3
Development of Sustainable High‑Performance Supercapacitor Electrodes from Biochar-Based Material
Kriti Shrivastava1* and Ankur Jain1,2†
1School of Applied Sciences, Suresh Gyan Vihar University, Jagatpura, Jaipur, India
2Centre for Renewable Energy & Storage, Suresh Gyan Vihar University, Jagatpura, Jaipur, India
Abstract
The future energy industry will be more electric, efficient, networked, and environment friendly as compared to the present one. Clean energy technology is rapidly becoming cost-effective, broadly usable, and a substantial new source of investment, job creation, and international collaboration, due to rapid technological breakthroughs and global legislative reforms. The increasing expansion of renewable generation resources necessitates the presence of energy storage systems which will ensure the energy security, and electrification of remote and rural areas and will also cut down the carbon emission. They can assure the reliability of electric power grids along with the continuous supply of electric power. In response to the rising need for energy-storage devices, supercapacitors, which are electrochemical capacitors, are being explored intensively. They have several advantages over standard secondary storage batteries, including faster charge propagation, which is intrinsically simpler and reversible, longer cycle life, and higher storage efficiency. In modern energy storage and conversion, carbon materials have the potential to be the most adaptive basic materials, and biochar, or bio carbon, can be easily chosen for commercial Electric Double Layer Capacitors (EDLCs) because of its larger surface area, high chemical stability, comparatively low cost, appropriate electrical conductivity, and availability. Plants residues, agricultural by-products, and waste biomass can be effectively utilized for biomass production as a carbon-negative process. This provides an effective and sustainable method for the manufacture of supercapacitor electrodes with major benefits coming in terms of performance with economic and environmental aspects.
Keywords: Biochar, supercapacitor, electric double layer capacitor, electrode, sustainable electrode material
3.1 Introduction
The economic recovery from Covid-induced recession is taking place rapidly and it is putting strain on all aspects of today’s energy system, which has resulted in a significant hike in the cost of traditional energy resources like natural gas, coal, and oil. Despite all the advances made in renewable energy and electric transportation, coal, and oil consumption increased dramatically in 2021, which resulted in a sharp increase in CO2 emission. The energy requirement of the future will be more electric, efficient, networked, and environmentally benign than the present one. With rapid technological advancements and worldwide policy changes, sustainable energy systems are gradually becoming cost-effective and widely applicable. Clean energy technology is also attracting significant new investments and creating job opportunities by enhancing the international collaborations between government and non-government organizations [1].
The unprecedented changes in the electric power systems are reflected by increasing attention over the development of smart grids and grid modernization [2]. To address the energy and environmental crises caused by using fossil fuels, a sustainable energy system must be developed and implemented on a broad scale, which can only be accomplished through the development and implementation of renewable energy. The promise of energy storage application is steadily appearing as the smart grid develops, aided by investment and government legislation. These applications span the whole power system spectrum from power generation to power transmission, its distribution, and consumption. They can address increasing the utilization of renewable energy sources at large scale, decreasing the overall construction cost of generation, and improving the efficiency of the power grid system, power quality, and power supply. Such applications also perform grid optimization through planning, management, and operational control [3].
Conventional utilization of fossil fuel resources has raised environmental concerns over a very long time along with the issues of limited availability and energy security. Being inexhaustible and environment-friendly, renewable energy generation has gained worldwide attention. Solar and wind energy are the two widely available infinite sources of energy, but they are not constant and reliable. In such conditions, thermal power plants are required to be used to balance the fluctuation of these renewable sources and therefore electric grid operators face considerable hurdles in such applications [4]. Some other important applications of energy storage are the integration of grid generating renewable energy, distribution, distributed generation, microgrid, and its auxiliary services [3].
As compared to the other existing energy storage technologies, supercapacitors are associated with advantages like quick charge-discharge, good cycle stability, and high-power density. Carbon is the most adaptable platform material which can be suitably applied in modern sustainable energy storage systems. They can be used in the form of activated carbon, and carbon nanostructures like fibers, tubes, and graphene. But their preparation involves energy-intensive complex synthetic procedures using coal or petrochemical compounds as the precursor. Several studies had been conducted to develop sustainable eco-friendly methods for efficient production of carbon-based materials using renewable resources [5].
Biochar or the bio-carbon is characterized by the presence of several surface functional groups, and easily adjustable porosity which makes it a suitable choice as a sustainable material having considerable application promise in energy storage and conversion during various recent studies. When compared to the other carbon material, Biochar is found to be a more efficient and active low-cost material [6, 7].
This chapter covers detailed information on the suitability of biochar-derived carbon nanomaterials for preparing electrodes of low-cost and effective supercapacitors for sustainable energy storage technologies. The major advantage is the control over their electrochemical activity by suitable modifications in the textural properties and surface functional groups, variation in the feedstock sources, and manufacturing procedures. This provides an effective method to the manufacture of supercapacitor electrodes which are economic, eco-friendly, and able to give high performance.
3.2 Role of Energy Storage Systems in Grid Modernization
Energy storage systems involve three major steps in energy storage [8]:
- The charge is the process of receiving electrical energy from the source.
- Storage is the process of conversion of electrical energy into some other form and its storage.
- Discharge is the process of receiving the stored electrical energy from the system.
Furthermore, all the storage systems consist of two parts:
- Central storage which is the repository of energy storage performs the power conversion.
- Being present between the central storage and the power system, the interface controls the bidirectional transfer and the level of charge or discharge of the stored energy by employing measuring devices like sensors. Because energy storage is merely a depository of energy rather than an ideal energy source, it is accompanied with energy losses at every stage of the storage process [9].
Energy storage is the most significant aspect in improving the electrical energy grid’s flexibility, economy, and security. As a result, energy storage is projected to support distributed power and microgrids, as well as encourage open sharing and flexible trading of energy production and consumption and achieve multi-functional coordination [6].
Electrical energy storage (EES) is an important part of the smart and modern future grid, which should be able to hold a huge amount of renewable energy. Generally, renewable energy resources are variable, intermittent, and located far away from the load centers. To tackle all these issues, an effective electrical energy storage system is required. It will also be beneficial for fueling hybrid and electric vehicles (i.e., electricity), even though the cost of establishing EES is a major problem [4].
In principle, an energy storage systems (ESS) should focus on its energy storage capacity during the time when it is easily available and its release during the period of its shortage. It should provide stability to the power output by combining the energy storage technology with renewable energy resources to enhance its reliability. It should also focus on increasing the resilience of the power system during natural disasters, weather variations, and the sudden onset of power shortage [10, 11].
In commercial applications, EES technology should be able to maintain a continuous and reliable power supply [12]. Numerous energy storage technologies are available to cater to the need of a modern electrical grid system (Figure 3.1). All these technologies can be classified into two broad categories on the basis of mode of electrical energy storage. Supercapacitors store electrical energy directly in the form of electrical charge and they are highly efficient due to their low energy density and shorter discharge time. Currently, they are being utilized for frequency regulation in power management.

Figure 3.1 Various energy storage technologies.
Alternatively, electrical energy can be converted into any of the other forms of energy like kinetic energy, potential energy, or chemical energy and afterwards it can be stored (Table 3.1). Mechanical energy storage systems like flywheels (FWs) store electrical energy by high-speed rotors spinning with their speed directly proportional to stored energy [13]. When the speed of rotors is slowed down, the mechanical energy can be converted again into electrical energy offering high power but low energy. They are also useful for power management. For bulk energy storage, electrical energy is stored in the form of potential energy by the pumped hydro and compressed air energy storage (CAES).
Most of the existing technologies are associated with the disadvantage of high cost due to the expenditure pertaining to operation, maintenance, replacement, and additional carrying costs. It can also be attributed to their unsatisfactory performance, high cost of raw materials and fabrication and the scale of production. To overcome this problem, technological advancements are required to improve the reliability, cycle life, and efficiency of conversion by using less expensive materials [4].
Table 3.1 Techno-economic parameters of various energy storage devices [9]. Reprinted with permission of Elsevier.
| Technologies | Capacity (MWh) | Power (MWh) | Response time | Discharge time | Maturity | Lifetime (Years) | Efficiency (%) | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|---|---|---|
| Electrochemical | Lead-Acid | 0.25-50 | ≤ 100 | millisecond | ≤ 4 h | Demo- Commercial | ≤ 20 | ≤ 85 | Inexpensive, High recyclable, Reality available | Very heavy, Limited usable energy, Poor energy density |
| Lithium Ion | 0.25-25 | ≤ 100 | ≤ 1 h | Demo | ≤ 15 | ≤ 90 | High Capacity, Great Stability in calendar and cycle life | |||
| NaS | ≤ 300 | ≤ 50 | ≤ 6 h | Commercial | ≤ 15 | ≤ 80 | High storage capacity, Inexpensive | Working only when the sodium and sulfur are liquids 290-390°C | ||
| Vanadium Redox | ≤ 250 | ≤ 50 | ≤ 10 min | ≤ 8 h | Demo | ≤ 10 | ≤ 80 | Possible to use for many different renewable energy sources | ||
| Mechanical | FES | ≤ 10 | ≤ 20 | ≤ 10 ms | ≤ 1 h | Demo-mature | ≤ 20 | ≤ 85 | High power density, non-polluting, high efficiency | not safe enough, Noisy, High-speed operation led to vibration |
| PHS (Small) | ≤ 5000 | ≤ 500 | sec-min | 6-24 hrs | mature | ≤ 70 | ≤ 85 | Remote operation is possible, low manpower factor, relatively low maintenance | Silt build-up, Impedance to the movement of environmental issues | |
| PHS (Large) | ≤ 14000 | ≤ 1400 | sec-min | |||||||
| CAES (underground, small) | ≤ 1100 | ≤ 135 | ≤ 15 min | ≤ 8 h | Demo- Commercial | ≤ 40 | ≤ 85 | High power capacity, low losses (can be storage energy for more than a year) Fast Start-up | It is not possible to install everywhere, and the location is dependent on a geological structure | |
| CAES (underground, large) | ≤ 2700 | ≤ 135 | ≤ 15 min | ≤ 20 h | Demo | |||||
| CAES (above ground) | ≤ 250 | ≤ 50 | ≤ 15 min | ≤ 5 h | ||||||
| Electrical | DLC | 0.1-0.5 | ≤ 1 | ≤ 10 ms | ≤ 1 min | Commercial | ≤ 40 | ≤ 95 | High power density, low resistance, high efficiency | Low energy density, low voltage per cell, incomplete capacity utilization |
| SMES | 1.0-3.0 | ≤ 10 | ≤ 10 ms | ≤ 1 min | Commercial | ≤ 40 | ≤ 95 | High power, high efficiency, environmentally safe | For sizing of high energy storage need to long loop, cooling system is needed, expensive | |
| Thermal | Thermal | ≤ 350 | ≤ 50 | ≤ 10 min | N/A | Mature | ≤ 40 | ≤ 90 | Non-polluting unlimited energy source | Expensive; depends on a geological structure |
Carbon-free source with operational adaptability can be utilized to facilitate the integration of multiple renewable energy sources and for the improvement of the usage of generation assets. Therefore, energy storage systems (EES) can help in attaining carbon neutrality in the power sector. EES could help generators to use less fossil fuel, resulting in lower standard discharge and GHG emissions [14]. This includes emissions from the entire energy production chain right from generation to transmission and distribution to the final user. Commercially modern energy storage can also help in the transition from centralized to more advantageous distributed generation system to improve energy access, availability in rural and remote locations along with the quality, dependability, and performance [13].
3.3 Overview of Current Developments of Supercapacitor-Based Electrical Energy Storage Technologies
The growing demand of efficient energy storage devices has caused extensive research on supercapacitors due to their advantages over traditional batteries, e.g., rapid charge propagation, extended cycle life, and higher storage efficiency. Due to these reasons, they can be easily applied in energy storage for electric vehicles, UPS (uninterrupted power supply), digital telecommunication and pulse lasers, etc. Although supercapacitors are known for their high power density, quick reaction, cheap maintenance, and wide range of operating temperature, they are usually employed in combination with other energy storage technologies [3, 11].
Various materials have been explored as potential electrode materials for supercapacitors and based on material selection supercapacitors can be classified into two parts: pseudocapacitors and electric double-layer supercapacitors (Figure 3.2). Pseudocapacitors generally contain materials like hydroxides, oxides, and polymers and exhibit improved specific capacitance and high energy density. However, mostly they depend on faradic redox processes and the active materials behave as insulators to slow down fast electron transport, which is necessary for higher rates. Therefore, these pseudocapacitors frequently compromise rate capability and reversibility [15]. On the other hand, electrochemical double-layer capacitors (EDLC) or ultracapacitors can efficiently replace batteries in energy storage systems and harvesting applications, which requires a high-rate power delivery and uptake. Small-scale supercapacitors can alone be utilized as power sources for microelectronic devices or they can be integrated with batteries and other energy harvesters for wider industrial applications [16].

Figure 3.2 Types of supercapacitors.
The electrochemical double-layer capacitors store electrical energy in an electrolytic double layer produced due to the separation of charged species, and this charge storage mechanism is fundamentally simpler and more reversible than secondary storage batteries. EDLCs’ electrode/electrolyte interfaces are perfectly polarizable and are not affected by faradaic reactions over the entire potential range of operation. But major problems associated with them is their restricted cycle life due to the device packaging rather than by device component deterioration [17].
Large-scale commercial utilization of the carbon materials in supercapacitors is due to their low cost, easy processability, and versatile existing forms (Figure 3.3). They follow charging–discharging through the electric double-layer mechanism. The carbon-based supercapacitor electrodes come in diverse shapes and sizes due to the numerous types of available carbon compounds. The thin-film electrodes are the most common and can be easily prepared by combining carbon compounds with a binder to form a slurry, and then printed or rolled into film electrodes [18]. Because of their chemical inertness and ease of processing, carbonaceous materials have piqued researchers’ interest in using them as supercapacitor electrodes in recent years. The well-developed surface area, size and shape distribution of pores, average pore size, wettability, conductivity, and presence of electroactive species are all important aspects while selecting suitable carbon materials for capacitor electrodes. Due to its amphoteric nature, carbon displays high electrochemical characteristics both as donor and acceptor, making it an appealing material for supercapacitors. Availability of enormous surface area, low density, and high electrical conductivity combined with exceptional chemical stability contribute to their excellent capacity to accumulate charges [19].

Figure 3.3 Advantages of carbon material for electrochemical energy storage.
3.4 Potential of Biochar as High-Performance Sustainable Material
Carbon materials have the potential to be the most adaptable base materials in modern energy storage and conversion. When such compounds are derived from coal and petrochemicals, it is typically energy-intensive to generate or involve difficult conditions for synthesis. The development of efficient ways for producing carbon materials from its renewable resources with excellent performance characteristics and minimal environmental impact is a top priority. Biochar is a type of bio-carbon with numerous surface functional groups and easily controllable porosity. Therefore it could prove to be an excellent candidate as a long-term carbon material in this case [7].
Recent studies show great applications of biochar-based materials in energy storage and conversion due to adaptable surface properties. Carbon aerogels have enormous (specific) capacities and capacitance densities due to their unique nanostructure. Because of the carbon electrode’s continuous structure, this stored energy may be released quickly and efficiently [17].
Generally activated carbon and carbon nanostructures of commercial importance are prepared from coal and petrochemicals via energy-intensive harsh synthetic processes. Different activation processes like steam activation, CO2 activation, and chemical activation by acid, base or salt are used to prepare activated carbon from conventional fossil fuel resources [20]. On the other hand, specific techniques like chemical vapor deposition and electric arc discharge are required for the synthesis of carbon nanomaterials, which require high temperature and complex operational procedures [21–23]. As a result, traditional methods are ineffective for large-scale manufacture and commercial use of functional carbon-based materials. New effective procedures must be developed to generate high-performance carbon materials with little environmental impact. Biomass is a viable raw material for the production of different biochar-based compounds in the context of sustainable carbon materials (biochar) production due to its renewable nature and natural availability [7].
Biochar is basically a solid residue that is formed by the thermal breakdown of biomass at moderate temperatures (350-700°C) either in the absence or limited supply of oxygen [24]. It can be considered as a specific type of biocarbon obtained from plant biomass through the methods of pyrolysis, carbonization, hydrothermal treatment, and gasification. Based on synthesis methods, biochar can be distinguished from other carbon compounds. Generally, carbon black and activated carbon are synthesized from fossil fuels whereas charcoal can be obtained from any biological resources like plants, animals, or microorganisms (Figure 3.4). Biochar and activated carbon are characterized by a porous amorphous carbon matrix but the surface of biochar is frequently packed with functional groups like C−O, C=O, -COOH, and -OH, etc. [25]. Therefore, as compared to the activated carbon, biochar shows high adaptability and serves as a framework for the creation of diverse functionalized carbon compounds [26].
Large-scale biochar production should be considered as a sustainable process as compared to the carbon black and activated carbon prepared from fossil fuels because it reduces anthropogenic CO2 emissions. Atmospheric CO2 is fixed into biomass by plants and becomes a part of the carbon matrix of biochar. This is a cost-effective way to reduce CO2 in the carbon cycle, hence slowing down global warming. By storing carbon in biochar, billions of tons of CO2 can be removed from the carbon cycle [28, 29].

Figure 3.4 Preparation of modified activated carbon electrode [27]. Reprinted under creative commons license
Energy storage and conversion technology is based on the combination of physical and chemical interactions occurring at the interface. Therefore biochar-based functional materials are particularly advantageous due to their easily tuned porosity and other surface properties. Functional groups on biochar surface enable the control over the thermodynamic features of surface interactions at the interface, while surface porosity can be changed according to the desired rate and kinetics of physical interactions and chemical reactions. Meanwhile, biochar’s easily adjustable surface characteristics make it a versatile platform for the development of a variety of nano-structured biochar composites which can be used for energy storage and conversion.
Biochar-based nanocomposites have been widely used as supercapacitor electrode materials. Raw biochar materials are useful because, in comparison to typical carbon sources, oxygenated functional groups are often abundant, e.g., -OH, >C=O, and -COOH. By adjusting the pyrolysis parameters, such as temperature and heating rate, the oxygen concentration can be fine-tuned [30]. Furthermore, appropriate selection of suitable biomass precursor, adjusting pyrolysis temperature and catalyst, the quantitative energy storage capacity of the supercapacitor can be increased. By enhancing the pore access and surface utilization, the wettability of the carbon electrode can be improved by some electrochemically inert oxygenated functional groups and, as a result, the specific capacitance can also be increased. In addition to this, when highly oxygenated functional groups are present on the surface, they can inhibit further oxidation of the carbon matrix over a varied potential range which improves the electrode materials’ cycle stability [31].
3.5 Overview of Recent Developments in Biochar-Based EDLC Supercapacitor
In the electrochemical double-layer capacitors (EDLCs), the amount of energy stored can be controlled through the charge separation at electrode/electrolyte interfaces (Figure 3.5). The charge separation distance is reduced to the dimensions equivalent to those of the ions within the electrolyte (few nanometers). This modification together with the availability of enormous surface area at the porous electrode surface supports the storage of much more energy than conventional capacitors. In comparison to batteries, the electrostatic mechanism of energy storage results in higher power densities, excellent reversibility, and longer cycle life. High surface area, easy availability, low cost, appropriate electrical conductivity, and chemical stability make biochar-based functional materials viable for commercial EDLCs.
In recent years, due to chemical inertness and ease of processing, carbonaceous materials have piqued researchers’ interest in their application as supercapacitor electrodes. For the preparation of supercapacitor electrodes, several biochar precursors have been utilized. Broadly they can be classified into three categories:
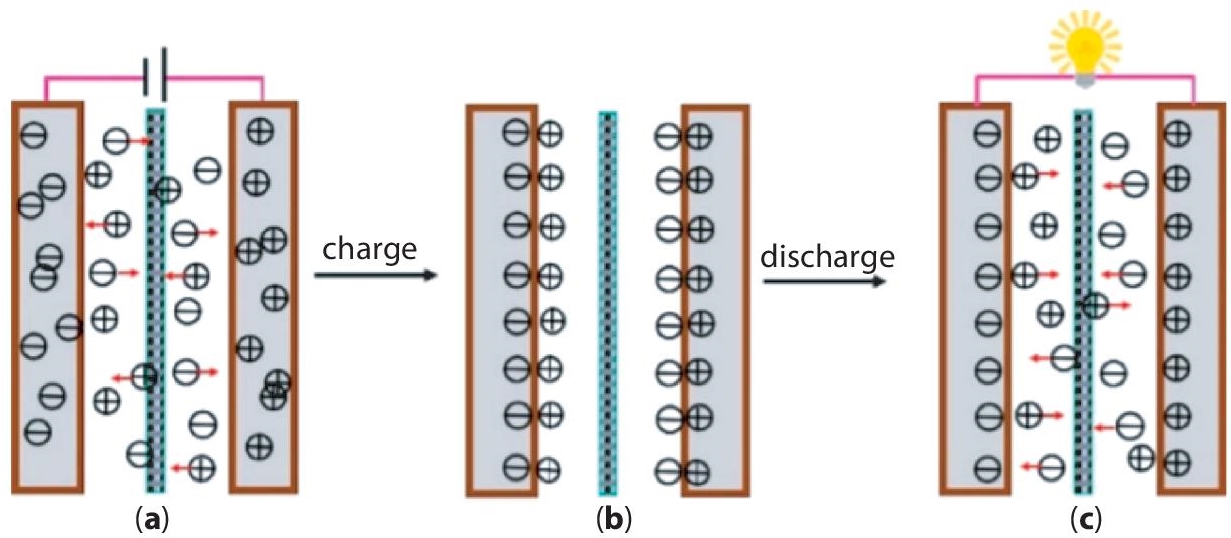
Figure 3.5 Mechanism of EDLC supercapacitors (a) the charging process, (b) EDLC after charging, and (c) the discharging process [32]. Reprinted under creative commons license.
- Biochar-based supercapacitors derived from wood and other plant residues.
- Biochar-based supercapacitors derived from waste biomass (industry, agriculture, solid waste).
- Biochar-based supercapacitors derived from other methods.
3.5.1 Wood & Plant Residues as Biochar Precursor for Supercapacitor Applications
Manufacturing of carbon materials from biomass is a cheap sustainable technology due to the abundancy of biomass. Biochar is a valuable product obtained from biomass through series of chemical reactions and this process of converting biomass to biochar is considered carbon negative. Various morphologies of biochar like fiber, sheet and honeycomb can be obtained from biomass. Plants are a rich source of lignin and cellulosic material which is proved to be a superior precursor of biomass (Table 3.2) with several advantages like high surface area, extensive pore structure, variety of surface functional groups and able to attain superior conductivity after appropriate treatment [33].
Woody biochar is a promising eco-friendly low-cost material due to its excellent performance as electrode with low environmental impacts for supercapacitor applications. Jiang et al. [5] carbonized red cedar wood via one pot pyrolysis and prepared woody biochar monolith having ultra-high carbon content and extensively ordered macropores. Structural studies by EDX and SEM revealed very high carbon content of approximately 98% along with a highly ordered microporous texture. When chemical activation of the biochar was performed in dilute nitric acid at room temperature, a significant 115 F.g-1 of capacitance was recorded which was seven times higher and this was attributed to the consideration due to the increase in the density of surface oxygen groups after activation.
Similarly, biochar was prepared by maple wood pyrolysis and three kinds of supercapacitor electrodes were prepared: mini-chunk, thin-film, and large-disk-chunk. The mini-chunk electrodes and thin film electrodes both showed outstanding performance characteristics and electrochemical behavior. The high specific capacitance (32 F.g-1) and high stability of mini-chunk supercapacitor indicate their suitability to be used as small-scale power source in different electronic devices. Furthermore, the mini-chunk electrode allows for a quick and easy evaluation of biochar materials as possible high-performance, low-cost, and environmentally friendly supercapacitor electrodes without using a binder or costly production techniques [16].
Table 3.2 Wood & plant residues as biochar precursor for supercapacitor applications [7, 12, 40].
| Biomass | Activation process | Surface area SBET (m2.g-1) | Specific capacitance (F.g-1) | Current density | Electrolyte used |
|---|---|---|---|---|---|
| Coconut kernel | KOH | 1200 | 173 | 10 A g-1 | H2SO4 |
| Microalgae | Hydrothermal KOH | 1800- 2000 | 200 | 0.25 A g-1 | LiCl |
| Willow catkins | Carbonization KOH | 1775.7 | 292 | 0.1 A g-1 | KOH |
| Tea Leaves | Activation analysis | 911.92 | 167 | 1 A g-1 | KOH |
| Ginko Leaves | Pyrolysis KOH | 1775 | 178 | 1 A g-1 | KOH |
| Broad Bean | KOH | 655.4 | 202 | 0.1 A g-1 | KOH |
| Wild rice stem | Hydrothermal KOH | 1228.8 | 301 | 1 A g-1 | |
| Bamboo | Hydrothermal KOH | 1472 | 301 | 0.1 A g-1 | KOH |
| Coffee Endocarp | Pyrolysis KOH, CO2, HNO3 | 89-1050 | 176 | 10 mA | H2SO4 |
| Corn Stalk | KOH | 2139 | 317 | 1 mVs-1 | KOH |
| Moringa leaves | Carbonization | 1327 | 323 | 1 A g-1 | KOH |
| Watermelon | KOH | 358 | 50 A g-1 | KOH | |
| Soybean root | KOH | 2143 | 276 | 0.5 A g-1 | KOH |
| Aloe vera | KOH | 1890 | 410 | 0.5 A g-1 | H2SO4 |
| Hyacinth | KOH | 2276 | 344.9 | 0.5 A g-1 | H2SO4 |
| Tobacco | Hydrothermal KOH | 2115 | 286.6 | 0.5 A g-1 | Aqueous |
| Elm samara | Pyrolysis KOH | 1947 | 470 | 1 A g-1 | KOH (6 M) |
| Bamboo | Hydrothermal KOH | 1472 | 301 | 0.1 A g-1 | KOH (6 M) |
| Hemp stems | Pyrolysis Steam MnO2 | 438 | 340 | 1 A g-1 | Na2SO4 (1 M) |
| Watermelon | Hydrothermal FeCl3 FeSO4 | 358 | 0.5 A g-1 | KOH (6 M) | |
| Soybean roots | Pyrolysis KOH | 2143 | 276 | 0.5 A g-1 | KOH (6 M) |
| Aloe vera | Pyrolysis KOH | 1890 | 410 | 0.5 A g-1 | H2SO4 (1 M) |
| Typha angustifolia | Pyrolysis KOH | 3062 | 257 | 0.5 A g-1 | K2SO4 (0.5 M) |
| Seaweeds | Pyrolysis | 3487 | 203 | 0.05 A g-1 | H2SO4 (1 M) |
| Neem leaves | Pyrolysis | 1230 | 400 | 0.5 A g-1 | H2SO4 (1 M) |
| Sisal | Pyrolysis KOH | 2289 | 415 | 0.5 A g-1 | KOH |
| Rose flower | Pyrolysis KOH/ KNO3 | 1980 | 350 | 1 A g-1 | KOH/ KNO3 |
| poplar anthers | Pyrolysis KOH | 3639 | 361.5 | 0.5 A g-1 | KOH |
| shaddock endothelium | Pyrolysis KOH | 1265 | 550 | 0.2 A g-1 | KOH |
| cornstalk | Pyrolysis K2C2O4. H2O | 2054 | 461 | 1 A g-1 | K2C2O4. H2O |
| cellulose | Pyrolysis NaOH | 1588 | 288 | 0.5 A g-1 | NaOH |
Pinecones biochar-based activated carbon with high specific surface area (up to 2450 m2.g-1) and pore structure developed especially for the adsorption of redox-active polyoxometalate (POM) clusters. This material was used for impregnation of the PMo12O403−(PMo12) and this hybrid material thus prepared possesses high redox activity leading to high areal capacitance of 1.19 F.cm-2 greater than the unmodified carbon material. This could be a potential way for designing high-performance hybrid energy storage electrodes at a low cost [34].
Eco-friendly wood-derived biochar (WDB) was prepared by Wan et al. [35]. They pyrolyzed wooden waste of agriculture and industry and then MnO2/WDB composite was prepared by in situ redox reaction with KMnO4. The core-shell structure thus prepared could be used as a free-standing, binder-free electrode with a specific capacitance of 101 F.g-1, coulombic efficiency of 98%‒100%, and enhanced cyclic stability along with a capacitance retention of 85.0% after 10,000 cycles. By blending it with other electrochemical active compounds, it can be employed as a unique, safe substrate material to produce high-performance energy storage devices.
High-temperature biochar supercapacitor was prepared with binder free biochar monolith and 1-butyl-3-methylimidazolium tetrafluoroborate-based liquid ionic electrolyte. It was discovered that raising the temperature up to 140°C could enhance the specific mass capacity and charge-discharge rate by ten times. Within a voltage range of 6V, cycle stability was obtained up to 1,000 cycles. Such supercapacitor electrodes have potential to be used in higher energy and power density energy storage devices [36].
A high-performance supercapacitor electrode was fabricated from the activated biochar of infested ash tree residue. Carbonization was done at 700°C to obtain the biochar which was chemically activated in an Ag2SO4/ HNO3 solution. This activated Ag/BC composite product was electrochemically compared with activated biochar (a-BC) chemically activated with HNO3. A 31.4% higher specific capacitance was obtained for Ag/BC composite electrode. Also, high cycling stability was demonstrated with specific capacitance retention of 98.6% after 2,000 cycles [37].
Y. Li et al. [38] utilized pomelo peel for the synthesis of super-hydrophilic biochar materials with microporous structure for supercapacitor application (Figure 3.6). Chemical activation was done by zinc nitrate (porogen) and urea (nitrogen source) which yielded biochar material with extensive microporous structure and a very large nitrogen and oxygen doping content (>20%). This method produced super-hydrophilicity to this functional material and enhanced the availability of the micropores for charge storage. Under moderate conditions of calcination, the biochar material can be imparted high conductivity without decreasing the super-hydrophilicity. The material (CNO700) exhibits high and stable specific capacitance of 391.0 F. g-1 at 0.5 A. g-1 and cycling stability more than 25,000 times at 10.0 A. g-1 displaying good capacitance retention due to the large effective pore volume (0.69 cm3g-1) synergetic effect, considerable doping of heteroatoms with proper elemental ratio and high conductivity value of 0.713 S.m-1.

Figure 3.6 Galvanostatic charge–discharge performances of (a) all samples at different rates, and (b) CNO700 at 10 A g-1 [38]. Reprinted with permission of Elsevier.
Biochar can also serve as eco-friendly low-cost precursor material for the large-scale manufacture of carbon nano structures which can be used for future supercapacitors with tailored characteristics. To attain high capacitance, biochar activation can be performed by the traditional method of mixing of biochar with any strong base followed by baking at high temperature. But this is a time-intensive procedure that is also inefficient in terms of energy consumption. An innovative process for low-temperature activation of biochar was developed by [39]. Their work involved low-temperature (<150°C) plasma treatment to activate yellow pine biochar. This oxygen plasma activation enhanced the biochar microstructure and significantly enhanced the specific capacitance as compared to the chemically activated biochar material. Due to the greater surface area, this results in the creation of widely scattered pore structures.
Agricultural waste like millet straw was utilized to prepare supercapacitor electrode material by thermal modification in the presence of 5:1 ratio of KOH and carbon. Cyclic voltammetry and ESR studies revealed specific capacitance of this activated biochar was larger with high BET specific surface area [27].
3.5.2 Biochar-Based Supercapacitors from Waste Biomass
As a result of population growth and economic activity, the increased generation and improper disposal of waste biomass has received more attention than in the past. Traditional dumping methods may pollute water and soil, causing environmental problems. The viability of producing biochar from waste biomass, which can be used in vivid applications, including the recently researched subject of supercapacitor electrode materials, must be investigated (Table 3.3).
Remediation of water and wastewater by using bio adsorbents generates heavy metal containing spent biochar. Its regeneration and disposal are very challenging, and are energy and cost intensive. Y. Wang et al. [41] obtained the biochar from the waste biomass of dairy manure and sewage and converted this biochar loaded with heavy metals into supercapacitor electrodes. Chemical activation was done by Ni adsorption followed by microwave treatment. In comparison to the original biochar supercapacitors, the specific capacitance of biochar supercapacitors improved after Ni loading. After microwave treatment of this hybrid material, the capacitance was further increased by more than 2 times. XRD and XPS studies reflected the conversion of Ni into NiO and NiOOH after microwave treatment which enhanced the capacitance of modified biochar. It also showed good stability of specific capacitance with a loss of less than 2% even after 1,000 cycles.
Biochar can also be obtained from the feedstock waste obtained after the thermochemical process of bio-oil production and it can be utilized for the preparation of the hierarchical carbon having high pore volume and specific surface area. After chemical activation by HNO3 treatment, specific capacitance of biochar material was found to be improved. It is interesting to note that these hierarchical carbons outperform bio-derived activated carbons, well-ordered mesoporous carbons, and commercially available graphene in terms of capacitive performance. As a result, this approach increases the economic feasibility of thermochemical biofuel processes by converting biochar to a high-value-added carbon material [42].
Organic waste obtained from poultry litter was used to derive biochar through the process of pyrolysis followed by the process of chemical activation. This resulted in a new hierarchically porous super-activated carbon having specific surface area above 3000 m2.g-1 and morphology like graphene. Minor concentration of Phosphorus and Sulphur present in this biochar-based activated carbon distinguishes it from the others, obtained from plant sources. Pontiroli et al. 2019, prepared a high-performance symmetric supercapacitor from this functional material which exhibited sufficiently high values of specific capacitance, current density, power density and reliability without using any conducting additives. Furthermore, these excellent results were achieved utilizing simple, environmentally friendly electrolytes such as aqueous solutions of KOH and Na2SO4. The low cost of the precursor materials, availability and biocompatibility combined with the low environmental effect of electrolyte, indicates a huge potential for large-scale applications for such devices in transportation, renewable energy grids, and in biomedicine [43].
Table 3.3 Waste biomass as biochar precursor for supercapacitor application [7, 12, 40, 44–46].
| Biomass | Activation process | Surface area SBET (m2.g-1) | Specific capacitance (F.g-1) | Current density | Electrolyte used |
|---|---|---|---|---|---|
| Coconut shell | FeCl3; ZnCl2 Pyrolysis, Steam | 1874 | 268 | 1 A g-1 | KOH |
| Garlic Skin | KOH | 2818 | 427 | 5 mA.s-1 | KOH |
| Coconut fibers | KOH | 2898 | 142 | 0.5 A g-1 | KOH/ EMIMBF4 |
| Mango stone | ZnCl2 & KOH | 1497.8 | 358.8 | 0.5 A g-1 | |
| Walnut Shell | Hydrothermal K2CO3 sol. | 62 | 255 | 0.5 A g-1 | PVA/KOH |
| Litchi Shell | KOH | 1122.6 | 222 | 0.1 A g-1 | KOH |
| Chestnut Shell | Melamine | 961 | 402.8 | 0.5 A g-1 | KOH |
| Coffee ground | Pyrolysis KOH | 404 | 0.5 A g-1 | EMIMTFSI | |
| Almond Shell | KOH/HNO3 | 1363.1 | 283 | 1 A g-1 | KOH |
| Corncob | KOH | 1210 | 120 | 1 A g-1 | KOH |
| Sugarcane bagasse | KOH, CaCl2 | 1982 | 142.1 | 0.5 A g-1 | KOH |

Figure 3.7 SEM Images showing surface morphology of carbonized leather waste biochar (a, c) and activated leather waste biochar (b, d) [47]. Reprinted with the permission of IOP Publishing.
Studies conducted by Martinez-Casillas et al. [47] indicated that footwear leather waste can also be used to generate activated biochar for energy storage devices. This can enhance supercapacitor cell performance as compared to commercially available carbons (Figure 3.7). They derived biochar by pyrolysis of footwear leather wastes at 700°C followed by chemical activation with KOH. This electrode material showed maximum specific capacitance in H2SO4 electrolyte. The prepared SC cell displayed high stability with a capacitance of 52 F.g-1 at 0.5 mA g-1 and loss of only 8% of capacitance even after 5,000 charge/discharge cycles.
3.5.3 Carbon-Based Supercapacitors from Other Methods
To generate high nitrogen biochar from biomass pyrolysis generally low temperature is used but such biochar usually possesses poor conductivity and is not suitable for any energy storage application. W. Zhang et al. [48] successfully prepared a biochar-based material with high nitrogen content with conductivity higher than sodium lignin sulfonate, graphene oxide and p-phenylenediamine. Sodium lignin sulfonate served as source of biochar and graphene oxide after reduction provided high conductivity. The biochar electrode possesses greater gravimetric as well as volumetric specific capacitance together with suitable cycle stability in 1M H2SO4 electrolyte. This novel approach paves the way for biochar-based carbon materials for their application as high-performance supercapacitors in energy storage applications.
Carbon nano fibers were synthesized electrochemically by reduction of chloroform in controlled conditions at room temperature and in situ functionalization of carbon fibers. Super capacitive behavior of this carbonaceous material was studied (Figure 3.8). Surface studies revealed extensive presence of functionalized species, i.e., -OH, =O, -COOH and amorphous as well as graphitic phase of carbon. Specific capacitance (28 F.g-1) with surface area of 78 m2.g-1 was measured. Thermal treatment caused the increase of surface area to 592 m2.g-1 with no apparent change in the surface morphology and capacitive performance [18].
Hierarchical porous biochar with sulfur and nitrogen co-doping was prepared using mantis shrimp-shell which involved self-activation at 750°C temperature. This material was found to have a distinctive dendritic and uniform surface structure containing regularly interconnected micropores, mesopores and macropores. Electrochemical analysis demonstrated the good specific capacitance of 201 F. g−1 at 1 A. g−1 in KOH electrolyte. The surface area was found to be 401 m2.g−1 with very high nitrogen (8.2 wt. %) and sulfur (1.16 wt. %). This is a type of in situ template approach from natural biomass which has displayed huge potential for preparing the active electrode materials of supercapacitors [49].
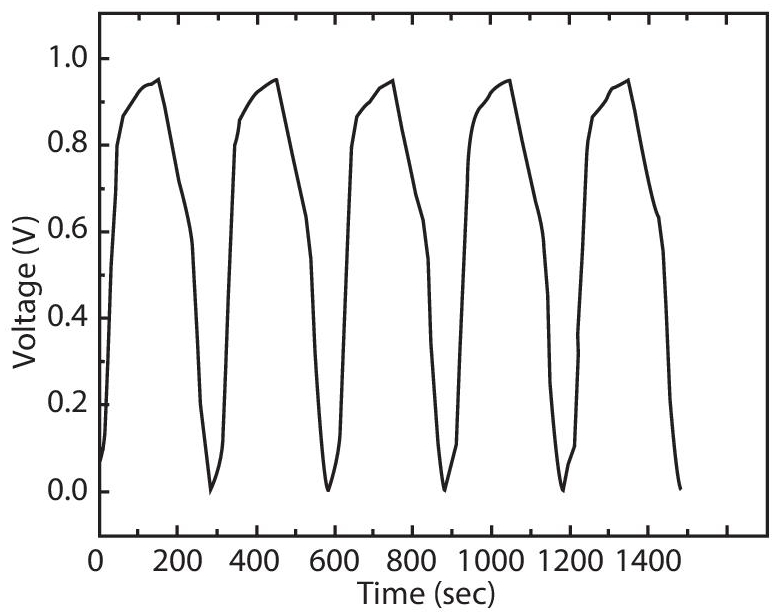
Figure 3.8 Charge-discharge curve of CF electrode [18]. Reprinted with the permission of Elsevier.
3.6 Current Challenges and Future Potential of Biochar-Based Supercapacitor
Electrical energy storage could help to decarbonize the electricity sector by providing a new, carbon-free source leading to the operational flexibility and increased utilization of generation assets, and facilitating the integration of different renewable energy sources. However, the future cost of energy storage technologies is unpredictable, and the value they can provide to the system is contingent on a variety of circumstances. Furthermore, the marginal benefit of storage diminishes when greater energy storage capacity is required. The impact of increasing energy storage capacity on power system operations and generation capacity investments must be investigated. Energy storage offers value by increasing the cost-effectiveness of renewable energy, maximizing the utilization of all installed capacity and lowering total investments in nuclear and gas-fired peaking units. Significant cost reductions in battery storage are necessary to allow large-scale employment. For decarbonization methods which depend on solar and wind energy, energy storage is a necessity, but it can be avoided for low carbon power sources and their varied mixture, e.g., flexible nuclear power [14]. The area of renewable energy not only addresses the issue of carbon reduction but also deals with a variety of different policy goals for example land usage, energy security, employment generation, waste management, etc. In general, it is believed that conflicting policy aims have hampered the growth of biomass utilization. The major environmental concern is related to the alarming concentrations of atmospheric carbon while the other factors like energy supply, waste, and other problems only make a minor contribution. Policymakers must understand that employing limited biomass resources cannot be a solution to battle atmospheric carbon and it is also not efficient or effective. Awareness needs to be raised among the other stakeholders as well, like farmers, industrial communities and societies, that processing their unused biomass resources may yield a better profit [29].
Developing future energy storage systems to increase the use of sustainable electricity like wind or solar in rural regions, as well as improving the use of electric autos, requires breakthroughs in supercapacitor technology. Finally, carbon materials used to fabricate electrodes should have a hierarchical mesoporous structure with suitable pore size distribution greater surface area and conductivity along with term cyclability to attain highly stable and reversible energy storage capacity with high-power density in supercapacitors.
It is sustainable and ecofriendly to obtain hierarchical carbon materials from biological precursors and recycled materials with scalable processes for the large-scale application of supercapacitor in energy storage and conservation. As compared to graphene and metal carbides, biochar feedstock is more economic for the preparation of activated carbon material. Additionally, the performance of electrode materials in supercapacitor can also be tested by well-established commercial current collectors, binders, conducting additives, separators and electrolytes. As a result, the high capacitance performance can be readily achieved in shorter duration in industrial environment [42].
Over the years, different energy storage solutions have been developed and utilized to overcome the unstable nature of renewable, alternative, and clean energy sources like wind, water, and solar energy. Supercapacitors have gained a lot of research attention in comparison to commercially employed lithium-ion batteries because of their higher power density and safer usage due to the prevalent usage of aqueous electrolytes. Biochar (BC) formed from numerous sources such as wood and plant residues, as well as biowastes, has a porous structure and contains N and O components, making it suitable for usage in supercapacitors [26, 38, 47].
Biochar supercapacitors are promising technology for supercapacitors for a great variety of energy and environmental applications due to their superior surface and electrochemical properties [5]. Furthermore, bio-waste-derived high-performance and stable materials, combined with a simple chemical activation process, are definitely a feasible option for generating economically affordable electrodes [37]. Accessible mesopores, which are created by entanglement and the central canal, are required in the best materials. Carbon structures must have mesopores to release a large amount of energy at a fast rate. Mesopores are created through activation mechanisms that include the addition of surface-active groups. In this circumstance, oxygenated groups’ pseudocapacitance redox processes also cause an increase in capacitance. In the electrochemical double-layer capacitors, the specific surface area (SSA) of the BC-derived carbon materials also plays a significant role. It makes the active sites available for the absorption and desorption of ionic electrolyte. This also helps in developing high SSA. Both these factors together provide channel for the quick transmission of electrolyte ions and hence increase the conductivity. An interesting future research path is the synthesis of biomass-derived carbon compounds with an interconnected and suitable pore structure. Meanwhile, introducing the heteroatom functional groups of N, P, and O atoms on the surface of biochar can improve both the wettability and capacitance of the electrode material. It can significantly increase their pseudocapacitance capabilities also. Further studies are required on maintaining and improving the cycle stability of biomass-derived carbon-based supercapacitors [12].
Apart from the biochar-based materials, some other carbon materials like multi-walled carbon nanotubes, graphene was also studied for energy storage applications of the supercapacitors. Carbon nanotubes have also been researched extensively due to their superior electrochemical characteristics as supercapacitor electrodes. However, because of their comparatively small surface area (usually less than 500 m2g-1), they have a poor energy density, which limits their practical use [31].
Multi-walled carbon nanotubes (MWNTs) can also be used for accumulation of charge due to their moderate surface area (Figure 3.9). Through the formation of micropores, activation of MWNTs should allow for larger capacitance values. Most of the surface area of carbon materials is made up of micropores, which are incapable of maintaining an electrical double layer. Ion migration through the pores allows ions to partially reach the surface, resulting in an increase in electrolyte resistance. As a result, only low frequencies or a DC technique can be used to extract the stored energy. The performance of capacitor devices is governed by the kinetics of the pseudo-faradaic reactions in three dimensions depending upon the activation energy of charge transfer processes. As a result, such capacitors’ rate capability is limited. The capacitance of a real capacitor is always twice that of single electrode materials [50].

Figure 3.9 Galvanostatic charge/discharge of a supercapacitor built from carbon nanotubes obtained at 7008C and modified by 69% nitric acid:152 mA, 6 M KOH [19]. Reprinted with the permission of Elsevier.
Similarly, graphene can be utilized to prepare supercapacitor electrodes by depositing it on glass and flexible substrates with very low surface resistance, and it can be technology of future in the field of optoelectronics. It can replace the brittle and expensive electrode material such as indium tin oxide (ITO) [21]. Graphene is an appealing material for supercapacitor applications due to its unique electrical characteristics and theoretical surface area (2620 m2g-1). Due to aggregation of graphene sheets, the surface area of graphene is generally significantly lower than the theoretical value (normally 500 m2g-1), resulting in lower capacitance values than expected (lesser than 200 F.g-1 in aqueous electrolyte, lesser than 120 F.g-1 for organic electrolyte, and lesser than 80 F.g-1 in ionic liquids) [51].
Modification of biochar-based material provides another approach to introduce desirable changes in the surface structure and functional groups. This can be done by different methods like self-doping, physical activation by modifying the extent and duration of pyrolysis temperature and by employing suitable chemical reagent for chemical modification of surface functional groups (Figure 3.10). It has been demonstrated that different mechanisms of carbon alteration result in samples with distinct chemical properties. As evidenced by data obtained from FTIR, sodium capacity, pH titration, and zeta potential studies, oxidation resulted in the fixing of weakly acidic functional groups. However, the oxidation process created some by-products in the form of humic compounds, which were removed using a sodium hydroxide solution. As indicated by the pore size distribution data, humic compounds tend to get accumulated in micropores, resulting in a loss of microporous structure. Elemental analysis showed presence of Nitrogen in the oxidized samples which was due to the nitrate ions stuck in the pores of the oxidized carbon material. The elimination of acidic functional groups that become unstable at high temperatures can be attributed to hydrogen treatment at elevated temperatures producing a sample with certain basic properties [20].
Self-activation and self-doping are effective methods to improve the electrochemical capacitance by modifying the carbon structure. It can produce high-value biochar from biomass utilization for supercapacitor application. This in situ template approach using naturally abundant biomass as a precursor held a lot of promise for supercapacitor active electrode materials [49]. Studies have also been done on the activation of biochar by plasma, which demonstrated the ability of oxygen plasma to efficiently create porous structures including micropores, mesopores, and macropores of different sizes. This study also reflected that a large surface area together with a proper pore-size distribution can be used to achieve higher specific capacitance [39].

Figure 3.10 SEM images of modified carbon samples, Source: [20] (a) F400 (coal-based commercial granular activated carbon), (b) AC1 (HNO3 oxidation, H2O wash, 363K, 9 Hrs.), (c) AC4 (Annealing of F400, 1173K, 3 Hrs.), (d) AC5 (HNO3/H2SO4/NH3 Na2S2O4/ (CH3CO)2O, 298K, 48 Hrs.). Reprinted with the permission of Elsevier.
Another promising application for the future modern grid is the high-temperature biochar supercapacitor, an energy-storage device with high energy and power density. Significant amount of research data is available to showcase that on increasing the temperature, various ionic liquids show significant increase in their viscosities, electrical conductivities, and diffusion coefficients (Figure 3.11). These enhancements are beneficial for enhancing the carbon/electrolyte interface, lowering the resistance associated with redox reactions occurring on carbon surfaces, and facilitating the ion mass transports in the porous structure of carbon materials. The pseudocapacitance associated with redox reactions of oxygen groups on carbon surfaces and electrochemical double layer capacitance can boost the capacity of a supercapacitor. Supercapacitors with a high temperature could store both thermal and electrical energy at the same time [36].

Figure 3.11 Changes of the high temperature biochar electrode as a function of charge passed during three successive constant-current charges and discharges at 0.2 A. g−1 and room temperature (black line); and at 1.4 A. g−1 and 140°C (red line) [36]. Reprinted under creative commons license.
Even after the tremendous progress in the field of biochar-based materials and their applications for different energy storage and conversion technologies, some existing key concerns still need the attention of future research [7].
- Surface oxidation, amination, and sulfonation are common methods for biochar functionalization, although they frequently involve complex procedures or harmful and toxic chemicals.
- It is challenging to keep the conversion efficiency of biomass into biochar without affecting the biochar’s subsequent treatments like the tuning of pores and surface chemistry together with the loading of metals and oxides.
- There is a need to develop a balanced strategy to overcome the harmful effect of contaminants in biochar material on the final performance of supercapacitor electrode because the predicted carbon structure may not necessarily have a good impact on the final performance characteristics.
- Although the surface characteristics of the biochar may be easily modified, there is no strong flexibility or the capacity to be composited with other different functional materials for additional performance improvement.
Several strategies can be developed and studied in future to find out an amicable and feasible solution of the issues related to the applications of biochar-based supercapacitor electrodes. More work should be put into customizing the precursor biomass or biochar raw material. Chemical structure of plant biomass could be modified with the help of biotechnology so as to enrich the biomass material in certain elements when it is pyrolyzed, and the resulting biochar with certain functions or heteroatom doping can be obtained immediately. This can be a more environmentally friendly technique of biochar functionalization as compared to the present traditional approaches. A cost-effective and readily scaled-up method can be developed to increase the conversion efficiency of biomass to biochar with respect to certain specific properties such as a surface porosity, presence of certain surface functional groups, and an effective surface structure. Carefully selecting and pre-treating biomass before turning it to biochar could be a possible low-cost approach to attain this goal. Theoretical models for the tailor-made biochar material’s design and synthesis can be developed. Emphasis should be given to the surface chemistry and molecular interactions so as to reduce the negative impacts of the presence of impurities on biochar’s carbon structure and its performance in energy conversion and storage.
To build high-performance biochar-based composites suitable for modern energy applications, components with acceptable physicochemical stability and compatibility with carbon must be chosen. To do this, the components must make full use of biochar’s large surface area and retain the structure and porosity of both the components and the biochar. Significant obstacles can be observed while increasing the use of biochar in energy applications; however, the current achievements are both inspirational and comprehensive. If continuous research contributions are produced in this field, there may be great chances to implement practical usage of biochar materials in the domains of renewable energy conversion and storage.
3.7 Conclusion
Carbon materials have the potential to be the most adaptable substrate material in modern energy storage and conversion. Being a carbon-negative process, it will not only aid in the mitigation of serious environmental issues like global warming and pollution but also provide a long-term solution for the storage and conversion of renewable, non-perennial, and dispersive, energy sources such as the sun, wind, water, geothermal, or biomass [52]. Wood, plant and agricultural residues, industrial waste biomass, and sewage, are all promising material for biochar production. Some good examples are red cedar wood, maple wood, pinecones, wooden waste of agriculture and industry, ash tree residue, pomelo peel, millet straw, waste biomass of dairy manure, and sewage, feedstock waste, organic waste obtained from poultry liter, footwear leather waste, etc. Other carbon materials like multi-walled carbon nanotubes, and graphene can also be utilized for supercapacitor applications.
There are two key concerns regarding carbon materials’ energy applications. The first difficulty is the process for mass-producing acceptable carbon materials, which determines the material’s electrical and electrochemical properties, cost, and environmental impact over its lifetime. Coal and petrochemical-derived carbon compounds are often energy-intensive to produce or involve difficult synthesis conditions. The development of efficient ways for producing carbon materials from renewable resources with excellent performance and minimal environmental impact is a top priority. Biochar, a bio-carbon with abundant surface functional groups and a porosity that can be easily controlled, could be a good contender as a sustainable carbon material in this regard [53].
The second point to consider is the efficiency and performance of carbon materials in a variety of physical situations for various applications. Biochar-based materials have recently been shown to have the potential for energy storage and conversion. Biochar-based materials perform well in supercapacitors, with high specific capacitance and cycle stability. The capacitive properties of biochar-based materials are influenced by a variety of parameters, including surface area, pore characteristics (pore size and distribution), electrical conductivity, and surface interactions. Surface doping with heteroatoms like N, P, and S, as well as surface combining with metal oxide nanostructures, can improve capacitive performance by introducing pseudocapacitive effects. Biochar-based materials perform well in supercapacitors, with high specific capacitance and good cycle stability. The capacitive properties of biochar-based materials are influenced by a variety of parameters, including surface area and pore characteristics (pore size and distribution), as well as electrical conductivity and surface functions. Heteroatom (N, P, S) doping of the surface and its combination with metal oxide nanostructures, can improve capacitive performance by introducing pseudocapacitive effects. In addition to this, specific physical and chemical surface morphologies impart great stability to the electrode materials during charge-discharge cycles. There are many obstacles to overcome while expanding the use of biochar in energy applications, but the existing achievements are both inspirational and comprehensive. It is likely to stimulate more research, allowing biochar-based materials to be employed effectively in modern sustainable energy storage and conversion applications.
References
- 1. World Energy Outlook 2021, International Energy Agency, Paris, 2021.
- 2. J. Romero Agüero, E. Takayesu, D. Novosel, and R. Masiello, Grid modernization: challenges and opportunities, Electricity Journal, 30(4), 1–6, 2017.
- 3. L. Yao, B. Yang, H. Cui, J. Zhuang, J. Ye, and J. Xue, Challenges and progresses of energy storage technology and its application in power systems, Journal of Modern Power Systems and Clean Energy, 4(4), 519–528, 2016.
- 4. Z. Yang et al., Electrochemical energy storage for green grid, Chemical Reviews, 111(5), 3577–3613, 2011.
- 5. J. Jiang et al., Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes, Electrochimica Acta, 113, 481– 489, 2013.
- 6. X. Li and S. Wang, Energy management and operational control methods for grid battery energy storage systems, CSEE Journal of Power and Energy Systems, 7(5), 1026–1040, 2021.
- 7. W. J. Liu, H. Jiang, and H. Q. Yu, Emerging applications of biochar-based materials for energy storage and conversion, Energy and Environmental Science, 12(6), 1751–1779, 2019.
- 8. A. K. Rohit, K. P. Devi, and S. Rangnekar, An overview of energy storage and its importance in Indian renewable energy sector: Part I – Technologies and Comparison, Journal of Energy Storage, 13, 10–23, 2017.
- 9. O. Palizban and K. Kauhaniemi, Energy storage systems in modern grids— Matrix of technologies and applications, Journal of Energy Storage, 6, 248– 259, 2016.
- 10. R. M. A., M. S. Palizban Omid, Proceedings of IEEE International Conference on Control System, Computing and Engineering, ICCSCE-2011, Penang, Malaysia, 601, 2011.
- 11. S. Sabihuddin, A. E. Kiprakis, and M. Mueller, A numerical and graphical review of energy storage technologies, Energies, 8(1), 172–216, 2015.
- 12. X. Li, J. Zhang, B. Liu, and Z. Su, A critical review on the application and recent developments of post-modified biochar in supercapacitors, Journal of Cleaner Production, 310, 2021.
- 13. A. K. Rohit and S. Rangnekar, An overview of energy storage and its importance in Indian renewable energy sector: Part II – energy storage applications, benefits and market potential, Journal of Energy Storage, 13, 447–456, 2017.
- 14. F. J. de Sisternes, J. D. Jenkins, and A. Botterud, The value of energy storage in decarbonizing the electricity sector, Applied Energy, 175, 368–379, 2016.
- 15. H. Wang, S. Casalongue, Y. Liang, and H. Dai, Ni(OH) 2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials, J. Am. Chem. Soc., 132(21), 7472-7477, 2010.
- 16. L. Zhang, J. Jiang, N. Holm, and F. Chen, Mini-chunk biochar supercapacitors, Journal of Applied Electrochemistry, 44 (10), 1145–1151, 2014.
- 17. S. T. Mayer, R. W. Pekala, and J. L. Kaschmitter, The Aerocapacitor: An Electrochemical Double-Layer Energy-Storage Device, Journal of Electrochemical Society, 140 (2), 446–451, 1993.
- 18. P. v Adhyapak, T. Maddanimath, S. Pethkar, A. J. Chandwadkar, Y. S. Negi, and K. Vijayamohanan, Application of electrochemically prepared carbon nanofibers in supercapacitors, Journal of Power Sources, 109, 105–110, 2002.
- 19. E. Frackowiak and F. Beguin, Carbon materials for the electrochemical storage of energy in capacitors, Carbon, 39, 937-950, 2001.
- 20. P. Chingombe, B. Saha, and R. J. Wakeman, Surface modification and characterisation of a coal-based activated carbon, Carbon N Y, 43 (15), 3132–3143, 2005.
- 21. R. Garg, S. Elmas, T. Nann, and M. R. Andersson, Deposition Methods of Graphene as Electrode Material for Organic Solar Cells, Advanced Energy Materials, 7(10), 2017.
- 22. A. Szabó, C. Perri, A. Csató, G. Giordano, D. Vuono, and J. B. Nagy, Synthesis methods of carbon nanotubes and related materials, Materials, 3(5), 3092– 3140, 2010.
- 23. A. R. Karaeva, N. v Kazennov, E. A. Zhukova, and V. Z. Mordkovich, Carbon nanotubes by continuous growth, pulling and harvesting into big spools, Material Today: Proceedings, 5, 25951-25955, 2018.
- 24. J. J. Manyà, Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs, Environmental Science and Technology, 46 (15), 7939–7954, 2012.
- 25. W. J. Liu, H. Jiang, and H. Q. Yu, Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material, Chemical Reviews, 115(22), 12251–12285, 2015.
- 26. W. J. Liu, H. Jiang, and H. Q. Yu, Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material, Chemical Reviews, 115(22), 12251–12285, 2015.
- 27. Y. Ding, T. Wang, D. Dong, and Y. Zhang, Using Biochar and Coal as the Electrode Material for Supercapacitor Applications, Frontiers in Energy Research, 7, 2020.
- 28. D. Woolf, J. E. Amonette, F. A. Street-Perrott, J. Lehmann, and S. Joseph, Sustainable biochar to mitigate global climate change, Nature Communications, 1(5), 2010.
- 29. M. Fowles, Black carbon sequestration as an alternative to bioenergy, Biomass and Bioenergy, 31 (6), 426–432, 2007.
- 30. Q. Zhang, E. Uchaker, S. L. Candelaria, and G. Cao, Nanomaterials for energy conversion and storage, Chemical Society Reviews, 42 (7), 3127–3171, 2013.
- 31. M. Sevilla and R. Mokaya, Energy storage applications of activated carbons: Supercapacitors and hydrogen storage, Energy and Environmental Science, 7(4), 1250–1280, 2014.
- 32. Y. Lin, H. Zhao, F. Yu, and J. Yang, Design of an extended experiment with electrical double layer capacitors: Electrochemical energy storage devices in green chemistry, Sustainability (Switzerland), 10 (10), 2018.
- 33. J. Wang, C. Ma, L. Su, L. Gong, D. Dong, and Z. Wu, Self‐Assembly/Sacrificial Synthesis of Highly Capacitive Hierarchical Porous Carbon from Longan Pulp Biomass, ChemElectroChem, 7(22), 4606–4613, 2020.
- 34. M. Genovese and K. Lian, Polyoxometalate modified pinecone biochar carbon for supercapacitor electrodes, Journal of Materials Chemistry A, 5(8), 3939–3947, 2017.
- 35. C. Wan, Y. Jiao, and J. Li, Core-shell composite of wood-derived biochar supported MnO2 nanosheets for supercapacitor applications, RSC Advances, 6(69), 64811–64817, 2016.
- 36. J. Jiang, High Temperature Monolithic Biochar Supercapacitor Using Ionic Liquid Electrolyte, Journal of the Electrochemical Society, 164 (8), H5043– H5048, 2017.
- 37. L. Kouchachvili and E. Entchev, Ag/Biochar composite for supercapacitor electrodes, Materials Today Energy, 6, 136–145, 2017.
- 38. Y. Li et al., Super-hydrophilic microporous biochar from biowaste for supercapacitor application, Applied Surface Science, 561, 2021.
- 39. R. K. Gupta, M. Dubey, P. Kharel, Z. Gu, and Q. H. Fan, Biochar activated by oxygen plasma for supercapacitors, Journal of Power Sources, 274, 1300– 1305, 2015.
- 40. C. Senthil and C. W. Lee, Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices, Renewable and Sustainable Energy Reviews, 137, 110464, 2021.
- 41. Y. Wang et al., Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents, Scientific Reports, 7, 2017.
- 42. H. Jin, X. Wang, Z. Gu, and J. Polin, Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation, Journal of Power Sources, 236, 285–292, 2013.
- 43. D. Pontiroli et al., Super-activated biochar from poultry litter for high-performance supercapacitors, Microporous and Mesoporous Materials, 285, 161–169, 2019.
- 44. H. Fu et al., Walnut shell-derived hierarchical porous carbon with high performances for electrocatalytic hydrogen evolution and symmetry supercapacitors, International Journal of Hydrogen Energy, 45 (1), 443–451, 2020.
- 45. J. Li, Q. Jiang, L. Wei, L. Zhong, and X. Wang, Simple and scalable synthesis of hierarchical porous carbon derived from cornstalk without pith for high capacitance and energy density, Journal of Materials Chemistry A, 8(3), 1469–1479, 2020.
- 46. J. Li, L. Wei, Q. Jiang, C. Liu, L. Zhong, and X. Wang, Salt-template assisted synthesis of cornstalk derived hierarchical porous carbon with excellent supercapacitance, Industrial Crops and Products, 154, 112666, 2020.
- 47. D. C. Martínez-Casillas, I. L. Alonso-Lemus, I. Mascorro-Gutiérrez, and A. K. Cuentas-Gallegos, Leather Waste-Derived Biochar with High Performance for Supercapacitors, Journal of the Electrochemical Society, 165(10), A2061– A2068, 2018.
- 48. W. Zhang, Y. Zou, C. Yu, and W. Zhong, Nitrogen-enriched compact biochar-based electrode materials for supercapacitors with ultrahigh volumetric performance, Journal of Power Sources, 439, 2019.
- 49. S. Huang, Y. Ding, Y. Li, X. Han, B. Xing, and S. Wang, Nitrogen and Sulfur Co-doped Hierarchical Porous Biochar Derived from the Pyrolysis of Mantis Shrimp Shell for Supercapacitor Electrodes, Energy and Fuels, 35(2), 1557– 1566, 2021.
- 50. E. Frackowiak and F. Beguin, Carbon materials for the electrochemical storage of energy in capacitors, 2001.
- 51. Y. Zhu et al., Carbon-Based Supercapacitors Produced by Activation of Graphene, Science, 332, 1537, 2011.
- 52. Y. Lin, F. Li, Q. Zhang, G. Liu, and C. Xue, Controllable preparation of green biochar based high-performance supercapacitors, Ionics (Kiel), 28 (6), 2525–2561, 2022.
- 53. Z. Li, D. Guo, Y. Liu, H. Wang, and L. Wang, Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors, Chemical Engineering Journal, 397, 125418, 2020.
Notes
- *Corresponding author: [email protected]; https://orcid.org/0000-0001-9690-4124
- †Corresponding author: [email protected]; https://orcid.org/0000-0002-9632-2682
