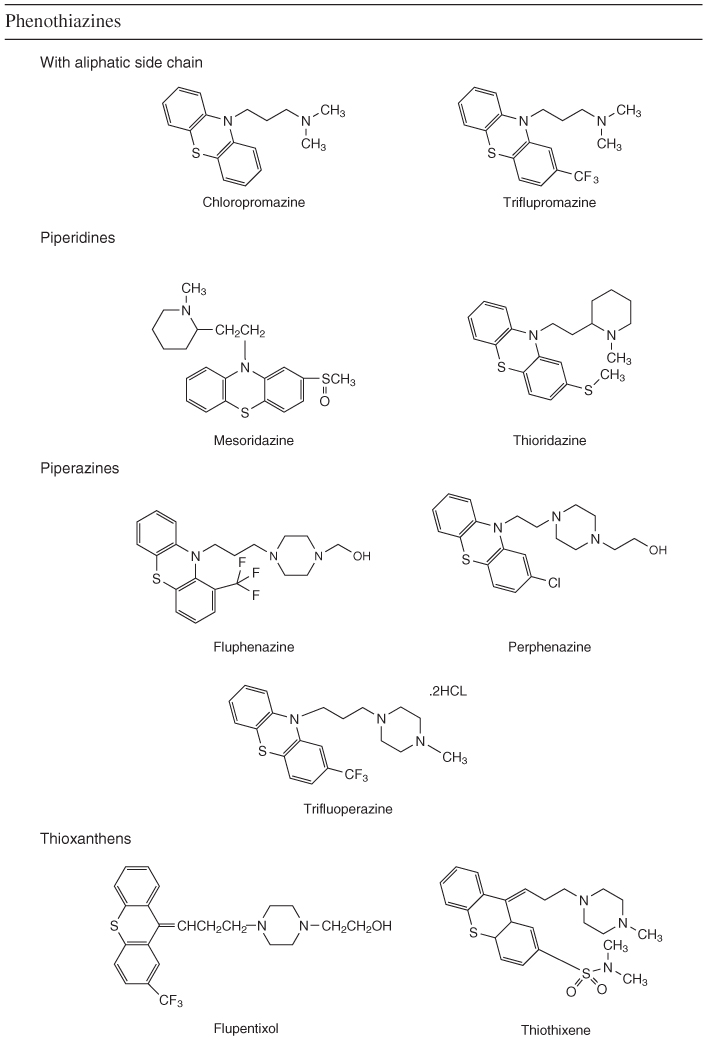
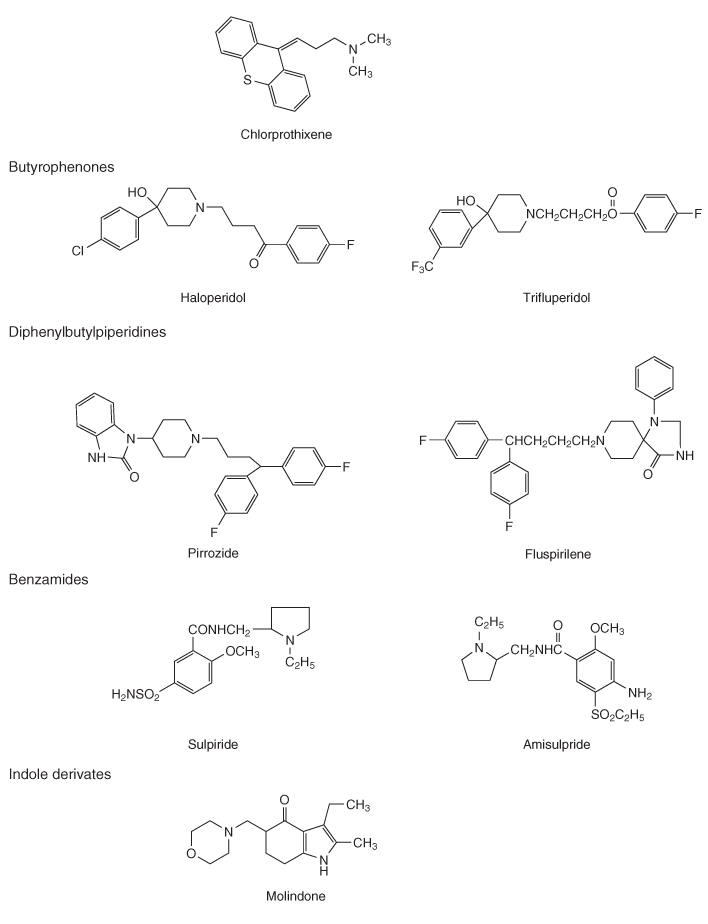
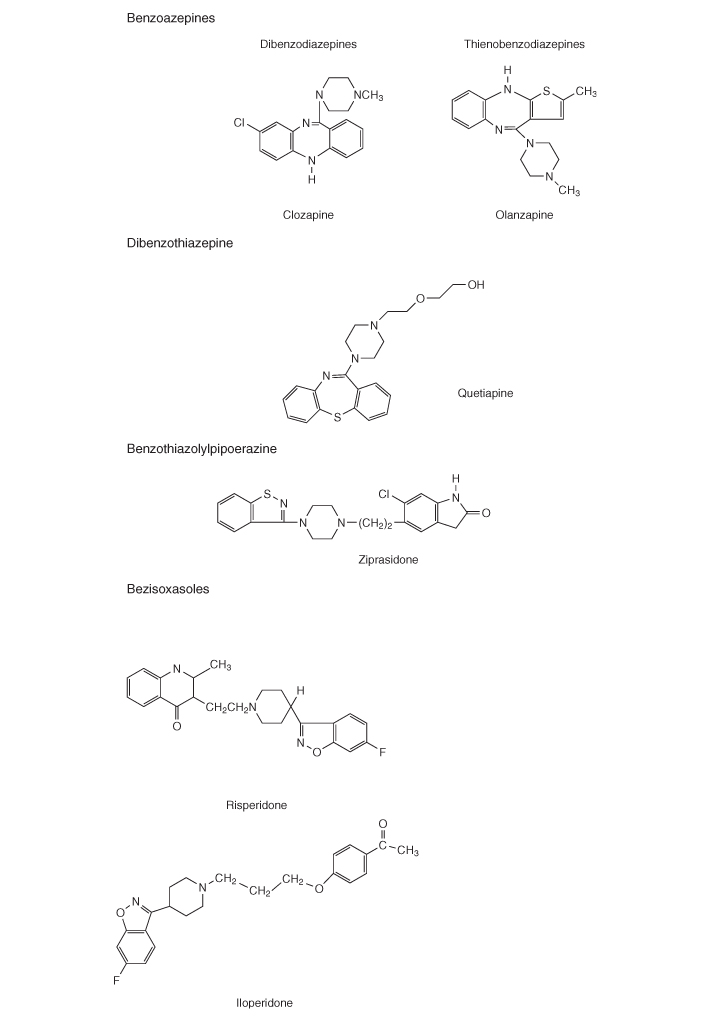
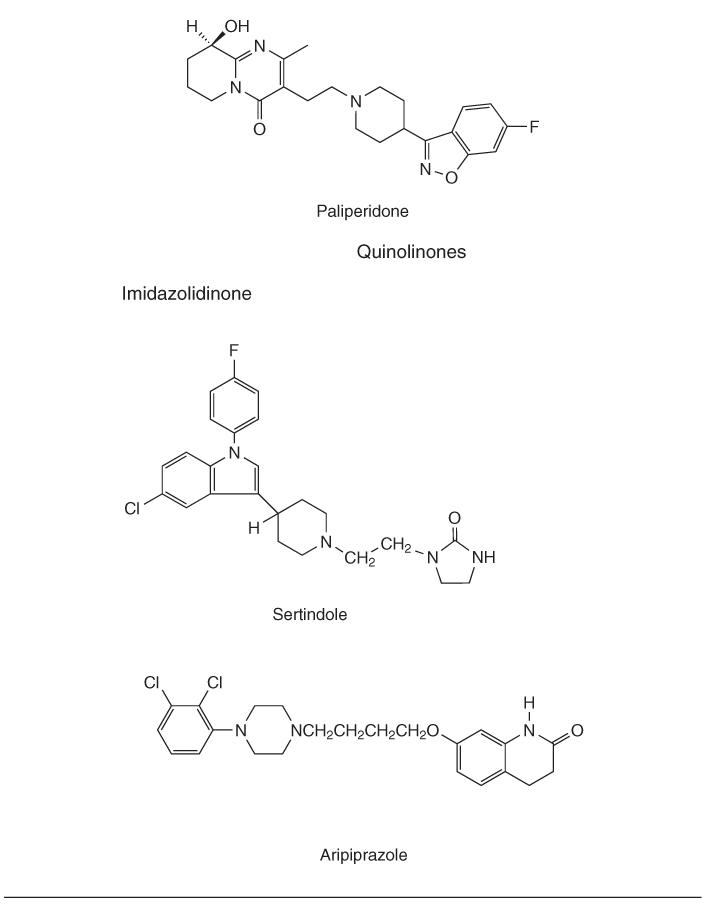
PET studies demonstrated the importance of DA D2 occupancy as a predictor of antipsychotic response and adverse events. The antipsychotic effect in conventional drugs requires 65–70% D2 receptor occupancy, but if it increased to more than 80% it increases significantly the risk of extrapyramidal symptoms. Atypical antipsychotics such as olanzapine do not show increased risk for development of extrapyramidal symptoms. Clozapine, at doses known to be effective in routine clinical settings, shows D2 receptor occupancy of 16–68%, which is lower than risperidone (63–89%) or olanzapine (43–89%) [11].
The strength of DA D2 receptor affinity (log Ki D2) showed a positive correlation with the clinically effective doses for conventional antipsychotics but not for atypical antipsychotics [12]. The most widely accepted hypothesis postulates that a relatively weaker D2 receptor affinity compared with 5HT2A receptor activity is the characteristic for compounds with robust antipsychotic activity. With none or minimal extrapyramidal symptoms in humans and in animal models, these drugs efficaciously block d-amphetamine-induced hyperlocomotion and have weak cataleptogenic effects [13]. Using the ratio of 5HT2C/D2 rather than 5HT2A/D2 receptor affinity [log Ki (5HT2A/D2)] plotted against the clinically effective dose (log average dose) provides a better correlation for conventional and atypical antipsychotics.
Clozapine, which shows minimal EPS liability and robust antipsychotic potential, has partial agonist properties at 5HT1A receptors. Several other atypical antipsychotics, such as aripiprazole and bifeprunix, have 5HT1A receptor agonist properties. The ability of clozapine to increase DA release in the PFC in rats is partly related to its 5HT1A antagonist properties.
Based on the receptor affinity analysis data, it is suggested that the antipsychotic potency is determined by three different factors as follows: 1) increased D2 receptor binding affinity enhances antipsychotic potency, 2) increased 5HT2A and 5HT2C receptor affinity improves antipsychotic effect, and 3) increased 5HT1A receptor binding reduces antipsychotic potential; however, partial or full agonism promotes an antipsychotic effect. Aripiprazole represent a somewhat unique profile: Both on D2 and 5HT1A receptors, it shows partial agonism. It was suggested that aripiprazole has “functional-selective” actions: Depending on the cellular milieu, a mixture of antagonist/partial agonist/agonist actions are likely. Agonist action might induce changes in receptor conformation, which causes changes in sensitivity.
An alternative hypothesis suggests that a faster dissociation rate (koff) from the DA D2 receptor is the key element, which results in a lower overall affinity for the D2 receptor [14]. However, the highly selective D2/3 receptor antagonist, amisulpride, shares a couple of criteria of atypicality with several 5HT2/D2 drugs. A simultaneous 5HT2A and 5HT2C receptor blockade might be more successful in mediating antipsychotic effect than inhibition of either of these receptors separately.
Several conventional and atypical antipsychotics are potent antagonists of the α1 and α2 receptors. The high α1 receptor affinity might explain the atypical feature of clozapine, olanzapine, risperidone, and paliperidone, which has D2 affinity profile similar to numerous conventional antipsychotics. The blockade of α1 receptors increases the DA release in the limbic system in the shell part of the nucleus accumbens, which indicates a limbic rather than striatal effect of α1 antagonism. Most of the atypical antipsychotics mentioned above are also potent α2 receptor antagonists. The combination of idazoxan, which is an α2 antagonist, with the conventional antipsychotic fluphenazine, resulted in improved antipsychotic treatment efficacy similar to clozapine in a treatment-resistant schizophrenic population [15].
18.3 Behavioral Phenotype
In rodents, locomotor activity changes are often used as a screening model for psychosis (schizophrenia). Animals treated with psychostimulants such as d-amphetamine and apomorphine (DA agonists) revealed locomotor hyperactivity as well as stereotyped or perseverative behaviors in higher doses. A blockade of d-amphetamine-induced hyperlocomotion is considered to be a predictor of antipsychotic effect via blockade of DA D2-receptors in the mesocortical—mesolimbical system.
The catalepsy test [16] is a behavioral measure (time spent immobile on an inclined plane) of nigro-striatal DA D2 receptor blockade.
The ratio of doses (ED0.5) of half-minute cataleptogenic effect and (ED50) dose of blunting hyperlocomotion followed by d-amphetamine exposure is considered a predictor of conventional versus atypical profile of an antipsychotic. The ED0.5 for cataleptogenic effect was >120 μmol/kg, >120 μmol/kg, 13.1 μmol/kg, and 0.3 μmol/kg for clozapine, chlorpromazine, risperidone, and haloperidol, respectively. The ED50 for cataleptogenic effect was 2.4 μmol/kg, 0.5 μmol/kg, 0.8 μmol/kg, and 0.2 μmol/kg, which provided a catalpetogenic/antihyperlocomotion ratio of 50.6, 42, 16.8, and 1.8, respectively. This model reliably differentiates clozapine from haloperidol, but chlorpromazine remained in the atypical range [17]. Several other drugs, which do not share a DA and/or 5HT antagonist profile, such as xanomeline (partial agonist to M1/M4 > M2/M3 > M5 and with modest interaction at 5HT1A, 5HT2A receptors), have shown an atypical profile. These drugs block dopamine agonist-induced behavioral disturbances in rodents, without producing catalepsy.
Of the available animal models, subchronic treatment with PCP or ketamine seems to provide the most valid model or schizophrenia-like alterations in the animal behavior, which include hyperlocomotion, stereotyped behavior, as well marked deficits in social behavior. These effects are reversed by clozapine and olanzapine but not by haloperidol.
18.4 Antipsychotics Clinical Efficacy Profile
Conventional versus atypical antipsychotic classification is based on numerous factors, which include behavioral phenotype in preclinical animal models, receptor-binding affinity, clinical efficacy and safety, and efficacy in “treatment-resistant” cases (see Table 18.2). The conceptualization of “atypicality” versus “conventionality” of antipsychotics from a clinical perspective was grossly determined by the fact, that clozapine—the archetype of atypical antipsychotics—provided clinically significant advantages to the established conventional antipsychotics, such as chlorpromazine or haloperidol. Controlled, double-blind studies suggest that clozapine, olanzapine, and risperidone are superior to haloperidol in controlling psychotic symptoms. Risperidone was superior to haloperidol in the treatment of positive and negative symptoms; olanzapine has demonstrated efficacy in the treatment of positive, negative, and mood symptoms of schizophrenia. In several trials, olanzapine was superior to risperidone and haloperidol in the improvement of negative symptoms; however, other studies—with different trial methodology showed a greater benefit of risperidone versus olanzapine in positive and mood symptoms [18]. Quetiapine, ziprasidone, and aripirazol seem to be comparable with chlorpromazine and/or haloperidol in both positive and negative symptoms [19–22]. With positive and negative symptoms, aripiprazole failed to show noninferiority in comparison with olanzapine in the positive and negative symptoms, as well in the depressive symptoms associated with schizophrenia. For further details of meta-analysis of efficacy of antipsychotics see systematic reviews in the Cochran database, and the WFSBP Treatment Guidelines for schizophrenia (Falkai et al., 2005) in the Further Readings.
Table 18.2 Classification of conventional and atypical antipsychotics by chemical structure
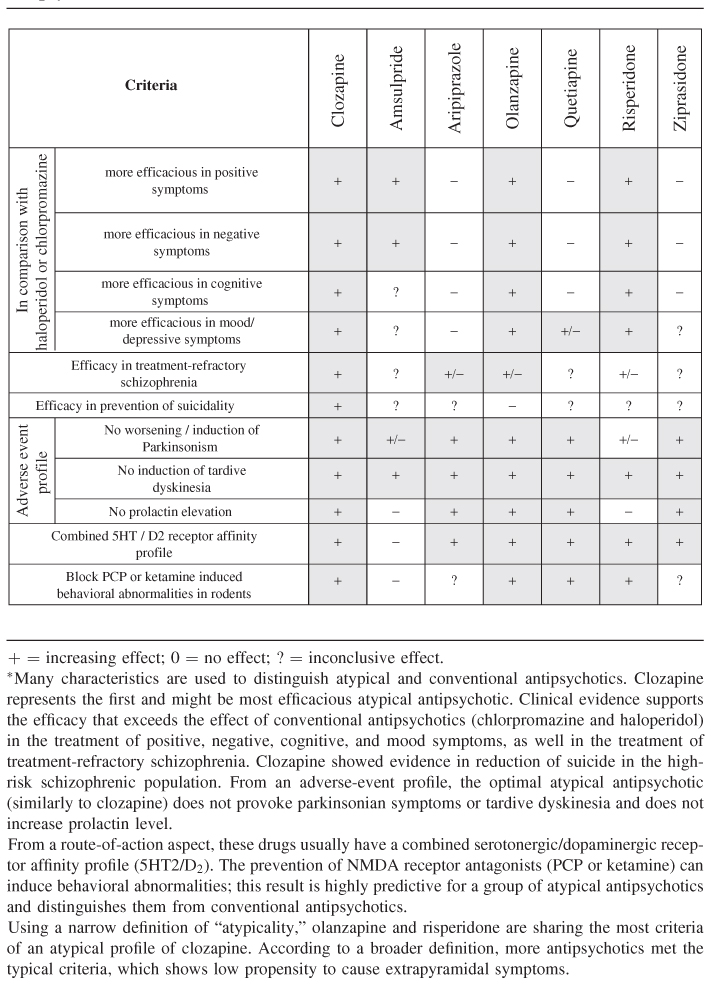
18.4.1 Treatment-resistant Schizophrenia
Clozapine is the golden standard of antipsychotics in the treatment of schizophrenia refractory to other antipsychotic administrations. Clozapine was superior compared with chlorpromazine in patients who showed no clinical response to three previous antipsychotic treatment regimens and 6 weeks of haloperidol administration. In a sample of 268 patients, 30% of patients in the clozapine group met response criteria at 6 weeks compared with 7% of patients treated with chlorpromazine [23]. Olanzapine failed to show superiority to chlorpromazine in a trial designed identically with the clozapine study mentioned above but was found more effective than haloperidol in another study [24]. No difference was observed between aripiprazole and perphenazine (a conventional antipsychotic) in treatment-resistant schizophrenia, where beyond case history, treatment resistance was confirmed by 4 to 6 weeks of open-label treatment with aripiprazole (15–30 mg/day) or perphenazine (8–64 mg/day). No differences were observed in the a priori predefined response rates.
18.4.2 Cognitive Impairments Associated with Schizophrenia
Cognitive symptoms are usually present during the prodromal phase (preceding the first psychotic episode) of schizophrenia and persist during the course of the illness. The impact of cognitive impairment in schizophrenia was well known for Kraepelin [1], but the pharmacotherapy remained ineffective in the cognitive domain until the introduction of clozapine and the newer atypical antipsychotics. Clozapine, risperidone, and olanzapine have shown superior efficacy compared with conventional antipsychotics; these drugs improve verbal fluency, attention, motor functions, and executive functions [25]. In comparison with conventional antipsychotics, the low frequency or absence of drug-induced parkinsonian symptoms in case of atypical antipsychotics might contribute to the cognitive benefits. Haloperidol has a significant negative impact on cognition, sustained attention, reaction time, and speed of information processing [26]. Comparing cognitive effects of various atypical antipsychotics in patients with poor response to conventional antipsychotic treatment clozapine, olanzapine and risperidone showed differences in their patterns of cognitive effects. However, in one study amisulpride was not inferior to olanzapine for any cognitive domain, comparing combined 5HT2A/D2 receptor blockade and D2/D3 receptor inhibition. This finding suggests that 5HT2A/D2 receptor blockade is probably not necessary for cognitive improvement by second-generation antipsychotics [27].
18.4.2.1 Prevention of Suicide
Suicide is one of the leading causes of death in schizophrenia. Four of six retrospective studies provide evidence for the ability of clozapine therapy to reduce suicidal behavior. So far, only one controlled clinical study has been conducted in the high-suicide-risk population, in which clozapine showed better efficacy in prevention of suicidal thoughts or suicide attempts compared with olanzapine [28].
18.5 Adverse Effects Associated with Antipsychotic Treatments
18.5.1 Extrapyramidal (Motor) Side Effects
Acute dystonia occurs secondary to D2 receptor blockade in the nigrostriatal system. Acute dystonia can appear within minutes or up to the first few days of conventional antipsychotic treatment in forms of involuntary and usually painful muscle spasms. These spasms occur particularly in the eye muscles (eyes roll upward, oculogyric crisis), spasm of the neck muscles (torticollis), spasm of laryngeal muscles (laryngospasm), or unilateral spasm of the paravertebral muscles. In addition, rotation and flexion of the upper trunk, neck, and head to one side (Pisa-syndrome) can occur.
18.5.1.1 Medium-term Adverse Events
Akathisia usually occurs within the first week of treatment and involves motor and/or psychic restlessness. Motor restlessness usually affects the lower limbs, with shifting from one foot to the other while standing or frequently crossing and uncrossing the legs while sitting. Akathisia is only partly the consequence of antidopaminergic effects; adrenergic, serotonergic and muscarineregic effects also play role in the development of adverse events.
Parkinsonism usually occurs after several days to weeks of antipsychotic treatment; this disorder mimics idiopathic Parkinson's disease with the typical symptoms of tremor, bradykinesia, rigidity of the muscles of extremities and face muscles (“poker face”), and loss of upper limb swing while walking. The symptomatology of parkinsonism is rather akinetic; rigid tremors (bilateral) occur less frequently.
18.5.1.2 Chronic Extrapyramidal Adverse Events
Tardive dyskinesia (TD) usually manifests after prolonged exposure of conventional antipsychotics. The main symptoms are the choreoathetoid involuntary movements of the tongue and mouth, as well as of the head, neck, and trunk. Movements are often suppressed by voluntary actions that involve the affected part. Based on the following facts, TD was considered as a consequence in supersensitivity of DA D2 receptors. First, TD is evoked by prolonged DA antagonist exposure. Second, dyskinesias increase when the dosage is reduced or the drug is discontinued. Also, movements are reduced by readministration or increase of the dose of the conventional antipsychotic, but increasing conventional neuroleptic dosage increases the risk and severity of tardive dyskinesia in the long run. Beyond upregulation of D2 receptors, degeneration in GABA-generating neurons is another significant pathway in tardive dyskinesia. Acetylcholine might play an important role in tardive dyskinesia as well. Degeneration of the striatal cholinergic neurons was observed in autopsies of patients treated with neuroleptics and who developed tardive dyskinesia. In fact anticholinergics, which are often used in the treatment of parkinsonian symptoms, worsen TD.
18.5.1.3 Neuroleptic Malignant Syndrome (NMS)
This syndrome is life threatening; it involves hyperthermia, muscle rigidity, severe instability of autonomic functions, and consciousness fluctuation. These symptoms are accompanied by extreme increase of creatinine phosphokinase and prolactin. NMS was rarely reported with atypical antipsychotics.
18.5.2 Nonmotor Side Effects of Antipsychotics
18.5.2.1 Hematologic Side Effects
Almost all groups of psychotropic drugs have been reported to cause dysfunctions in the hemopoietic system. In case of phenothiazines (chlorpromazine), the risk of developing agranulocytosis is approximately 0.13%; in case of clozapine, the risk is 0.85%. Reports of agranulocytosis led to the withdrawal of clozapine in the 1970s, and in most countries, clinical administration of clozapine is restricted only to treatment-resistant schizophrenia. Metabolism of clozapine, nor-clozapine, and/or clozapine-N-oxide to electrophilic nitrenium ions is the initial step in the events that lead to agranulocytosis. Nitrenium ions may bind to neutrophils causing either cell death or neutrophil apoptosis via oxidative stress. Antineutrophil antibody formation against the neutrophil protein + nitrenium ion hapten may also be involved in the pathophysiology of agranulocytosis induced by clozapine.
18.5.2.2 Weight Gain and Metabolic Effects
Concerns about extrapyramidal symptoms induced by conventional antipsychotics have been replaced by weight gain, glucose deregulation, and dyslipidemia caused by atypical antipsychotics. Weight gain during atypical antipsychotic treatment varies by the length of administration and by body mass index (BMI) at baseline: Patients with low BMI (<23) tend to gain more weight compared with patients with BMI greater than 27 who saw less weight gain. Psychotropic drugs with significant histaminergic activity (such as the antidepressant mirtazapine or the low potential conventional antipsychotics) have well-known effects of weight gain. Weight gain after short-term atypical antipsychotic exposure significantly correlates with H1 histaminergic affinity of the drugs, as well with α1A, 5HT2C, and 5HT6 receptor bindings. Other analysis shows correlation between weight increase and affinity to D2, α2, 5HT1A, and 5HT2C receptors. Dopamine produces a robust decrease in feeding behavior in animal models. Blockade of D2 receptors in the lateral-perifornical hypothalamus seems to increase feeding, but it decreases appetite in nucleus accumbens [29]. Antipsychotics might influence the energy homeostasis indirectly or directly. Leptin and ghrelin play major roles in the control system for energy balance in humans. Elevation of adipocyte-derived hormone leptin levels has been observed in schizophrenic patients during several atypical antipsychotic treatment (olanzapine and clozapine) early after the onset of medication. Changes in the gut-derived hormone ghrelin levels were observed as well in a biphasic manner depending on the treatment duration; a decrease at was observed earlier time intervals and an increase was observed in longer ones.
The use of (atypical) antipsychotic medications is associated with an increase in adipose mass and the development of diabetes. Characteristic hormonal findings in patients with insulin-resistance syndromes include elevated levels of plasma insulin. In studies conducted on schizophrenic patients, plasma insulin levels are markedly increased compared with the control population. The changes in insulin levels associated with the use of antipsychotic medications are not acutely observed. The increase in insulin levels and insulin resistance in the absence of weight gain may be the result of an increase in the secretion of insulin counter-regulatory hormone glucagon. DA D2 receptors are expressed in β cells of both rats and humans and are believed to mediate glucose-stimulated insulin secretion with insignificant effects on the basal secretion of insulin (see Table 18.3) [30].
Table 18.3 Metabolic effects of haloperidol and atypical antipsychotics [30]
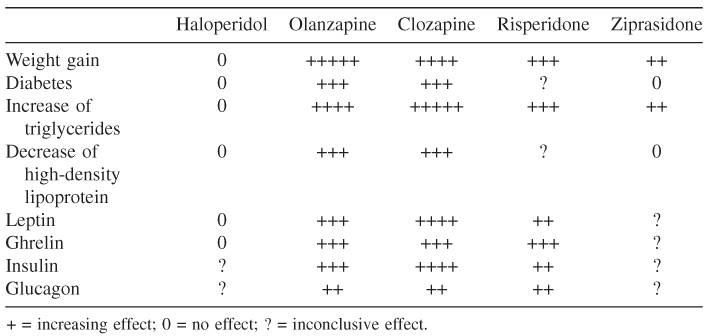
The comparison of antipsychotic-induced weight change after 10 weeks of standard drug dose showed the rank of clozapine > olanzapine > thioridazine > sertindol > chlorpromazine > risperidone > haloperidol > fluphenazine > ziprasidone (weight neutral) > molindine (weight loss) > placebo (weight loss) [31]. Genetic variation such as polymorphism of 5HT2C receptor gene influences antipsychotic-induced weight changes [32].
Patients with schizophrenia are 2–3 times more likely to have Type 2 diabetes than adults in the general population. Large population studies found that patients treated by atypical antipsychotics, such as clozapine, quetiapine, and olanzapine are at higher risk for developing Type 2 diabetes than patients treated by conventional antipsychotics. Decreased pancreatic beta-cell responsiveness as well as decreased insulin sensitivity that is possibly mediated by antagonism of the H1 5HT2C receptor, as well M3 muscarinic receptors, might lead to the development of Type 2 diabetes.
Clozapine, olanzapine, and to a lesser extent quetiapine are associated with an increase of triglyceride and serum cholesterol levels, whereas risperidone and aripirazol have minimal or no effect. The antipsychotics route of action in deregulation of lipid metabolism is unclear, but peroxisome proliferator-activated receptors might play a role [33].
18.6 Cholinergic Drugs (Positive Modulators of Muscarinic Receptors)
The hypothesis that positive allosteric modulation of muscarinic receptors might lead to reduction of psychosis symptoms of schizophrenia was raised by a study of xanomeline in Alzheimer's Disease (AD). Xanomeline showed dose-dependent improvement in cognitive–behavioral disturbances associated with AD, which include reductions in vocal outbursts, suspiciousness, delusions, hallucinations, paranoia, and other behavioral disturbances that share similarities with the positive symptoms observed in schizophrenia [34]. However, a 4-week placebo-controlled proof-of-concept trial of with xanomeline that consisted of 20 schizophrenic patients (with a dose of 225 mg/day) showed significant improvement in both positive and negative cognitive domains of schizophrenia [35].
18.7 Gulatamatergic Drugs in the Treatment of Schizophrenia
From a mechanistic perspective, if NMDA antagonists (ketamine, PCP, and MK-801) provoke or worsen psychotic symptoms by increase of glutamate release, drugs that as agonists on the NMDA receptor complex might provide an antipsychotic effect. Direct NMDA agonists provoke excessive excitation and seizure should not be feasible to develop due to their toxicity. However, positive allosteric modulators of NMDA receptor glycine site, such as glycine, full agonist D-serine, and partial agonist D-cycloserine, are potential candidates. In a behavioral model, PCP-induced hyperactivity in rodents was blocked by glycine and glycine uptake inhibitor gycinedodecyclaminde [36]. Studies have demonstrated the putative benefits of a D-serine and D-cycloserine in reduction of negative symptoms and the benefit of D-serine, D-alanine, and glycine treatment for improvement of cognitive symptoms. A meta-analysis of adjuvant glycine-site agonist treatments show a moderate amelioration of negative symptoms based on the data of 19 relatively heterogeneous trials [35].
Lamotrigine is a broad-spectrum anticonvulsant with a route of action that includes inhibition of glutamate release. In a placebo-controlled trial of a lamotrigine add-on to clozapine, administration in treatment-resistant schizophrenic patients showed significant improvement of positive symptoms. Lamotrigine adjuvant therapy in 400 mg was efficacious in another trial of treatment-resistant schizophrenia by adding it on to conventional and atypical antipsychotics.
Like atypical antipsychotics (e.g., clozapine and olanzapine), agonists for mGlu2/3 receptors (e.g., LY354740 and LY379268) attenuate some of the behavioral effects of PCP in animals and block increased glutamate release [36]. The ability of mGlu2/3 receptor agonists to block PCP-induced behaviors seems to be linked to suppression of glutamate transmission, as it can still be observed in monoamine-depleted rats [37].
LY2140023 (the methionine prodrug of the potent mGlu2/3 receptor agonist LY404039) showed statistically significant superiority compared with placebo in monotherapy of schizophrenia, both in the positive and negative symptoms of the illness. An adverse-event profile of LY2140023 differed from any existing conventional or atypical antipsychotics. A lack of prolactin increase or worsening of extra pyramidal symptoms along with a significant reduction in akathisia, was observed in patients treated with the mGlu2/3 agonist when compared with placebo. In addition, the investigational new drug was weight neutral. This study provided the first clinical evidence about efficacy of a new, nondopaminergic monotherapy paradigm in schizophrenia [38].
After the breakthrough of the first efficacious antipsychotics during the mid-twentieth century, followed by the clinical use of atypical antipsychotics with a better efficacy/extrapyramidal adverse event profile in the 1990s, the discovery and development of new antipsychotics with novel routes of action is still in the main focus of psychopharmacology with the goal of full recovery of symptoms.
References
1. Kraepelin E. Dementia Praecox and Paraphrenia. 1971. Krieger Publishing, Huntington, New York.
2. Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol. Psychiatry 2005;10:27–39.
3. Bleich A, Brown SL, Kahn R, van Prag HM. The role of serotonin in schizophrenia. Schizophr. Bull. 1988;14:111–123.
4. Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism, J. Neurosci. 2001;21:9856–9866.
5. Cameron AM, Oram J, Geffen GM, Kavanagh DJ, McGrath JJ, Geffen LB. Working memory correlates of three symptom clusters in schizophrenia. Psychiatry Res. 2002;110:49–61
6. Frankle WG, Lombardo I, Kegeles LS, Slifstein M, Martin JH, Huang Y, Hwang DR, Reich E, Cangiano C, Gil R, et al. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology 2006;189:155–164.
7. Krystal JH, D'Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Cassello K, Bowers MB Jr, Vegso S, Heninger GR, et al. Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology 1999;145:193–204.
8. Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol. Psychiatry 2006;11:118–119
9. Deutsch, SI, Rosse, RB, Schwartz, BL, Mastropaolo J. A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin. Neuropharmacol. 2001;24:43–49.
10. Baldessarini RJ, Katz B, Cotton P. Dissimilar dosing with high-potency and low-potency neuroleptics. Am. J. Psychiatry 1984;141:748–752.
11. Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am. J. Psychiatry 1999;156:286–293.
12. Richtand NM, Welge JA, Logue AD, Keck PE Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology 2007;32:1715–1726.
13. Meltzer HY, Fang V, Young MA. Clozapine-like drugs. Psychopharmacol. Bull. 1980;16:32–34.
14. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no Parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol. Psychiatry 1998;3:123–134.
15. Litman RE, Su TP, Potter WZ, Hong, WW, Pickar D. Idazoxan and response to typical neuroleptics in treatment resistant schizophrenia. Comparison with the atypical neuroleptic clozapine. Br. J. Psychiatry 1996;168:571–579.
16. Hoffman D, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal liability. Psychopharmacology 1995;120:128–133
17. Jackson DM, Mohell N, Bengtsson A, Malmberg A. What are atypical neuroleptics and how do they work. In Brunello N, Mendelewitz J, Rcagni G eds. New Generation of Antipsychotic Drugs: Novel Mechanism of Action. 1993. Karger, Basel, Switzerland.
18. Conley RR, Mahmoud R, Risperidone Study Group. Efficacy of risperidone vs olanzapine in the treatment of patients with schizophrenia or schizoaffective disorder. Am. J. Psychiatry 2001;158:765–774.
19. Peuskens J, Link CGG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Pscyh. Scand. 1997;96:4:265–273.
20. Arvanitis LA, Miller BG, Seroquel Trial 13 Study Group. Multiple fixed doses of seroquel (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol. Psychiatry 1997;42:233–246.
21. Goff DC, Posever T, Herz L, Simmons J, Kletti N, Lapierre K, Wilner KD, Law CG, Ko GN. An exploratory haloperidol-controlled dose finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective psychosis. J. Clin. Psychopharm. 1998;18:296–304.
22. Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. Efficacy and safety of aripiprazol and haloperidol vs placebo in patients with schizophrenia and schizoaffective disorder. J. Clin. Psychiatry 2002;63:763–771.
23. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenia. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988;45:789–796.
24. Breier AF, Hamilton SH. Comparative efficacy of olanzapine and haloperidol for patients with treatment-resistant schizophrenia. Biol. Psychiatry 1999;45:403–411.
25. Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effect of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bull. 1999;25:201–222.
26. Saeedi H, Remington G, Christensen BK. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr. Res. 2006;85:222–231.
27. Wagner M, Quednow BB, Westheide J, Schlaepfer TE, Maier W, Kühn KU. Cognitive improvement in schizophrenic patients does not require a serotonergic mechanism: randomized controlled trial of olanzapine vs amisulpride. Neuropsychopharmacology 2005;30:381–390.
28. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S, International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia. International Suicide Prevention Trial (InterSePT) Arch. Gen. Psychiatry 2003;60:82–91.
29. Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 2000;16:843–857.
30. Elias AN, Hofflich HH. Abnormalities in glucose metabolism in patients with schizophrenia treated with atypical antipsychotic medications. Am. J. Med. 2008;121:98–104.
31. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic induced weight gain: a comprehensive research synthesis. Am. J. Psychiatry 1999;156:1686–1696.
32. Reynolds GP, Zhang ZJ, Zhang XB. Association of antipsychotic drug-induced weight gain with 5HT2c receptor gene polymorphism. Lancet 2002;359:2086–2087.
33. Arulmozhi DK, Dwyer DS, Bodhankar SL. Antipsychotic induced metabolic abnormalities. Life Sci. 2006;79:1865–1872.
34. Boidick NC, Offen WW, Shannon HE, Satterwhite J, Lucas R, Van Lier R, Paul SM. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1997;11:S16–22.
35. Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dubé S, Mallinckrodt C, Bymaster FP, McKinzie DL, Felder CC. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008;165:1033–1039
36. Javitt DC, Sershen H, Hashim A, Lajtha A. Reversal of phencyclidine-induced hyperactivity by glycine and the glycine uptake inhibitor gycildodeccyclamide. Neuropsychopharmacology 1997;17:202–204.
37. Tuominen HJ, Tiihonen J, Wahlbeck K. Glutaminergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophrenia Res. 2005;72:225–234.
38. Cartmell J, Monn JA, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology 2000;148:423–429.
39. Swanson CJ, Schoepp DD. The group II metabotropic glutamate receptor agonist (-)-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylate (LY379268) and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J. Pharmacol. Exp. Ther. 2002;303:919–927.
40. Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. Metabotropic glutamate 2/3 receptor agonism represents a novel therapeutic approach in treating schizophrenia: results from a randomized, placebo-controlled Phase 2 clinical trial. Nature Med. 2007;13:1102–1107.
Further Reading
Cochrane database of systematic reviews. Amisulprid for schizophrenia. Cochrane Database Syst. Rev. 2002;2:CD001357.
Cochrane database of systematic reviews. Olanzapine for schizophrenia. Cochrane Database Syst. Rev. 2005;2:CD001359.
Cochrane database of systematic reviews. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst. Rev. 2003;2:CD000440.
Cochrane database of systematic reviews. Sertindol for schizophrenia. Cochrane Database Syst. Rev. 2005;3:CD001715.
D'Haenen H, den Boer JA, Willner P, eds. Biological Psychiatry. 2002. Wiley, New York.
Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller HJ, WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, Part 1: acute treatment of schizophrenia. World J. Biol. Psychiatry 2005;6:132–191.
Kenneth L, Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: The Fifth Generation of Progress. 2002. Lippincott Williams & Wilkins, Philadelphia, PA.
