Chapter 7. Heat Transfer
We know all about heat transfer. Everyone has touched that hot pot and had our neurons awoken—ouch! We pay a lot of money to heat and cool buildings and houses. We buy fashionable coats to keep us warm and hats to keep the sun out of our eyes and off our heads (yes, and to keep us warm too!). We have touched sun-soaked objects and been sadly startled at how hot they were. We are fearful of boiling grease and hissing steam. We watch dogs pant and have no idea how small birds can survive frigid cold. We are happiest at a certain temperature and humidity but the world around us often wants us to be much hotter or colder.
In products and systems, heat is often created by friction and has to be removed. If you pull and squeeze a kneaded eraser you will be surprised how much heat builds up. Hopefully you have never touched a brake rotor after you park your car—the frictional heat produced by braking a car is enormous. Moreover, materials and our bodies behave differently when hot or cold. Studying heat transfer tells us why water evaporates at room temperature, why shiny seat belt buckles get so hot, why dogs pant, and why the SR 71 Blackbird airplane is painted black. It is for all of these reasons that the behavior of heat is important for a designer.
What Is Heat?
Heat is the movement of molecules—the faster molecules vibrate, the higher the measured temperature. The second law of thermodynamics tells us that this vibration energy wants to be shared; that is, heat flows to cooler objects. Heat transfer is accomplished by three mechanisms, although it is easier to simply think of five types of heat exchange: conduction, convection, radiation, infiltration, and internal heat.
The three heat transfer mechanisms—conduction, convection, and radiation—can be demonstrated by holding your hand at different positions relative to a flame. Sticking your finger in a flame produces conductive heat transfer from the flame to your finger (ouch!), placing your hand above the flame produces convective heat transfer from the flame to your hand, and finally, holding your hand to the side of the flame (away from the rising hot gases) produces radiant heat transfer from the flame to your hand.
Conduction
Conduction heat transfer is produced by direct contact of two surfaces. The more insulated the material, the slower the heat transfers through it. In other words, aluminum with its high thermal conductivity will allow heat to transfer quickly through it, while air with its low thermal conductivity slows the rate of heat transfer.
For noncrystalline materials, conductivity principally relies on the movement of electrons for transferring energy. Therefore, materials with low thermal conductivity generally have low electrical resistance.
If constant heat is applied to one side of a block, a thermometer on the other side, and the whole arrangement is perfectly insulated (referred to as adiabatic), given enough time the temperature measured on the far side of the block will be the same as the applied temperature. However, if the measured temperature was plotted against time, the rapidity of the temperature rise would be directly proportional to the thermal conductivity. That is, a high conductivity would produce a rapid temperature rise. In practice, we rarely have adiabatic conditions; heat is always leaking out somewhere.
Thermal conductivity can be given per unit of material thickness, or as the total value per surface as seen in the thermal resistance (or R) values of house insulation. The R value gives the insulation of the total material per square foot. So a 1-inch (25-mm) thick foam panel has an R value of 3.5 per square foot (0.32 per square meter). If two panels were stacked on top of each other, the total R value would be 3.5 x 2 = 7. This is a convenient measurement because we can obtain R values for various types of building construction without having to add up the individual material conductivities and thicknesses. The U value is more commonly used in thermal load calculations. U is a measure of conductance and is equal to the inverse of R. Fourier’s law describes how conductive heat transfer is equal to the multiplication of temperature difference, area, and the inverse of thermal resistance.
Temperature has other effects also. Most importantly for the designer is to recall that a material’s expansion and contraction are directly related to temperature and the material’s thermal expansion coefficient. The expansion and contraction of a material can tear apart materials and assemblies and was previously described in Chapter 3.
Convection
Convective heat transfer is produced by flowing liquids or gases. This is the principal method of heat transfer in many heat exchangers, including car radiators. The rate of convective heat transfer from a surface is related to the temperature difference and area (as it was with conduction) as well as the film conductance.
The film conductance, or convective heat transfer coefficient, is difficult to obtain. The best values are those based on experimental data, but the coefficient is proportional to the thermal conductivity of the fluid and inversely proportional to the thickness of the boundary layer. However, the general ranges of these coefficient values are as follows:
-
Natural Convection — Air: 0.9–4.4 Btu/h ft2 R (5–25 W/m2K)
-
Forced Convection — Air: 1.8–35 Btu/h ft2 R (10–200 W/m2K)
-
Natural Convection — Water: 3.5–18 Btu/h ft2 R (20–100 W/m2K)
-
Forced Convection — Water: 8.8–1762 Btu/h ft2 R ( 50–10000 W/m2K)
Natural convection arises from differing air density and the pressure it produces. Hot air is less dense than cold air and will rise due to the pressure differences. Forced convection means a mechanical system, such as a fan, drives the fluids.
Radiation
Radiation heat transfer is an interesting mechanism because it occurs by invisible electromagnetic radiation over vast distances without requiring a medium to travel through as with conduction and convection. Radiation heat transfer can be experienced when you feel the sun warming up your face on a cold, winter day, or the heat from a black road under the onslaught of sun. Interestingly, the propensity to absorb radiation is the same as that to emit radiation. If you put a piece of black paper and white paper in the sun, you will observe the black paper heats up faster. If you put the papers in the shade, you will see the black paper cools off faster.
Radiation will always be emitted when an object is hotter than its surroundings. The amount of radiation transferred from the hot surface to another surface is dependent on the temperature difference, emissivity of the surface (which will be presented later), and the orientation of the radiating surfaces with respect to one another. If a hot and a cold surface are parallel (therefore showing a lot of area to each other), they will transfer more heat by radiation than if they are askew. Almost no radiation transfer will occur if they are perpendicular.
Nature of Electromagnetic Radiation
Electromagnetic radiation is massless energy that radiates outward from a source. Unlike vibration and other mechanical wave phenomena, electromagnetic waves do not require a medium and can travel in a pure vacuum, such as outer space. In space, electromagnetic waves travel at “the speed of light” or 186,000 miles/s (300,000,000 m/s), while mechanical waves, used in ultrasonic thickness gauges, travel at only 2.8 miles/s (4.5 km/s) in structural steel and only a third of this velocity in rubber.
Visible light, X-rays, and radio waves are examples of electromagnetic radiation. Light is unique simply because our retina is sensitive to the range of frequencies known as visible light, with red light at the low frequency end and violet light at the high frequency end. Green lies in the middle band of visible light and the human eye can best discern the frequencies associated with green light.
Other frequencies outside the visible light band have important features. Infrared light warms skin while ultraviolet light causes sunburns. Microwaves heat water and X-rays let us see through solid objects.
A full description of electromagnetic radiation is seen through a description of its energy, wavelength, and frequency. High-frequency waves have a higher energy content than do low-frequency waves. Wavelength is an inverse function of frequency. Electromagnetic radiation has both wave and particle properties. Radiation behaves as particles, called photons, because they have defined length. That is, a light of one color will produce radiation of a certain frequency, but it will emit a barrage of photons.
The nerves in our skin respond to infrared frequencies and feel them as heat. However, our retina responds to the visual spectrum allowing us to see light. For temperatures below 1300°F (700°C), almost all the emissions are in the infrared spectrum and cannot be seen with the naked eye. The relationships between the frequency of electromagnetic radiation and some of its consequences are shown in Figure 7-1.
Where do colors come from then? Colors are a result of the reflection and absorption properties of a surface. Leaves are green because they absorb all the colors but green and reflect the green wavelengths. Black appears if the material absorbs all the visible wavelengths, and conversely, white appears if all the visible wavelength frequencies are reflected. However, we need to recognize that visible light is a very small portion of the spectrum and the surface properties of a material may be quite different at other frequencies.
The best example is snow, which is white and therefore is reflecting all the visible light frequencies. However, at the longer, infrared wavelengths, snow is an efficient absorber. Therefore, if we could see infrared radiation, snow would appear “black.” Fortunately, at the high temperatures of incandescent lightbulbs and flames, radiation occurs in the visible light spectrum so lightbulbs illuminate things and we can usually see fires. Incandescent lights, for example, operate at about 4500°F (2500°C) and the sun is at approximately 10,000°F (5500°C).

Figure 7-1. Frequency of electromagnetic radiation and some of its consequences
Radiation Heat Transfer
Radiation properties are characterized by a balance of three material properties. These interconnected properties are transmission, reflection, and absorption. Transmission is a measure of how well radiation can transmit through a material. Transparent materials have high transmission while opaque materials have no transmission of light.
Reflection is a measure of how well a surface reflects—and therefore, rejects—heat energy. The nature of the reflected energy varies. The reflected radiation can be a specular reflection where the angle of reflected radiation is equal to the angle of incident irradiation, such as with a mirror. Alternatively, the reflections can be diffuse and uniformly distributed in all directions. Albedo is the climatological term used to describe the reflectivity of an object. It represents the ratio of scattered to incident radiation. Snow, for example, has a higher albedo at visual light frequencies than does grass, but they are nearly the same at infrared frequencies, as shown in Figure 7-2.
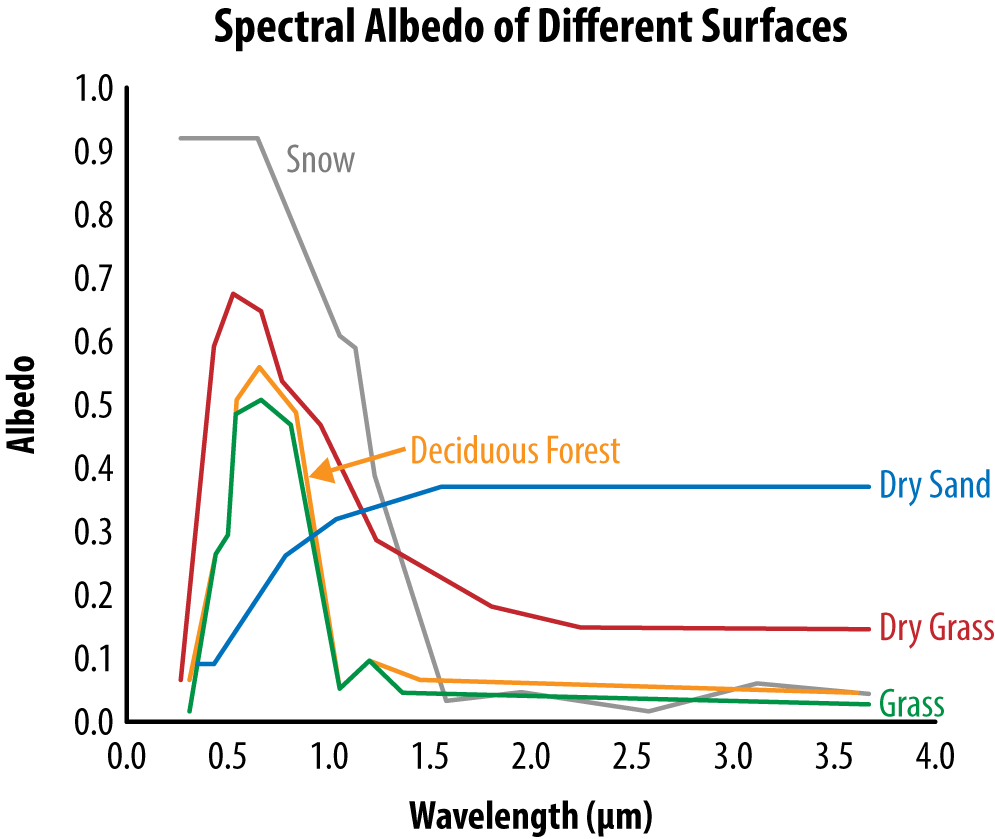
Figure 7-2. Albedo of materials changes with frequency, so that snow is very reflective at visible light (wavelengths from 0.4 to 0.7 nm) but not at infrared frequencies (wavelengths greater than 0.7 nm)
Absorption is a measure of how well a surface absorbs (as opposed to emits) heat energy. Reflection and absorption are surface properties and are affected principally by color and surface finish.
A unitless value between 0 and 1 is assigned to reflection, transmission, and absorption. These values indicate how much of the incoming radiation is divided among these phenomena. The summation of these phenomena is equal to 1 (or 100%):
absorption + reflection + transmission = 1
This means incoming energy is divided into three responses. It is divided in a manner dictated by material properties between absorption, reflection, and transmission. For example, in a nearly perfectly transparent material like glass, transmission is almost 100%. Therefore both reflection and absorption are equal to zero. If a material was perfectly absorptive, like a black brick, and did not reflect or transmit radiation, both reflection and transmission would be equal to zero and absorption would be equal to 100%. However, if a material was opaque but had some reflection, like a red brick, the transmission would be equal to 0 and therefore the reflection plus absorption is equal to 100%.
Absorption and reflection are properties of the surface of an object. That is, except for transmission, the only factor in considering the radiation properties of an object is its surface. Therefore, painting an object can change its radiation properties. For example, highly reflective roofs keep buildings cool, while highly absorptive roofs keep buildings warm. Besides painting roofs white (or other reflective materials), commercial buildings will sometimes use “spectral blue” windows that allow visible light to penetrate, but block the infrared light. This allows dwellers to enjoy the view without allowing the solar heating to drive up the cooling load.
Emission
Emission measures the ability of a surface to radiate. For a gray body, where reflected radiation is diffuse and the radiation properties are independent of wavelength, the emissivity equals the absorptivity. Emission is the ratio of energy radiated between one thing and a black body/object. A black body is the most efficient emitter and absorber of energy—both the emission and absorption are equal to one. That is, it absorbs 100% of the radiation that lands on it (there is no reflection and no transmission). Interestingly, human skin is a very good absorber and emitter with an emissivity equal to 95%. Figure 7-3 illustrates the response of an object to irradiation and internal heat.
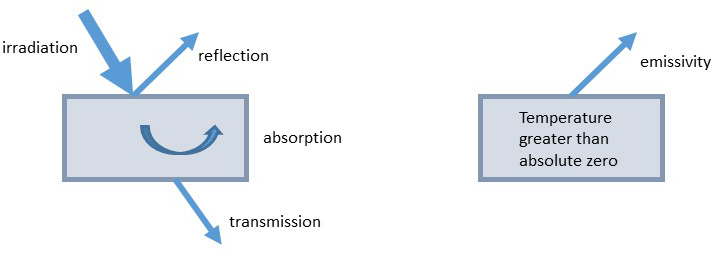
Figure 7-3. An object responds to incoming radiation by some combination of reflection, transmission, and absorption; an object emits heat as a function of its temperature and emissivity
To keep a surface cool, the material should have both high reflectivity and low absorptivity. It should also have high emissivity to get rid of any heat from its surface. However, we note for thermally opaque objects, where transmission equals zero, the reflection plus absorption equals 100%. That is, increasing one parameter decreases the other. Furthermore, absorption equals emission (Kirchoff’s law of thermal radiation) so the interplay between reflectivity, absorption, and emission depend on the specifics of the problem being addressed. Table 7-1 provides the absorptivity and emissivity, at visible light frequencies, of various surfaces.
| Surface material | Absorptivity |
|---|---|
Aluminum |
15% |
White paint |
25% |
Concrete |
60% |
Colored paints |
60% |
Black polyethylene |
94% |
Black paint |
94% |
While radiation is emitted by everything with a temperature above absolute zero, the net amount of radiation between two objects is related to their temperature difference and area as well as the propensity to emit and absorb radiation.
If you put a small heated box inside another box at room temperature, both boxes emit radiation (because they are above absolute zero) but the net radiation will be outward from the hot box to the cool box. If they were at the same temperature but different emissivity and absorptivity, the inner box will receive radiation from the outer box plus reflections of its own radiation.
The temperature difference and surface properties explain why the ground loses less heat to a cloudy sky than a clear, dark sky. With a cloudy sky, the clouds are both warmer and have a higher reflection than the night sky. Therefore, the heat transfer is lower due to the decrease in temperature difference and the reflected energy radiated back from the clouds down to the earth’s surface. Figure 7-4 provides an overview of the earth’s response to solar irradiation.
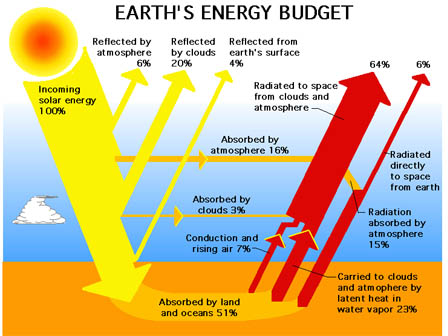
Figure 7-4. Earth’s response to solar irradiation
Combined Effects with Radiation
Heat transfer is produced by the multiple mechanisms of conduction, convection, and radiation. However, radiation heat transfer is driven by temperature difference, area, and surface properties. Therefore, the conditions at the surface of an object are influenced by what is going on around the surface. Is the surface being continually heated or is it cooling off? Is the temperature much different than the surface it faces? Let’s consider some of these questions.
Radiation, Conduction, and Specific Heat
The relationships between absorption and reflection raise the question of why, when your car sits in the sun, the seat belt buckle is much hotter to the touch than the seat belt webbing. You would think that due to the high reflection of the shiny buckle, it should not absorb much heat (absorption + reflection =100%). This is true. Because of its higher reflection, as shown in Figure 7-5, the buckle does not absorb as much heat as the webbing. However, the specific heat of the steel buckle is much lower than the webbing (about one-third the value). Understanding specific heat is the key to understanding the hot buckle problem.
Specific heat measures the increase in temperature per energy added. That is, specific heat measures the temperature response of a material to added heat. Therefore, the temperature of steel increases more quickly than does the temperature of the webbing for the amount of radiation they absorb.
Another factor in this story is that the steel buckles have much higher thermal conductivity than does the webbing. Consequently, when you touch the buckle, the heat travels very quickly from the hot buckle to your hand. The webbing will transfer the heat at its surface immediately but the heat travels slowly through the webbing so the cool spot you made with your hand stays cooler than the highly conductive steel seat buckle.

Figure 7-5. Watch out for hot metal seat buckles!
Radiation and Convection
Heat exchangers, such as car radiators, both radiate and convect. However, convection is the dominant heat transfer mechanism in these cases. Some devices, including infrared heaters, radiant panels, and floors, are intended to heat by radiant heat transfer. While radiation is the dominant heat transfer mode, natural convection cycles occur and contribute to the heat transfer from these devices to the surroundings.
Sometimes heat transfer devices do not even try to get help from radiation. Electrical heat sinks, for example, rely almost entirely on convection to reject the heat of electrical components (see Figures 7-6 and 7-7). In this design, the large flat areas of the heat sink face each other. Because the two surfaces are at the same temperature, there is no temperature differential to drive radiation heat transfer. In reality, the radiation emissions are diffuse, so some of the radiation “escapes” the fin.
Note that fins must be parallel to gravity to take advantage of natural convection. If they are turned horizontal, convective flow and its accompanying heat transfer will be virtually eliminated.
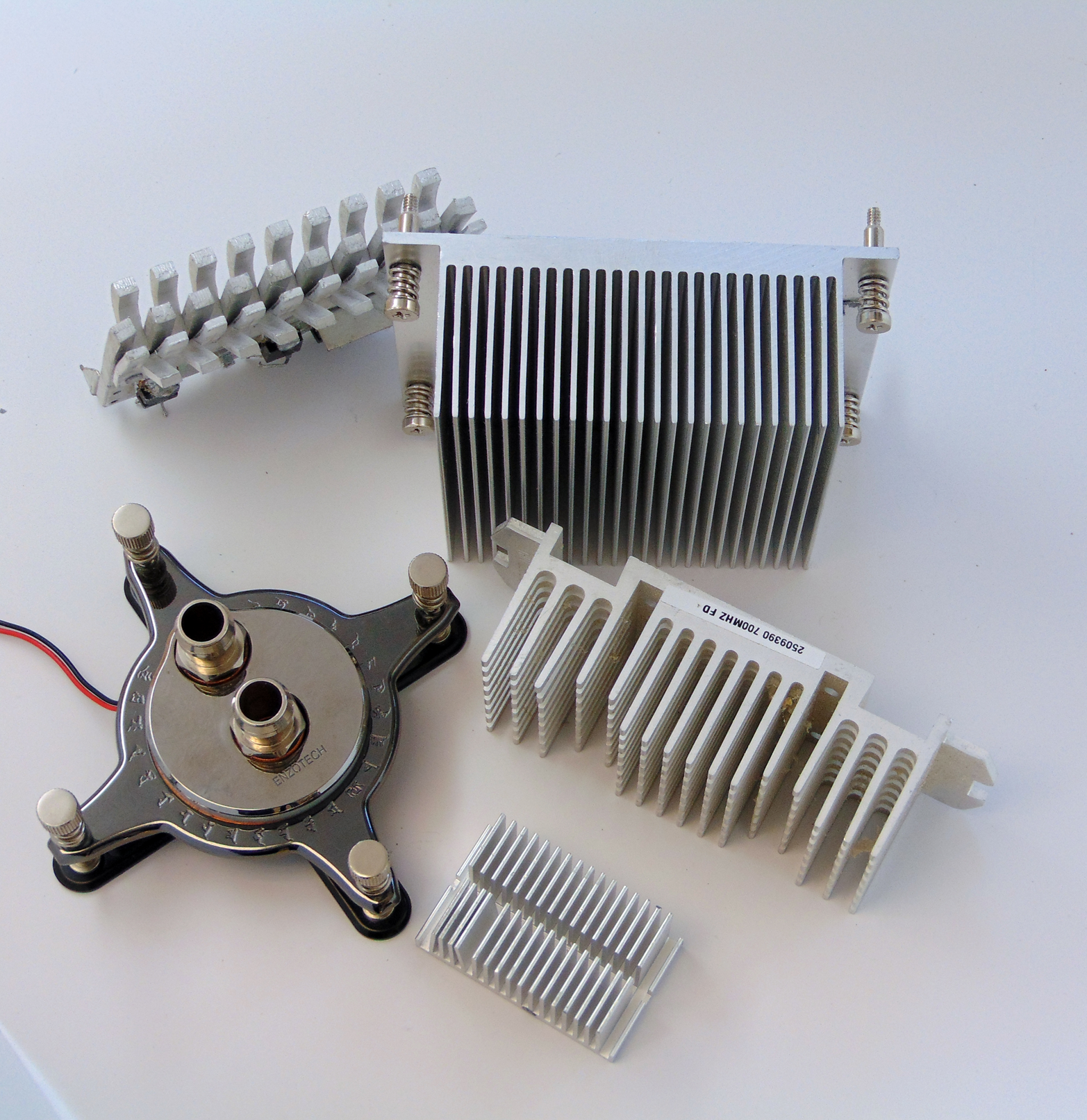
Figure 7-6. Heat sinks that rely on natural convection except the one on the bottom left, which uses a liquid coolant
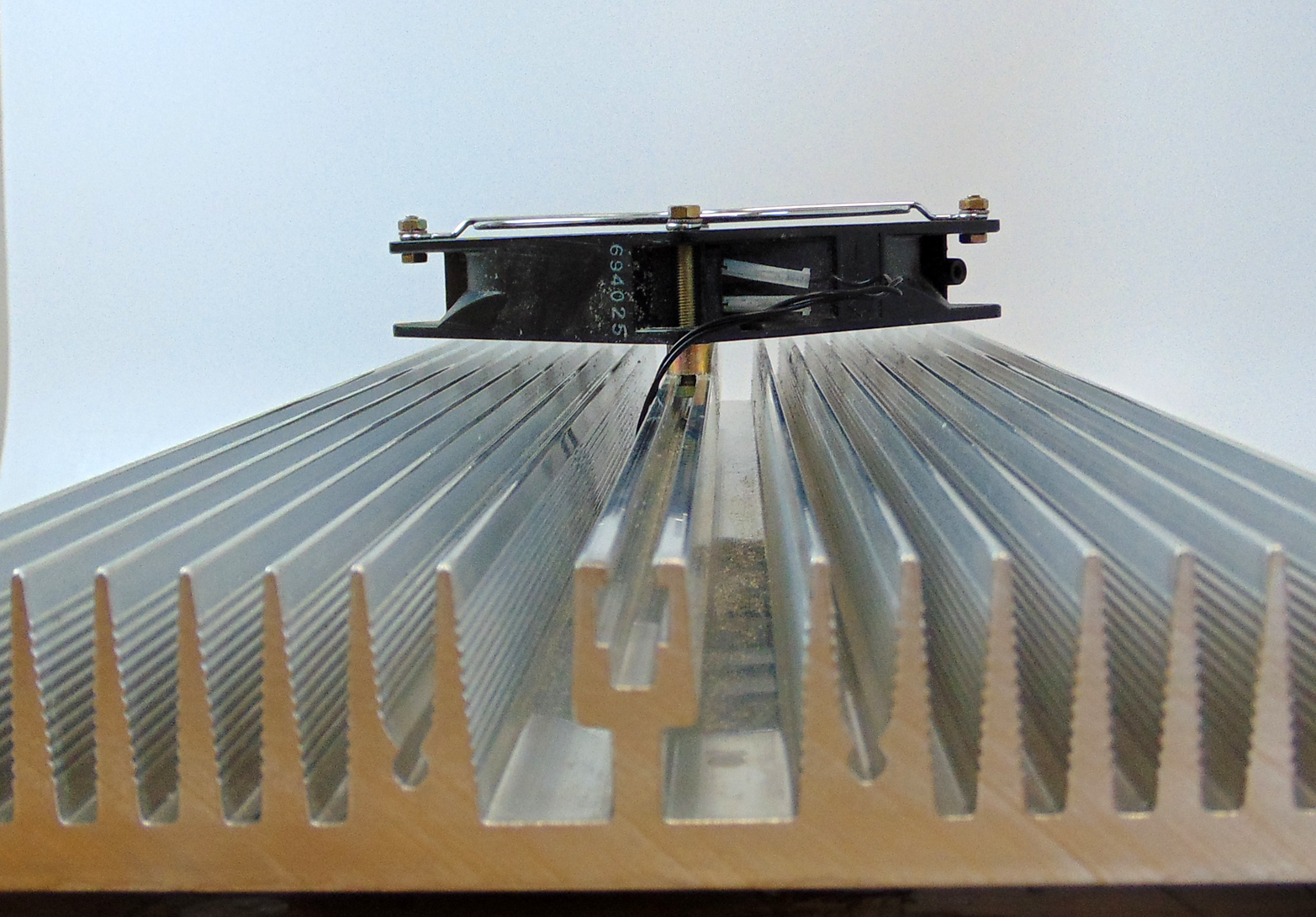
Figure 7-7. This heat exchanger uses a fan to increase convective heat transfer
Other Heat Transfer Considerations
Heat doesn’t just transfer; it can be created by phenomena such as chemical reactions, friction, and physical transfer of heated molecules from one place to another. The physical movement of matter is a common form of heat transfer and can be influenced by design features, just as convection can be encouraged by taking advantage of the buoyancy of heated gases. Heat energy can also be stored in helpful ways. Storing heat is vital for many designs and we will look at the interesting relationship between heat storage and temperature.
Mass Transfer
Mass transfer is an important source of heat loss and gain. This is not an energy transfer in the sense just described, but rather the actual exchange of molecules between spaces. Infiltration, unlike heat transfer that is driven by temperature differences, depends only on pressure differences. Air (or another fluid) is pushed into a new location. Infiltrated heat is not transferred between molecules as with conduction and convection, rather the molecules are actually exchanged. Infiltration is like dumping buckets of cold air into a warm room. Mass transfer describes evaporation also. In the case of evaporation, high-energy liquid water moves into the gas phase taking not only its mass away but also its energy. This is the process that people, dogs, and many other animals use to keep cool.
Cause of Infiltration
The pressure difference that produces infiltration can be created by wind. This happens when a building develops high pressure on the side facing the wind and a low pressure downstream of the wind. The air then enters (infiltrates) through the windward cracks and exits (exfiltrates) through cracks on the downwind (or lee) side. The difference between the air coming in and the air going out establishes the building pressure.
A positive pressure makes doors hard to close but keeps contaminants out. Therefore, positive pressure is used for such applications as clean rooms and radon abatement. A negative pressure will slam doors shut but keeps the air from leaving a certain space. Therefore, negative pressure is used for such applications as kitchens and bathrooms to prevent malodorous air from escaping. Reference Chapter 6 for further exploration of this phenomenon.
In addition to wind, a pressure difference can also be produced by the buoyancy of warm air venting a building. The higher pressure of cold air forces warm air up, then viscosity and inertia entrain all the air in the upward movement called the stack effect. The stack effect increases with building height, as illustrated in Figure 7-8.
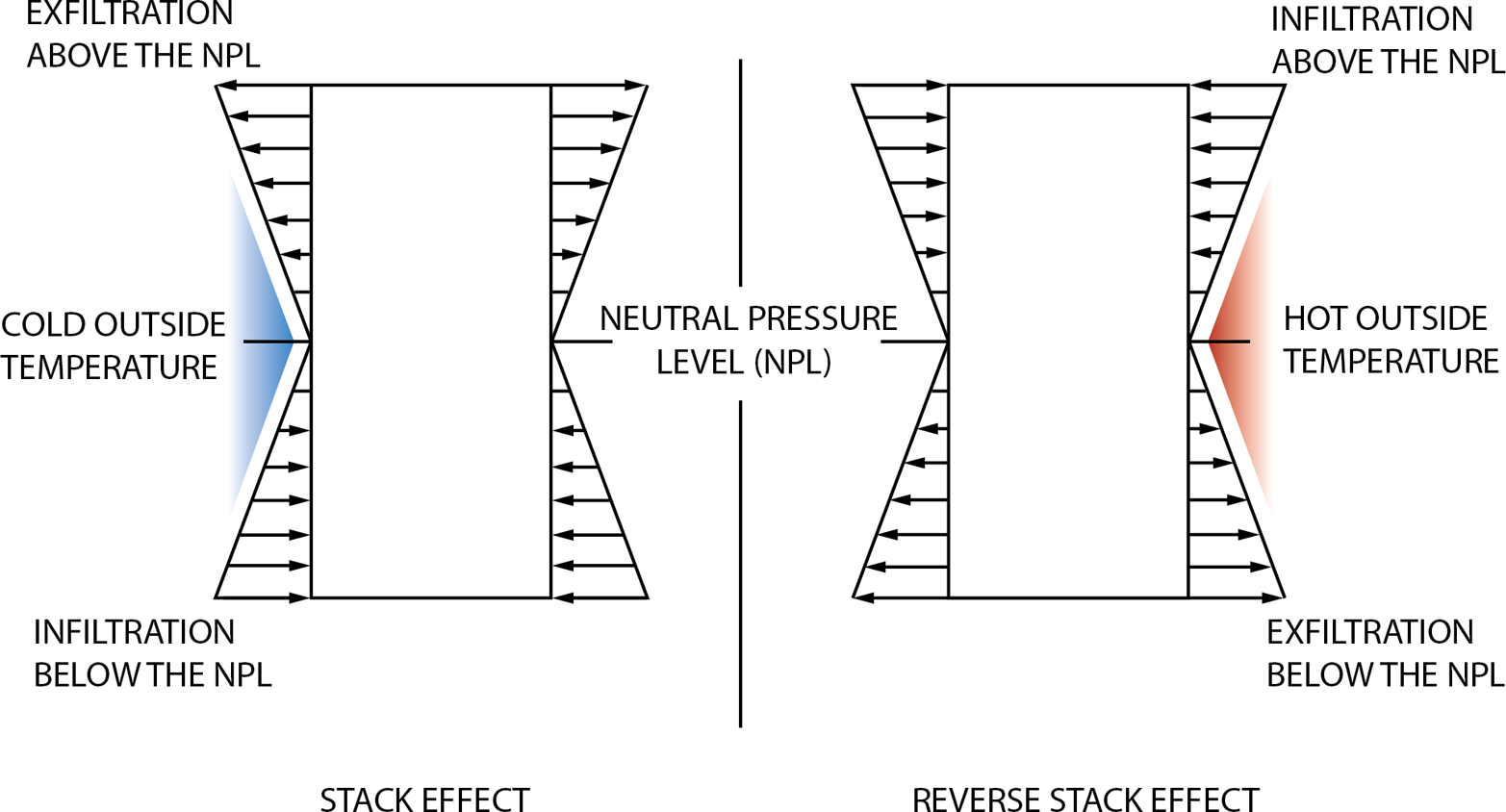
Figure 7-8. Rising warm air changes the pressure in a building so that it can draw air in at the bottom, called the “stack effect” (Image used with permission from ©ASHRAE www.ashrae.org HVAC Design Guide for Tall Commercial Buildings, Chapter 2, 2010.)
Pressure changes can also be produced by outside air. For example, wind blowing on a building will produce a higher pressure that can promote infiltration. An example of pressure change from wind being used advantageously can be seen in termite nests, which have a large central chimney that produces a cool nest, as indicated in Figure 7-9.
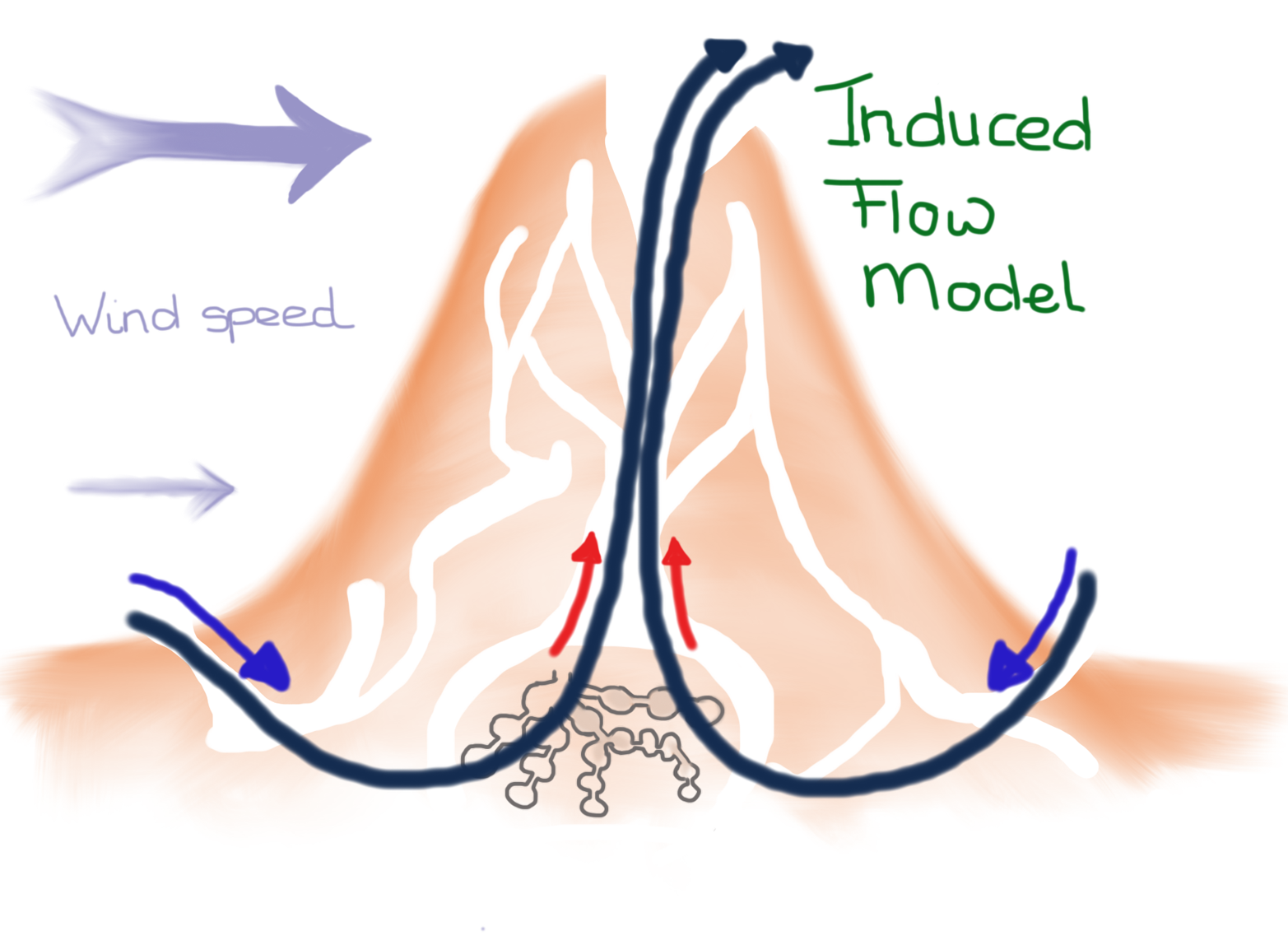
Figure 7-9. Wind blowing over the nest draws air out of the termite nest
Thermal Storage
The energy stored is a function of the specific heat, temperature change, and mass. We had considered specific heat in the earlier discussion of the hot seat belt buckle.
Specific heat is a measure of heat storage per degree temperature change of a material. For example, the specific heat of water is over 10 times that of copper. Therefore, it takes a lot more heat to elevate the temperature of water 1° than it does copper of equal mass. Because the temperature change is also a function of mass, it can take more heat to heat up a huge mass of copper than a small mass of water. Figure 7-10 shows the disconnect and wide range of variability between the specific heat and thermal conductivity.
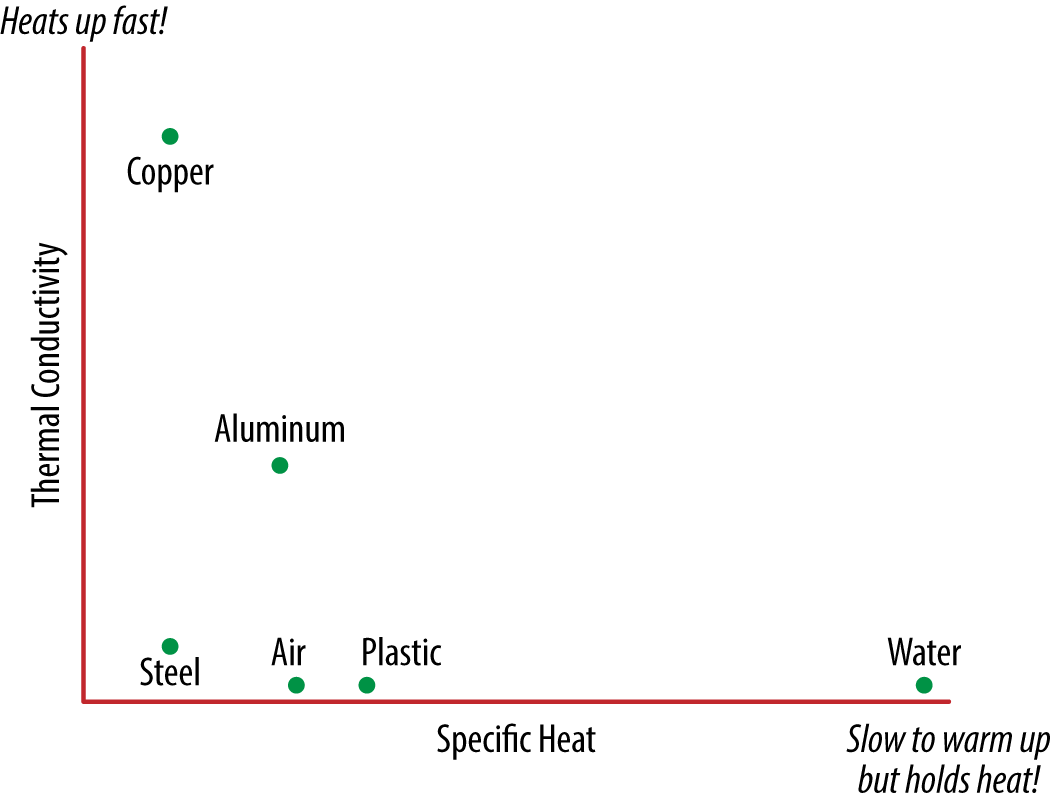
Figure 7-10. Plot of thermal properties for common materials
Internal Sources of Heat
Heat is generated by such things as people and electronics. As discussed previously, heat is produced in two forms: sensible and latent. Sensible heat can be “sensed” and measured with a thermometer, while latent heat is the addition of moisture to the air. Lights, for example, produce only sensible heat, while people produce both sensible and latent heat. Well-insulated commercial buildings, such as offices and stores, have such high internal loads that they are actually self-heating until the temperature falls below around 35°F (2°C).
Closing Thoughts
Heat transfer is one of the more intuitive subjects we have discussed. Heat transmits by contact with a material or via radiation. You know heat can be produced internally by such things as chemical reactions, living organisms, and friction. If you are designing an electrical circuit that will reside inside a cabinet, the best way to get rid of the heat is through a cooling liquid that directly contacts the component within the circuit that is producing the most heat. The transfer is increased by having very cold coolant (giving a high temperature difference), a lot of contact surface area, and thermally conductive materials. If you cannot route a liquid coolant, convection will help. You know good convective cooling needs cool, high-velocity fluid and a lot of surface area. If this is not an option, you know you can rely on a natural convection—that is, taking advantage of the movement of warm air to run across your heat exchanger. However, you know cooling fins need to be oriented up and down so they are aligned with the air flow. If you have to rely on radiation, you remember the hot component has to have something cool to radiate to and requires lots of area as well as a high-emissivity surface finish.
If the frame that holds your automatic pancake flipping invention is getting too hot, you might want to check the rotating surfaces to see if you have a lot of frictional heating. If that doesn’t fix the problem, maybe the device is receiving too much conductive heat from the pan and you need some thermally insulating materials. Perhaps your frame is getting too much radiant heat from the stove and you need to reduce the exposed area and paint it a shiny, non-absorptive color.
