CARBON, C
THIS “VITAL FORCE” OF LIFE
see-oh-two see-aytch-ohs see-oh-two
From CO2 to CHOs and back to CO2,
And so this “vital force” of life transfers from me to you.
GROUP 14 NON-METAL
Photosynthesis is the process whereby plants harness the sun’s energy to drive the reaction between water (H2O) and atmospheric carbon dioxide gas (CO2), producing breathable oxygen (O2) and edible carbohydrates—chemical compounds made up of carbon (C), hydrogen (H), and oxygen (O). These CHOs are the primary source of energy for your body’s cells, where they react with O2 from the air you inhale.
CHOs + O2 → CO2 + H2O + Energy
You exhale the CO2 and H2O vapor, which are waste products, thus returning the CO2 to the atmosphere. The CO2 is then taken up again by plants to make more CHOs for someone else to eat, and the carbon cycle begins all over again.
In 1828, the German chemist Friedrich Wöhler (1800–1882) successfully synthesized urea (NH2CONH2) in his laboratory. This marked the first time that an otherwise naturally occurring, or organic, chemical had been artificially created without involving living organisms. Naturally occurring urea is a by-product of protein metabolism in the body and is excreted in urine. Until then, the so-called vital force theory had held that such organic chemicals could only be produced by living organisms with the aid of some mysterious vital force within them. Wohler’s synthesis of urea sounded the death knell for this vital force theory and heralded the birth of organic chemistry, the chemistry of carbon.
pet, as in “a pet dog”
Its PET scans pinpoint cancers,
Positron emission tomography (PET) is a nuclear magnetic imaging technique that identifies the location of cancer cells in the body. In one application of the technique, a small amount of the vitamin choline, doped with radioactive carbon-11 isotope, is injected into a vein. Cancer cells take up this radioactively labeled choline more readily than normal cells and can be detected with a PET scan.
While its “dating” undoes chancers.
[chancers: noun, plural (slang) — a confidence trickster, “con man,” or rogue]
The carbon dating process measures quantities of carbon-14 to find out the approximate age of some types of archaeological artifacts. Carbon-14 is a radioactive isotope of carbon that forms in minute amounts when nitrogen in the upper atmosphere is bombarded by cosmic rays. It then makes its way (via atmospheric CO2) into the structures of all living trees and plants during photosynthesis, accumulating to extremely low yet detectable levels.
Once dead, the tree or plant no longer incorporates any “fresh” carbon-14. The remaining carbon-14 gradually decays radioactively with a half-life of 5,730 years. So, you can estimate the age of an item by measuring how much carbon-14 it still contains. To carbon-date an ancient artifact, such as a wooden-handled knife, you’d first measure the residual amount of carbon-14 within the wooden handle. Then, by comparing this result with the amount of the isotope that would have been present if the wood were still attached to a living tree, you can estimate how long it’s been since the piece of wood was cut from the tree to make the handle.
Bond-friendly with its neighbors so, each day brings compounds new.
Carbon’s position at the top of group 14 of the periodic table makes it a particularly versatile element. It is capable of forming bonds not only with other carbon atoms but also with atoms of many other elements, such as hydrogen, oxygen, nitrogen, sulfur, phosphorus, and the halogens.
To carbon our world’s fate is bound through gas and oil and coal,
Organic to our way of life, our true synthetic soul.
From plastics to medicines (not to mention fuels and energy), today’s world relies heavily on the synthetic organic chemistry of carbon. That reliance is destined to be challenged in the years ahead as the planet’s finite reserves of carbon-based fossil fuels become ever scarcer.
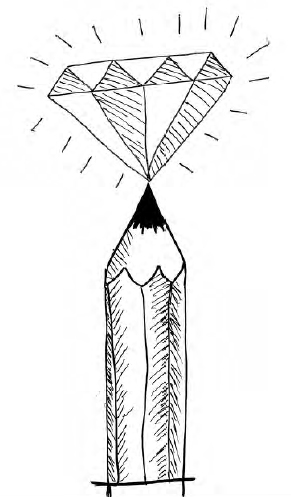
From nanotubes and fullerene,
To diamonds, graphite, thin graphene,
Depending on how its individual atoms are bonded, carbon can adopt different physical forms (known as allotropes). For example, diamond consists of a rigid, semi-infinite, 3D array of carbon atoms; each atom is bonded to four others in a tetrahedral arrangement. In graphene, each carbon atom is bonded to three others in a hexagonal, 2D, semi-infinite, single layer. You can stack multiple sheets of graphene on top of each other to produce graphite, the “lead” you find in pencils (see “Molybdenum, Mo” on page 123 for more about pencil lead). Or you can roll the sheets into cylinders to make nanotubes, which are a type of fullerene. Alternatively, 60 carbon atoms shaped as a hollow spherical shape (so the hexagonal bonding pattern resembles the stitching on a soccer ball) makes buckminsterfullerene, C60, the poster child of the fullerenes!
This rhyme’s too short by far for all its virtues to extol.
This line speaks for itself!