8
Recycling Technologies – Hydrometallurgy
Denise C. R. Espinosa, Rafael P. de Oliveira, and Thamiris A. G. Martins
University of São Paulo. Polytechnic School, Department of Chemical Engineering, Av. Prof. Luciano Gualberto, 380 – Butantã, São Paulo, SP 05508-010, Brazil
8.1 Background
Electronic waste (e-waste) has in its composition polymers, ceramics, and metals. The metallic fraction can contain critical metals, such as rare-earth-elements (REE), and valuable metals such as silver and gold. Furthermore, e-waste can also contain hazardous materials, such as lead, cadmium, polybrominated diphenyl ethers, and arsenic. Therefore, recycling end-of-life (EoL) electronic devices has economic and environmental benefits (Chauhan et al. 2018; EASAC 2016). E-waste utilization as a secondary raw material is a complementary route of metal production included in urban mining. These activities also promote necessary techniques to a circular economy, through recovery of metals, which were lost in waste discharge or landfilling. However, a challenge to close the loop in life cycle of products is the collection of e-waste. Problems are the storage of small electronic devices and the lack of collection strategy. People must realize that it is better to dispose of the old smartphone in a collection point for recycling instead of storing it (Chauhan et al. 2018; Tesfaye et al. 2017).
One challenge with e-waste recycling is related to technological advancement and the programmed obsolescence of this equipment that generate an increasing amount of residues with a heterogeneous composition (Babu et al. 2007; Raele et al. 2017; Wang et al. 2017). Although unit operations for secondary source processing are similar to primary sources, recycling of e-waste has advantages, such as reduction of extraction of raw materials from nonrenewable resources, a higher metal content when compared with natural ores and the collaboration to waste management, reducing contamination of environment with inappropriate release of metals, and energy savings due to lower-energy requirements to process waste instead of using natural ores (Tesfaye et al. 2017).
Currently, e-waste recycling is done initially by pyrometallurgical route, which provides viable recovery of metals in industrial scale. However, the following hydrometallurgical process is necessary for a possible total separation and recovery of metals. Thus, hybrid routes of e-waste processing are more common than pyrometallurgical exclusive route. In this context, Umicore can be mentioned. Located in Belgium, Umicore is a company that industrially recovers metals from waste electrical and electronic equipment (WEEE). Initially, electronic scrap undergoes pretreatment (preprocessing or mechanical processing), pyrometallurgical route, and, later, recovery/refining by hydrometallurgical process (Cui and Anderson 2016).
Steel recycling uses entirely pyrometallurgy to recover iron from large household appliances as refrigerators. This overview indicates that there is a technological opportunity to search for sustainable hydrometallurgical e-waste recycling. Tailing generation, toxic and expensive reagents are difficulties to be overcome. If these problems are solved, hydroprocessing can be applied industrially for recovering different metals from the heterogeneous e-waste composition (Chauhan et al. 2018; Tesfaye et al. 2017).
Hydrometallurgical techniques consist of chemical reactions in aqueous medium. Extraction of metals is performed by chemical leaching, usually followed by stages of purification and recovery of the pregnant leaching solution (PLS) (Gupta 2003; Perez et al. 2019; Tesfaye et al. 2017). In the leaching process, the transfer of metals to the liquid phase occurs. The employed leaching solution may be acidic (e.g. H2SO4, HNO3, and HCl solution), alkaline (e.g. NaOH, NH3, and Na2CO3 solution), saline (e.g. CuCl2), or even water (Gupta and Mukherjee 1990; Jackson 1986; Swain and Mishra 2019). Other lixiviants (e.g. cyanide, halide, thiourea, and thiosulfate) are significant, due to efficacy in precious metal recovery (Namias 2013). The purification and recovery steps can occur by selective chemical precipitation/cementation, solvent extraction, ion-exchange resins, and electrorefining methods (Gupta and Mukherjee 1990; Perez et al. 2019; Silvas et al. 2015; Tesfaye et al. 2017).
The first step in hydrometallurgical processing before the leaching of metals from electronic is the dismantling, separating, and comminuting. Dismantling and manual separation can concentrate metals in e-waste, thereby reducing the use of chemical reagents during leaching. Magnetic separation can separate iron before leaching, thereby reducing the use of reagents and facilitates purification of leaching solution (Chauhan et al. 2018; Tesfaye et al. 2017; Valix et al. 2017). Polymers or ceramic materials present in e-waste could hinder contact of metals with leaching solution, as presented by Valix et al. (2017).
Hydrometallurgical processing of e-waste, in contrast to pyrometallurgy, can be controlled to selectively recover different metals, even those present in ppm concentration. Aqueous processing also reduces gas emitted and requires less energy than smelting metals in furnaces (Gupta 2003; Swain and Mishra 2019).
The optimal condition for leaching metals from e-waste depends on pulp density, temperature, time, particle size, percentage of magnetic fraction, and mixing velocity. Polymer coating hinders the contact with leaching solution, also influencing hydroprocessing, and is related with parameters such as particle size, solubility in the solution, and degradation. Some authors show a desoldering step that facilitates the leaching of copper, gold, and base metals from electronics. Desoldering is made by leaching with fluoroboric acid, which removes lead and tin, and separates the nonmetallic components. The residue is then leached to recover copper, zinc, nickel, silver, palladium, and gold (Ashiq et al. 2019; Kamberović et al. 2009; Namias 2013; Valix et al. 2017).
Several studies have been carried out on the recycling of WEEE through hydrometallurgical routes and/or hybrid routes. Even in hybrid routes, the hydrometallurgical technique is used in the metal recovery and purification stage, as it manages to treat several metals present in solution, even in low concentrations (on a ppm scale). Thus, this technique is used for recycling WEEE such as printed circuit boards (PCBs), photovoltaic modules (PV), batteries, and light-emitting diodes (LEDs). Due to the constant change in the composition and amount of waste generated each year, hydrometallurgical processing must always be in constant adaptation and study. This technique allows the recovery of several materials present at the same time in solution, in different concentrations.
8.2 Waste Printed Circuit Boards (WPCBs)
PCBs are present in almost all electronics products, such as air conditioners, televisions, computers, mobile phones, and printers (Ning et al. 2017; Zhou and Qiu 2010). They are nonconductive substrates that electrically connect the circuit components, and they can be made up of about 30% polymeric materials, 30% ceramic materials, and up to 40% metals (Li et al. 2004; Kaya 2016; Silvas et al. 2015). Studies estimate that the fraction of PCBs in e-waste (by mass) can vary from 3% to 8% (Cucchiella et al. 2015; Raele et al. 2017).
Several studies report routes for characterization and recovery/concentration of metals in waste printed circuit boards (WPCBs) such as mobile phones (Camelino et al. 2015; Jing-ying et al. 2012; Petter et al. 2014), digital video discs (DVD) players and vacuum cleaners (VCs) (Kumar et al. 2015), printers (Silvas et al. 2015), and computers (Veit et al. 2005; Yamane et al. 2011).
Table 8.1 shows the mass percentage of the main metals in different WPCBs. Due to this complexity and heterogeneity of the composition of different types of existing WPCBs, the release and recovery of the metals present have become a challenge for the existing studies. Different routes hydrometallurgical can be adopted depending on the materials of interest (Cui and Anderson 2016). Rocchetti et al. (2018) presented a study with various patents on recycling WPCBs. The survey and search for these patents were updated until July 2017. The main processes based on the analysis of these patents are shown in Figure 8.1.
Figure 8.1 presents a flowchart illustrating a general outline based on the study that was carried out on existing and updated patents until 2017 (Rocchetti et al. 2018). The main processes that can be included in the recycling of WPCBs have been shown. However, these steps can be modified, depending on the metal of interest, the available technologies, and the type of WPCB. Generally, metal recycling/recovery in WPCBs starts with a pretreatment that is directed toward the removal of components (e.g. capacitors and transistors) from WPCBs through physical and manual processes (Silvas et al. 2015). Pretreatment may lead to the recovery of electronic components and the manufacture of Sn (Rocchetti et al. 2018).
Table 8.1 Mass percentage of main metals in different WPCBs.
| WPCB sample | Yamane et al. (2011) | Silvas et al. (2015) | Kasper et al. (2011) | Guo et al. (2011) | Behnamfard et al. (2013) | Yamane et al. (2011) | Kumar et al. (2015) | Kumar et al. (2015) |
|---|---|---|---|---|---|---|---|---|
| Mobile phones | Printer | Mobile phones (other brand) | Computer motherboards | Computer | Personal computer | DVD | Vacuum cleaner | |
| Ag | 0.21 | 0.31 | 0.05 | 0.0026 | 0.0704 | 0.16 | – | – |
| Pd | – | – | – | – | 0.0028 | – | – | – |
| Al | 0.26 | 3.73 | 0.61 | 1.32 | 4.011 | 5.7 | 10.10 | 3.26 |
| Au | 0.00 | 0.004 | 0.09 | 0.006 | 0.013 | 0.13 | – | – |
| Cu | 34.49 | 32.50 | 37.81 | 27.5 | 19.187 | 20.19 | 17.80 | 7.08 |
| Fe | 10.57 | 1.42 | 4.85 | 1.6 | 1.133 | 7.33 | 5.51 | 4.40 |
| Ni | 2.63 | 0.34 | 2.54 | 0.4 | 0.165 | 0.43 | 0.36 | 0.26 |
| Pb | 1.87 | 0.00 | 1.23 | 2.91 | 0.385 | 5.53 | 3.33 | 3.71 |
| Sn | 3.39 | 0.96 | 2.55 | 3.43 | 0.689 | 8.83 | 2.57 | 2.75 |
| Zn | 5.92 | 0.64 | 1.82 | 2.86 | 0.840 | 4.48 | 1.99 | 5.54 |
Processing and/or concentration are stages that may include physical–mechanical processes such as comminution; granulometric classification; and magnetic, electrostatic, and density separation. These processes are performed for size reduction and particle separation, and they are often a prerequisite for additional treatments for metal extraction techniques (hydrometallurgical, pyrometallurgical, biohydrometallurgical, and electrohydrometallurgical, in addition to hybrid or combined routes) (Veit et al. 2006 2014; Zhang and Xu 2016). Dismantling and physical–mechanical processes should be used when there is a need to release and separate metallic components from WPCB to expose metals to subsequent chemical processes (Cui and Anderson 2016).
Kumar et al. (2015) reported the use of physical processing for the generation of a concentrate enriched with the metals of interest for subsequent selective extraction through hydrometallurgical processing. The authors processed WPCBs of DVD and VCs by dismantling, comminution, homogenization, pneumatic separation, and froth flotation. Metal concentrations in DVD WPCBs obtained in waste characterization were 43 (wt%), 88 (wt%) in concentrates from froth flotation, and 75 (wt%) from pneumatic separation. For VC WPCBs, through applications, these methods, the total quantity of the metals in characterization was 30 (wt%), and in concentrates from froth flotation and pneumatic separation was 90 and 65 (wt%) total metals. Therefore, this work obtained an enriched metal concentrate according to studied conditions, and this material can help the stages of extraction, recuperation, and purification of the metals.
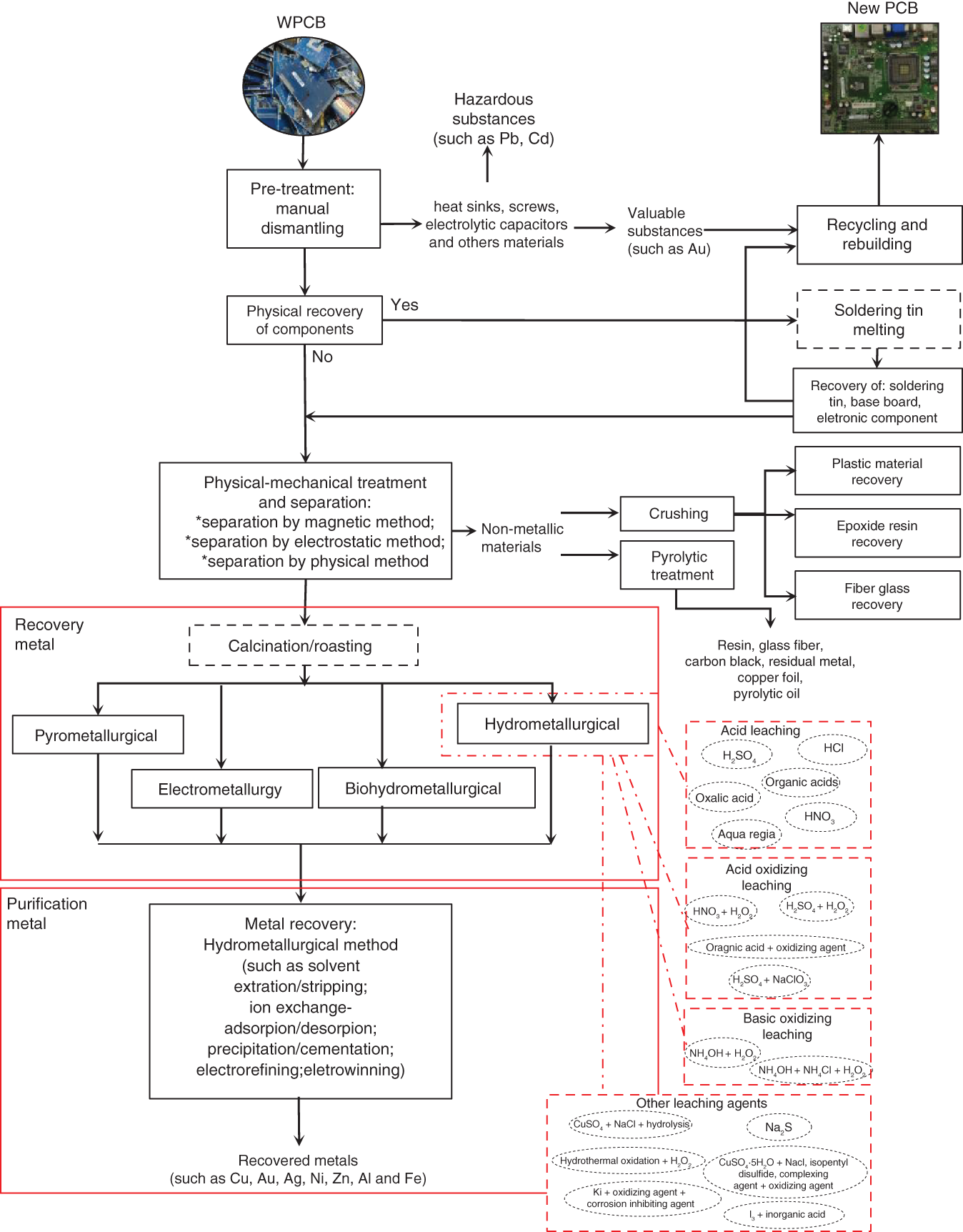
Figure 8.1 General flowchart illustrating the whole recycling value chain of the WPCB.
Source: Adapted from Rocchetti et al. (2018).
The recovery/purification stage is carried out to recover elements of interest through hydrometallurgical techniques. This stage can occur by selective chemical precipitation or cementation, solvent extraction, ion-exchange resins, and electrorefining/electroplating methods (Kaya 2016; Perez et al. 2019; Silvas et al. 2015; Tesfaye et al. 2017).
The extraction and recovery of metals can be carried out using various leaching agents. Awasthi and Li (2017) presented a revision with different leaching agent studies for extraction, recovery, and purification of WPCBs metals. Some of them reached recoveries of more than 90% of Au, Ag, Cu, Pd, and Zn. Some of these leaching agents checked were sulfuric, nitric, and hydrochloric acid, ammonia, and thiosulfate. These reagents were also applied in combinations intended to be synergetic for metal extraction. The authors also compared the leaching rates, cost, toxicity, corrosion behavior, and reliabilities of lixiviants. Thiourea was well assessed in all categories, and cyanide had the disadvantage of toxicity. Chloride and aqua regia were badly rated because they have aggressive corrosive behavior. Therefore, the application of chloride and aqua regia can be a problem in industries.
Table 8.2 presents a compilation of results for the extraction and recovery of WPCBs by leaching processes that have been reported in several studies. Jing-ying et al. (2012) studied the effects of particle size on the extraction of metals (Au and Ag) from WPCBs of mobile phones. They also verified the best conditions for the concentration of leaching agents (thiourea and Fe3+) and temperature. The study was able to obtain, in two hours of reaction, with particles of 100 mesh, in a leach solution with 24 g/l of thiourea and 0.6% of Fe3+ and room temperature, about 90% of Au and 50% of Ag.
Silvas et al. (2015) described a hydrometallurgical extraction process for copper recovery from printer WPCBs. The process was performed in two subsequent leaching steps: the first in sulfuric medium and the second in oxidant sulfuric medium. The metallic fraction present in the waste was 44.0 wt%, whose main metal was copper 32.5 wt%. In the sulfuric leaching, 90 wt% Al, 40 wt% Zn, and 8.6 wt% Sn were extracted. In the oxidative leaching, the extraction percentage was 100 wt% Cu, 60 wt% Zn, and 10 wt% Al. Thus, at the end of the proposed hydrometallurgical processing, the extraction results were 100% Cu. The recovery was 98.46%, which would correspond to 32 kg of Cu for 100 kg of residues of these WPCBs.
Complementing the studies about Cu leaching techniques, Correa et al. (2018) studied a route for purification and recovery of this metal of interest. This route was composed of an extraction step (oxidative sulfuric leaching) and another subsequent solvent extraction step for purification and recovery of Cu. In the extraction step, the nonmagnetic fraction of the WPCBs was ground and leached, according to Table 8.2. This step was similar to the study of Silvas et al. (2015). Extraction results were 60 wt% Al; 94 wt% Cu; Zn, Ni, and residual Fe. Subsequently, the solvent extraction step (applying D2EHPA) was performed in two steps: (i) separation of residual Zn, Al, and Fe; and (ii) copper separation. In the first stage, metals were removed from the solution 100 wt% Zn, Fe, and Al. pH 3.5, 2 : 1 aqueous/organic ratio (A/O), 10% (v/v) D2EHPA were used. In the second stage, 100% of the copper was extracted at pH 3.5, 1 : 1 A/O, 20% (v/v) D2EHPA.
Wang et al. (2019) performed the separation and recovery of Cu from PCB extraction solution because the composition of the leach solution (mainly Fe) could affect this recovery. The solvent extraction technique also was used, in which the concentration of the organic extractor Acorga M5640, pH of the aqueous phase, phase ratio (O/A), contact time, and the concentration of H2SO4 as a stripping reagent were studied for recovery Cu. Thus, in the extraction solution containing Cu (6.166 g/l) and Fe (57.5 g/l), under defined ideal extraction conditions (16% M5640, pH = 1.1, O/A = 1/1, contact time three minutes and 25 °C) and stripping (2.5 mol/l H2SO4, O/A = 1/1, contact time three minutes, 25 °C and five stages), more than 88.6% Cu and less than 2.4% Fe were transferred to the recovery solution for these e-waste.
Table 8.2 Results compilation for extraction and recovery from WPCBs by leaching processes.
| References | WPCB sample | Leaching medium | Conditions | Extraction (wt%) |
|---|---|---|---|---|
| Correa et al. (2018) | WPCBs fraction non-magnetic | H2SO4 (1 M) + H2O2 (10 ml were added every 30 min, total added 120 ml) | 1 : 10 solid/liquid rate; 75 °C; for 6 h | 60 Al, 94 Cu, 76 Zn; 50 Ni; and residual iron |
| Silvas et al. (2015) | Printer | 1st leaching: H2SO4 (1 M); 2nd leaching: H2SO4 (1 M) + H2O2 (30 ml were added every 30 min); | 1:10 solid/liquid rate; 75 °C; 1st for 4 h; 2nd for 4 h | 100 Al; 100 Cu; 8.6 Sn; 100 Zn |
| Camelino et al. (2015) | Mobile phones (particle size <2 mm) | H8N2O3S2 (0.13 M) | 10 pH; 20 °C; 180 rpm | 70 Au |
| Jing-ying et al. (2012) | Mobile phones (particle size <100 mesh) | 24 g/l of thiourea +0.6% of Fe3+ | Room temperature; for 2 h; | 90 Au; 50 Ag |
| Petter et al. (2014) | Mobile phones (particle size <1 mm) | (i) for leaching Ag: HNO3 (1/3 v/v); | 1 : 20 solid/liquid rate; 25 °C and 60 °C; for 2 h | 100 Ag |
| (ii) for leaching Au: commercial cyanide (Galvastripper concentrate); | 1 : 20 solid/liquid rate; 25 °C; for 2 h and 4 h; pH ∼ 12.5 | 60 Au | ||
| Havlik et al. (2010) | Personal computer (after thermal pretreatment) | 400 ml HCl (1 M) | Sample 3 g; 80 °C; for 180 min | 98 Cu |
| Neto et al. (2016) | Motherboards <0.250 mm | HNO3 (2 mol/l) | 1 : 10 solid/liquid rate; 50 °C; por 210 min; 200 rpm | 59 Cu |
| Birloaga and Vegliò (2016) | Computers | 1.7 M H2SO4 (98wt./vol%) + 17% (v/v) H2O2 (30 wt./vol%), | 1 : 15 solid/liquid rate; room temperature; 200 rpm; por 1 h | 98 Cu |
| Behnamfard et al. (2013) | Computers | 1st leaching: H2SO4 (2 M/l) + H2O2 (35%) 20 V% | 1 : 10 solid/liquid rate; ∼25 °C; 200 rpm; por 3 h | 85.76 Cu 0.86 Ag |
| 2nd leaching: H2SO4 (2 M/l) + H2O2 (35%) 20 V% | 1 : 10 solid/liquid rate; ∼25 °C; 200 rpm; por 3 h | 13.99 Cu 11.30 Ag | ||
| 3rd leaching: Thiourea (20 g/l) + Ferric Iron (6 g/l) + H2SO4 (10 g/l) | 1:10 solid/liquid rate; ∼25 °C; 200 rpm; por 3 h | 84.31 Au 71.36 Ag 2.13 Pd | ||
| 4th leaching: HCl (5 M/l) + H2O2 (1%V) + NaClO (10%V) | 1 : 10 solid/liquid rate; ∼63 °C; 300 rpm; por 3 h | 97.87 Pd 6 Au 16.48 Ag |
Different from Correa et al. (2018) and Wang et al. (2019), Neto et al. (2016) studied extraction, recovery, and purification of Cu in motherboards with ion-exchange resins. Initially, they obtained a multielement leaching solution with 78% of the total amount of Cu of the residue. After this leaching, a bispicolylamine resin was used to recover 59.0% Cu, and a final Cu solution with high purity (99.0%) was obtained after eluting, with H2SO4 (4 mol/l) in the column.
Kasper et al. (2011) also recovered Cu from mobile phone WPCBs. They used mechanical processing techniques for concentration of iron in the magnetic fraction, copper in the conductive fraction, and another fraction with polymers and ceramics. Magnetic separation concentrated 60% of Cu. This metal fraction was extracted through leaching in aqua regia (solid/liquid ratio of 1 : 20, under magnetic stirring, heating at 60 °C, for two hours). Subsequently, the solution resulting from the extraction step was electrowinned for Cu recovery. The electrolytic cell was assembled with a Pt anode and a Cu cathode, and different variations of current densities (1, 3, and 6 A/dm2) and deposition times (30, 60, 90, 120, and 180 minutes) were studied. Thus, 92.8% of the dissolved copper was recovered.
Table 8.2 also presents the study of Behnamfard et al. (2013) that proposes a new hydrometallurgical process for the selective recovery of Cu, Ag, Au, and Pd from PCBs. They propose a hybrid route that is composed of leaching processes and precipitation/cementation (Figure 8.2).
As shown in Figure 8.2, about 99% of the Cu content was extracted after the two consecutive leaching steps in an oxidizing sulfuric medium. The solid residue from the second leaching step was treated (acid thiourea + ferric iron as an oxidizing agent), and an extraction of 85.76% Au and 71.36% Ag was obtained. The precipitation of Au and Ag from this third lixiviation was investigated in which sodium borohydride (SBH) was used as a reducing agent. The leaching of Pd and gold (from the solid residue of the third stage) was carried out in the leaching system NaClO–HCl–H2O2. The total amount of Pd and Au resulting from the chloride leachate was precipitated using 2 g/l of SBH. Thus, they proposed a process flow chart for the recovery of Cu, Ag, Au, and Pd from the PCB.
These studies show that the quantity and diversity of metals present in WPCBs can also vary according to the type and year of manufacture of the original equipment (Kasper et al. 2011; Veit et al. 2006). This metal fraction can transform PCB scrap into an interesting raw material according to the environmental and economic points of view, assigning high-added value to the e-waste through reusing it in the manufacture of other products (Hadi et al. 2015; Mdlovu et al. 2018).
8.3 Photovoltaic Modules (PV)
Since the 2000s, the photovoltaic module technology has been extensively used due to the increase in cell efficiency and the decrease in production costs. The total world production of photovoltaic energy rose from 278 MW (megawatt) in 2000 to 56 000 MW in 2012 (Klugmann-Radziemska and Kuczynska-Łazewska 2020; Tammaro et al. 2015). These PV modules have a life span of around 25–30 years; hence, it is estimated that the amount of waste will increase from 2030 (Klugmann-Radziemska & Kuczynska-Łazewska, 2020; Tammaro et al., 2015; Dias et al., 2016b).
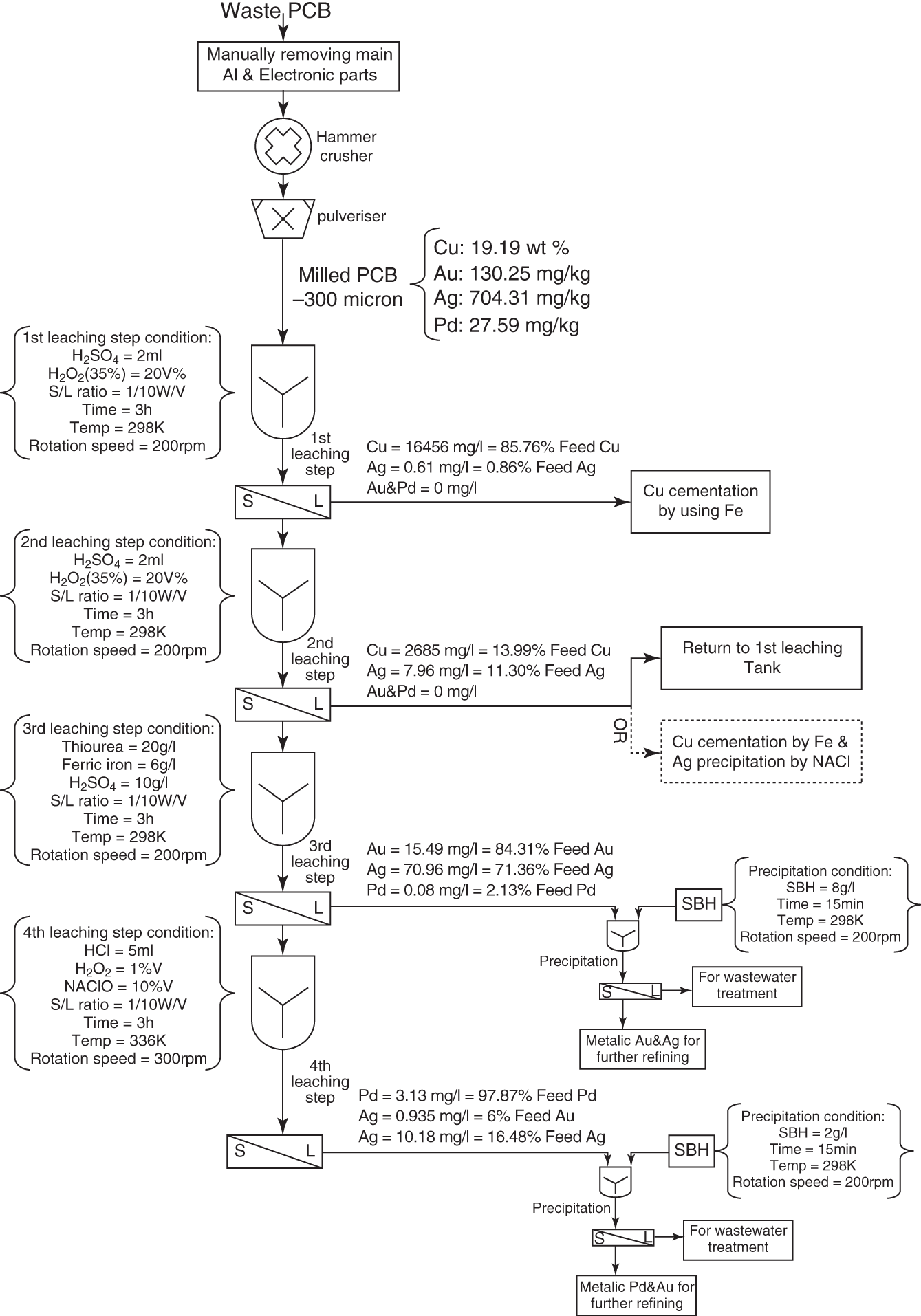
Figure 8.2 The proposed route for extraction of Cu, Ag, Au, and Pd from WPCB of computers.
Source: Behnamfard et al. (2013).
Types of PV modules can differ according to production technologies and semiconductor materials. PV modules can be classified as first, second, or third generation, depending on the production technology. The first generation is composed of crystalline silicon (c-Si), which can be monocrystalline, and polycrystalline or ribbon sheets (approximately 80% of world production). Second generation is formed by thin-film amorphous silicon (a-Si), cadmium telluride (CdTe), multijunction cells (a-Si-lc-Si), copper indium gallium diselenide (CIGS), and copper indium diselenide (CIS). Third generation is composed of photovoltaic concentrator (CPV) and emerging technologies (Dias et al. 2016a; Tammaro et al. 2015; Tao and Yu 2015).
Industrial-scale recycling processes silicon photovoltaic modules crystalline (c-Si) at SolarWorld, First Solar, and ANTEC Solar GmbH companies were cited in Tao and Yu (2015) studies. SolarWorld recycles silicon through calcination (600 °C) of these modules (for thermal decomposition of ethylene-vinyl acetate [EVA]), and, subsequently, the solids are manually separated into fractions with glass, Si-cells, and metals. Then, the semiconductor is purified by etching, and recovering the glass and semiconductor. First Solar is a manufacturer that recycle CdTe thin-film modules. The modules are comminuted (shredding + hammer milling), and, later, they are leached in oxidizing sulfuric medium in stainless steel drum. The company recovers 90% of glass, 95% of semiconductors, and 80% of tellurium. ANTEC Solar GmbH has a pilot plant in Germany for recycling CdTe and CdS modules. The initial step is also physical disintegration, which is followed by calcination at 300 °C in an oxygen atmosphere. An etching step occurs when exposing the resulting fragments in a chlorine gas atmosphere and the condensation of CdCl2 and TeCl4 occurs, and these compounds can be precipitated by cooling.
Latunussa et al. (2016b) summarize the SASIL S.p.A. project in which it was called full recovery end-of-life photovoltaic (FRELP). SASIL S.p.A. created a pilot-scale plant for recycling c-Si modules in which FRELP uses mechanical and chemical separation steps. This project became an industrial-scale plant with a processing capacity of 1 t/h up to 8000 t/year of crystalline-silicon waste PV modules (Latunussa et al. 2016a). Figure 8.3 presents a proposal for the recovery process of silicon photovoltaic waste in FRELP.
The photovoltaic waste recycling process proposed/developed by the SASIL photovoltaic waste treatment project (Figure 8.3) consists of a sequence of processes (transport of waste PV modules, unloading of the waste panels, disassembly, cable treatment, incineration of cable polymers, glass separation and refinement, cutting of modules, incineration of encapsulation, and back-sheet layer with energy recovery, sieving, acid leaching, filtration, electrolysis, neutralization, and filter press) that are described in detail in Latunussa et al. 2016a. SASIL can recover about 94% Ag, 99% Cu, and 99% Al (Latunussa et al. 2016a, 2016b).
Studies of Wang et al. (2012) have proposed a thermal method to separate materials (such as silicon, glass, and metal) from conventional crystalline silicon modules. Two steps of thermal processing were carried out, and the EVA was burnt. The glass plate was recovered without breaking, and the copper could be recovered (85%) in further acid treatment.

Figure 8.3 Recycling process of c-Si modules proposed by the FRELP method of SASIL.
Source: Latunussa et al. (2016a).
Dias et al. (2016a) studied characterization of PV waste modules (c-Si modules), and they checked if Ag could be leached by hydrometallurgical procedures. To obtain the semiconductor material, the modules were cut and immersed, for two days, in H2SO4 (95%), at room temperature and constant agitation. This dry and ground material was leached with HNO3 (64%), for two hours at room temperature, and later analyzed chemically. In these modules, the Ag content was 600 g/t.
The estimated amount of silver is 10 g of silver/m2 of photovoltaic panel produced. Silver scrap recycling plays an important role in the silver market as it accounts for a third of the total market (Latunussa et al. 2016b). Even so, in the literature consulted, few published works addressing the recovery of silver from solar modules were found.
The extraction of silver from crystalline silicon modules was performed at 20 °C and 40 °C by Yi et al. (2014). H2SO4, HCl, and HNO3 (the concentrations varied from 1 to 3 mol/l) were used as leaching agents with a solid/liquid ratio of 1 : 5 for two hours. Nitric acid extracted about 100% of the available silver, while sulfuric and hydrochloric acids recovered 10% less silver in two hours, even at a concentration of 3 mol/l.
The recycling of several metals present in PV modules, such as Ag, Cu, Al, Ga, In, Ge, and Te is not yet explored. Latunussa et al. (2016b) report that glass, Al, and Cu make up most of the PV mass. However, despite the untapped economic potential, these materials are lost in the EoL of crystalline PV modules. Yi et al. (2014) affirm that Ag, Cu, and Al will be the metals of interest in PV recycling between 2030 and 2050 and that these metals correspond to US$ 0.54–1.70/W of the cost of crystalline silicon modules. Hence, in future, recycling strategies will no longer focus mainly on Si recovery, but on metal recovery. In this context, hydrometallurgy routes for recovering these metals from PV modules should be further studied and explored.
8.4 Batteries
Batteries are present in many electronic devices to supply energy, thereby allowing autonomy. Mobile phones, laptops, tablets, cameras, and recently electric cars require batteries, which use critical materials and generate a great amount of waste. Batteries can cause a potential environmental impact due to their composition of hazardous metals, such as cadmium, lead, and mercury (Bernardes et al. 2004). There are two main groups of batteries: primary and rechargeable. The rechargeable batteries include lead–acid, nickel–cadmium, nickel–metal hydride (NiMH), and lithium-ion (Zeng et al. 2014).
The recycling processes for batteries can be applied through pyrometallurgical routes, hydrometallurgy, or a combination of these two extractive methodologies. Examples of hydrometallurgical processes for batteries are TNO process for NiCd batteries; BATENUS process for most batteries types; ZINCEX process for Zn-bearing materials; and RECUPYL for most batteries types. UMICORE process for Li-ion and NiMH batteries is a hybrid process (Goodship and Stevels 2012).
TNO process initiates sorting batteries and selecting NiCd to comminution and hydrometallurgical step. The fraction with granulometry above 3 mm is Fe rich, then is submitted to magnetic separation, and the magnetic and non-magnetic portions are washed in HCl to remove Cd. The washing solution is used to leach comminuted fraction (with granulometry below 3 mm) at 90 °C. Leaching liquor is purified with solvent extraction to remove Cd and then Fe is removed by precipitation. The final purified leaching solution is used to recover Ni by electrolysis process. Stripping solution of Cd solvent extraction is also submitted to electrolysis to recover this metal (Erkel 1995).
In BATENUS process, batteries pass through a sorting step with sieving. Button batteries are separated and sent to be treated in another process. The remaining are shredded, sieved, and magnetically separated. Fine fraction is leached and solution purified to recover Zn, Hg, Cu, Ni, Cd, and Mn (Fröhlich and Sewing 1995).
Hydrometallurgical processing is commonly executed after the discharging and dismantling of spent batteries. In the case of lithium-ion, dismantled parts are composed of separators, cathodes, anodes, and shells; electrolyte salts and cathodes are the lithium-containing components; anodes are made of a carbon material, copper, active substances, and organic binder. Cathode-active materials are commonly leached by acids to selectively extract metals, such as cobalt, nickel, manganese, and lithium. However, some studies indicate the use of NaOH leaching during dismantling step to dissolve cathode aluminum foil. The powder collected after filtration is then heated to burn organics, and exhausted gas is managed due to its hazardous content. The calcined material is grounded and leached by acid to extract metals. Organic acids are preferable in environmentally sustainable methods, such as lactic, gluconic, and tartaric acids (Ashiq et al. 2019; Nayaka et al. 2019; Roshanfar et al. 2019; Siqi et al. 2019).
Existing industrial recycling processes for spent lithium batteries, which involves hydrometallurgy, are the Toxco process, which was developed to avoid risks with Li explosion, through cryogenic treatment; and Umicore VAL’EAS process, which is a hybrid pyro–hydro process of spent Li-ion and NiMH batteries to recover Ni, Co, Cu, Fe, and Mn, with exception of Al and Li that are lost (Georgi-Maschler et al. 2012).
Li et al. (2019) presented a review on clean recovery of lead–acid battery, based on alkaline or acid leaching to extract lead. These batteries represented more than 50% market share in 2015. Some processes involve organic acid leaching–calcination, which is the main technology published for batteries. CLEANLEAD, PLACID, and PLINT processes are examples of Pb recycling from spent batteries. PLACID and PLINT processes consist of leaching battery pastes, purification of leaching solution, and metallic lead production, which differ in electrowinning for PLACID process and precipitation for PLINT. CLEANLEAD just produces desulfurized pastes through leaching for further processing (Andrews et al. 2000). Discussion suggests that that hydrometallurgical process is preferably applied, if compared with pyrometallurgy, due to its environment-friendly characteristics. Other technologies are reagent leaching–electrowinning and alkaline leaching–crystallization (Li et al. 2019).
Provazi et al. (2011) studied the separation of metals from a solution composed of a mixture of the main types of household batteries. This solution was prepared by grinding several batteries, and the reduction and elimination of volatile metals took place in an oven. The extraction step was performed by acid leaching (solid/liquid ratio 1 : 10, H2SO4–1 M, at room temperature, for 24 hours). Thus, two hydrometallurgical routes were applied: selective precipitation with sodium hydroxide (NaOH) and solvent extraction using Cyanex 272 acid. In the selective precipitation step, a solution of NaOH (2 M) was added to the liquor resulting from the sulfuric acid leaching step to increase the pH of this solution, so selective precipitation can occur. For solvent extraction, Cyanex 272 (0.6 M-extractor) was used and kerosene was used as a diluent. In this comparative study, solvent extraction obtained the highest recovery rates: 99% for Zn (pH 2.5); above 95% Fe, 90% Ce, 88% Mn, Cr, and Co (pH 7.0); more than 85% Ni (pH 3.0); and more than 80% Cd and La (pH 3.5).
8.5 Light-Emitting Diodes (LEDs)
LEDs are dominating lighting market, with expectations of 74% of market share in 2030, and are present in various types of equipment (displays from smartphones, TVs, laptops, tablets, and lamps), due to their energy efficiency (Mizanur Rahman et al. 2017).
Hydrometallurgical processing is also expected for metal recovery in LED waste. Elements such as yttrium, cerium, europium, and gallium are the focus, because they are critical and gold is a precious metal. As a lamp phosphor LED rare earth elements (REE) may have a distinct leaching behavior. Yttrium and europium from fluorescent lamps are leached easily when compared with other rare-earth elements. In these cases, leaching efficiency is more significant than leaching rate. Other benefits from diverse leaching kinetics are the selectivity. Leaching of REE may be performed with the duration of more than 24 hours with separation of rare-earth phosphors in different leaching steps (Jha et al. 2016; Tunsu et al. 2015). Contribution of Fang et al. (2018) assessed criticality and recyclability of LEDs in different colors and products, based on cost for processing, supply risk, environmental impact, technology availability, composition, and total amount of materials.
Ruiz-Mercado et al. (2017) proposed a process of metal recovery from LEDs, which used hydrochloric acid and ammonia as leachate. At the initial leaching with HCl, the REE were dissolute and the solids were releached with NH3 to separate silver from indium and gallium nitrides. The solution from initial leaching precipitated cerium oxide and in the remaining solution rare-earth metals are recovered by solvent extraction. Aqueous strip solution containing Eu and Y was separated by selective reduction and precipitation, producing respective oxalates, which were calcined to oxides. Figure 8.4 shows schematic flowchart with proposed route to recover rare-earth metals from the LED.
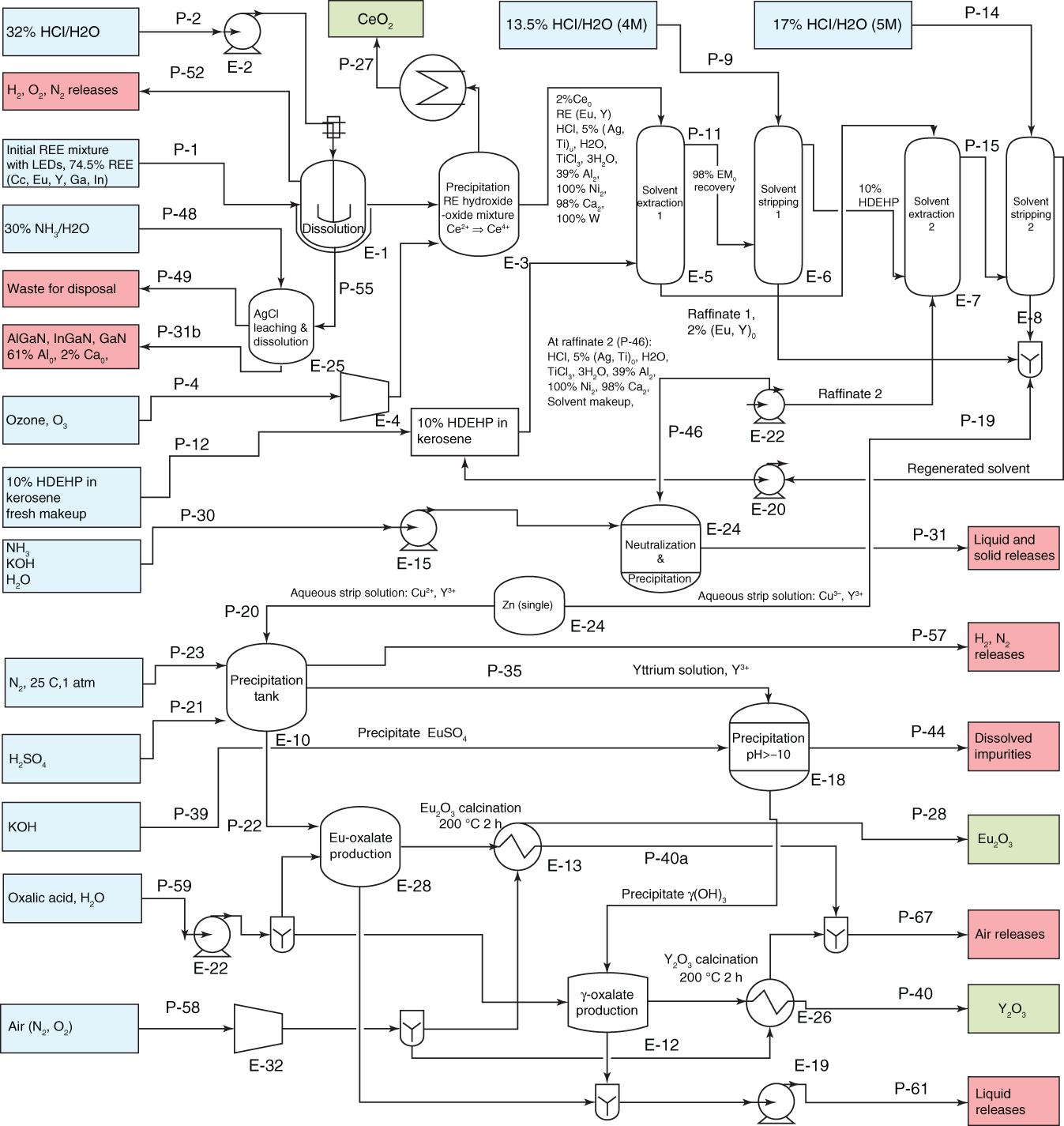
Figure 8.4 Recycling flowchart of REE recovery from LED waste.
Source: Ruiz-Mercado et al. (2017).
Maarefvand et al. (2020) studied the recovery of gallium from LED waste based on a process of oxidation and subsequent leaching. Gallium in LED is present as nitride. However, leaching gallium oxide is preferable due to refractory characteristics of gallium nitride. Oxidation step at 1100 °C facilitates leaching, which reaches 91.4% of Ga recovery using 4 M hydrochloric acid, at 93 °C. Increasing oxidation temperature may unprivilege leaching, because surface area decreases and grain size increases, which reduces the contact with acid. Zhou et al. (2019) treated the LED waste through a pyrolysis step at 460 °C before leaching. Escaping from inorganic acids, leaching was performed with oxalic, citric, and DL-malic acids, oxalic acid being a better option due to 83.42% of Ga recovery, which was higher and with modifications reached 90.36% of efficiency at optimal conditions (90 °C, pulp density of 10 g/l, 0.7 M of acid concentration and particles size between 48 and 150 μm). In the same study, a possible mechanism of gallium reaction with oxalic acid and precipitation of iron oxalate was proposed (Maarefvand et al. 2020; Zhou et al. 2019).
Leaching can also be performed under pressure in autoclaves, reduce the consumption of acid, and give better leaching efficiencies. Studies affirmed an increase of extraction in pressurized leaching, such as the recovery of yttrium and europium from spent fluorescent lamp tubes during four hours of leaching, at 125 °C and 5 MPa (Jha et al. 2016), and gallium leaching from GaN waste of LED industry, at 23 atm, 220 °C, in four hours (Chen et al. 2018).
Murakami et al. (2015) studied the recovery of gold by ion-exchange method from LED leaching solution, obtained by leaching with aqua regia in an autoclave, at 80 °C, 1 atm, during 24 hours. The adsorption resin needed to be used in low aqua regia concentrations to avoid decomposition of the amine group and improve the recovery efficiency. Elution was made by thiourea solution, and gold was precipitated from this solution using sodium borohydride to reduce gold. Gold was produced with a purity of 100%.
8.6 Trends
E-waste processing keeps changing as long as there are technological advances of electronic products. New equipment, such as electric vehicles batteries, compact PCBs, organic light-emitting diodes (OLEDs), and flexible smartphones, will require renewed recycling processes. Hybrid car batteries, as an example, may contain cobalt, nickel, and rare earths in a NiMH battery, which has an average weight of 50 kg, and is necessary to develop a metal recovery process (Petranikova et al. 2017; Sethurajan et al. 2019).
A novel and potentially greener technology applied to extract and recover metals from electronic wastes and their leaching solution is the use of ionic liquid (IL). ILs have low volatility, low combustibility and high extractability, and thermal stability, making them environmental friendly (Chauhan et al. 2018). These liquids have been used in studies to separate REE and transitional metals also applied in WEEE. Common functional groups in IL constitution are imidazolium, pyridinium, pyrrolidinium, ammonium, sulfonium, and phosphonium, which can functionalize IL to extract specific metals (Huang et al. 2014; Sethurajan et al. 2019; Tunsu et al. 2015; Zhou et al. 2018).
Supercritical fluid technology is a recent method to recycle metals and deteriorate polymers in e-waste. Supercritical fluid is an efficient extraction media due to its properties of low viscosity, low surface tension, high mass transport coefficient, high solubility and diffusivity for organics. These fluids are pressurized in 25–40 MPa pressures with temperatures varying from 350 to 550 °C. Studies with the recovery of metals from WPCBs using supercritical fluid show metals concentrating on solid products of the treatment. Another variation of this process is the combination of supercritical water oxidation and electrodeposition process, which decomposes organics with oxidative environment and turns metals into oxides; these oxides are soluble in chloride media and deposition occurs in electrochemical cells (Ram et al. 2014; Zhang and Xu 2016).
Magnetic nanohydrometallurgy is a potential technique for metal recovery from waste electric and electronic equipment. This novel process applies magnetic nanoparticles, which are designed with functionalized organic compounds to extract specific metals from leaching solution. These particles ensure selectivity, being capable of recovering the metal through electrowinning combined with its magnetic attraction. When deposition is completed, nanoparticles are recomposed, being liberated to close the loop (Condomitti et al. 2012).
Magnetic nanoparticles are versatile, with many benefits such as the use of compact reactors, low-energy demand, non-aggressive conditions of use, reduced consumption of solvents, multiple design possibilities giving selectivity to capture metals of interest. It is important to highlight that magnetic nanoparticles may separate rare-earth elements, such as lanthanum and neodymium, in three extraction stages (Almeida and Toma 2016; Condomitti et al. 2018).
References
- Almeida, S.D.N. and Toma, H.E. (2016). Neodymium(III) and lanthanum(III) separation by magnetic nanohydrometallurgy using DTPA functionalized magnetite nanoparticles. Hydrometallurgy 161: 22–28. https://doi.org/10.1016/j.hydromet.2016.01.009.
- Andrews, D., Raychaudhuri, A., and Frias, C. (2000). Environmentally sound technologies for recycling secondary lead. Journal of Power Sources 88 (1): 124–129. https://doi.org/10.1016/S0378-7753(99)00520-0.
- Ashiq, A., Kulkarni, J., and Vithanage, M. (2019). Hydrometallurgical recovery of metals from E-waste. Electronic Waste Management and Treatment Technology: 225–246. (Issue March). https://doi.org/10.1016/B978-0-12-816190-6.00010-8.
- Awasthi, A.K. and Li, J. (2017). An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE. Resources, Conservation and Recycling 126: 228–239. https://doi.org/10.1016/j.resconrec.2017.07.014.
- Babu, B.R., Parande, A.K., and Basha, C.A. (2007). Electrical and electronic waste: a global environmental problem. Waste Management and Research 25: 307–318. https://doi.org/10.1177/0734242X07076941.
- Behnamfard, A., Mehdi, M., and Veglio, F. (2013). Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Management 33: 2354–2363.
- Bernardes, A.M., Espinosa, D.C.R., and Tenório, J.A.S. (2004). Recycling of batteries: a review of current processes and technologies. Journal of Power Sources 130 (1–2): 291–298. https://doi.org/10.1016/j.jpowsour.2003.12.026.
- Birloaga, I. and Vegliò, F. (2016). Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver and copper from waste printed circuit boards. Journal of Environmental Chemical Engineering 4: 20–29. https://doi.org/10.1016/j.jece.2015.11.021.
- Camelino, S., Rao, J., Padilla, L.R., and Lucci, R. (2015). Initial studies about gold leaching from printed circuit boards (PCB’s) of waste cell phones metals recycling from PCB’s and ion-lithium batteries view project. Procedia Materials Science 9: 105–112. https://doi.org/10.1016/j.mspro.2015.04.013.
- Chauhan, G., Jadhao, P.R., Pant, K.K., and Nigam, K.D.P. (2018). Novel technologies and conventional processes for recovery of metals from waste electrical and electronic equipment: challenges & opportunities – a review. Journal of Environmental Chemical Engineering 6 (1): 1288–1304. https://doi.org/10.1016/j.jece.2018.01.032.
- Chen, W.-S., Hsu, L.-L., and Wang, L.-P. (2018). Recycling the GaN waste from LED industry by pressurized leaching method. Metals 8 (10): 861. https://doi.org/10.3390/met8100861.
- Condomitti, U., Zuin, A., Silveira, A.T. et al. (2012). Magnetic nanohydrometallurgy: a promising nanotechnological approach for metal production and recovery using functionalized superparamagnetic nanoparticles. Hydrometallurgy 125–126: 148–151. https://doi.org/10.1016/j.hydromet.2012.06.005.
- Condomitti, U., Almeida, S.N., Silveira, A.T. et al. (2018). Green processing of strategic elements based on magnetic nanohydrometallurgy. Journal of the Brazilian Chemical Society 29 (5): 948–959. https://doi.org/10.21577/0103-5053.20180009.
- Correa, M.M.J., Silvas, F.P.C., Aliprandini, P. et al. (2018). Separation of copper from a leaching solution of printed circuit boards by using solvent extraction with D2EHPA. Brazilian Journal of Chemical Engineering 35 (3): 919–930. https://doi.org/10.1590/0104-6632.20180353s20170144.
- Cucchiella, F., D’Adamo, I., Lenny Koh, S.C., and Rosa, P. (2015). Recycling of WEEEs: an economic assessment of present and future e-waste streams. Renewable and Sustainable Energy Reviews 51: 263–272. https://doi.org/10.1016/j.rser.2015.06.010.
- Cui, H. and Anderson, C.G. (2016). Literature review of hydrometallurgical recycling of printed circuit boards (PCBs). Journal of Advanced Chemical Engineering 6 (1): 1–11. https://doi.org/10.4172/2090-4568.1000142.
- Dias, P., Javimczik, S., Benevit, M. et al. (2016a). Recycling WEEE: extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Management 57: 220–225. https://doi.org/10.1016/j.wasman.2016.03.016.
- Dias, P.R., Benevit, M.G., and Veit, H.M. (2016b). Photovoltaic solar panels of crystalline silicon: characterization and separation. Waste Management and Research 34 (3): 235–245. https://doi.org/10.1177/0734242X15622812.
- EASAC (2016). Priorities for critical materials for circular economy: Policy report 19. (Issue November).
- Erkel, V. (1995). Recovery of Cd and Ni from batteries. US Patent 5,407,463.
- Fang, S., Yan, W., Cao, H. et al. (2018). Evaluation on end-of-life LEDs by understanding the criticality and recyclability for metals recycling. Journal of Cleaner Production 182: 624–633. https://doi.org/10.1016/j.jclepro.2018.01.260.
- Fröhlich, S. and Sewing, D. (1995). The BATENUS process for recycling mixed battery waste. Journal of Power Sources 57 (1–2): 27–30. https://doi.org/10.1016/0378-7753(95)02234-1.
- Georgi-Maschler, T., Friedrich, B., Weyhe, R. et al. (2012). Development of a recycling process for Li-ion batteries. Journal of Power Sources 207: 173–182. https://doi.org/10.1016/j.jpowsour.2012.01.152.
- Goodship, V. and Stevels, A.L.N. (2012). Waste electrical and electronic equipment (WEEE) handbook. In: Waste Electrical and Electronic Equipment (WEEE) Handbook, 1e (eds. V. Goodship, A. Stevels and J. Huisman), 752. Woodhead Publishing https://doi.org/10.1533/9780857096333.
- Guo, C., Wang, H., Liang, W. et al. (2011). Liberation characteristic and physical separation of printed circuit board (PCB). Waste Management 9–10: 2161–2166. https://doi.org/10.1016/j.wasman.2011.05.011.
- Gupta, C.K. (2003). Chemical Metallurgy Principles and Practice. Wiley-VCH https://doi.org/10.1002/anie.200385071.
- Gupta, C.K. and Mukherjee, T.K. (1990). Hydrometallurgy in Extraction Processes, 1e, vol. 2. CRC Press https://doi.org/10.1201/9780203751404.
- Hadi, P., Xu, M., Lin, C.S.K. et al. (2015). Waste printed circuit board recycling techniques and product utilization. Journal of Hazardous Materials 283: 234–243. https://doi.org/10.1016/j.jhazmat.2014.09.032.
- Havlik, T., Orac, D., Petranikova, M. et al. (2010). Leaching of copper and tin from used printed circuit boards afterthermal treatment. Journal of Hazardous Materials 183: 866–873. https://doi.org/10.1016/j.jhazmat.2010.07.107.
- Huang, J., Chen, M., Chen, H. et al. (2014). Leaching behavior of copper from waste printed circuit boards with Brønsted acidic ionic liquid. Waste Management 34: 483–488. https://doi.org/10.1016/j.wasman.2013.10.027.
- Jackson, E. (1986). Hydrometallurgical Extraction and Reclamation. Ellis Horwood Limited.
- Jha, M.K., Kumari, A., Panda, R. et al. (2016). Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 161: 77–101. https://doi.org/10.1016/j.hydromet.2016.01.035.
- Jing-ying, L., Xiu-li, X., and Wen-quan, L. (2012). Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Management 32 (6): 1209–1212. https://doi.org/10.1016/j.wasman.2012.01.026.
- Kamberović, Ž., Korać, M., Ivšić, D. et al. (2009). Hydrometallurgical process for extraction of metals from electronic waste-part I: material characterization and process option selection. Metalurgija-Journal of Metallurgy 15 (4): 231–243. https://doi.org/10.30544/382.
- Kasper, A.C., Berselli, G.B.T., Freitas, B.D. et al. (2011). Printed wiring boards for mobile phones: characterization and recycling of copper. Waste Management 31 (12): 2536–2545. https://doi.org/10.1016/j.wasman.2011.08.013.
- Kaya, M. (2016). Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Management 57: 64–90. https://doi.org/10.1016/j.wasman.2016.08.004.
- Klugmann-Radziemska, E. and Kuczynska-Łazewska, A. (2020). The use of recycled semiconductor material in crystalline silicon photovoltaic modules production – a life cycle assessment of environmental impacts. Solar Energy Materials and Solar Cells 205, 110259 Contents. https://doi.org/10.1016/j.solmat.2019.110259.
- Kumar, V., Lee, J.c., Jeong, J. et al. (2015). Recycling of printed circuit boards (PCBs) to generate enriched rare metal concentrate. Journal of Industrial and Engineering Chemistry 21: 805–813. https://doi.org/10.1016/j.jiec.2014.04.016.
- Latunussa, C.E.L., Ardente, F., Blengini, G.A., and Mancini, L. (2016a). Life cycle assessment of an innovative recycling process for crystalline silicon photovoltaic panels. Solar Energy Materials and Solar Cells 156: 101–111. https://doi.org/10.1016/j.solmat.2016.03.020.
- Latunussa, C. E. L., Mancini, L., Blengini, G. A., Ardente, F., Pennington, D. (2016b). Analysis of material recovery from silicon photovoltaic panels (p. 45). https://doi.org/10.2760/378123.
- Li, J., Shrivastava, P., Gao, Z., and Zhang, H.-C. (2004). Printed circuit board recycling methods. Workshop Materials on WEEE Management in Taiwan 27: 33–42.
- Li, M., Yang, J., Liang, S. et al. (2019). Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. Journal of Power Sources 436 (July): 226853. https://doi.org/10.1016/j.jpowsour.2019.226853.
- Maarefvand, M., Sheibani, S., and Rashchi, F. (2020). Recovery of gallium from waste LEDs by oxidation and subsequent leaching. Hydrometallurgy 191 (June 2019): 105230. https://doi.org/10.1016/j.hydromet.2019.105230.
- Mdlovu, N.V., Chiang, C.L., Lin, K.S., and Jeng, R.C. (2018). Recycling copper nanoparticles from printed circuit board waste etchants via a microemulsion process. Journal of Cleaner Production 185: 781–796. https://doi.org/10.1016/j.jclepro.2018.03.087.
- Mizanur Rahman, S.M., Kim, J., Lerondel, G. et al. (2017). Missing research focus in end-of-life management of light-emitting diode (LED) lamps. Resources, Conservation and Recycling 127: 256–258. https://doi.org/10.1016/j.resconrec.2017.04.013.
- Murakami, H., Nishihama, S., and Yoshizuka, K. (2015). Separation and recovery of gold from waste LED using ion exchange method. Hydrometallurgy 157: 194–198. https://doi.org/10.1016/j.hydromet.2015.08.014.
- Namias, J. (2013). The future of electronic waste recycling in the united states: obstacles and domestic solutions. July.
- Nayaka, G.P., Zhang, Y., Dong, P. et al. (2019). An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. Journal of Environmental Chemical Engineering 7 (1): 102854. https://doi.org/10.1016/j.jece.2018.102854.
- Neto, I.F.F., Sousa, C.A., Brito, M.S.C.A. et al. (2016). A simple and nearly-closed cycle process for recycling copper with high purity from end life printed circuit boards. Separation and Purification Technology 164: 19–27.
- Ning, C., Sze, C., Lin, K. et al. (2017). Waste printed circuit board (PCB) recycling techniques. Topics in Current Chemistry 375: 43. https://doi.org/10.1007/s41061-017-0118-7.
- Perez, J.P.H., Folens, K., Leus, K. et al. (2019). Progress in hydrometallurgical technologies to recover critical raw materials and precious metals from low-concentrated streams. Resources, Conservation and Recycling 142: 177–188. https://doi.org/10.1016/j.resconrec.2018.11.029.
- Petranikova, M., Herdzik-Koniecko, I., Steenari, B.M., and Ekberg, C. (2017). Hydrometallurgical processes for recovery of valuable and critical metals from spent car NiMH batteries optimized in a pilot plant scale. Hydrometallurgy 171: 128–141. https://doi.org/10.1016/j.hydromet.2017.05.006.
- Petter, P.M.H., Veit, H.M., and Bernardes, A.M. (2014). Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Management 34 (2): 475–482. https://doi.org/10.1016/j.wasman.2013.10.032.
- Provazi, K., Campos, B.A., Espinosa, D.C.R., and Tenório, J.A.S. (2011). Metal separation from mixed types of batteries using selective precipitation and liquid-liquid extraction techniques. Waste Management 31 (1): 59–64. https://doi.org/10.1016/j.wasman.2010.08.021.
- Raele, M.P., De Pretto, L.R., and Zezell, D.M. (2017). Soldering mask laser removal from printed circuit boards aiming copper recycling. Waste Management 68: 475–481. https://doi.org/10.1016/j.wasman.2017.07.019.
- Ram, C.G., Kislik, V.S., and Vladimir, S.K. (2014). Solvent Extraction: Classical and Novel Approaches. Elsevier (Issue 1). https://doi.org/10.1007/s13398-014-0173-7.2.
- Rocchetti, L., Amato, A., and Beolchini, F. (2018). Printed circuit board recycling: a patent review. Journal of Cleaner Production 178: 814–832. https://doi.org/10.1016/j.jclepro.2018.01.076.
- Roshanfar, M., Golmohammadzadeh, R., and Rashchi, F. (2019). An environmentally friendly method for recovery of lithium and cobalt from spent lithium-ion batteries using gluconic and lactic acids. Journal of Environmental Chemical Engineering 7 (1): 102794. https://doi.org/10.1016/j.jece.2018.11.039.
- Ruiz-Mercado, G.J., Gonzalez, M.A., Smith, R.L., and Meyer, D.E. (2017). A conceptual chemical process for the recycling of Ce, Eu, and Y from LED flat panel displays. Resources, Conservation and Recycling 126: 42–49. https://doi.org/10.1016/j.resconrec.2017.07.009.
- Sethurajan, M., van Hullebusch, E.D., Fontana, D. et al. (2019). Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes – a review. Critical Reviews in Environmental Science and Technology 49 (3): 212–275. https://doi.org/10.1080/10643389.2018.1540760.
- Silvas, F.P.C., Jiménez Correa, M.M., Caldas, M.P.K. et al. (2015). Printed circuit board recycling: physical processing and copper extraction by selective leaching. Waste Management 46: 503–510. https://doi.org/10.1016/j.wasman.2015.08.030.
- Siqi, Z., Guangming, L., Wenzhi, H. et al. (2019). Recovery methods and regulation status of waste lithium-ion batteries in China: a mini review. Waste Management and Research 37 (11): 1142–1152. https://doi.org/10.1177/0734242X19857130.
- Swain, N. and Mishra, S. (2019). A review on the recovery and separation of rare earths and transition metals from secondary resources. Journal of Cleaner Production 220: 884–898. https://doi.org/10.1016/j.jclepro.2019.02.094.
- Tammaro, M., Rimauro, J., Fiandra, V., and Salluzzo, A. (2015). Thermal treatment of waste photovoltaic module for recovery and recycling: experimental assessment of the presence of metals in the gas emissions and in the ashes. Renewable Energy 81: 102–112. https://doi.org/10.1016/j.renene.2015.03.014.
- Tao, J. and Yu, S. (2015). Review on feasible recycling pathways and technologies of solar photovoltaic modules. Solar Energy Materials & Solar Cells 141: 108–124.
- Tesfaye, F., Lindberg, D., Hamuyuni, J. et al. (2017). Improving urban mining practices for optimal recovery of resources from e-waste. Minerals Engineering 111: 209–221. https://doi.org/10.1016/j.mineng.2017.06.018.
- Tunsu, C., Petranikova, M., Gergorić, M. et al. (2015). Reclaiming rare earth elements from end-of-life products: a review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 156: 239–258. https://doi.org/10.1016/j.hydromet.2015.06.007.
- Valix, M., Loo, Y.S., Bucknell, J. et al. (2017). Effect of FR-4 decomposition in the hydrometallurgical recovery of copper from electronic waste. Hydrometallurgy 173: 199–209. https://doi.org/10.1016/j.hydromet.2017.08.012.
- Veit, H.M., Diehl, T.R., Salami, A.P. et al. (2005). Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Management 25 (1): 67–74. https://doi.org/10.1016/j.wasman.2004.09.009.
- Veit, H.M., Bernardes, A.M., Ferreira, J.Z. et al. (2006). Recovery of copper from printed circuit boards scraps by mechanical processing and electrometallurgy. Journal of Hazardous Materials 137 (3): 1704–1709. https://doi.org/10.1016/j.jhazmat.2006.05.010.
- Veit, H.M., Juchneski, N.C.d.F., and Scherer, J. (2014). Use of gravity separation in metals concentration from printed circuit board scraps. Metallurgy and Materials 67 (1): 73–79.
- Wang, T. Y., Hsiao, J. C., & Du, C. H. (2012). Recycling of materials from silicon base solar cell module. Conference Record of the IEEE Photovoltaic Specialists Conference, 2355–2358. https://doi.org/10.1109/PVSC.2012.6318071.
- Wang, F., Zhao, Y., Zhang, T. et al. (2017). Metals recovery from dust derived from recycling line of waste printed circuit boards. Journal of Cleaner Production 165: 452–457. https://doi.org/10.1016/j.jclepro.2017.07.112.
- Wang, L., Li, Q., Sun, X., and Wang, L. (2019). Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Separation Science and Technology 54 (8): 1302–1311. https://doi.org/10.1080/01496395.2018.1539106.
- Yamane, L.H., de Moraes, V.T., Espinosa, D.C.R., and Tenório, J.A.S. (2011). Recycling of WEEE: characterization of spent printed circuit boards from mobile phones and computers. Waste Management 31 (12): 2553–2558. https://doi.org/10.1016/j.wasman.2011.07.006.
- Yi, Y.K., Kim, H.S., Tran, T. et al. (2014). Recovering valuable metals from recycled photovoltaic modules. Journal Ofthe Air & Waste Management Association 64 (7): 797–807. https://doi.org/10.1080/10962247.2014.891540.
- Zeng, X., Li, J., and Singh, N. (2014). Recycling of spent lithium-ion battery: a critical review. Critical Reviews in Environmental Science and Technology 44 (10): 1129–1165. https://doi.org/10.1080/10643389.2013.763578.
- Zhang, L. and Xu, Z. (2016). A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. Journal of Cleaner Production 127: 19–36. https://doi.org/10.1016/j.jclepro.2016.04.004.
- Zhou, Y. and Qiu, K. (2010). A new technology for recycling materials from waste printed circuit boards. Journal of Hazardous Materials 175: 823–828. https://doi.org/10.1016/j.jhazmat.2009.10.083.
- Zhou, H., Wang, Y., Guo, X. et al. (2018). The recovery of rare earth by a novel extraction and precipitation strategy using functional ionic liquids. Journal of Molecular Liquids 254: 414–420. https://doi.org/10.1016/j.molliq.2018.01.078.
- Zhou, J., Zhu, N., Liu, H. et al. (2019). Recovery of gallium from waste light emitting diodes by oxalic acidic leaching. Resources, Conservation and Recycling 146 (March): 366–372. https://doi.org/10.1016/j.resconrec.2019.04.002.
