9
Recycling Technologies – Biohydrometallurgy
Franziska L. Lederer and Katrin Pollmann
Helmholtz-Zentrum Dresden-Rossendorf, Helmholtz Institute Freiberg for Resource Technology, Biotechnology Division, Bautzner Landstraße 400, 01328 Dresden, Germany
9.1 Introduction
Biohydrometallurgy is one of many different processes for metal recovery. As a highly interdisciplinary field, biohydrometallurgy combines microorganisms and their metabolites (−bio) in a mainly aquatic environment (−hydro) and the treatment of metal-containing materials or solutions (−metallurgy) for metal production and treatment. It is applied to many different metal-rich materials from primary mineral sources, secondary mining products, and numerous manufactured resources (Watling 2015). Biohydrometallurgy uses biological tools for the processing of primary ores for many years – especially in case of bioleaching. Besides that, special biological tools can enhance the metal recovery from manufactured resources such as technical waste products, processing wastes, industrial waste waters, and other secondary sources (Pollmann et al. 2018). In nature, multiple processes exist that influence biogeochemical cycles of elements. These microorganism-driven processes contribute to bioaccumulation, bioweathering, biomineralization, and precipitation or microbial reduction. Using these bio-inspired processes promotes biological recycling strategies as well as several clean industrial processes, bio-based materials, and bioremediation. Modern bio-based approaches that are currently being developed for the recycling of valuable elements found in technical products contribute to a “green” circular economy. Main processes in biohydrometallurgy are bioleaching, biosorption, bioflotation, and bioreduction.
9.2 Bioleaching: Metal Winning with Microbes
Bioleaching is defined as the extraction of metals by the metabolic activity of bacteria (direct bioleaching) or metabolic compounds (indirect bioleaching). It is applicable to metal extraction from low-grade ores, beneficiation of ores or coal, removal of toxic metals, and recovery of metals from waste materials.
Many bioleaching studies concentrate on the metal extraction from ores, but the processes can be applied principally also to other sources such as industrial residues and waste materials that can be considered as an artificial ore. During the last years, numerous studies were published that describe the application of bioleaching approaches for the recovery of metals from various technical and industrial waste products, e.g. fly ash from municipal waste incineration, electronic scrap such as printed circuit boards from computers and mobile phones, spent catalysts and batteries, and others. Bioleaching approaches on industrial residues and waste materials are based on the use of chemolithoautotrophic bacteria, heterotrophic bacteria, yeasts and fungi, and cyanogenic bacteria. Most studies with chemolithoautotrophic bacteria used the acidophilic organisms Acidithiobacillus ferrooxidans or A. thiooxidans (e.g. Brombacher et al. 1998; Gholami et al. 2011; Karwowska et al. 2014; Mishra et al. 2008). These approaches obtained relatively high leaching efficiencies (in many cases of >80% of metals) but required the addition of sulfur and acidification of the cultivation media to maintain bacterial growth and solubilization process. Studies describing approaches with heterotrophic microorganisms used bacteria, fungi, or yeasts that produce diverse organic acids such as citric acid, gluconic acid, or acetic acid as lixiviant for the respective metals. Especially the fungus Aspergillus niger was used for a broad range of materials (Bosshard et al. 1996; Brandl et al. 2001; Qu et al. 2015). Depending on growth conditions, this organism produces huge amounts of diverse organic acids (e.g. citric acid, gluconic acid, oxalic acid). The organism is applied commercially for organic acid production and cultivation as well as its metabolism has been studied in detail. In some cases, more than 90% of metals could be mobilized by application of this organism (Brandl et al. 2001). Biogenic cyanide produced by Chromobacter violaceum was used for the mobilization of gold and other noble metals, copper, and nickel from shredded printed circuit boards and automotive catalysts by using direct or indirect leaching approaches (Campbell et al. 2001; Chi et al. 2011; Faramarzi et al. 2004; Shin et al. 2015b). In indirect bioleaching processes, more than 90% of metals were mobilized. These results were comparable with the usage of commercial sodium cyanide (NaCN) demonstrating the principal suitability of such approaches for commercial applications (Shin et al. 2015b).
Most studies aimed at the recovery of valuable metals such as Cu, Ni, Au, or other noble metals. These elements can be found in high concentrations in diverse electronic wastes or in residues from mineral processing, e.g. smelter dust, fly ash, or incineration slag (Auerbach et al. 2019; Bosshard et al. 1996; Brandl et al. 2001; Brandl et al. 2008; Brombacher et al. 1998; Klink et al. 2016; Oliazadeh et al. 2006; Ramanathan and Ting 2016). However, starting with the resource crises in 2009, an increasing number of studies have been published that concentrate on other valuable elements. Several research groups investigated the mobilization of rare-earth elements (REE) that are an essential component in most modern technologies, from different solid materials. Most of these studies used a variety of heterotrophic bacteria for the extraction of REE from REE-bearing minerals such as monazite (Hassanien et al. 2014; Shin et al. 2015a). However, some recent studies investigated bioleaching approaches for different REE-bearing secondary resources such as red mud from alumina production (Cizkova et al. 2019; Qu and Lian 2013a; Qu et al. 2013b) or electronic waste material, e.g. from waste phosphors (Hopfe et al. 2017, 2018; Reed et al. 2016). In most cases, REE are mobilized either by different organic acids or by enzymatic activity of phosphate solubilizing microorganisms.
As consequence of increasing electromobility, lithium and cobalt as components of energy storage devices moved into the center of attention. Some studies investigated the extraction for Li and Co from waste lithium ion batteries either by a combination of chemical treatment with citric acid and bioactivity (Dolker and Pant 2019) or by A. niger (Horeh et al. 2016) or A. ferrooxidans (Mishra et al. 2008).
Maneesuwannarat et al. (2016) used a strain of Cellolosemicrobium funkei, which was isolated from cadmium- and arsenic-contaminated soil for bioleaching of GaAs (Maneesuwannarat et al. 2016). It was supposed that proteins are involved in Ga mobilization, indicating a new mechanism for metal dissolution (Maneesuwannarat et al. 2019).
These studies demonstrate the great potential that microorganisms offer for the transformation of materials that can be used for new recycling routes. It can be expected that the ongoing growing demand of other elements, and development and growth of new technologies such as renewable energies will promote further studies.
Although all these studies gave a proof of principle for the application of microorganisms for metal mobilization from various waste materials, only few of these approaches have been implemented in industrial processes just yet. Major challenges are selectivity, efficiency, and economy of bioprocesses. More recent studies developed stepwise approaches by combining different chemical or biological leaching steps. For example, Pourhossein and Mousavi obtained high leaching rates of >80% for different elements (Cu, Ni, Ga) from waste light-emitting diodes (WLEDs) by applying a stepwise indirect bioleaching approach using a biogenic ferric agent (Pourhossein and Mousavi 2019). Rizki et al. (2019) combined chemical leaching using thiourea with bioleaching by a thiourea-tolerant Fe-oxidizing microorganism to extract gold from electronic waste (Rizki et al. 2019). Huang et al. (2019) reported on a bio-electro-hydrometallurgical process that combines bioleaching by using sulfur-oxidizing bacteria with an electrokinetic recovery process (Huang et al. 2019). It can be expected that such approaches overcome current barriers in using biological approaches.
9.3 Biosorption: Selective Metal Recovery from Waste Waters
Biosorption is defined as the property of biomass or certain biomolecules to bind and concentrate selected ions or other molecules from aqueous solutions (Volesky 2007). It is a passive process and independent from metabolic activities. Therefore, nutrients are not required, and processes can be performed in environments with high toxicity. Biosorption has been mainly applied for the removal of toxic metals from polluted waters, such as arsenic, chromate, cadmium, or uranium (Volesky and Holan 1995).
Another attractive application is the recovery of valuable metals such as gold, platinum, palladium, or others from solutions (Das 2010; Pollmann et al. 2006b). Conventional pyrometallurgical or hydrometallurgical methods (e.g. adsorption by ion-exchange resin, activated carbon, or minerals, solvent extraction, chemical precipitation) require high amounts of energy and addition of chemical agents, thus generating secondary wastes, or are inefficient especially for highly diluted solutions (Das 2010). Biosorption is an environment-friendly alternative to these methods because it uses biodegradable compounds that can be easily produced in high amounts. Further, biomass is considered as carbon-neutral and petrochemical-independent process as it does not emit extra carbon dioxide when burned (Maruyama et al. 2007; Ritter 2004). Various types of biomass have been reported to bind and concentrate metal ions from industrial effluents and aqueous solutions.
Metal-containing solutions such as industrial waste waters, leachates, and mining waters are often acidic with pH < 3, have a complex composition containing different competing elements, and contain toxic chemicals or organic compounds that influence biosorptive properties. Therefore, major challenges of biosorptive approaches are the stability of materials, selectivity, effectivity, and cost efficiency. Several approaches address these challenges. Most studies concentrated on the use of bacterial cells, fungi, yeast (Volesky and Holan 1995), algae (reviewed by He and Chen (2014)), seaweed biomass (Figueira et al. 2000), or biocomponents such as crab shells (Daubert and Brennan 2007), plant fibers (Salamun et al. 2015), etc. as biosorptive components that can be easily produced or are waste materials (e.g. in case of crab shells). Especially biopolymers such as cellulose, chitin, or chitosan materials are chemically resistant. However, these materials possess no selectivity and bind a broad range of different elements. This is a drawback for their application in metal recovery processes because these applications are intended only the concentration of metals from highly diluted solutions but also the selective recovery of metals of interest.
A different approach was propagated by Bonificio and Clarke (2016). These authors described a selective recovery of REE by biosorption on immobilized bacterial biomass followed by a selective desorption as a function of pH. This approach enabled the separation of the three heaviest lanthanides Tm, Lu, and Yb from a mixture of different lanthanides.
Other recent developments concentrate on the use of defined proteins from biomass or the direct engineering of improved microbes and enzymes. Maruyama et al. (2007) tested different model peptides, proteins, and protein-rich biomass regarding their capability to selectively bind different precious metals from model solutions, metal-refining solutions, and industrial wastes at acidic conditions (Maruyama et al. 2007). All tested biomasses as well as proteins selectively adsorb Pd and Au ions in the presence of transition elements. Further, it was possible to remove Au, Pd, and Pt from plating wastes using protein-rich chicken egg-shell membrane.
Other approaches use the metal-binding motifs of natural proteins, e.g. metallothioneins, as biosorptive component. Metallothioneins (MT) are cysteine-rich proteins that bind different metals such as Cd, Hg, Cu, and Pb. MTs from different natural sources have been expressed in Escherichia coli and Pseudomonas putida and used as biosorbent, mainly for removal of heavy metals (reviewed by Chen et al. (1999)) and Mejare and Bulow (2001). However, these proteins are also attractive for the recovery of valuable metals. Terashima et al. (2002) produced a fusion protein composed of the maltose-binding protein and human MT and immobilized it on Chitopearl resins (Terashima et al. 2002). These materials were used for binding of Cd and Ga in a concentration range of 0.2–1.0 mM. Further, the biosorbents could be used several times without loss of binding activity.
CadR, which is a Cd-binding protein first isolated from rhizobacterium P. putida, has been expressed on the surface of E. coli cells. These engineered cells show a high Cd2+ adsorption capacity of 19.5 μmol Cd(II) g−1 cells (Liu et al. 2015).
Phytochelatins (PCs) are naturally occurring metal-binding peptides, which contain multiple repeats of the γGlu-Cys moiety terminated by a Gly residue. Various researchers have expressed different synthetic PCs onto the surface of bacterial cells to improve metal uptake and biosorption. For example, recently, Tan et al. (2019) displayed the synthetic phytochelatin EC20 onto the surface of E. coli. The obtained constructs showed an increased biosorption of Pt(IV) accompanied by the formation of platinum nanoparticles (Tan et al. 2019).
Proteinaceous bacterial surface layers that envelope many bacterial cells are other interesting biomolecules that have been used for the binding of different elements such as U, Pd, Au, or Cu (Allievi et al. 2011; Merroun et al. 2005; Pollmann et al. 2006b). The binding of U, Pd, and Au has been investigated in more detail in case of the S-layers from Lysinibacillus sphaericus JG-A12 and NCTC 9602 (Fahmy et al. 2006; Jankowski et al. 2010; Merroun et al. 2005). These elements were coordinated by phosphate and carboxyl groups (Fahmy et al. 2006; Merroun et al. 2005); in case of Au(III), it was assumed that amine groups were involved in complexation (Jankowski et al. 2010). Due to their self-assembling properties that enable the formation of nanostructured protein arrays on various technical surfaces (Sleytr et al. 2014; Toca-Herrera et al. 2005; Weinert et al. 2015), S-layer proteins are attractive biomolecules for the construction of biosorptive composites (Suhr et al. 2014). For example, so-called biocers were produced by entrapping S-layer carrying cells or S-layers in porous ceramics using sol–gel technology and used for the removal of U from contaminated waters (Soltmann et al. 2002). Pollmann et al. (Pollmann and Matys 2007) constructed modified His-tagged S-layer proteins that exhibited enhanced Ni-binding capacities while self-assembling to a nanoporous protein meshwork (Pollmann and Matys 2007).
Peptides are other less complex and easily synthesizable biomolecules that have been used for the design of various biosorbents. Stair et al. (Stair and Holcombe 2005) synthesized and immobilized various peptides of different lengths composed of Gly, Asp, and Cys residues on commercial Tentagel resins and used it as biosorbents for the binding of Ni2+, Cd2+, Co2+, and Mg2+ (Stair and Holcombe 2005).
All the previously discussed biomolecules are able to interact with a specific number of ions. A selectivity to the target ion is not given in the above shown approaches.
9.3.1 Biosorption Via Metal Selective Peptides
The lack of selectivity in separation processes can be solved by using a novel, very promising approach for the selection of metal-binding peptides by phage surface display. With this technique, peptides selective for several metallic surfaces or metal ions were identified (Sarikaya et al. 2003; Seker and Demir 2011). Cetinel and coworkers describe the technique appropriately as “the directed evolution of peptides with specific interactions toward technologically relevant materials” based on combinatorial bio-based libraries (Cetinel et al. 2012). The functional groups presented by individual amino acids of the identified peptides and the interaction with neighbor functional groups are responsible for the specific and strong interaction with the target material. Insertion, deletion, or exchange of one amino acid can change the peptide–target interaction drastically. The peptide–target bonding usually occurs via long-range interactions (physisorption) or short-range interactions (chemisorption) (Schwaminger et al. 2018). Fundamental knowledge of the occurring peptide–target interactions is necessary to improve and control these bio-based interactions for an optimization of separation and recycling processes.
Material-selective peptides are used currently mainly for the development of nanomaterials and composites, but they are also attractive as biosorbents. Nian et al. (2010) selected Pb2+-specific peptides and identified one bacteriophage-expressed peptide (TNTLSNN) with high affinity and specificity to Pb2+ as proven by cross-binding assay to different metal ions (Nian et al. 2010). In a follow-up study, Nguyen et al. (2013) constructed a recombinant E. coli displaying the peptide on its cell surface thus obtaining a highly selective E. coli-based biosorbent (Nguyen et al. 2013). Similarly, Yang et al. (2015) selected Cr(III) binding phages from a phage display library (Yang et al. 2015). A phage expressing the heptameric peptide YKASLIT was immobilized on cytopore beads for Cr(III) preconcentration. Sawada et al. (2016) selected Nd(III) binding bacteriophages via phage surface display technology (Sawada et al. 2016). These phages were used as adsorbent for the selective recovery of Nd(III) from mixed solutions of Nd(III) and Fe(III), mimicking the dissolved solution of neodymium–iron–boron alloys (Nd2Fe15B) indicating a high potential to be applied in recycling strategies. In another approach, lanthanide oxide particles were used as target to select peptides that induce the precipitation of lanthanide hydroxides (Hatanaka et al. 2017). Three peptides (SCLWGDVSELDFLCS, SCLYPSWSDYAFCS, SCPVWFSDVGDFMVCS) were identified that mediate the mineralization of lanthanide ions. The researchers proposed that such peptides have a potential for the separation of lanthanides via selective mineralization. Yunus et al. (Yunus and Tsai 2015) immobilized genetically engineered fusion proteins composed of palladium-binding peptides and cellulose-binding domains on cellulose materials (Yunus and Tsai 2015). These constructs were used as biosorbents for the selective binding of Pd(II). The materials were able to selectively bind Pd(II) from a mixture of Pd(II) and Pt(IV) with a maximum adsorption capacity of 175.44 mg/g. Further, it was possible to remove the bound Pd and reuse the biosorbent several times without losing the binding capacity. The materials were working at a wide range of different pH (pH 1.8–11) and temperatures (10–40 °C); therefore, they can be applied at different conditions. Yang et al. (2018) identified arsenic (III)-binding peptides with the ability to induce the aggregation of gold nanoparticles in the absence of arsenic (III). These peptides can be applied as colorimetric detection sensors for arsenic (III) (Yang et al. 2018). Schönberger and coworkers presented several linear gallium-binding peptides identified via phage surface display and used afterwards a cysteine-scanning methodology to introduce structures in one preferred peptide. The changed binding affinity of the modified peptides were tested in subsequent biosorption experiments for the peptides future application in biorecovery approaches for gallium (Schönberger et al. 2019a, b). In 2020, Matys and coworkers identified peptides that selectively interact with nickel (CNAKHHPRC) and cobalt (CTQMLGQLC) using sol–gel coated glass–fiber fabrics for the future application in new element-specific biosorptive materials (Matys et al. 2020). Arsenic-binding peptides were identified in the study of Braun and coworkers in 2020 for the decontamination of industrial wastewater. This group developed a new combined approach of phage display and next-generation sequencing for the identification of the strongest target-binding peptides (Braun et al. 2020). These examples demonstrate that phage surface display technology is a promising strategy to identify highly selective peptides for different elements that can be used for the construction of biosorbents not only for bioremediation but also for the recovery of valuable metals.
In numerous studies, metal-binding motifs and peptides were expressed and anchored on the surface of microbial cells via fusion with outer membrane proteins. In many approaches, the peptides were anchored to the outer membrane protein LamB, thus obtaining engineered microbes that worked as an efficient adsorbent. For example, hexa-His chains were expressed on the surface of E. coli by construction of LamB hybrids. These cells exhibited high affinity to Ni ions (Sousa et al. 1996). Other researchers expressed metallothioneins or metal-binding peptides as fusions to membrane or membrane associated proteins in E. coli, P. putida, yeasts, or other microorganisms (Kotrba et al. 1999a, b; Nishitani et al. 2010; Sousa et al. 1998; Valls et al. 2000a, b; Valls et al. 1998). Park et al. (2016) produced fusion proteins comprising the surface (S-layer) protein of Caulobacter crescentes and peptides that have been used as lanthanide-binding tags for protein purification, biosensing, and nuclear magnetic resonance (NMR) spectroscopy (Liang et al. 2013; Martin et al. 2007; Nitz et al. 2003; Park et al. 2016). These hybrid proteins were expressed in the cell surface of C. crescentes in high density (Park et al. 2016). The engineered cells exhibited an enhanced sorption of REE and a high specificity for REE. Further, it was possible to desorb the bound REE enabling a repeatable reuse of the bioadsorbents. Li and coworkers developed a Ni-ion biosorption process based on nickel-binding peptides presented by surface engineered yeast (Li et al. 2019). Immobilized Saccharomyces cerevisiae EBY100 expressing three different Ni-binding peptides at the same time showed the selective biosorption of up to 68.62% of all the Ni-ions in the system. Other heavy metals like As(III), Pb(II), Cr(III), and Cd(III) were not or were in very small dimensions bound to the yeast surface. Thus, cell surface display is an attractive approach for implementation in recycling processes. Other approaches use metabolic products as complexing agents.
9.3.2 Chelators Derived from Nature
Very interesting biomolecules are siderophores. These small organic molecules are iron chelators that are produced and secreted by bacteria and are used for the uptake of iron. In addition to iron, other metals, e.g. Ga, Co, different actinides, can be complexed by the siderophores (Brainard et al. 1992; Gascoyne et al. 1991b; Gascoyne et al. 1991a; Harrington et al. 2012). These properties make them attractive for biotechnological applications. For example, Jain et al. (2019) used the siderophores desferrioxamine A and E for Ga complexation and developed a chromatography method enabling the selective recovery of Ga from industrial waste waters. Regeneration and multiple reuse of the biomolecules was possible, which is the requirement for an economic application of the technology (Jain et al. 2019). In another study, the siderophore yersinobactin, a metal-chelating peptide derived from Yersinia pestis, was adsorbed on a resin within a packed-bed column. With this material, it was possible to remove >80% of copper from field water mixed with copper (Ahmadi et al. 2016).
Biopolymers are another attractive group of metabolites. Bacterial poly(γ-glutamic acid) has been used for the adsorption of toxic Hg(II) (Inbaraj et al. 2009), Pb(II) (Mu et al. 2011), and Fe(III) (Bodnar et al. 2013). Varshini constructed a modified biohydrogel and used it for the removal of the rare-earth element Ce(III) from industrial effluents (Varshini et al. 2015). Different extracellular polymeric substances have been used for the removal of Co(II), Cu(II), and other elements (Dobrowolski et al. 2017; Mona and Kaushik 2015; Perez et al. 2008). Natural polysaccharides such as alginate, chitin, chitosan, starch as well as their derivatives and polysaccharide-based composites have been widely used for the removal of not only different heavy metals (reviewed by Crini (2005)) but also precious metals (Donia et al. 2007).
To enable a low-cost usage, the biocompounds should be recycled and reused after adsorption. The repeatable use of biomolecules for biosorption requires the immobilization of the molecules to an appropriate surface. The combination of biocompounds with inorganic materials brings together advantages of both materials. Soltmann et al. (2002) developed uranium-binding composites, so-called bio-ceramics (biocers), by immobilization of bacterial cells or surface (S) layer proteins via sol–gel techniques (Soltmann et al. 2002). These composites were used not only for the removal of uranium from waters but also for the binding of Pd(II) and copper (Raff et al. 2003; Pollmann et al. 2006a, b). Yunus et al. (Yunus and Tsai 2015) immobilized fusion proteins composed of palladium-binding peptides and cellulose-binding domains on cellulose (Yunus and Tsai 2015). These complexes were used for the adsorption of Pd(II) from model solutions at various conditions. In addition, it was possible to desorb the Pd(II) using 1 M thiourea, thus creating a reusable Pd(II) selective biosorbent. Other approaches entrap the biocompounds in polyvinyl alcohol, chitosan, hydrogels, or alginate (Ting and Sun 2000).
9.4 Bioflotation: Separation of Particles with Biological Means
Microbial cells, cell components, metabolites, or other biomolecules can interact with solid substrates and modify surface properties, e.g. by introducing hydrophobic properties by adhesion to the surfaces (Das et al. 1999; Patra and Natarajan 2006). These properties can be used for mineral beneficiation. For example such biocompounds have been reported as environment-friendly collectors or depressants and were applied as flotation reagents in selective mineral separation (reviewed by Behera and Mulaba-Bafubiandi (2017)). Most of these approaches concentrate on the use of bacterial cells or their products that have been described to specifically interact with minerals such as Acidithiobacillus or Leptospirillum ferrooxidans or Rhodococcus opacus or on model organisms (Behera and Mulaba-Bafubiandi 2017). However, newer investigations demonstrate the applicability of a much broader range of microorganisms beyond the classical bioleaching bacteria or model organisms. Luque Consuegra et al. investigated the influence of different marine bacteria on bioflotation of pyrite and chalcopyrite and identified strains of Halobacillus sp. and Marinococcus sp. to depress pyrite in artificial sea water conditions while improving the flotation of chalcopyrite (Consuegra et al. Consuegra et al. 2019). These studies prove the high potential of the application of various bacteria in diverse environments for particle separation, thus opening up not only new perspectives in mineral separation technologies but also in recycling technologies and other industrial applications.
Biopolymers and so-called extracellular polymeric substances (EPS) mediate the attachment of bacterial cells to surfaces and biofilm formation (Gehrke et al. 1998; Kinzler et al. 2003; Vu et al. 2009). They form the structure and architecture of the biofilm matrix. The EPS are composed of an undefined complex mixture of biopolymers primarily consisting not only of polysaccharides, but also lipids, proteins, humic acids, and nucleic acids (reviewed by Vu et al. (2009)). Their composition depends on type of microorganisms, age of biofilm, and environmental conditions, including surface properties (Donlan 2002). Their affinity to surfaces makes them interesting for their application in flotation processes. Consequently, several studies investigated the effect of EPS as bioreagent in mineral separation (Figure 9.1).
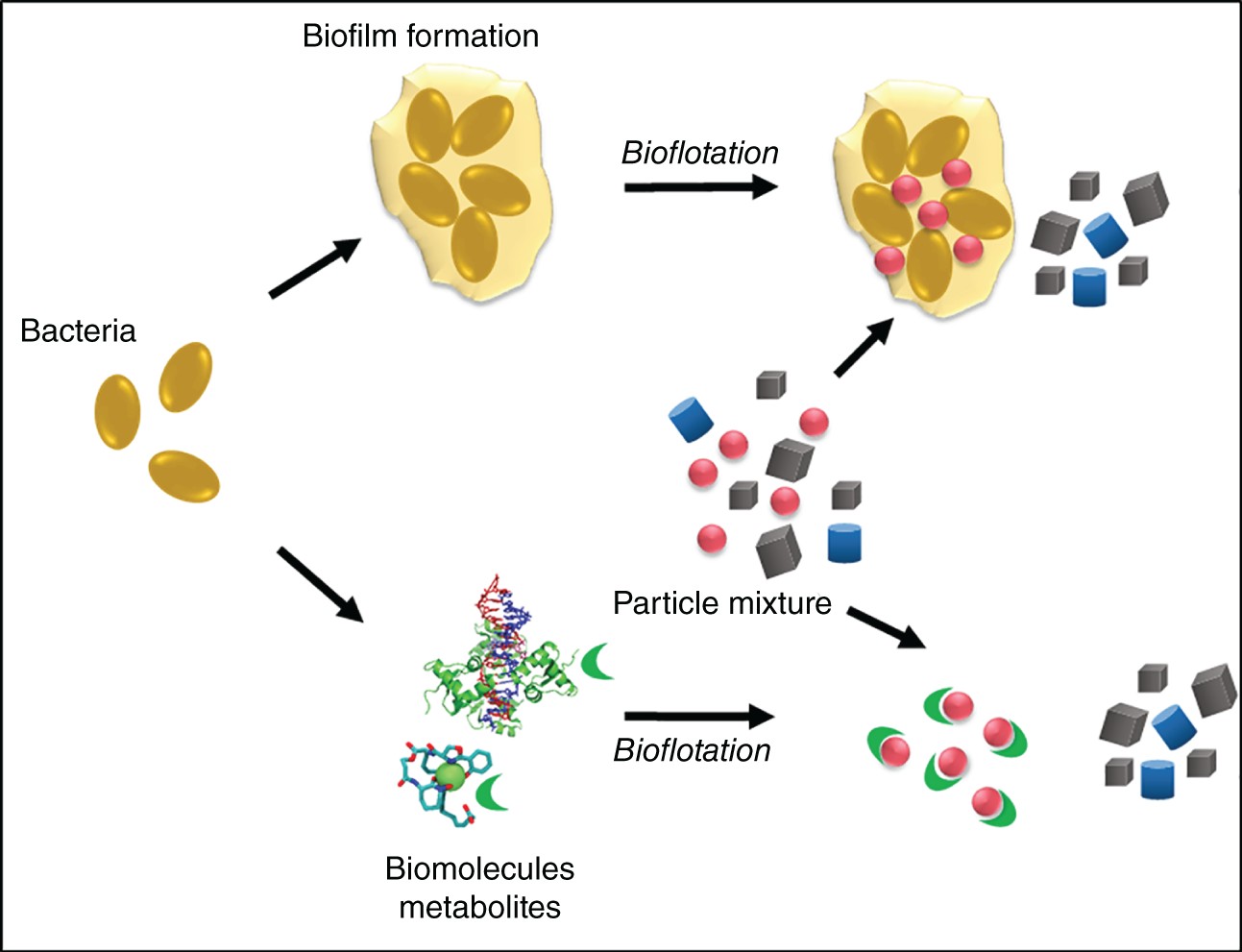
Figure 9.1 Use of extracellular polymeric substances (EPS) producing bacteria and biomolecules for particle separation.
Besides EPS, other biomolecules have been investigated regarding their application in mineral separation. Especially biosurfactants are interesting compounds that have been applied as frothers in many flotation experiments. Biosurfactants are surface-active organic molecules that are produced by many microorganisms. They have been attributed to lowering the surface tension at the interfaces of solid, liquid, and gases. In contrast to common commercial chemical surfactants, they are less toxic, biodegradable, and effective under extreme conditions. Consequently, there are numerous potential fields of application, including pharmaceutical industry, environmental remediation, and petroleum industry (reviewed by Saha and Rao (2017)). Amphiphilic siderophores are another class of surface-active compounds produced by microorganisms. The amphiphilic siderophore marinobactin is composed of a hydrophilic chelating head group and a hydrophobic fatty acid tail of different lengths and interacts with iron minerals. These properties make the molecules attractive as flotation agents for mineral separation in froth flotation as propagated by Schrader et al. (2017) (Schrader et al. 2017).
Hacha et al. (2018) combined innovative electroflotation processes that reduces bubble sizes, with R. opacus cells as bioreagent (Hacha et al. 2018). By this, it was possible to separate fine hematite particles from a mixture. The combination of different methodologies from different fields enables innovations in classical flotation procedures, thus overcoming current limitations.
All these approaches include living cells or natural biocompounds that were isolated from living cells. Principally, these developments could be transferred to the separation of fine particles that are released during recycling processes and cannot be targeted by existing processes. Lederer and coauthors described the selection of phages displaying LaPO4:Ce3+, Tb3+ (LAP), and CeMgAl11O19:Tb3+ (CAT)-specific peptides on their surface (Lederer et al. 2017; Braun et al. 2018). Moreover, the researchers introduced modifications and reached a > 5000 fold higher binding strength to LAP in comparison to the wild type. The directed modification of individual amino acids was proven to increase or decrease the binding specificity and affinity of a peptide to the target material drastically. These phages bind to several components of compact fluorescent lamps (LAP, CAT), but not or only weak to Y2O3:Eu3+ (YOX), LaPO4, SiO2, and BaMgAl10O17:Eu2+ (BAM). This proof of principle shows that the researchers are able to identify perfectly fitting biomolecules for target particles by using the improved phage surface display techniques. The authors proposed an application as collectors in bioflotation processes for the separation and recycling of fluorescence phosphor components from electronic scrap (Lederer et al. 2019).
Other approaches anchored ZnO-, Au-, or TiO2-binding peptides or organic molecules to magnetic particles and separated the respective particles from colloidal mixtures (Essinger-Hileman et al. 2013; Shen et al. 2017; Vreuls et al. 2011). Given the high number of peptides that have been described to selectively interact with various inorganic surfaces and that were mostly applied for material syntheses or sensory applications (reviewed by Care et al. (2015); Seker and Demir 2011)), one can assume that these separation technologies can be easily transferred to other materials.
9.5 Bioreduction and Bioaccumulation: Nanomaterials from Waste
Bioaccumulation and bioreduction are often accompanied by the formation of nanoparticles. These properties make the processes attractive for the creation of novel nanomaterials. Bioaccumulation describes the accumulation and enrichment of metals in the cells, relative to the environment. This mechanism can be applied for the recovery and concentration of valuable elements from diluted solutions. For example, different microorganisms have been described to accumulate Ga and were used for Ga removal (Gascoyne et al. 1991b). In this case, the accumulation is mediated by siderophores that are complexing with Ga.
The transformation of metal ions into nanoparticles is one strategy to overcome the toxic effects of the metals. Nanoparticles are formed either via bio-precipitation or biologically catalyzed metal reduction (bioreduction). Among the various described biologically produced inorganic nanoparticles, precious metals are the most interesting metals for many applications.
Precious metals of the platinum group metals such as platinum, palladium, rhodium, and ruthenium are widely used in medicine, electronics, for optical devices, and catalysis (Yong et al. 2002, 2003). Especially it is commonly used as catalyst, e.g. in automotive catalytic converters or as catalyst in chemical syntheses. Consequently, significant amounts of Pd are released during production processes, consumption and recycling processes. For minimizing loss of Pd and enabling a circular economy, Pd-efficient recycling processes while avoiding secondary waste streams of toxic chemicals as well as an efficient recovery of Pd from industrial waste waters are mandatory. The application of Pd(II)-reducing microorganisms is an attractive approach that combines the removal of Pd from waste streams, thus minimizing the loss of Pd, with the synthesis of nanocatalysts via bio-reduction and deposition of Pd-nanoparticles on biomass using nontoxic biological means (De Corte, De Corte et al. 2012). The catalysts themselves can be used for the degradation of different recalcitrant pollutants or chemical syntheses.
Different microorganisms have been described that mediate the reduction of Pd(II). In case of the extensively studied anaerobic sulfur-reducing bacteria Desulfovibrio desulfuricans and Geobacter sulfurreducens and the facultative anaerobic iron-reducing Shewanella oneidensis, it has been proposed that hydrogenases and cytochrome c3 are involved in bioreduction of Pd(II) and nanoparticle deposition (De Corte et al. 2012; De Windt et al. 2005; Lloyd et al. 1998; Pat-Espadas et al. 2013; Yates et al. 2013). These reactions require H2 or formate as electron donor. In other cases (e.g. different cyanobacteria, E. coli), it was assumed that other enzyme systems such as nitrogenase enzyme or molybdenum-containing enzyme systems are responsible for Pd(II) reduction and deposition of Pd(0) in the medium (Foulkes et al. 2016). The majority of the formed Pd(0) particles were formed outside the cell. In other cases, Pd(0) formation is not based on enzyme activities and it was assumed that organic functional groups of the cell wall are responsible for the bioreductive process. For example, Pd(0) nanoparticles could be deposited on S-layer carrying Gram–positive bacteria as well as on the S-layer proteins following the array structure (Pollmann et al. 2006a, b; Wahl et al. 2001). Experiments with native and dead cells of E. coli, S. oneidensis, and P. putida and artificial systems demonstrated that the presence of amine groups mediates the reduction of Pd(II) bound to cell surfaces suggesting the use of amine-rich biomaterials rather than native cells for Pd-recovery (Rotaru et al. 2012). Consequently, De Corte et al. (2013) replaced the bacteria and used amine-functionalized surfaces as target for the synthesis of Pd(0) nanocatalysts (De Corte et al. 2012, 2013).
The application of metal-reducing bacteria for the removal of precious metals from industrial waste streams is a quite attractive alternative to conventional methods, because it requires less toxic chemicals and new products (nano-catalysts) are formed as “byproducts.” A wide range of natural or genetically engineered metal-reducing bacteria were successfully applied by several authors for recovery of precious metals from synthetic solutions or scrap leachates (Creamer et al. 2006; Ito et al. 2016; Konishi et al. 2006, 2007a, b; Mabbett et al. 2006; Maes et al. 2016, 2017; Martins et al. 2013; Pat-Espadas et al. 2013). Metal reduction was accompanied by nanoparticle formation. In these approaches, recovery rates of up to 99% were obtained.
In all cases, the formed bio-Pd was catalytically active. It was especially applied to transform a wide range of pollutants, mainly by reduction (Cr(VI), ClO4−) (Tuo et al. 2013; Mabbett et al. 2006; Humphries et al. 2007) or dehalogenation (e.g. printed circuit boards (PCBs), trichloroethylene, pharmaceuticals) (Baxter-Plant et al. 2004; De Windt et al. 2005; Hennebel et al. 2009a, b, 2010). Further, bio-Pd was used as a catalyst for diverse chemical syntheses, e.g. the hydrogenation of organic molecules or for coupling reactions in synthetic organic chemistry (Creamer et al. 2007). The doping of bio-Pd with other metals, e.g. Au, thus producing bimetallic catalysts, significantly enhanced catalytic activity. This relatively new approach will extend the applicability of metallic biocatalysts.
Besides catalytic applications, bio-Pd has been applied in microbial fuel cells, e.g. proton-exchange membrane fuel cells, for the generation of energy. In these approaches, biologically produced Pd(0) particles were deposited onto the anode, e.g. carbon papers, of the fuel cells and used for energy generation (Yong et al. 2009, 2010; Quan et al. 2015a). The now-modified anode possessed both electrooxidation and biodegradation capability (Quan et al. 2015a, b).
Most studies concentrated on precious metals. However, some newer publications studied the reduction and recovery of other valuable metals or used bioreduction for removal of toxic elements from industrial waste waters. For example, Lv et al. (2018) synthesized copper nanoparticles via bioreduction by a Shewanella loihica strain and discussed their use as antibacterial material (Lv et al. 2018). Maleke et al. (2019) described the reduction and intracellular accumulation of the REE europium by a Clostridium strain, probably mediated by active transport and intracellular precipitation (Maleke et al. 2019). The authors suggested an application for REE recovery from waste materials. Moreno-Benavides et al. (2019) used a Bacillus cereus strain for reduction of toxic Cr(VI) from electroplating wastewater (Moreno-Benavides et al. 2019).
9.6 Conclusion
In conclusion, many efforts have been done to recover metals from solutions by biological means ranging from the application of different biomasses, construct biosorptive composites, engineering of chelators, and use of different metabolic microbial processes. These approaches were used especially for the removal of toxic elements from waters. There are some reports on the removal of precious metals but only few studies describing the recovery of other valuable metals, e.g. REE, Ga, In. It can be assumed that many technologies developed for heavy metal removal can be transferred to other elements. Most current approaches concentrate on the usage of well-studied microorganisms such as chemolithoautotrophic sulfur-oxidizing bacteria like A. ferrooxidans, bioreducing bacteria such as Shewanella strains, or heterotrophic citric acid–producing fungi such as A. niger. However, there are some reports exploring the potential of novel microorganisms from diverse environments. It can be expected that especially extreme habitats such as salt lakes, volcanoes, deep sea, etc. bear many microorganisms with new metabolic properties that can be used for metal recovery also from e-wastes. Another approach is the smart design of new biomolecules, e.g. of new metal-chelating agents. For example, peptides can be designed interacting with numerous inorganic target materials. Such bioreagents can be integrated into various resource technologies such as metal extraction, fine particle flotation, and metal complexing. First results in these fields are highly promising. The combination of diverse biotechnological methods with classical resource technologies leads to new opportunities to find more environment-friendly and efficient solutions for metal extraction. Thus, a high potential for future applications also in recycling technologies can be expected. Opening up to these new multidisciplinary ideas offers new chances for a green economy.
References
- Ahmadi, M.K., Ghafari, M., Atkinson, J.D. et al. (2016). A copper removal process for water based upon biosynthesis of yersiniabactin, a metal-binding natural product. Chemical Engineering Journal 306: 772–776.
- Allievi, M.C., Florencia, S., Mariano, P.A. et al. (2011). Metal biosorption by surface-layer proteins from Bacillus species. Journal of Microbiology and Biotechnology 21: 147–153.
- Auerbach, R., Ratering, S., Bokelmann, K. et al. (2019). Bioleaching of valuable and hazardous metals from dry discharged incineration slag. An approach for metal recycling and pollutant elimination. Journal of Environmental Management 232: 428–437.
- Baxter-Plant, V.S., Mikheenko, I.P., Robson, M. et al. (2004). Dehalogenation of chlorinated aromatic compounds using a hybrid bioinorganic catalyst on cells of Desulfovibrio desulfuricans. Biotechnology Letters 26: 1885–1890.
- Behera, S.K. and Mulaba-Bafubiandi, A.F. (2017). Microbes assisted mineral flotation a future prospective for mineral processing industries: a review. Miner Process Extractive Metallurgy 38: 96–105.
- Bodnar, M., Hajdu, I., Rothi, E. et al. (2013). Biopolymer-based nanosystem for ferric ion removal from water. Separation and Purification Technology 112: 26–33.
- Bonificio, W.D. and Clarke, D.R. (2016). Rare-earth separation using bacteria. Environmental Science & Technology Letters 3 (4): 180–184.
- Bosshard, P.P., Bachofen, R., and Brandl, H. (1996). Metal leaching of fly ash from municipal waste incineration by Aspergillus niger. Environmental Science & Technology 30: 3066–3070.
- Brainard, J.R., Strietelmeier, B.A., Smith, P.H. et al. (1992). Actinide binding and solubilization by microbial siderophores. Radiochimica Acta 58–9: 357–363.
- Brandl, H., Bosshard, R., and Wegmann, M. (2001). Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 59: 319–326.
- Brandl, H., Lehmann, S., Faramarzi, M.A. et al. (2008). Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy 94 (1–4): 14–17.
- Braun, R., Bachmann, S., Schönberger, N. et al. (2018). Peptides as biosorbents – promising tools for resource recovery. Research in Microbiology 169: 649–658.
- Braun, R., Schönberger, N., Vinke, S. et al. (2020). Application of next generation sequencing (NGS) in phage displayed peptide selection to support the identification of arsenic-binding motifs. Viruses 12 (12): 1360. https://doi.org/10.3390/v12121360.
- Brombacher, C., Bachofen, R., and Brandl, H. (1998). Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Applied and Environmental Microbiology 64 (4): 1237–1241.
- Campbell, S.C., Olson, G.J., Clark, T.R. et al. (2001). Biogenic production of cyanide and its application to gold recovery. Journal of Industrial Microbiology & Biotechnology 26: 134–139.
- Care, A., Bergquist, P.L., and Sunna, A. (2015). Solid-binding peptides: smart tools for nanobiotechnology. Trends in Biotechnology 33: 259–268.
- Cetinel, S., Dincer, S., Cebeci, A. et al. (2012). Peptides to bridge biological-platinum materials interface. Bioinspired Biomimetic and Nanobiomaterials 1: 143–153.
- Chen, W., Bruhlmann, F., Richins, R.D. et al. (1999). Engineering of improved microbes and enzymes for bioremediation. Current Opinion in Biotechnology 10: 137–141.
- Chi, T.D., Lee, J.C., Pandey, B.D. et al. (2011). Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Minerals Engineering 24: 1219–1222.
- Cizkova, M., Mezricky, D., Rucki, M. et al. (2019). Bio-mining of lanthanides from red mud by green microalgae. Molecules 24 (7): 1356. https://doi.org/10.3390/molecules24071356.
- Consuegra, G.L., Kutschke, S., Rudolph, M. et al. (2019). Halophilic bacteria as potential pyrite bio-depressants in Cu–Mo bioflotation. Minerals Engineering 145: 106062.
- Creamer, N.J., Baxter-Plant, V.S., Henderson, J. et al. (2006). Palladium and gold removal and recovery from precious metal solutions and electronic scrap leachates by Desulfovibrio desulfuricans. Biotechnology Letters 28: 1475–1484.
- Creamer, N.J., Mikheenko, I.P., Yong, P. et al. (2007). Novel supported Pd hydrogenation bionanocatalyst for hybrid homogeneous/heterogeneous catalysis. Catalyt Tod 128: 80–87.
- Crini, G. (2005). Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Progress in Polymer Science 30 (1): 38–70.
- Das, N. (2010). Recovery of precious metals through biosorption – a review. Hydrometallurgy 103: 180–189.
- Das, A., Rao, K.H., Sharma, P. et al. (1999). Surface chemical and adsorption studies using Thiobacillus ferrooxidans with reference to bacterial adhesion to sulfide minerals. Process Metallurgy 9: 697–707. https://doi.org/10.1016/S1572-4409(99)80072-8.
- Daubert, L.N. and Brennan, R.A. (2007). Passive remediation of acid mine drainage using crab shell chitin. Environmental Engineering Science 24: 1475–1480.
- De Corte, S., Hennebel, T., De Gusseme, B. et al. (2012). Bio-palladium: from metal recovery to catalytic applications. Microbial Biotechnology 5: 5–17.
- De Corte, S., Bechstein, S., Lokanathan, A.R. et al. (2013). Comparison of bacterial cells and amine-functionalized abiotic surfaces as support for Pd nanoparticle synthesis. Colloid Surface B 102: 898–904.
- De Windt, W., Aelterman, P., and Verstraete, W. (2005). Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidenis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environmental Microbiology 7: 314–325.
- Dobrowolski, R., Szczes, A., Czemierska, M. et al. (2017). Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresource Technology 225: 113–120.
- Dolker, T. and Pant, D. (2019). Chemical-biological hybrid systems for the metal recovery from waste lithium ion battery. Journal of Environmental Management 248: 109270, ISSN 0301-4797, https://doi.org/10.1016/j.jenvman.2019.109270.
- Donia, A.M., Atia, A.A., and Elwakeel, K.Z. (2007). Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy 87 (3–4): 197–206.
- Donlan, R.M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases 8: 881–890.
- Essinger-Hileman, E.R., Popczun, E.J., and Schaak, R.E. (2013). Magnetic separation of colloidal nanoparticle mixtures using a material specific peptide. Chemical Communications 49: 5471–5473.
- Fahmy, K., Merroun, M., Pollmann, K. et al. (2006). Secondary structure and Pd(II) coordination in S-layer proteins from Bacillus sphaericus studied by infrared and X-ray absorption spectroscopy. Biophysical Journal 91: 996–1007.
- Faramarzi, M.A., Stagars, M., Pensini, E. et al. (2004). Metal solubilization from metal-containing solid materials by cyanogenic Chromobacterium violaceum. Journal of Biotechnology 113: 321–326.
- Figueira, M.M., Volesky, B., Ciminelli, V.S.T. et al. (2000). Biosorption of metals in brown seaweed biomass. Water Research 34: 196–204.
- Foulkes, J.M., Deplanche, K., Sargent, F. et al. (2016). A novel aerobic mechanism for reductive palladium biomineralization and recovery by Escherichia coli. Geomicrobiology Journal 33 (3–4): 230–236.
- Gascoyne, D.J., Connor, J.A., and Bull, A.T. (1991a). Isolation of bacteria producing siderophores under alkaline conditions. Applied Microbiology and Biotechnology 36: 130–135.
- Gascoyne, D.J., Connor, J.A., and Bull, A.T. (1991b). Capacity of siderophore – producing alkalophilic bacteria to accumulate iron, gallium and aluminum. Applied Microbiology and Biotechnology 36: 136–141.
- Gehrke, T., Telegdi, J., Thierry, D. et al. (1998). Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Applied and Environmental Microbiology 64: 2743–2747.
- Gholami, R.M., Borghei, S.M., and Mousavi, S.M. (2011). Bacterial leaching of a spent Mo-Co-Ni refinery catalyst using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 106 (1–2): 26–31.
- Hacha, R.R., LeonardoTorem, M., Merma, A.G. et al. (2018). Electroflotation of fine hematite particles with Rhodococcus opacus as a biocollector in a modified partridge-Smith cell. Minerals Engineering 126: 105–115.
- Harrington, J.M., Parker, D.L., Bargar, J.R. et al. (2012). Structural dependence of Mn complexation by siderophores: donor group dependence on complex stability and reactivity. Geochim Cosmochim Ac 88: 106–119.
- Hassanien, A.G., Desouky, O.A.N., and Hussien, S.S.E. (2014). Bioleaching of some rare earth elements from Egyptian monazite using Aspergillus ficuum and Pseudomonas aeruginosa. Walailak Journal of Science and Technology 11 (9): 809–823.
- Hatanaka, T., Matsugami, A., Nonaka, T. et al. (2017). Rationally design mineralization for selective recovery of the rare earth elements. Nature Communications 8: 15670. https://doi.org/10.1038/ncomms15670.
- He, J.S. and Chen, J.P. (2014). A comprehensive review on biosorption of heavy metals by algal biomass: materials, performances, chemistry, and modeling simulation tools. Bioresource Technology 160: 67–78.
- Hennebel, T., Simoen, H., De Windt, W. et al. (2009a). Biocatalytic dechlorination of trichloroethylene with bio-palladium in a pilot-scale membrane reactor. Biotechnology and Bioengineering 102 (4): 995–1002.
- Hennebel, T., Verhagen, P., Simoen, H. et al. (2009b). Remediation of trichloroethylene by bio-precipitated and encapsulated palladium nanoparticles in a fixed bed reactor. Chemosphere 76 (9): 1221–1225.
- Hennebel, T., De Corte, S., Vanhaecke, L. et al. (2010). Removal of diatrizoate with catalytically active membranes incorporating microbially produced palladium nanoparticles. Water Research 44 (5): 1498–1506.
- Hopfe, S., Flemming, K., Lehmann, F. et al. (2017). Leaching of rare earth elements from fluorescent powder using the tea fungus Kombucha. Waste Management 62: 211–221.
- Hopfe, S., Konsulke, S., Barthen, R. et al. (2018). Screening and selection of technologically applicable microorganisms for recovery of rare earth elements from fluorescent powder. Waste Management 79: 554–563.
- Horeh, N.B., Mousavi, S.M., and Shojaosadati, S.A. (2016). Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. Journal of Power Sources 320: 257–266.
- Huang, T., Liu, L.F., and Zhang, S.W. (2019). Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy 188: 101–111.
- Humphries, A.C., Penfold, D.W., and Macaskie, L.E. (2007). Cr(VI) reduction by bio and bioinorganic catalysis via use of bio-H-2: a sustainable approach for remediation of wastes. Journal of Chemical Technology and Biotechnology 82 (2): 182–189.
- Inbaraj, B.S., Wang, J.S., Lu, J.F. et al. (2009). Adsorption of toxic mercury(II) by an extracellular biopolymer poly(gamma-glutamic acid). Bioresource Technology 100 (1): 200–207.
- Ito, R., Kuroda, K., Hashimoto, H. et al. (2016). Recovery of platinum(0) through the reduction of platinum ions by hydrogenase-displaying yeast. AMB Express 6: 88. https://doi.org/10.1186/s13568-016-0262-4.
- Jain, R., Fan, S.Y., Kaden, P. et al. (2019). Recovery of gallium from wafer fabrication industry wastewaters by Desferrioxamine B and E using reversed-phase chromatography approach. Water Research 158: 203–212.
- Jankowski, U., Merroun, M.L., Selenska-Pobell, S. et al. (2010). S-layer protein from Lysinibacillus sphaericus JG-A12 as matrix for Au(III) sorption and Au-nanoparticle formation. International Journal of Spectroscopy 24 (1–2): 177–181. https://doi.org/10.3233/SPE-2010-0408.
- Karwowska, E., Andrzejewska-Morzuch, D., Lebkowska, M. et al. (2014). Bioleaching of metals from printed circuit boards supported with surfactant-producing bacteria. Journal of Hazardous Materials 264: 203–210.
- Kinzler, K., Gehrke, T., Telegdi, J. et al. (2003). Bioleaching – a result of interfacial processes caused by extracellular polymeric substances (EPS). Hydrometallurgy 71: 83–88.
- Klink, C., Eisen, S., Daus, B. et al. (2016). Investigation of Acidithiobacillus ferrooxidans in pure and mixed-species culture for bioleaching of Theisen sludge from former copper smelting. Journal of Applied Microbiology 120 (6): 1520–1530.
- Konishi, Y., Tsukiyama, T., Ohno, K. et al. (2006). Intracellular recovery of gold by microbial reduction of AuCl4-ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 81 (1): 24–29.
- Konishi, Y., Ohno, K., Saitoh, N. et al. (2007a). Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. Journal of Biotechnology 128 (3): 648–653.
- Konishi, Y., Tsukiyama, T., Tachimi, T. et al. (2007b). Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochimica Acta 53 (1): 186–192, ISSN 0013-4686, https://doi.org/10.1016/j.electacta.2007.02.073.
- Kotrba, P., Doleckova, L., De Lorenzo, V. et al. (1999a). Enhanced bioaccumulation of heavy metal ions by bacterial cells due to surface display of short metal binding peptides. Applied and Environmental Microbiology 65: 1092–1098.
- Kotrba, P., Pospisil, P., de Lorenzo, V., and Ruml, T. (1999b). Enhanced metallosorption of Escherichia coli cells due to surface display of beta- and alpha-domains of mammalian metallothionein as a fusion to lamb protein. Journal of Receptor and Signal Transduction 19 (1–4): 703–715. https://doi.org/10.3109/10799899909036681.
- Lederer, F.L., Curtis, S.B., Bachmann, S. et al. (2017). Identification of lanthanum-specific peptides for future recycling of rare earth elements from compact fluorescent lamps. Biotechnology and Bioengineering 114: 1016–1024.
- Lederer, F.L., Braun, R., Schöne, L.M. et al. (2019). Identification of peptides as alternative recycling tools via phage surface display – how biology supports geosciences. Minerals Engineering 132: 245–250.
- Li, H., Dong, W., Liu, Y. et al. (2019). Enhanced biosorption of nickel ions on immobilized surface-engineered yeast using nickel-binding peptides. Frontiers in Microbiology 10: 1254.
- Liang, H.H., Deng, X., Bosscher, M. et al. (2013). Engineering bacterial two-component system PmrA/PmrB to sense lanthanide ions. Journal of the American Chemical Society 135: 2037–2039.
- Liu, Q., Yuan, F., Liang, Y. et al. (2015). Cadmium adsorption by E. coli with surface displayed CadR. RSC Advances 5: 16089–16092.
- Lloyd, J.R., Yong, P., and Macaskie, L.E. (1998). Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Applied and Environmental Microbiology 64: 4607–4609.
- Lv, Q., Zhang, B.G., Xing, X. et al. (2018). Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. Journal of Hazardous Materials 347: 141–149.
- Mabbett, A.N., Sanyahumbi, D., Yong, P. et al. (2006). Biorecovered precious metals from industrial wastes: single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application. Environmental Science & Technology 40: 1015–1021.
- Maes, S., Claus, M., Verbeken, K. et al. (2016). Platinum recovery from industrial process streams by halophilic bacteria: influence of salt species and platinum speciation. Water Research 105: 436–443.
- Maes, S., Props, R., Fitts, J.P. et al. (2017). Biological recovery of platinum complexes from diluted aqueous streams by axenic cultures. PLoS One 12 (1): e0169093. https://doi.org/10.1371/journal.pone.0169093.
- Maleke, M., Valverde, A., Gomez-Arias, A. et al. (2019). Anaerobic reduction of europium by a Clostridium strain as a strategy for rare earth biorecovery. Scientific Report 9: 14339. https://doi.org/10.1038/s41598-019-50179-z.
- Maneesuwannarat, S., Vangnai, A.S., Yamashita, M., and Thiravetyan, P. (2016). Bioleaching of gallium from gallium arsenide by Cellulosimicrobiurn funkei and its application to semiconductor/electronic wastes. Process Safety and Environmental Protection 99: 80–87. https://doi.org/10.1016/j.psep.2015.10.008.
- Maneesuwannarat, S., Kudpeng, K., Yingchutrakul, Y. et al. (2019). A possible protein model involved in gallium arsenide leaching by Cellulosimicrobium funkei. Minerals Engineering 137: 207–216.
- Martin, L.J., Hahnke, M.J., Nitz, M. et al. (2007). Double-lanthanide-binding tags: design, photophysical properties, and NMR applications. Journal of the American Chemical Society 129: 7106–7113.
- Martins, M., Assuncao, A., Martins, H. et al. (2013). Palladium recovery as nanoparticles by an anaerobic bacterial community. Journal of Chemical Technology and Biotechnology 88: 2039–2045.
- Maruyama, T., Matsushita, H., Shimada, Y. et al. (2007). Proteins and protein-rich biomass as environmentally friendly adsorbents selective for precious metal ions. Environmental Science & Technology 41: 1359–1364.
- Matys, S., Schönberger, N., Lederer, F.L. et al. (2020). Characterization of specifically metal-binding phage clones for selective recovery of cobalt and nickel. Journal of Environmental Chemical Engineering 8: 103636.
- Mejare, M. and Bulow, L. (2001). Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends in Biotechnology 19: 67–73.
- Merroun, M., Raff, J., Rossberg, A. et al. (2005). Complexation of uranium by cells and S-layer sheets of Bacillus sphaericus JG-A12. Applied and Environmental Microbiology 71: 5532–5543.
- Mishra, D., Kim, D.J., Ralph, D.E. et al. (2008). Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Management 28 (2): 333–338.
- Mona, S. and Kaushik, A. (2015). Chromium and cobalt sequestration using exopolysaccarides produced by freshwater cyanobacterium Nostoc linckia. Ecological Engineering 82: 121–125.
- Moreno-Benavides, J.A., Pena-Salamanca, E.J., and Benitez-Campo, N. (2019). Reducing Cr6+ in electroplating wastewater with Bacillus cereus strain B1. Universitas Scientiarum 24 (1): 73–89.
- Mu, R.M., Ma, G.X., and Zhao, X. (2011). Removal efficiency of lead(II) by a biopolymer poly-gamma-glutamic acid. Applied Mechanics and Materials 94–96: 995–998.
- Nguyen, T.T.L., Lee, H.R., Hong, S.H. et al. (2013). Selective lead adsorption by recombinant Escherichia coli displaying a lead-binding peptide. Applied Biochemistry and Biotechnology 169: 1188–1196.
- Nian, R., Kim, D.S., Thuong, N. et al. (2010). Chromatographic biopanning for the selection of peptides with high specificity to Pb2+ from phage displayed peptide library. Journal of Chromatography. A 1217: 5940–5949.
- Nishitani, T., Shimada, M., Kuroda, K. et al. (2010). Molecular design of yeast cell surface for adsorption and recovery of molybdenum, one of rare metals. Applied Microbiology and Biotechnology 86: 641–648.
- Nitz, M., Franz, K.J., Maglathlin, R.L. et al. (2003). A powerful combinatorial screen to identify high-affinity terbium(III)-binding peptides. Chembiochem 4: 272–276.
- Oliazadeh, M., Massinaie, M., Bagheri, A.S. et al. (2006). Recovery of copper from melting furnaces dust by microorganisms. Minerals Engineering 19 (2): 209–210.
- Park, D.M., Reed, D.W., Yung, M.C. et al. (2016). Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environmental Science & Technology 50: 2735–2742.
- Pat-Espadas, A.M., Razo-Flores, E., Rangel-Mendez, J.R. et al. (2013). Reduction of palladium and production of nano-catalyst by Geobacter sulfurreducens. Applied Microbiology and Biotechnology 97: 9553–9560.
- Patra, P. and Natarajan, K.A. (2006). Surface chemical studies on selective separation of pyrite and galena in the presence of bacterial cells and metabolic products of Paenibacillus polymyxa. Journal of Colloid and Interface Science 298: 720–729.
- Perez, J.A.M., Garcia-Ribera, R., Quesada, T. et al. (2008). Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World Journal of Microbiology and Biotechnology 24 (11): 2699–2704.
- Pollmann, K. and Matys, S. (2007). Construction of an S-layer protein exhibiting modified self-assembling properties and enhanced metal binding capacities. Applied Microbiology and Biotechnology 75: 1079–1085.
- Pollmann, K., Merroun, M., Raff, J. et al. (2006a). Manufacturing and characterization of Pd-nanoparticles formed on immobilized bacterial cells. Letters in Applied Microbiology 43: 39–45.
- Pollmann, K., Raff, J., Merroun, M. et al. (2006b). Metal binding by bacteria from uranium mining waste piles and its potential applications. Biotechnology Advances 24: 58–68.
- Pollmann, K., Kutschke, S., Matys, S. et al. (2018). Bio-recycling of metals: recycling of technical products using biological applications. Biotechnology Advances 36 (4): 1048–1062.
- Pourhossein, F. and Mousavi, S.M. (2019). A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). Journal of Hazardous Materials 378: 120648, ISSN 0304-3894, https://doi.org/10.1016/j.jhazmat.2019.05.041.
- Qu, Y. and Lian, B. (2013a). Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresource Technology 136: 16–23.
- Qu, Y., Lian, B., Mo, B.B. et al. (2013b). Bioleaching of heavy metals from red mud using Aspergillus niger. Hydrometallurgy 136: 71–77.
- Qu, Y., Li, H., Tian, W.J. et al. (2015). Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Minerals Engineering 81: 1–4.
- Quan, X.C., Sun, B., and Xu, H.D. (2015a). Anode decoration with biogenic Pd nanoparticles improved power generation in microbial fuel cells. Electrochimica Acta 182: 815–820.
- Quan, X.C., Zhang, X., and Xu, H.D. (2015b). In-situ formation and immobilization of biogenic nanopalladium into anaerobic granular sludge enhances azo dyes degradation. Water Research 78: 74–83.
- Raff, J., Soltmann, U., Matys, S. et al. (2003). Biosorption of uranium and copper by biocers. Chemistry of Materials 15: 240–244.
- Ramanathan, T. and Ting, Y.P. (2016). Alkaline bioleaching of municipal solid waste incineration fly ash by autochthonous extremophiles. Chemosphere 160: 54–61.
- Reed, D.W., Fujita, Y., Daubaras, A.L. et al. (2016). Bioleaching of rare earth elements from waste phosphors and cracking catalysts. Hydrometallurgy 166: 34–40.
- Ritter, S.K. (2004). Biomass or bust. Chemical and Engineering News 82: 31–34.
- Rizki, I.N., Tanaka, Y., and Okibe, N. (2019). Thiourea bioleaching for gold recycling from e-waste. Waste Management 84: 158–165.
- Rotaru, A.E., Jiang, W., Finster, K. et al. (2012). Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnology and Bioengineering 109: 1889–1897.
- Saha, P. and Rao, K.V.B. (2017). Biosurfactants – a current perspective on production and applications. Nature, Environment and Pollution Technology 16: 181–188.
- Salamun, N., Triwahyono, S., Jalil, A.A. et al. (2015). Acid-vacuo heat treated low cost banana stems fiber for efficient biosorption of Hg(II). RSC Advances 5: 14129–14137.
- Sarikaya, M., Tamerler, C., Jen, A.K.Y. et al. (2003). Molecular biomimetics: nanotechnology through biology. Nature Materials 2: 577–585.
- Sawada, T., Asada, M., and Serizawa, T. (2016). Selective rare earth recovery employing filamentous viruses with chemically conjugated peptides. ChemistrySelect 1: 2712–2716.
- Schönberger, N., Braun, R., Matys, S. et al. (2019a). Chromatopanning for the identification of gallium binding peptides. Journal of Chromatography. A 1600: 158–166.
- Schönberger, N., Zeitler, C., Braun, R. et al. (2019b). Directed evolution and engineering of gallium-binding phage clones – a preliminary study. Biomimetics 4: 35.
- Schrader, S., Kutschke, S., Rudolph, M. et al. (2017). Production of amphiphilic hydroxamate siderophores marinobactins by Marinobacter sp. DS40M6 for bioflotation process. Solid State Phenomena 262: 413–416.
- Schwaminger, S., Blank-Shim, S.A., Borkowska-Panek, M. et al. (2018). Experimental characterization and simulation of amino acid and peptide interactions with inorganic materials. Engineering in Life Sciences 18: 84–100.
- Seker, U.O.S. and Demir, H.V. (2011). Material binding peptides for nanotechnology. Molecules 16: 1426–1451.
- Shen, L.F., Zhu, Y.Z., Zhang, P.F. et al. (2017). Capturing of nano-TiO2 from complex mixtures by bisphosphonate-functionalized Fe3O4 nanoparticles. ACS Sustainable Chemistry & Engineering 5: 1704–1710.
- Shin, D., Kim, J., Kim, B. et al. (2015a). Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore. Minerals-Basel 5 (2): 189–202.
- Shin, D., Park, J., Jeong, J. et al. (2015b). A biological cyanide production and accumulation system and the recovery of platinum-group metals from spent automotive catalysts by biogenic cyanide. Hydrometallurgy 158: 10–18.
- Sleytr, U.B., Schuster, B., Egelseer, E.M. et al. (2014). S-layers: principles and applications. FEMS Microbiology Reviews 38: 823–864.
- Soltmann, U., Raff, J., Selenska-Pobell, S. et al. (2002). Biosorption of heavy metals by sol-gel immobilized Bacillus sphaericus cells, spores and S-layers. Journal of Sol–Gel Science and Technology 26: 1209–1212.
- Sousa, C., Cebolla, A., and deLorenzo, V. (1996). Enhanced metalloadsorption of bacterial cells displaying poly-his peptides. Nature Biotechnology 14: 1017–1020.
- Sousa, C., Kotrba, P., Ruml, T. et al. (1998). Metalloadsorption by Escherichia coli cells displaying yeast and mammalian metallothioneins anchored to the outer membrane protein LamB. Journal of Bacteriology 180: 2280–2284.
- Stair, J.L. and Holcombe, J.A. (2005). Metal remediation and preconcentration using immobilized short-chain peptides composed of aspartic acid and cysteine. Microchemical Journal 81: 69–80.
- Suhr, M., Unger, N., Viacava, K.E. et al. (2014). Investigation of metal sorption behavior of Slp1 from Lysinibacillus sphaericus JG-B53: a combined study using QCM-D, ICP-MS and AFM. BioMetals 27: 1337–1349.
- Tan, L., Cui, H., Xiao, Y. et al. (2019). Enhancement of platinum biosorption by surface-displaying EC20 on Escherichia coli. Ecotoxicology and Environmental Safety 169: 103–111.
- Terashima, M., Oka, N., Sei, T. et al. (2002). Adsorption of cadmium ion and gallium ion to immobilized metallothionein fusion protein. Biotechnology Progress 18: 1318–1323.
- Ting, Y.P. and Sun, G. (2000). Comparative study on polyvinyl alcohol and alginate for cell immobilization in biosorption. Water Science and Technology 42: 85–90.
- Toca-Herrera, J.L., Krastev, R., Bosio, V. et al. (2005). Recrystallization of bacterial S-layers on flat polyelectrolyte surfaces and hollow polyelectrolyte capsutes. Small 1: 339–348.
- Tuo, Y., Liu, G.F., Zhou, J.T. et al. (2013). Microbial formation of palladium nanoparticles by Geobacter sulfurreducens for chromate reduction. Bioresource Technology 133: 606–611.
- Valls, M., Gonzalez-Duarte, R., Atrian, S. et al. (1998). Bioaccumulation of heavy metals with protein fusions of metallothionein to bacterial OMPs. Biochimie 80: 855–861.
- Valls, M., Atrian, S., de Lorenzo, V. et al. (2000a). Engineering a mouse metallothionein on the cell surface of Ralstonia eutropha CH34 for immobilization of heavy metals in soil. Nature Biotechnology 18: 661–665.
- Valls, M., de Lorenzo, V., Gonzalez-Duarte, R. et al. (2000b). Engineering outer-membrane proteins in Pseudomonas putida for enhanced heavy-metal bioadsorption. Journal of Inorganic Biochemistry 79: 219–223.
- Varshini, J.S.C., Das, D., and Das, N. (2015). Recovery of cerium (III) from electronic industry effluent using novel biohydrogel: batch and column studies. Der Pharmacia Lettre 7 (6): 166–179.
- Volesky, B. (2007). Biosorption and me. Water Research 41: 4017–4029.
- Volesky, B. and Holan, Z.R. (1995). Biosorption of heavy-metals. Biotechnology Progress 11: 235–250.
- Vreuls, C., Genin, A., Zocchi, G. et al. (2011). Genetically engineered polypeptides as a new tool for inorganic nano-particles separation in water based media. Journal of Materials Chemistry 21: 13841–13846.
- Vu, B., Chen, M., Crawford, R.J. et al. (2009). Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14: 2535–2554.
- Wahl, R., Mertig, M., Raff, J. et al. (2001). Electron-beam induced formation of highly ordered palladium and platinum nanoparticle arrays on the S-layer of Bacillus sphaericus NCTC9602. Advanced Materials 13: 736–740.
- Watling, H.R. (2015). Review of biohydrometallurgical metals extraction from polymetallic mineral resources. Minerals 5: 1–60.
- Weinert, U., Vogel, M., Reinemann, C. et al. (2015). S-layer proteins as an immobilization matrix for aptamers on different sensor surfaces. Engineering in Life Sciences 15: 710–720.
- Yang, T., Zhang, X.Y., Zhang, X.X. et al. (2015). Chromium(III) binding phage screening for the selective adsorption of Cr(III) and chromium speciation. ACS Applied Materials & Interfaces 7: 21287–21294.
- Yang, T., Zhang, X.X., Yang, J.Y. et al. (2018). Screening arsenic(III)-bining peptide for colorimetric detection of arsenic(III) based on peptide induced aggregation of gold nanoparticles. Talanta 177: 212–216.
- Yates, M.D., Cusick, R.D., and Logan, B.E. (2013). Extracellular palladium nanoparticle production using geobacter sulfurreducens. ACS Sustainable Chemistry & Engineering 1: 1165–1171.
- Yong, P., Rowson, N.A., Farr, J.P. et al. (2002). Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnology and Bioengineering 80: 369–379.
- Yong, P., Rowson, N.A., Farr, J.P. et al. (2003). A novel electrobiotechnology for the recovery of precious metals from spent automotive catalysts. Environmental Technology 2003: 289–297.
- Yong, P., Mikheenko, I.P., Deplanche, K. et al. (2009). Biorecovery of precious metals from wastes and conversion into fuel cell catalyst for electricity production. Advanced Materials Research-Switzerland 71–73: 729–732.
- Yong, P., Mikheenko, I.P., Deplanche, K. et al. (2010). Biorefining of precious metals from wastes: an answer to manufacturing of cheap nanocatalysts for fuel cells and power generation via an integrated biorefinery? Biotechnology Letters 32 (12): 1821–1828.
- Yunus, I.S. and Tsai, S.L. (2015). Designed biomolecule-cellulose complexes for palladium recovery and detoxification. RSC Advances 5: 20276–20282.
