Chart Man’s ascent, probe Earth’s extent, find iron at both their cores,
With coke ’n’ flux its pig we make by smelting Fe’s ores.
With zinc, ’gainst rust it’s galvanized,
Chart Man’s ascent, probe Earth’s extent, find iron at both their cores,
With coke ’n’ flux its pig we make by smelting Fe’s ores.
With zinc, ’gainst rust it’s galvanized,
GROUP 8 TRANSITION METAL
Used in tools, weapons, industrial machinery, and construction materials, iron has played a major role in humanity’s development in many ancient societies and in the present day. If it were possible, a journey to Earth’s center would reveal a mostly molten core composed mainly of iron.
eff-ees
Obtained through mining, iron ores are solid mixtures of iron oxides, silicates, carbonates, hydrates, sulfides, and impurities. The first step in the industrial production of iron involves the smelting of iron ore in a blast furnace to produce pig iron. The process involves heating the iron ore in the presence of coke (a carbon-rich solid residue obtained when certain coals are strongly heated in the absence of air to remove all their volatile constituents) and a suitable flux (such as calcium carbonate, CaCO3), which bonds with impurities in the ore so they can be removed. By blasting plenty of fresh air up through the base of the furnace, the oxygen in the air reacts with the carbon in the coke to form carbon monoxide (CO). In turn, the CO reduces the iron oxide in the ore to molten iron. The calcium in the flux reacts with silicates in the ore to form a calcium silicate slag that floats atop the molten iron and is removed. The pig iron that remains is the raw material used to produce the wrought irons, cast irons, and steels that have made iron the most important and versatile material in the history of civilization.
Iron has an Achilles’ heel. In the presence of oxygen (O2) in the air and help from some moisture (H2O), iron is electrochemically converted to a hydrated form of iron(III) oxide (that is, Fe2O3 combined with some H2O), which is better known as rust. Ironically, the electrochemical basis for forming rust provides a convenient way of controlling it. Galvanizing involves coating vulnerable iron with a layer of metallic zinc, which protects it from contact with air and moisture. Because the zinc reacts more readily than iron with oxygen, any scratches that develop in this protective layer won’t promote rusting of the underlying iron. The zinc is “sacrificed” to spare the iron. This process is further described in the section “Zinc, Zn” on page 87.
The atoms of most transition metal elements have some unpaired d-electrons. These unpaired electrons account for the phenomena of temporary magnetism (paramagnetism) and permanent magnetism (ferromagnetism). The reason is that each atom or ion with unpaired electrons has a magnetic moment associated with it; a magnetic moment is a bit like a tiny bar magnet with a north pole at one end and a south pole at the other—they’re represented by the arrows in the following diagram.
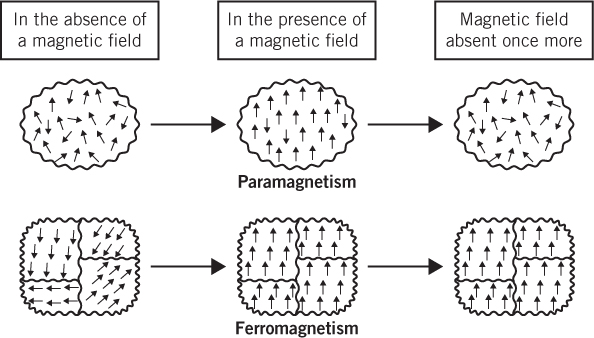
The difference between paramagnetism and ferromagnetism—the arrows represent the magnetic moments (that is, the tiny bar magnets) associated with individual atoms or ions that have unpaired electrons; only a few of the many magnetic moments are shown.
In a sample of a paramagnetic substance, the bar magnets are randomly oriented throughout. But when the sample is placed within a magnetic field, most of these bar magnets align in the direction of that field, causing the sample to be drawn toward the field. Remove the field and the bar magnets become randomly oriented once more.
Ferromagnetic substances consist of regions called domains. Each domain contains a large number of atoms that already have their individual bar magnets aligned in a given direction, although this direction varies from one domain to the next. When a ferromagnetic substance is placed within a magnetic field, all the bar magnets in all the domains line up in the direction of the field, and the substance becomes magnetized. In addition, this total alignment persists even after the field is removed, thus leaving the substance permanently magnetized. Ferromagnetism only occurs in substances when the distances between their constituent atoms are such that they allow the atoms to be arranged into domains. Iron is one of the few elements that meets this requirement (iron’s close neighbors, cobalt and nickel, are two others). This gives the element and many of its compounds their famous permanent magnetic properties.
oh-two
Iron is central to the process of transporting oxygen (as the diatomic molecule, O2) to every cell in the body via the hemoglobin in red blood cells. Hemoglobin molecules bind O2 molecules in groups of four because each molecule of hemoglobin contains four heme groups within its globular protein structure. At the heart of each heme is an atom of iron in the +2 oxidation state, and each Fe2+ ion can reversibly bind a single molecule of life-sustaining O2.
