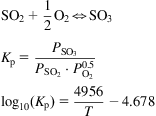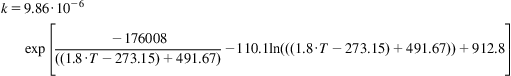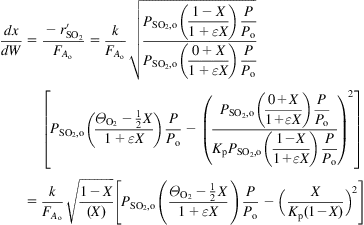7.3.3.3.2 Oxidation kinetics
In the previous section, only the equilibrium is evaluated to design the reactor. However, the bed depth depends on the kinetics, which is a function of the catalysts—platinum, iron oxide, and V2O5 (Fogler, 1997).
The mechanism of the reaction is suggested to be the following:
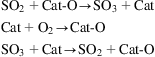
The kinetics of the reaction were established by Eklund (1956):
(7.4)
where k is a function of the catalyst and the geometry, and Pi is the partial pressure of each species. For the example, based on the data from Eklund’s paper, the catalyst is V2O5 supported on volcanic rock with a volumetric density of 33.8 lb/ft3. In the following example, we determine the depth of a catalytic bed in a converter for SO3 production.
7.3.3.3.3 SO3 absorption
The SO3 produced is sent to absorption columns where it is put into contact with water. Typically, the water remains in a solution of sulfuric acid. It is a chemical absorption:
The process is highly exothermic, and the formation enthalpy of sulfuric acid is as follows:
Apart from the formation itself, if the sulfuric acid is diluted, solution enthalpies must be considered too. In this section we design one such a column.
Apart from the kinetics, the flow through a tower has a pressure drop. The pressure drop across the absorption tower is given by the Ergun equation, and depends on the type of packing. An empirical correlation was developed by Cameron and Chang (2010):
(7.5)
The pressure drop given by Eq. (7.5) must be corrected to include that of the loading region so that:
(7.6)
where C2, C3, and C4 are adjustable parameters that can be seen in Table 7.4. VL is the irrigation rate (ft/s) based on an empty tower. Fs is the ratio between the gas velocity across an empty tower and the density as V/ρ0.5, and ε is the porosity of the tower, typically around 0.75.
Table 7.4
Coefficients for Pressure Drop Estimation (Cameron and Chang, 2010)
| Packing Type | Size (in) | C2 | C3 | C4 |
| Standard saddle | 1 | 0.69 | 22.33 | 1.6 |
| Standard saddle | 1.5 | 0.38 | 22.33 | 1.6 |
| Standard saddle | 2 | 0.21 | 22.33 | 1.6 |
| Standard saddle | 3 | 0.15 | 22.33 | 1.6 |

The diameter is computed (as in typical absorption towers) using the loading line for determining the K parameter, and the following set of equations (Sinnot, 1999):
(7.7)
 (7.7)
(7.7)
7.3.3.3.4 Mixing tanks: heat of solution
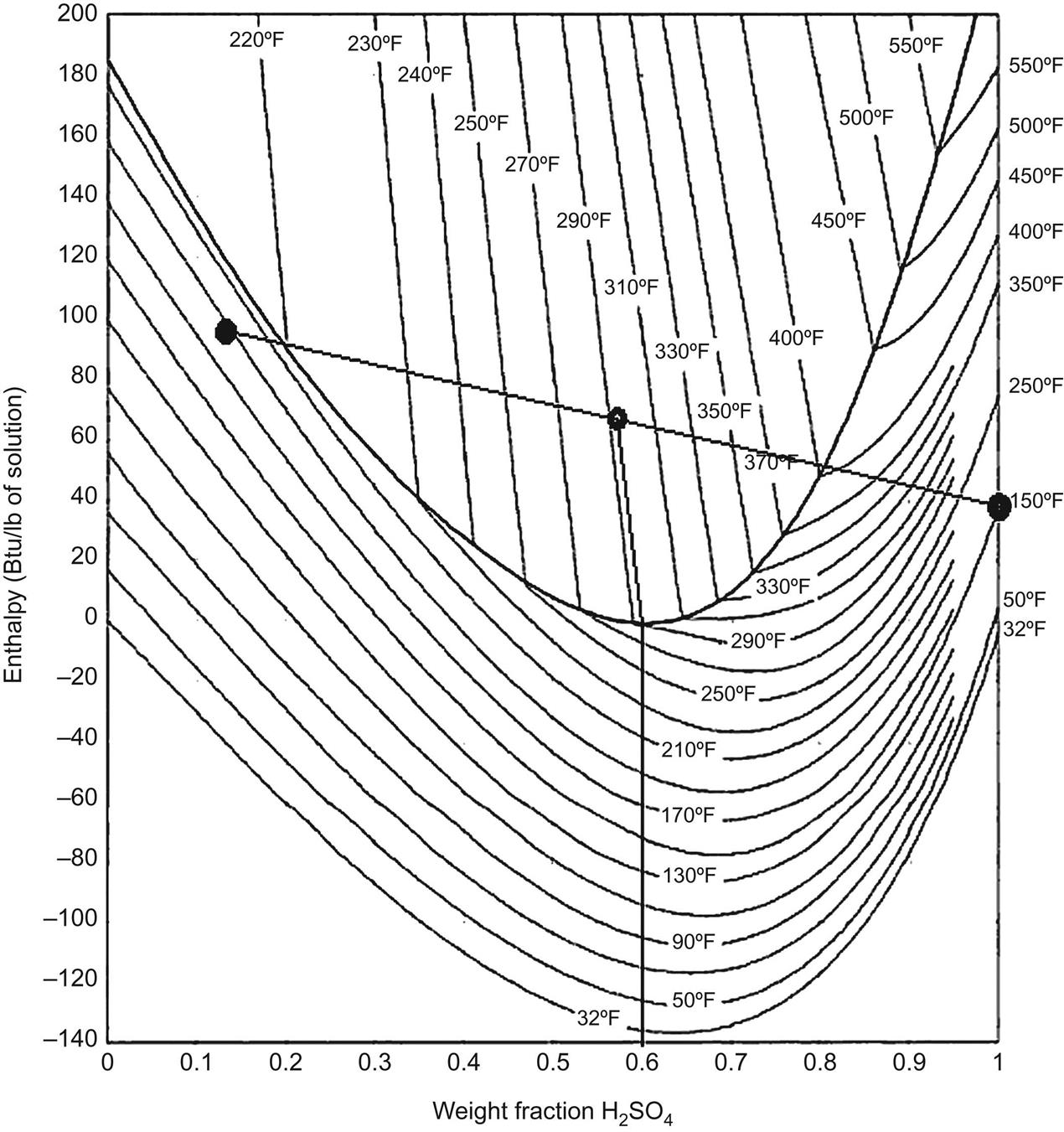
When mixing acids or acid solutions there is a certain energy involved. In Fig. 7.11 the integral heat of solution for sulfuric acid is presented at 25°C. The mixture is exothermic and can result in the evaporation of water. Therefore, apart from the health and safety issues related to acid solution mixing, the concentration of the resulting mixture is to be carefully computed to account for that (Houghen et al., 1959). Example 7.10 presents such a case. We compute the energy involved in the mixing using enthalpy concentration diagrams.
7.4 Problems
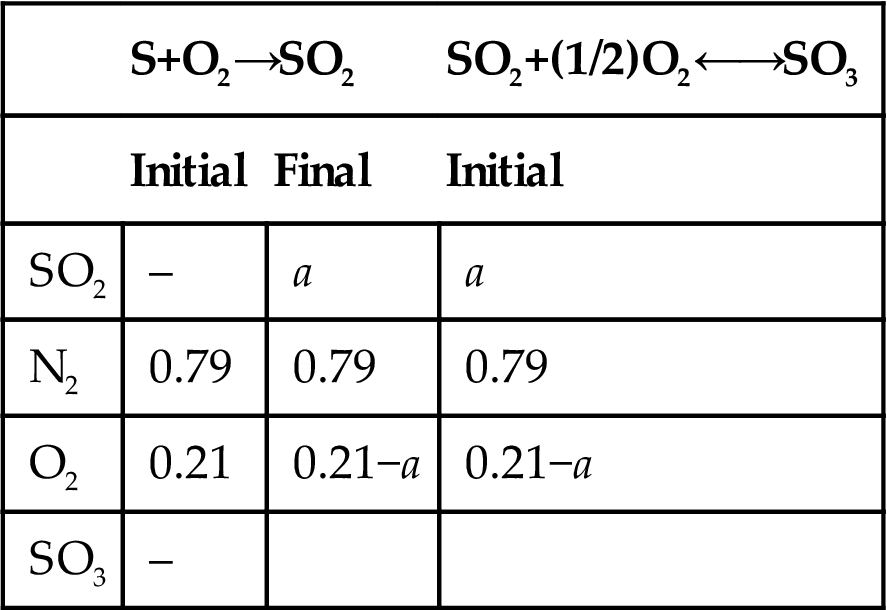
P7.1. A mixture of SO2, O2, and N2 from sulfur burning with dry air is fed to a converter. The mixture contains 8% per volume of SO2. The gas is fed to the converter at 415°C and 1 atm. The converter oxidizes SO2 to SO3 using two beds with intercooling. Assume that the equilibrium conversion is reached at each of the beds (which operate adiabatically). Select between both equilibrium curves below (Fig. P7.1A and B). Determine the composition of the gases exiting each bed and the cooling temperature after the second. Compute the temperature at which the gases from the first bed must be cooled down to so that the final conversion is 95%.

P7.2. A mixture of SO2, O2, and N2 from sulfur burning with dry air is fed to a converter. The converter oxidizes SO2 to SO3 using two beds with intercooling. The gas is fed to the converter at 415°C and 1 atm. The conversion obtained is 95% and the gases exit at 767K. The gases are fed to the second bed at 450°C. Assume that the equilibrium conversion is achieved at each bed and that both operate adiabatically. Plot the equilibrium curve under the operating conditions, and compute the conversion reached after the first bed and the composition of the gases entering and exiting the converter.

P7.3. A mixture of SO2, O2, and N2 from sulfur burning using dry air is fed to a converter. The mixture contains 10% per volume of SO2. The gas is fed to the converter at 415°C and 1 atm. The converter oxidizes SO2 to SO3 using two beds with intercooling. Assume that the equilibrium conversion is reached at each one of the beds (which operate adiabatically). After the first bed, the stream is sent to an absorption tower where all the SO3 is removed. The gases are recycled to the reactor and fed to the second bed at 450°C. Determine the equilibrium curve for each bed and the conversion after the first bed as well as the global conversion of the converter.


P7.4. Sulfur dioxide for the production of sulfuric acid is produced from pyrite roasting. The furnace is fed with pyrite (FeS2) that contains moisture and inert slag. The facilities manager believes that the composition in the contract is not correct based on the products obtained. Air is fed at 25°C and 700 mmHg with a relative humidity of 40%. The gas product has a dew point of 12.5°C and the furnace is operated with twice the stoichiometric oxygen. The slag collected after 10 min contains 0.36 t of FeS, 36 t of Fe2O3, and 6.6 t of inert slag. Determine the composition of the mineral that was sold to the plant and the heat losses, assuming cp,slag=0.16 kcal/kg K and the pyrite is fed to the furnace at 25°C.
P7.5. The gases from pyrite burning are fed to a Glover Tower where a fraction of the SO2 is transformed into H2SO4. Together with the gas, a stream consisting of 592 kg (77% H2SO4, 22% H2O, and 1% N2O3) comes from a Gay-Lussac Tower, and another one from lead chambers (178 kg of H2SO4, 64%); we feed 1.32 kg of HNO3 as catalyst makeup. From the tower we obtain 770 kg of acid (78%); see Fig. P7.5. Determine the conversion of SO2 and the composition of the product gas.
P7.6. Determine the conversion reached in a 25 m tall tower for the absorption of SO3 to produce H2SO4. Assume that the kinetic rate is given by the following equation:
P7.7. The feed to the chambers (at 91°C) is given in Table P7.7. Water is fed at 25°C. The unconverted gases have a dew point of 2°C at 1 atm. These gases are fed to the Gay-Lussac Tower at 40°C. The liquid product, 178 kg of sulfuric acid (64% by weight), exits the chambers at 91°C. Compute the conversion of SO2 in the lead chambers, the water added, and an energy balance to the lead chambers. See Fig. P7.7 for the process scheme.
P7.8. A stream of 467.1292 mol/s at 2 atm and 750K is fed to a catalytic multibed converter. The gas comes from burning sulfur, and 51.42 mol/s of the stream is SO2. Determine the equilibrium conversion and the final temperature after the first catalytic bed. The converter has a diameter of 6.650 m; the catalyst features are given below:
P7.9. Compute the amount of sulfuric acid 98% required to absorb 99.9% of the SO3 in the gases from the third catalytic bed (see Table P7.9), and the acid temperature. The product has a composition of 99%, the gases are at 200°C, and the acid is fed at 90°C. The unabsorbed gases exit at 90°C and we remove 6500 kcal/s using cooling.
P7.10. Evaluate a sulfuric acid production facility that uses the contact method with one absorber. Humid air at 25°C and relative humidity of 0.55 is dried using sulfuric acid 99%, the product. The acid gets diluted to 98% and is used to absorb SO3 produced at the converter. Assume that the molar ratio of dry air to sulfur is 10:1. Sulfur is burned with the dry air to produce SO2. The gas mixture is sent to the converter, which achieves 98% conversion to SO3. This stream is sent to the absorber. In the absorber, water, sulfuric acid 98%, and the gas are put into contact so that sulfuric acid 99% is produced. Compute the amount of sulfuric acid produced (99%), the flowrate of H2SO4 98%, the flow rate of water fed to the absorption tower, and the fraction of sulfuric acid used to dry the initial air. Determine the temperature of operation at the converter assuming isothermal operation at 1 atm.
P7.11. Evaluate a sulfuric acid production facility that uses the contact method with one absorber. Humid air at 25°C and relative humidity of 0.55 is dried using sulfuric acid 99%, the product. The acid gets diluted to 98% and is used to absorb SO3 produced at the converter. Assume that the molar ratio of dry air to sulfur is 10:1. Sulfur is burned with the dry air producing SO2. The gas mixture is sent to the converter, which operates isothermally at 1.5 atm and 700K. This stream is sent to the absorber. In the absorber, water, sulfuric acid 98%, and the gas are put into contact so that sulfuric acid 99% is produced. Compute the amount of sulfuric acid produced (99%), the flowrate of H2SO4 98%, the flow rate of water fed to the absorption tower, and the fraction of sulfuric acid used to dry the initial air.
P7.12. To produce sulfuric acid using the lead chamber process, SO2 is produced by burning sulfur. Sulfur (100 kg/h) with 10% inert slag are fed to the furnace. The sulfur is fed at 25°C. Humid air—100% excess with respect to the stoichiometric one—is fed at 25°C and 700 mmHg. The gases leaving the furnace have a dew point of 12.5°C and are at 450°C; the slag is collected at 400°C (cp=0.16 kcal/kg). Determine the composition of the gases if all the sulfur is burned to SO2, the air moisture, and the heat loss of the burner.
P7.13. The gas leaving the furnace from P7.12 is fed into the chamber system. The aim is to produce sulfuric acid 64%. 0.005 mol of HNO3 per mol of SO2 are also fed in the form of an aqueous solution 40% w. Determine the gas composition and the water fed for a conversion of SO2 to sulfuric acid of 95%. The gas product has a dew point of 10°C at 760 mmHg.
P7.14. Using a process simulator (ie, CHEMCAD, ASPEN), model a four bed reactor for the oxidation of SO2 to SO3 assuming that equilibrium is reached after each bed. The feed consists of 10% SO2, 11% O2, and 79% N2 at 750K and 1 atm. Assume that equilibrium is reached at each bed:
a. Determine the cooling needs after each bed and the conversion after each bed to reach at least 98% conversion after the four beds.
b. Explain what happens if after the third bed, SO3 is removed from the system.

Table P7.7
| kmol | kg | |
| N2 | 15.5612727 | 435.715636 |
| O2 | 1.95656918 | 62.6102137 |
| H2O | 1.41367395 | 25.4461312 |
| SO2 | 1.1921243 | 76.2959555 |
| NO | 0.16332356 | 4.89970694 |
| Total | 20.2869637 | 604.967644 |
| Temp (K) | 364 |