9
Industrial Waste Minimization Methodology: Industrial Ecology, Eco‐Industrial Park and Manufacturing Process Intensification and Integration
9.1 Introduction
In the United States, roughly 12 billion T of nonhazardous waste is generated and disposed of by the US industries. That amount is about is over 200 lb of industrial waste per person per day. The largest industrial contributors to nonhazardous waste are manufacturing industries (~7600 million T/Y), oil and gas production (2100–3600 million T/Y), and the mining industry (>1400 million T/Y). Contributors of lower amounts are electricity generators (fly ash and flue‐gas desulfurization waste), construction waste, hospital infectious waste, and waste tires. The rate of industrial hazardous waste generation in the United States is approximately 750 million T/Y (Allen and Shonnard 2012; Allen and Rosselot 1997). Once these materials are designated as hazardous, the costs of managing, treating, storing, and disposing of it increase dramatically. This chapter describes some specific industrial waste minimization processes and technologies that have been successfully operating and provides other methodologies, including industrial ecology, eco‐industrial park (EIP), manufacturing process intensification, and integration. The wastes (in air, water, or as solid) or by‐products generated during manufacturing process are recovered. The materials and energy recovered from waste streams either are reused in the plant or are sold to another plant as feedstock. It is in practice, as well as in theory, possible to isolate some industrial facilities almost completely from the environment by recycling all wastes into materials that can then be manufactured into consumer products. An example of such a facility is a coal‐fired power plant. An electron beam–ammonia conversion unit adds ammonia to the effluent gases, which is then irradiated electronically, producing ammonium nitrate and ammonium sulfate that are sold as feedstock to fertilizer manufacturing; enhanced recovery of mercury from flue gas by adsorption and mercury recovery from a coal‐fired plant. The details of these two processes are given as case studies later in this chapter. Also, two separate case studies have been presented that highlight a profitable industrial “by‐product‐to‐energy” recovery generating electricity and heat, and making chemicals and energy from gasification of black liquor, as by‐product of pulping process.
Our goal is to modify industrial processes so that services and manufactured goods can be produced without waste. But it is important to understand that some manufacturing processes inherently produce wastes, even after all reasonable efforts at pollution prevention. Thus, in some cases, the use of a conversion technology may be more appropriate than a program of pollution prevention: many industrial wastes can be processed to render them viable as material inputs to another industry or to part of an industrial cluster of several connected industries – as part of the movement of “industrial ecology.”
9.2 Industrial Ecology
Industrial ecology (IE) is the study of material and energy flows through industrial systems. The global industrial economy can be modeled as a network of industrial processes that extract resources from the Earth and transform those resources into commodities which can be bought and sold to meet the needs of humanity. IE seeks to quantify the material flows and document the industrial processes that make modern society function. Industrial ecologists are often concerned with the impacts that industrial activities have on the environment, with use of the planet's supply of natural resources, and with problems of waste disposal. IE is a growing multidisciplinary field of research which combines aspects of engineering, economics, sociology, toxicology, and the natural sciences (Allenby 2006; Ashton 2009; Jensen 2011).
IE provides the theoretical scientific basis upon which understanding, and reasoned improvement, of current practices can be based. It incorporates, among other things, research involving energy supply and use, new materials, new technologies, basic sciences, economics, law, management, and social sciences. It encompasses concurrent engineering, design for the environment (DFE), dematerialization, process pollution prevention, waste conversion, waste exchange, by‐product utilization to a new product, waste minimization, and recycling, with Zero Emissions as an important subset. IE can also be a policy tool. It is a view of a system in which one seeks to optimize the total materials cycle from virgin material to finished material, to component, to product, to obsolete product, and to ultimate disposal. Factors to be optimized include resources, energy, and capital.
IE is a dynamic, systems‐based framework that enables management of human activity on a sustainable basis by minimizing energy and materials usage, ensuring acceptable quality of life for people, minimizing ecological impacts of human activity to levels natural systems can sustain, and maintaining economic viability of systems for industry, trade, and commerce. The IE approach involves (i) application of science to industrial systems, (ii) defining the system boundary to incorporate the natural world, and (iii) seeking to optimize that system. In this context, industrial systems apply not just to private sector manufacturing and service but also to government operations, including provision of infrastructure.
IE is a framework for designing and operating industries as living systems interdependent with natural systems, and therefore it is essential to grasp the concept of industrial metabolism. Whereas the biosphere's metabolism is a near‐perfect recycling system because so many of its components are capable of biological regeneration, in industry, metabolism depends largely on the combustion of fossil fuels that are not regenerated within the system.
Thus, students of industrial metabolism investigate the mass flows of key industrial materials of environmental significance and their associated emissions. With this information in hand, industrial ecologists are better able to balance environmental concerns and economic performance, factoring in the real value of nonrenewable resources and the real costs of environmental pollution. These efforts are enhanced by improvements in understanding of local and global ecological constraints.
IE supports coordination of design over the life cycle of products and processes. IE enables creation of short‐term innovations in a long‐term context. While much of the initial work in IE has focused on manufacturing, a full definition of industrial systems includes service, agricultural, manufacturing, military, and other public operations, as well as infrastructure such as landfills, water and sewage systems, and transportation systems.
The concept of an “industrial ecosystem” received wide attention when Scientific American published an article by two General Motors researchers who suggested that the days of finding an “open space beyond the village gates” for the by‐products of industrial activity were quickly fading (Frosch and Gallopoulos 1989). The concept of IE has spawned an ever increasing amount of research and activities. At the most basic level, IE describes a system where one industry's wastes (outputs) become another's raw materials (inputs). Within this “closed loop, illustrated in Figure 9.1,” fewer materials would be wasted. Thus, if businesses were able to turn waste into food, they could sharply reduce pollution and the need for raw materials (Van der Ryn and Cowan 1996).
Many industries have long had symbiotic relationships where wastes and materials are transformed internally or by others. For example, metal industries use scrap materials in the production process; the advent of the electric arc furnace (EAF) increased the ability of steel manufacturers to use scrap materials. Petrochemical and chemical companies are adept at finding new production uses or markets for waste materials (Richards et al. 1994). The growth in rubber, plastics, paper, and glass recycling has generated new uses for previously discarded materials. As Ernest Lowe suggests, IE is a broad holistic framework for guiding the transformation of the industrial systems. The shift from the linear model (mine pit to producer, to consumer, to dump) to a closed‐loop model, more closely resembling the cyclical flows of ecosystems, has stimulated new ways of thinking in forward‐looking companies, in a number of universities, and in governmental agencies like the Environmental Protection Agency and the Department of Energy in the United States (Low and Warren 1996; Lowe 1995, 2001).
9.2.1 What Is EIP?
According to Ernest Lowe, John Warren, Andreas Hein, and others, an EIP is a community of manufacturing and service businesses seeking enhanced environmental and economic performance through collaboration in managing environmental and resource issues, including energy, water, and materials (Lowe and Warren 1996). By working together, the community of businesses seeks a collective benefit that is greater than the sum of the individual benefits each company would realize if it optimized its individual performance only. The goal of an EIP is to improve economic performance of the participating companies while minimizing their environmental impact.
Industrial activity releases wastes into local, regional, and eventually global ecosystems. Since the 1970s, myriad laws and regulations have been promulgated to limit emissions to air and water, and to regulate solid and hazardous waste disposal. Further, industrial activity results in nonpoint‐source pollution caused by general runoff, spills, or illegal dumping. Business and the environment have traditionally been considered natural enemies. The assumption has long been “more environmental protection corresponds to higher costs for business”; however, new developments in research and business operations are challenging this assumption. Many companies such as 3 M have long realized the economic benefits of applying environmental principles to business operations. The goal of an EIP is to minimize the ecological impact of industrial activity and to improve business performance. In the United States and Canada, several projects are testing this idea, which has already demonstrated its effectiveness in Europe, most spectacularly perhaps, in Kalundborg, Denmark, as discussed by Hein et al. (2015); EIO 2012; Lowe (2001), and others.
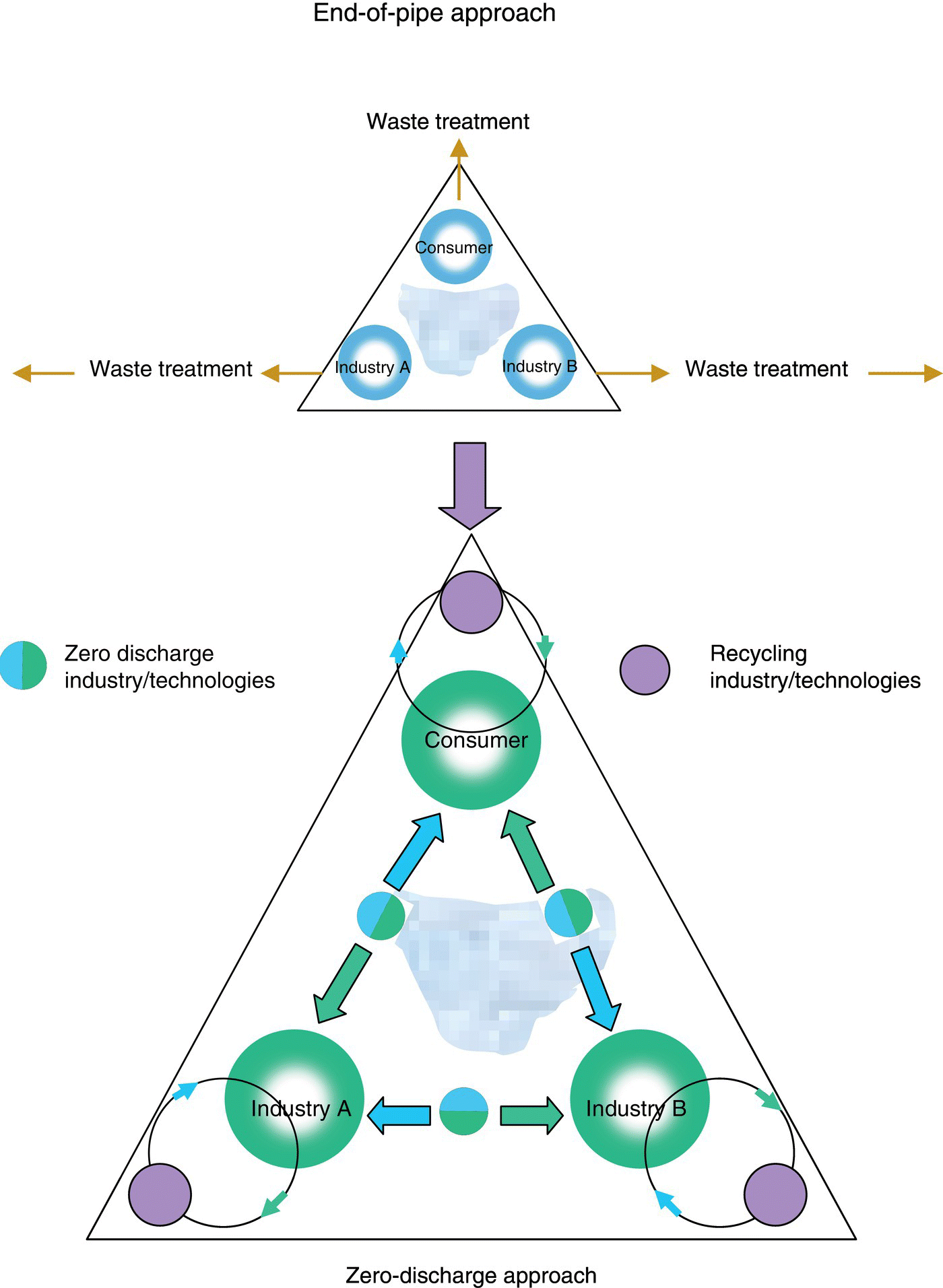
Figure 9.1 Schematic diagram of end‐of‐pipe and Zero‐Emission approaches.
Source: From Ebara Corporation, Tokyo, Japan (2010) and Das (2005).
An EIP is a set of businesses that share resources in order to increase profitability and reduce environmental impact. The implementation of EIPs may significantly contribute to the creation of a sustainable economy. Despite this prospect, the actual development of EIPs is challenging, as a variety of factors must be considered. Not only technical, economic, and environmental factors are relevant but numerous stakeholder relationships as well, such as between firms, governmental bodies, and local communities. Hein et al. (2015) presents a conceptual framework that is used to capture these diverse aspects and the relationships between them. The Unified Modeling Language is used for modeling its concepts and relationships. First, based on a literature survey, relevant concepts of EIPs are identified. One central concept is “industrial symbiosis.” A novel value‐based interpretation of industrial symbiosis is presented. Second, the park's economic, local, and regional development context, as well as its internal technical components and their relationships, are modeled. Finally, the framework is used for modeling a concrete EIP, in this case part of the Kalundborg EIP (Hein et al. 2015).
9.2.2 EIP Development
EIP development (EIPD) is a new paradigm for achieving excellence in business and environmental performance. It opens up innovative new avenues for managing businesses and conducting economic development. By creating linkages among local “resources,” including businesses, nonprofit groups, governments, unions, and educational institutions, communities can creatively foster dynamic and responsible growth. Antiquated business strategies, based on isolated enterprises, are no longer responsive enough to market, environmental, and community requirements.
Economic development is a never‐ending challenge for communities. As the global marketplace has become increasingly competitive, municipalities, counties, states, and regions seek new strategies for attracting good investments with good jobs. Moreover, communities everywhere are demanding improvements in local ecosystems. In the past, economic development and environmental protection were seen as mutually exclusive. However, new practices and activities are challenging that assumption. One broad category of activities falls under the umbrella of EIPD.
Sustainable EIPD looks systematically at development, business, and the environment attempting to stretch the boundaries of current practice. On one level, it is as directly practical as making the right connections between wastes and resources needed for production. At another level, it is a whole new way of thinking about doing business and interacting with communities. The eco‐industrial approach has many ways of being applied. At a most basic level, each organization seeks higher performance internally. However, most eco‐industrial activity is moving ahead by increasing interconnections between companies.
Just as in nature interconnected systems work together to ensure survivability and efficient use of resources and energy, in the business world, strategic partnerships, networked manufacturing, and preferred supplier arrangements assist companies to grow, to contain costs, and to reach for new opportunities. Eco‐industrial development can help to achieve these goals by offering businesses access to cost‐effective, quality resources for producing products or delivering services.
The vital task of securing community support for eco‐industrial projects calls for multi‐stakeholder engagement in setting a vision for local development. Companies that offer attractive models in their community outreach will have an edge in obtaining popular support. Attention to getting wide backing for a project in the early planning stages can reduce delays due to the reflexive community opposition that often crops up when a major change is proposed. A positive and proactive stance in anticipating and overcoming residents' concerns can be most helpful.
The three mini‐case studies presented in Section 9.2.3 illustrate the range of results that are possible when an attempt is made to husband the resources and improve the environment of communities dominated by a single industry.
9.2.3 EIPs – The Ebara Process: Mini Case Study 9.1 in Japan
The first fertilizer plant using the Ebara process using SO2 and NOx gases from a nearby coal‐fired power station was developed and marketed by the Tokyo‐based Ebara International Corporation (Das 2005; Ebara 2010). Electron beam irradiation of a flue gas containing both SO2 and NOx (about 95% NO and 5% NO2) acts on these pollutants by oxidizing them to nitrogen dioxide and sulfur trioxide anions. Addition of water and ammonia to these ionized gases yields solid products, ammonium nitrate (NH4NO3), and ammonium sulfate ((NH4)2SO4) that can be separated and sold as fertilizers. The simplified chemical reactions are as follows:
The main attraction of the Ebara process (Figure 9.2) is the simultaneous removal of both SO2 and NOx. The complex reaction mechanism entails ionization, the formation of excited electronic states, the transfer of excitation energy between molecules, molecular dissociation, electron capture, neutralization, and radical reactions. The flue gas is first cooled and humidified, then irradiated by means of a high‐intensity electron beam. The flue gas is routed first through electrostatic precipitators (ESPs) or other mechanical collectors to remove particulates, such as fly ash. The gas is next piped to a spray cooler and humidified and cooled to a temperature in the 160–200 °F range, the optimal temperature. Anhydrous ammonia is introduced in stoichiometric amounts at this point. The ammoniated flue gas is passed to an electron‐beam reactor chamber, where it is irradiated (1.5 Mrad dose), producing nitrate and sulfate anions. These species react with ammonia to yield a dry, particulate mixture of NH4NO3 and (NH4)2SO4.

Figure 9.2 Schematic diagram of the electron beam–ammonia conversion of SO2 and NOx to fertilizer (Das 2005).
9.2.3.1 Advantages and Disadvantages
There are at least five advantages of the electron beam process:
- SO2 and NOx are removed together in the same piece of equipment by the addition of one chemical. The method can meet all New Source Performance Standards (NSPS) for SO2 removal because it has the potential to remove 98% of all SO2 and 70–90% NOx depending on the process condition (CFR‐40 1999). High SO2 removal can be achieved with very low power consumption (1% of the block). Most of the energy is consumed for NOx reduction. NSPS standards are complex, but in the case of 3–6% sulfur coal, a 90% reduction is required.
- Volatile organic compounds are removed in the process as well.
- Since no waste is produced, there is no need to devise a means of disposal.
- Since the process produces a dry product, high maintenance costs associated with abrasive and corrosive slurries are avoided.
- A salable product results.
Among the corresponding disadvantages and challenges associated with this process is the need to keep ammonia injection close to the stoichiometric point to alleviate any ammonia slip. In addition, large electron‐beam generators are needed for a large‐scale plant because the current electron beam has a limited range. Technical concerns about this process include questions about the ability to scale up the electron accelerators for a full‐scale application and the associated power requirements for the technology. To accelerate radiation, a combined microwave‐electron‐beam process has been developed and patented, and this hybrid radiation system will be suitable for a large‐scale plant (Das 2005; Ebara 2010).
9.2.3.2 Cost Analysis
Table 9.1 presents an annualized cost analysis, including a projected overall return on investment for the installation and operation of a system of E‐beam–ammonia conversion of SO2 and NOx. The cost analysis shows that the coal‐fired power‐generating industry can earn about $11 million/year from this process. The system itself is much cheaper than conventional flue‐gas treatments – with 25% less construction costs and 20% less running costs – and requires considerably less space.
Table 9.1 Return on investment calculations: electron beam–ammonia SO2 and NOx conversion.
Source: From Ebara Corporation (2010).
| Sample calculation | Total profit/cost ($ million) |
| Profit from product sales Fertilizer: 110 000 T/Y (400 T/day) × $100/T |
11/year profit |
| Operating cost Personnel expenses, etc. Ammonia: 29 000 T/Y (105 T/day) × $130/T |
−0.9/year −3.8/year −4.7/year cost |
| Facility construction cost | −38.1 cost |
At an annual interest rate of 12%, facility construction cost would have been paid back within 15 years.
But there are caveats in all such calculations. For starters, the Ebara process would be sensitive to the cost of anhydrous ammonia. To control this variable, research is being conducted to discover a way to extract ammonia from municipal wastewater (Ebara 2010). If ammonia were available from a nearby municipal wastewater treatment plant, it would be even more economical to run this process. In any event, the fertilizer‐producing variant of the Ebara process would be economically beneficial only in locations well sited with respect to markets for the fertilizer materials produced as by‐products, and variability in capital and electricity costs.
9.2.4 Mini‐Case Study 9.2: Seshasayee Paper and Board Ltd. in India
As a success example, consider one where a corporate group devised and adopted a growth strategy whereby a majority of the wastes from one activity were converted to feedstock for another activity.
Seshasayee's original enterprise was a paper mill. To ensure a regular supply of raw material, the company then set up a sugar mill. Bagasse, a waste from the sugar mill, was used as a raw material for papermaking. Another waste product, molasses, was used in a distillery for the production of ethyl alcohol. To guarantee a regular supply of sugarcane, the company took interest in the cultivation of this crop by organizing the farmers in the region. Seshasayee struck long‐term agreements with the farmers to buy back their produce and, in turn, took the responsibility of supplying them with water. Part of the water for cultivation was treated wastewater from the paper manufacturing operations. The company also used bagasse pith (a waste after the papermaking) and other combustible agricultural wastes in the region as energy sources. The agro industrial eco‐complex is illustrated in Figure 9.3.
This is particularly important to the theme of a process integration and intensification work because it involves the production of a good (paper) and a human food (sugar), and the sustainable generation of energy (from bagasse – e.g. waste‐to‐energy) and sustainable use of water. In this case, the discussion of the mini‐case study shows that it is possible to combine the production of a food and the generation of energy with the efficient use of water resources. This is important because the production of a commercial food (sugar) is economically and socially important, especially if it can be produced as sustainably as possible.

Figure 9.3 An agroindustrial eco‐complex.
9.2.5 Mini‐Case Study 9.3: Materials and Energy Flow in an EIP in North Texas, USA
Consider, for example, an EIP in North Texas where the central facility is a steel mill. This facility, shown conceptually in Figure 9.4, utilizes scrap cars as the primary feed material. The steel from the vehicles goes to an electric arc furnace (EAF), producing a variety of steel products. The furnace also produces a significant quantity of EAF dust, which contains significant quantities of zinc, lead, and other metals. In the North Texas facility, the EAF dust is sent to a cement kiln where the trace metals (copper, chromium, manganese, nickel, lead, and other metals) have values. Automobile shredder residue (ASR) can be burned for energy recovery, or some of the plastics in the residue can be separated.
Another alternative for EAF dust, currently being exploited in Europe, is as a feed for zinc and lead recovery operations. The recovered zinc can then be used in producing galvanized steel products and batteries can be used as an alternative source of zinc.
These case studies illustrate the basic principle of EIP – integrating flows of materials, energy, and water in diverse industrial operations, increasing mass and energy efficiency. The three case studies examined in this section involved exchanges between facilities that are located adjacent to each other; however, co‐location of facilities in an EIP is not always necessary.
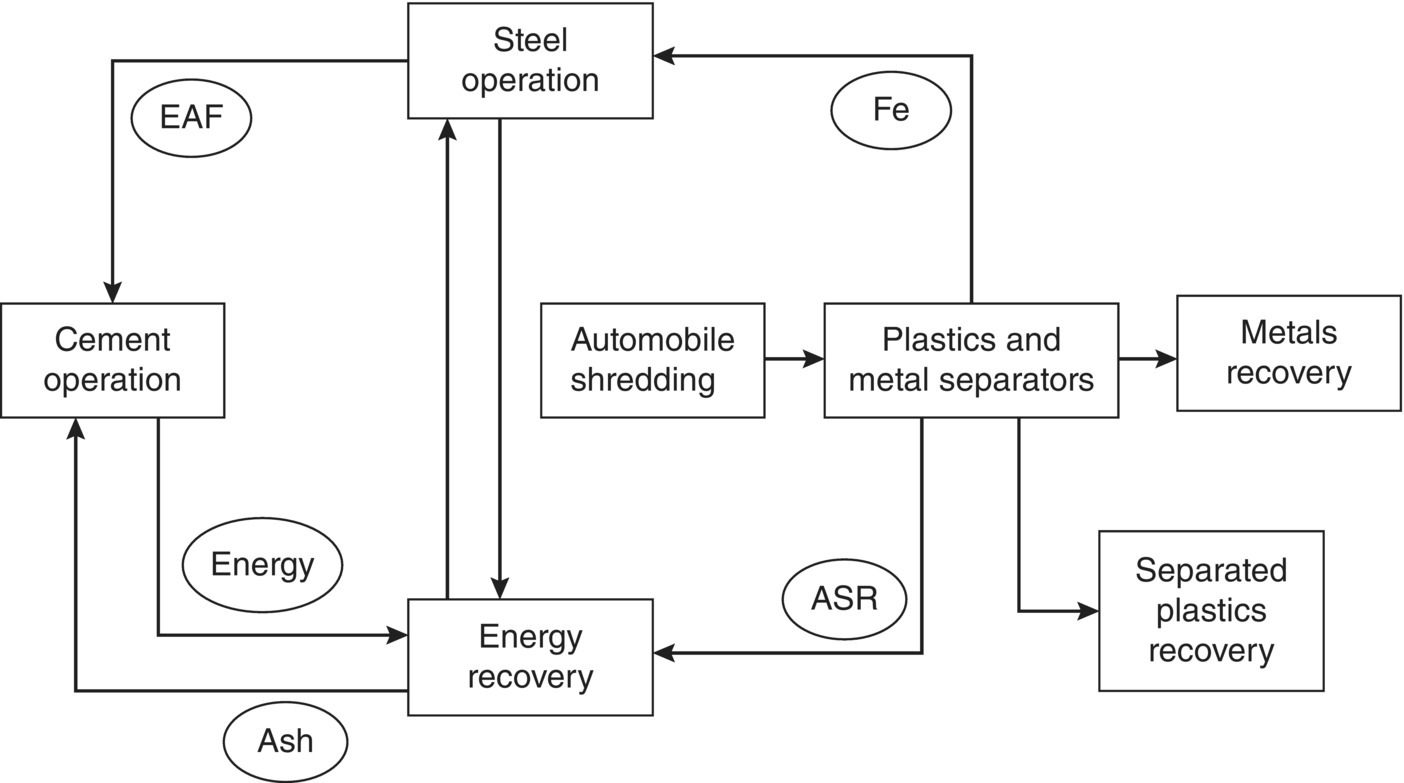
Figure 9.4 Materials and energy flow in an EIP in North Texas, USA.
9.2.6 Mini‐Case Study 9.4: EIP Including Numerous Symbiotic Factories for Manufacturing Very Large Scale Photovoltaic System
The global climate destabilization underway is primarily due to human combustion of fossil fuels for energy and the resultant greenhouse gas (GHG) emissions (IPCC 2007). There is a large consensus among scientists that if current trends continue in climate destabilization, the earth will reach a point of no return. The challenge of reducing atmospheric GHG like carbon dioxide (CO2) emissions is significant – with the Kyoto Protocol and the recent Paris Agreement, an entire order of magnitude below those values necessary to stabilize the global climate. To obtain a stable climate, GHG emissions would have to be reduced to a point equal to the natural absorption of CO2. After this stabilization occurs, the level of natural absorption will gradually fall as the vegetation sink is exhausted so GHG emissions would need to fall to the level of ocean uptake alone. This level is not well quantified, but it may demand emissions reductions to 5 Gt CO2 equiv/year (more than 80% below current levels) by the second half of the next century (Hansen and Sato 2004; Prentice et al. 2001).
In order to increase both the economic and environmental performance of the manufacturing sector, the government could introduce a second set of policies with the large‐scale PV (photovoltaic) manufacturing in order to facilitate the widespread adoption of industrial symbiosis. In industrial symbiosis, traditionally separate industries are considered collectively to gain competitive advantage by instituting the mutually beneficial physical exchange of materials, energy, water, and/or by‐products. Such a system collectively optimizes material and energy use at efficiencies beyond those achievable by any individual process alone. The key benefits of industrial symbiosis are collaboration and the synergies offered by geographic proximity (Chertow 2000). Industrial symbiotic systems such as the now‐classic network of companies in Kalundborg, Denmark, have spontaneously evolved from a series of micro‐innovations over a long time scale. In order to accelerate the process and demonstrate the possibilities for mutually beneficial collaboration, the government could engineer the design of the new solar PV manufacturing plant using industrial symbiosis.
The multi‐GW PV factory could sit at the center of a next generation EIP. The EIP would be made up of at least eight symbiotic factories as seen in Figure 9.5. These could be located outside of a major population center to provide raw materials, labor, and a ready market. The first factory would be a conventional recycling facility (1). In this way, the glass and aluminum needed to fabricate the solar cell could be recovered from recycled materials and thus have a lower embodied energy (95% lower for aluminum and 20% for glass) (Milne and Readon 2005).
The raw glass from the recycling plant will be fed to a sheet glass factory (2) and melted using natural gas. Generally, the high‐quality requirements of flat glass prohibit the use of post‐consumer waste glass. However, the glass industry is exploring a method to eliminate problems with color contamination by using thin plastic coatings, which can be made with a variety of colors and that would vaporize during remelting without affecting the quality of the new glass. This would benefit the industry considerably because using recycled glass, called cullet, has several important benefits that include (i) lowering the consumption of raw materials, (ii) reducing the release of CO2 formed in the chemical reaction of raw materials, (iii) increasing the life of the furnace by up to 30% due to lower melting temperatures, (iv) reducing energy use during the melting stage of production and thus reducing additional GHGs and operating costs, and (v) reducing the costs associated with pollution abatement due to lower emissions of NOx, SO2, and particulates (Pearce 2008). The factory will output cut sheets of 3 mm thick glass with seamed edges and low‐iron content in order to obtain a high‐solar transparency. Finally, the glass will be tempered for mechanical strength and coated with a transparent conductor such as tin oxide, zinc oxide, or indium tin oxide to be used as the substrates.
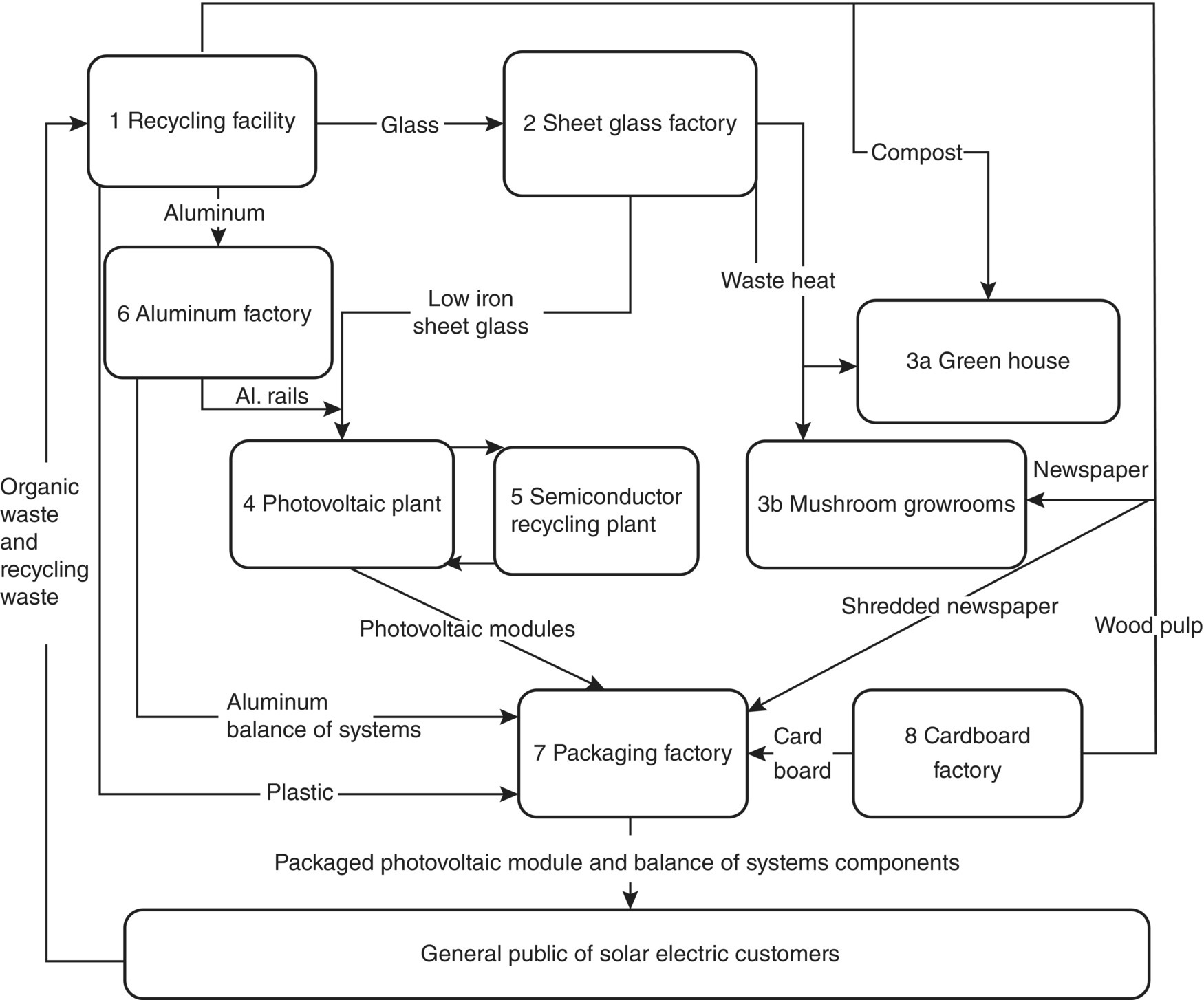
Figure 9.5 Schematic diagram of an EIP to produce giga‐watt solar PV.
Source: From Pearce (2008).
The production stages in the glass factory that utilize large amounts of heat will have integrated thermal recovery to provide lower grade heat for the other facilities and a greenhouse complex (3a). In the greenhouse complex, exotic plants can be grown year round in even northern climates utilizing the waste heat from the manufacturing plants in the EIP. Similarly, waste heat could be utilized to provide grow rooms for mushrooms (3b). In both agricultural plants, the food or other agricultural products will be sold outside of the park and the growing medium will be provided by the recycling facility (1), compost for the greenhouse (3a), and wood pulp or compost for the mushroom‐growing facility (3b). In warmer climates, the waste heat could be used to drive absorption chillers perhaps providing cooling for an office park.
The substrates will then be fed directly into the PV module plant (4). Then a group of semiconductor and metal thin‐film deposition systems will create and pattern the active layers of the solar cells. All waste semiconductors and metals will be captured and returned to a semiconductor recycling plant (5) to supplement the incoming and generally expensive high‐purity materials going into the deposition systems. The output of the PV deposition and patterning lines will be PV solar panels ready for protective coatings and packaging.
The aluminum extracted from common drinking cans in the recycling center (1) will be fed to an aluminum fabrication factory (6) that will produce coated aluminum rails for holding the glass solar panels. The aluminum rails will be extruded and used to provide a simple and inexpensive means of attachment to rooftops, ground mounted systems, or building integrated PV. In addition, the extruded aluminum rails could be designed into ground and flat roof mounting balance of system components. Similar to the glass manufacturing plant (2), waste heat will be recovered and used in the symbiotic collective or to heat the greenhouse (3a) or mushroom grow rooms (3b).
Next, in the packaging factory (7), the solar panels are interconnected if necessary and are sprayed with a protective polymer coating to seal them to the environment. The primary constituents of the polymer coating could again be acquired from the recycling plant (1) and common plastics. The panels would then be wired with quick connects so they can be easily installed in the field by connecting to each other an inverter or battery bank. Finally, the panels would be packaged for shipment to prevent damage in cardboard boxes and cushioned with shredded newspaper. The newspapers would again come from the recycling plant (1) and the cardboard could come from a cardboard plant (8), which would gain its raw materials from the recycling factory (1).
By co‐locating these factories in the EIP, the transportation costs and energy between them can be minimized and many of the inputs for the solar PV plant can literally come from waste products in the surrounding population centers. It should be noted that each factory will be scaled appropriately for the symbiotic system and should be individually profitable so that independent businesses can replicate this model by co‐locating and benefit from industrial symbiosis in future facilities.
9.3 Water–Energy Nexus
Present day water and energy systems are tightly intertwined. Water is used in all phases of energy production and electricity generation. Energy is required to extract, convey, and deliver water of appropriate quality for diverse human uses, and then again to treat wastewaters prior to their return to the environment. Historically, interactions between energy and water have been considered on a regional or technology‐by‐technology basis. At the national and international levels, energy and water systems have been developed, managed, and regulated independently. Water and energy are critical, mutually dependent resources – the production of energy requires large volumes of water and water infrastructure requires large amounts of energy (Das 2017; Das and Cabezas 2018; EPRI 2011; USDOE 2014a, 2013a, b).
Water is required to generate energy: Thermoelectric cooling, hydropower, energy mineral extraction and mining, fuel production (including fossil fuels, biofuels, and other nonconventional fuels), and emission controls all rely on large amounts of water. In the United States, the thermoelectric generating industry is the largest withdrawal user of water. According to USGS (United State Geological Survey), 349 billion gallons of freshwater were withdrawn per day in the United States in the year 2005. The largest use, thermoelectric, accounted for 41% of freshwater withdrawn at 143 billion gal/day. However, freshwater consumption for thermoelectric purposes is low (only 3%) when compared to other use categories such as irrigation, which was responsible for 81% of water consumed.
- Water withdrawal: The total volume removed from a water source such as a lake or river. Often, a large portion of this water is returned to the source and is available to be used again.
- Water consumption: The amount of water removed for use and not returned to its source.
Water supply also requires energy use: A large amount of energy is needed to extract, convey, treat, and deliver potable water. Additionally, energy is required to collect, treat, and dispose of wastewater. In 2010, the US water system consumed over 600 billion kWh, or approximately 12.6% of the nation's energy according to a study by researchers at the University of Texas at Austin. The study found water systems use about 25% more energy than is used for residential or commercial lighting in the United States.
Water and energy are both multifaceted issues with many variables impacting their supply, demand, and management. Lawmakers should consider the following variables which add complexity to the management of water and energy:
- Growing population: According to a 2012 United States Census Bureau projection, the US population could reach 400 million people by 2051. Population growth affects energy use through increases in housing, commercial floor space, transportation, and economic activity. The US Energy Information Administration (EIA) estimates that total electricity consumption will grow from 3841 billion kWh in 2011 to 4930 billion kWh in 2040, an average annual rate of 0.9%. With a higher generating capacity, the United States will require additional water withdrawals.
- Agriculture: Feeding a growing population may require greater agricultural water use. Agriculture accounts for approximately 37% of total freshwater withdrawals in the United States, and 81% of water consumption.
- Geographical water demand: Water supply and demand are not geographically linked. From 1990 to 2010, the second largest regional population growth, 13.8%, occurred in the west, which is one of the most water‐deficient regions in the United States. Additionally, water consumption in the western United States is much higher than other regions due to agricultural demands. It is estimated that it takes over one million gallons of water a year to irrigate one acre of farmland in arid conditions. In other words about 86% of irrigation water withdrawals were in western states in 2000.
- Climate change: Climate change could affect water supply and electricity use. Warmer or colder weather patterns could result in increases or decreases in energy use. Changes in precipitation in a region could increase or decrease the ability to store water, agricultural production and water use, and overall water supply.
States are beginning to assess their energy options and promote policies that allocate financial support to a diverse range of technologies to encourage responsible, sustainable energy production. States are also becoming aware of the limitations to accessible water, and as our energy demands grow, competition for water among municipalities, farmers, industrial, and power suppliers will increase. Water and energy are linked at both the supply side (electric generation and water/wastewater facilities) and the end‐use side (residential, commercial, industrial, and agriculture sectors) (Figure 9.6). In order to sustain energy production and a dependable water supply, the United States must gain a detailed understanding of the interdependencies of water and energy systems and balance the needs of all users. State lawmakers and constituents will be critical in this process given their responsibility formulating policy, convening stakeholders, facilitating negotiations, and ratifying reached agreements.

Figure 9.6 Examples of interrelationships between water and energy. Source: Adopted from USDOE (2014b).
Flows of energy and water are intrinsically interconnected, in large part due to the characteristics and properties of water that make it so useful for producing energy and the energy requirements to treat and distribute water for human use. This interconnectivity is illustrated in the Sankey diagram in Figure 9.7, which captures the magnitude of energy and water flows in the United States on a national scale. As shown in the diagram, thermoelectric power generation withdraws large quantities of water for cooling and dissipates tremendous quantities of primary energy due to inefficiencies in converting thermal energy to electricity. The intensity of water use and energy dissipated varies with generation and cooling technology.
As the largest single consumer of water, agriculture competes directly with the energy sector for water resources. However, agriculture also contributes indirectly to the energy sector via production of biofuels. Both connections will be strained by increasing concerns over water availability and quality. In addition, water treatment and distribution for drinking water supply and municipal wastewater also require energy.
Significant aspects of water and energy flows do not appear in Figure 9.6. First, flows will change over time, and anticipated changes in flows are important to consider when prioritizing investment in technology and other solutions. Increased deployment of some energy technologies in the future, such as carbon capture and sequestration, could lead to increases in the energy system's water intensity, whereas deployment of other technologies, such as wind and solar photovoltaics, could lower it. In addition, there is significant regional variability in the water and energy systems, their interactions, and resulting vulnerabilities. For example, producing oil and natural gas through horizontal drilling and hydraulic fracturing has the potential for localized water quantity and quality impacts that can be mitigated through fluid life cycle management. Large volumes of water produced from oil and gas operations in general present both localized management challenges and potential opportunities for beneficial reuse. The energy requirements for water systems also have regional variability, based on the quality of water sources and pumping needs.

Figure 9.7 Hybrid Sankey diagram of 2011 US interconnected water and energy flows.
Source: From USDOE (2014a).
Water availability will affect the future of the water–energy nexus. While there is significant uncertainty regarding the magnitude of effects, water availability and predictability may be altered by changing temperatures, shifting precipitation patterns, increasing variability, and more extreme weather. Shifts in precipitation and temperature patterns – including changes in snowmelt – will likely lead to more regional variation in water availability for hydropower, biofeedstock production, thermoelectric generation, and other energy needs. Rising temperatures have the potential to increase the demand for electricity for cooling and decrease the efficiency of thermoelectric generation, as well as increase water consumption for agricultural crops and domestic use. These changes and variations pose challenges for energy infrastructure resilience.
Water and energy needs will also be shaped by population growth and migration patterns, as well as changes in fuels used and energy technologies deployed. For example, projected population growth in the arid southwest will amplify pressure on water and energy systems in that region. Increased production of oil and gas may increase both localized demand for water and generation of produced water that requires management. According to EIA data, planned retirements and additions of electricity generation units and cooling systems will likely decrease water withdrawals, increase water consumption, and increase the diversity of water sources used. While many of the forces affecting the water–energy nexus are out of the federal government's direct control, the future of the nexus hinges on a number of factors that are within the DOE's scope of influence, including technology options, location of energy activities, and energy mix.
The decision‐making landscape for the nexus is shaped by political, regulatory, economic, environmental, and social factors, as well as available technologies. The landscape is fragmented, complex, and changing; the incentive structures are overlapping but not necessarily consistent. Water is inherently a multi‐jurisdictional management issue and is primarily a state and local responsibility. States and localities vary in philosophies regarding water rights. There is also variation across states in relevant energy policies, including renewable portfolio standards, regulation of oil and gas development activities, and regulation of thermoelectric water intake and discharge. Regulations for both oil and gas development and thermoelectric water use are currently undergoing substantial change. Energy for water is also the subject of policy activity at multiple scales, from appliance standards to municipal water treatment funding mechanisms. A more integrated approach to the interconnected energy and water challenges could stimulate the development and deployment of solutions that address objectives in both domains (Table 9.2; AGU 2012; Clark and Veil 2009; DOE 2013a, b; EPRI 2011).
9.3.1 Technology Roadmaps and R&D
There are a number of technologies that support water‐efficient energy systems or energy‐efficient water systems. These technologies are at various stages of research, development, demonstration, and deployment. Figure 9.8 illustrates a range of technologies optimizing water use for energy in waste heat recovery, cooling, alternate fluids, and process water efficiency.
Cooling for thermoelectric generation is an important target for water efficiency because it withdraws large quantities of water for cooling and dissipates tremendous amounts of primary energy. One approach to reduce thermoelectric and other cooling requirements, along with associated water use, is to reduce the generation of waste heat through more efficient power cycles (e.g. the recompression closed‐loop Brayton cycle). Another option is to increase the productive use of the waste heat, such as through thermoelectric materials, enhancements in heat exchanger technologies, or low‐temperature co‐produced geothermal power. A third approach to improve the water efficiency of cooling systems is through advancements in technologies, including air flow designs, water recovery systems, hybrid or dry cooling, and treatment of water from blowdown.
Opportunities to optimize water use also exist in other parts of the overall energy system. With further research, alternative fluids may replace freshwater in hydraulic fracturing, geothermal operations, and power cycles. Process freshwater efficiency can be improved in carbon capture, bioenergy feedstock production, and industrial processes. Many of the technologies that improve water efficiency are enhanced by advances in materials, including thermoelectric properties, heat‐driven state change, scaling/fouling resistance, and temperature and pressure tolerance.
Figure 9.9 shows water treatment technologies that can potentially enhance energy efficiency of water systems and enable the productive, economical, and safe use of nontraditional water resources for energy and nonenergy applications. Such improvements in water treatment and management have particular use for treating oil‐ and gas‐produced waters, as well as saline aquifers, brackish groundwater, brines, seawater, and municipal wastewater. For saline sources, promising water treatment technologies include membrane distillation, forward osmosis, evaporation, nanomembranes, and capacitive deionization. For municipal wastewater, treatment technologies include anammox systems, anaerobic pretreatments, and anaerobic membrane bioreactors. In addition, the biosolids contained in wastewater can be a source of methane energy.
Table 9.2 Comparison of the water withdrawal and water consumption factors (in gal/MWh) for fuel‐based electricity‐generating technologies.
Source: From National Renewable Energy Laboratory (NREL) (2011).
| Fuel type | Cooling | Technology | Median withdrawal | Median consumption |
| Nuclear | Tower | Generic | 1 101 | 672 |
| Once‐through | Generic | 44 350 | 269 | |
| Pond | Generic | 7 050 | 610 | |
| Natural gas | Tower | Combined cycle | 225 | 205 |
| Steam | 1 203 | 826 | ||
| Combined cycle with CCS | 506 | 393 | ||
| Once‐through | Combined cycle | 11 380 | 100 | |
| Steam | 35 000 | 240 | ||
| Pond | Combined cycle | 5 950 | 240 | |
| Dry | Combined cycle | 2 | 2 | |
| Coal | Tower | Generic | 1 005 | 687 |
| Supercritical | 634 | 493 | ||
| IGCC | 393 | 380 | ||
| Supercritical with CCS | 1 147 | 846 | ||
| IGCC with CCS | 642 | 549 | ||
| Once‐through | Generic | 36 350 | 250 | |
| Supercritical | 15 046 | 103 | ||
| Pond | Generic | 12 225 | 545 | |
| Supercritical | 15 046 | 42 | ||
| Biopower | Tower | Steam | 878 | 553 |
9.3.2 Circular Economy
There are various definitions of a circular economy (CE): An idea for a truly sustainable future that works without waste, in symbiosis with our environment and resources. A future where every product is designed for multiple cycles of use, and different material or manufacturing cycles are carefully aligned, so that the output of one process always feeds the input of another. Rather than seeing emissions, manufacturing by‐products, or damaged and unwanted goods as “waste,” in the CE they become raw material, nutrients for a new production cycle (Figure 9.10).
Waste Resources Action Program in the United Kingdom defines it as an alternative to a traditional linear economy (make, use, dispose) in which we keep resources in use for as long as possible, extract the maximum value from them while in use, then recover and regenerate products and materials at the end of each service life. It is also described as a regenerative system in which resource input and waste, emission, and energy leakage are minimized by slowing, closing, and narrowing energy and material loops. This can be achieved through long‐lasting design, maintenance, repair, reuse, remanufacturing, refurbishing, and closed recycling loops (Geissdoerfer et al. 2017). This is in contrast to a linear economy which is a “take, make, dispose” model of production. The Ellen MacArthur Foundation (2012) works with business, governments, and education to help explain the concepts and benefits of a CE. The Foundation's “butterfly diagram” is often used to illustrate a CE, but for those new to the concept, it can be difficult to understand.
9.3.2.1 Origins
As early as 1966 Kenneth Boulding already raised awareness of an “open economy” with unlimited input resources and output sinks in contrast with a “closed economy,” in which resources and sinks are tied and remain as long as possible a part of the economy. The concept of a CE was raised by two British environmental economists David W. Pearce and R. Kerry Turner in 1989. In Economics of Natural Resources and the Environment (Pearce and Turner 1989), they pointed out that a traditional open‐ended economy was developed with no built‐in tendency to recycle, which was reflected by treating the environment as a waste reservoir. Other early schools of thought include Professor Walter Stahel, Gunter Pauli, William McDonough, and Michael Braungart, and complementary approaches such as IE, Permaculture, and The Natural Step.

Figure 9.8 Representative problem/opportunity spaces in water for energy.
Source: Adapted from USDOE (2014a).
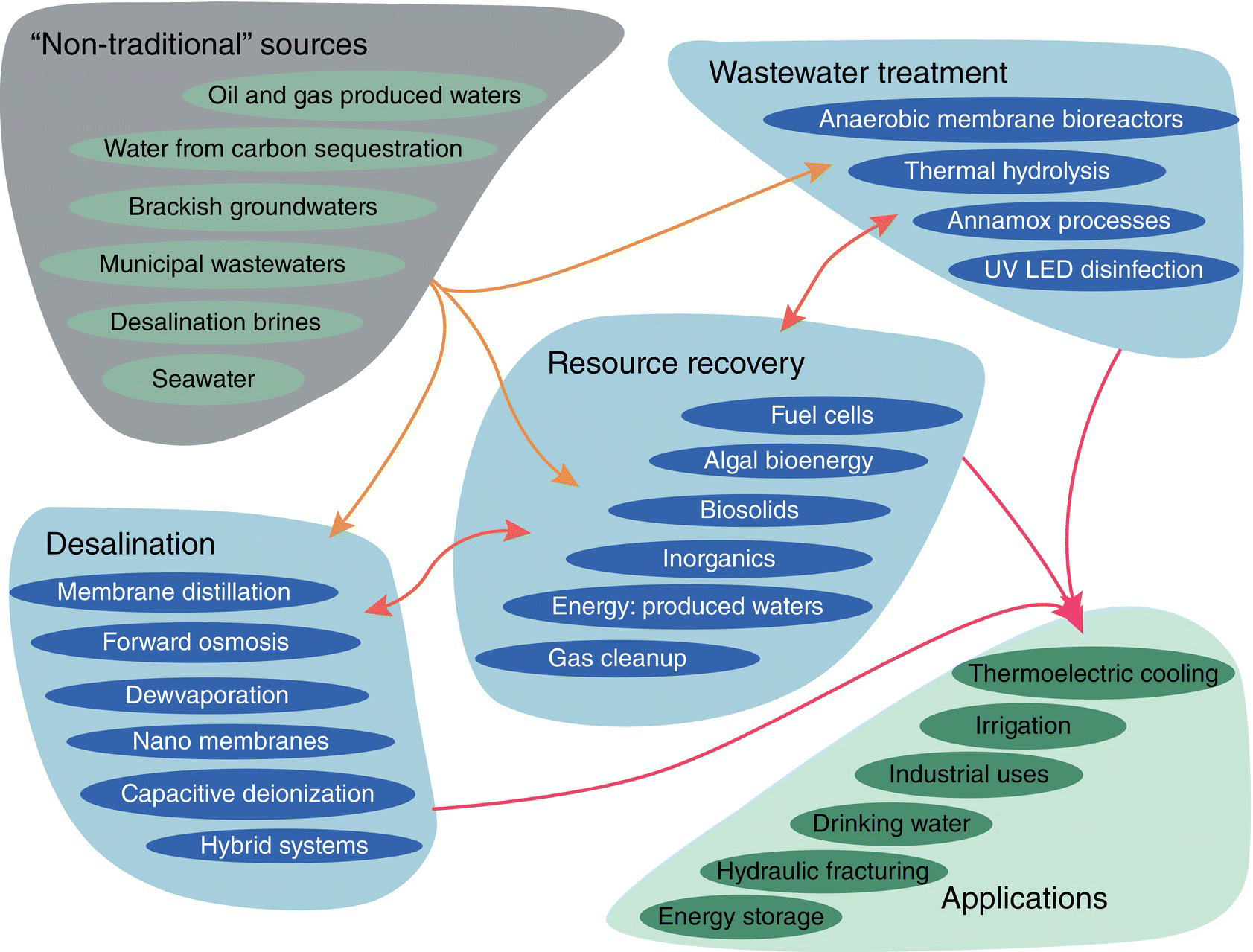
Figure 9.9 Representative problem/opportunity spaces in energy for and from water.
Source: From USDOE (2014a).

Figure 9.10 A schematic depicting the “take, make, waste” linear approach to the CE.
9.3.2.2 Moving Away from the Linear Model
Linear “take, make, dispose” industrial processes and the lifestyles that feed on them deplete finite reserves to create products that end up in landfills or in incinerators. This realization triggered the thought process of a few scientists and thinkers, including Walter Stahel, an architect, economist, and a founding father of industrial sustainability. Credited with having coined the expression “cradle to cradle” (in contrast with “cradle to grave,” illustrating our “resource to waste” way of functioning), in the late 1970s, Stahel worked on developing a “closed‐loop” approach to production processes, co‐founding the Product‐Life Institute in Geneva more than 25 years ago. In the United Kingdom, Steve D. Parker researched waste as a resource in the UK agricultural sector in 1982, developing novel closed‐loop production systems mimicking, and integrated with, the symbiotic biological ecosystems they exploited.
9.3.2.3 Emergence of the Idea
In their 1976 Hannah Reekman research report to the European Commission, “The Potential for Substituting Manpower for Energy,” Walter Stahel and Genevieve Reday sketched the vision of an economy in loops (or CE) and its impact on job creation, economic competitiveness, resource savings, and waste prevention. The report was published in 1982 as the book Jobs for Tomorrow: The Potential for Substituting Manpower for Energy.
Considered as one of the first pragmatic and credible sustainability think tanks, the main goals of Stahel's institute are product‐life extension, long‐life goods, reconditioning activities, and waste prevention. It also insists on the importance of selling services rather than products, an idea referred to as the “functional service economy” and sometimes put under the wider notion of “performance economy,” which also advocates “more localization of economic activity” (Clift and Allwood 2011).
In broader terms, the circular approach is a framework that takes insights from living systems. It considers that our systems should work like organisms, processing nutrients that can be fed back into the cycle – whether biological or technical – hence the “closed loop” or “regenerative” terms usually associated with it. The Ellen MacArthur Foundation, an independent charity established in 2010, has more recently outlined the economic opportunity of a CE. As part of its educational mission, the Foundation has worked to bring together complementary schools of thought and create a coherent framework, thus giving the concept a wide exposure and appeal (Ellen MacArthur Foundation 2012).
Most frequently described as a framework for thinking, its supporters claim it is a coherent model that has value as part of a response to the end of the era of cheap oil and materials and can contribute to the transition to a low‐carbon economy. In line with this, a CE can contribute to meet the COP 21 Paris Agreement. The emissions reduction commitments made by 195 countries at the COP 21 Paris Agreement are not sufficient to limit global warming to 1.5 °C. To reach the 1.5 °C ambition, it is estimated that additional emissions reductions of 15 billion T CO2/year need to be achieved by 2030. Circle Economy and Ecofys estimated that CE strategies may deliver emissions reductions that could basically bridge the gap by half (Blok et al. 2018). However, we have to keep in mind that economic and business goals and environmental goals are two distinct goal sets (see Appendix I).
9.3.2.4 Sustainability
The CE seems intuitively to be more sustainable than the current linear economic system. The reduction of resource inputs into and waste and emission leakage out of the system reduces resource depletion and environmental pollution. However, these simple assumptions are not sufficient to deal with the involved systemic complexity and disregards potential trade‐offs. For example, the social dimension of sustainability seems to be only marginally addressed in many publications on the CE, and there are cases that require different or additional strategies, like purchasing new, more energy efficient equipment. By reviewing the literature, a team of researchers from Cambridge and TU Delft could show that there are at least eight different relationship types between sustainability and the CE (Geissdoerfer et al. 2017)
- Conditional relation
- Strong conditional relation
- Necessary but not sufficient conditional relation
- Beneficial relationship
- Subset relation (structured and unstructured)
- Degree relation
- Cost‐benefit/trade‐off relation
- Selective relation
9.3.2.5 Use Waste as a Resource
The second element aims to utilize waste streams as a source of secondary resources and recover waste for reuse and recycling and is grounded on the idea that waste does not exist. It is necessary here to design out waste, meaning that both the biological and technical components (nutrients) of a product are designed intentionally in such a way that waste streams are minimalized. Closed recycling loops are key here, one for manufacture (production‐waste recycling) and two for disposal of the product (product and material recycling) (Lienig and Bruemmer 2017). This follows a cradle‐to‐cradle design rather than a cradle‐to‐grave process.
9.3.2.6 Design for the Future
Account for the systems perspective during the design process, to use the right materials, to design for appropriate lifetime, and to design for extended future use. Meaning that a product is designed to fit within a materials cycle, can easily be dissembled, and can easily be used with a different purpose. In addition to strategies like emotionally durable design, it involves anticipating product recycling (the reuse and further use of the product), and material recycling (the reuse and further use of its constituent materials) during the design process (Lienig and Bruemmer 2017). It should be stressed that there is not something like one ideal blueprint for future design. Modularity, versatility, and adaptiveness are to be prioritized in an uncertain and fast‐evolving world, meaning that diverse products, materials, and systems, with many connections and scales, are more resilient in the face of external shocks, than monotone systems built simply for efficiency.
9.3.2.7 Preserve and Extend What's Already Made
While resources are in use, maintain, repair, and upgrade them to maximize their lifetime and give them a second life through take back strategies when applicable. This could mean that a product is accompanied with a pre‐thought maintenance program to maximize its lifetime, including a buyback program and supporting logistics system. Leasing programs (“purchase the usage” instead of “purchase the product”), secondhand sales, or product recycling also falls within this element (Lienig and Bruemmer 2017).
9.3.2.8 Collaborate to Create Joint Value
Within a CE, one should work together throughout the supply chain, internally within organizations and with the public sector to increase transparency and create joint value. For the business sector this calls for collaboration within the supply chain and cross‐sectoral, recognizing the interdependence between the different market players. Governments can support this by creating the right incentives, for example via common standards within a regulatory framework and provide business support.
9.3.2.9 Incorporate Digital Technology
Track and optimize resource use and strengthen connections between supply chain actors through digital, online platforms, and technologies that provide insights. It also encompasses virtualized value creation and delivering, for example via 3D printers, and communicating with customers virtually.
9.3.3 Rethink the Business Model
Consider opportunities to create greater value and align incentives through business models that build on the interaction between products and services. Do we need to own products or is one satisfied by using it as service? Can the current business model, for example, be modified to lease its products? This also includes rethinking circular production processes (McDonough and Braungart 2002)
9.3.3.1 Framework
The various approaches to “circular” business and economic models have slightly different emphasis on the key components. They share several common principles aiming to
- extend the life of materials and products, where possible over multiple “use cycles”;
- use a “waste = food” approach to help recover materials and ensure those biological materials returned to earth are benign, not toxic;
- retain the embedded energy, water, and other process inputs in the product and the material for as long as possible;
- use systems‐thinking approaches in designing solutions;
- regenerate or at least conserve nature and living systems;
- push for policies, taxes and market mechanisms that encourage product stewardship, for example “polluter pays” regulations (Weetman 2016).
The many different “understandings” of the CE are evidenced by a recent review of 114 different publications. Some of the key aspects are outlined in the following.
9.3.3.2 Systems Thinking
System thinking is the ability to understand how things influence one another within a whole. Elements are considered as “fitting in” their infrastructure, environment, and social context. While a machine is also a system, systems thinking usually refers to nonlinear systems: systems where through feedback and imprecise starting conditions, the outcome is not necessarily proportional to the input and where evolution of the system is possible – the system can display emergent properties. Examples of these systems are all living systems and any open system such as meteorological systems or ocean currents, even the orbits of the planets have nonlinear characteristics.
Understanding a system is crucial when trying to decide and plan (corrections) in a system. Missing or misinterpreting the trends, flows, functions of, and human influences on, our socio‐ecological systems can result in disastrous results. In order to prevent errors in planning or design, an understanding of the system should be applied to the whole and also to the details of the plan or design. The Natural Step created a set of systems conditions (or sustainability principles) that can be applied when designing for (parts of) a CE to ensure alignment with functions of the socio‐ecological system.
The concept of the CE has previously been expressed as the circulation of money versus goods, services, access rights, valuable documents, etc., in macroeconomics. This situation has been illustrated in many diagrams for money and goods circulation associated with social systems. As a system, various agencies or entities are connected by paths through which the various goods etc., pass in exchange for money. However, this situation is different from the CE described above, where the flow is unilinear – in only one direction, that is until the recycled goods are again spread over the world.
9.3.4 Biomimicry
Janine Benyus, author of Biomimicry: Innovation Inspired by Nature, defines her approach as “a new discipline that studies nature's best ideas and then imitates these designs and processes to solve human problems. Studying a leaf to invent a better solar cell is an example. I think of it as ‘innovation inspired by nature’” (Benyus 2003). Biomimicry relies on three key principles:
- Nature as model: Biomimicry studies nature's models and emulates these forms, processes, systems, and strategies to solve human problems.
- Nature as measure: Biomimicry uses an ecological standard to judge the sustainability of our innovations.
- Nature as mentor: Biomimicry is a way of viewing and valuing nature. It introduces an era based not on what we can extract from the natural world but on what we can learn from it.
9.3.4.1 Cradle to Cradle
Created by Walter R. Stahel, a Swiss architect who graduated from the Swiss Federal Institute of Technology Zürich in 1971. He has been influential in developing the field of sustainability by advocating philosophies of “service‐life extension of goods – reuse, repair, remanufacture, upgrade technologically” as they apply to industrialized economies. He cofounded the Product Life Institute in Geneva, Switzerland, a consultancy devoted to developing sustainable strategies and policies, after receiving recognition for his prize‐winning paper “The Product Life Factor” in 1982. His ideas and those of similar theorists led to what is now known as the CE, in which industry adopts the reuse and service‐life extension of goods as a strategy of waste prevention, regional job creation, and resource efficiency in order to decouple wealth from resource consumption; in other words, to dematerialize the industrial economy. Recent technical developments such as the recyclebot, which profitably converts postconsumer plastic waste to 3D printing feedstock to make higher‐value products, (upcycling) provide financial incentives to tighten the loop of the CE (McDonough and Braungart 2002; Zhong 2018).
9.3.4.2 Toward the CE
In January 2012, a report was released entitled Towards the Circular Economy: Economic and business rationale for an accelerated transition. The report, commissioned by the Ellen MacArthur Foundation and developed by McKinsey & Company, was the first of its kind to consider the economic and business opportunity for the transition to a restorative, circular model. Using product case studies and economy‐wide analysis, the report details the potential for significant benefits across the EU. It argues that a subset of the EU manufacturing sector could realize net materials cost savings worth up to $630 billion annually toward 2025 – stimulating economic activity in the areas of product development, remanufacturing, and refurbishment. Towards the Circular Economy also identified the key building blocks in making the transition to a CE, namely in skills in circular design and production, new business models, skills in building cascades and reverse cycles, and cross‐cycle/cross‐sector collaboration (Ellen MacArther Foundation 2012).
In January 2015 a Definitive Guide to The Circular Economy (Ellen MacArthur Foundation 2015) was published by Cowes with the specific aim to raise awareness among the general population of the environmental problems already being caused by our “throwaway culture.” Waste Electrical and Electronic Equipment, in particular, is contributing to excessive use of landfill sites across the globe in which society is both discarding valuable metals and dumping toxic compounds that are polluting the surrounding land and water supplies. Mobile devices and computer hard drives typically contain valuable metals such as silver and copper but also hazardous chemicals such as lead, mercury, and cadmium. Consumers are unaware of the environmental significance of upgrading their mobile phones, for instance, on such a frequent basis but could do much to encourage manufacturers to start to move away from the wasteful, polluting linear economy toward are sustainable CE.
9.3.4.3 Circular Business Models
A CE calls upon chances to make more prominent esteem and adjust motivating forces through plans of action that expand on the connection among items and administrations. Essentially, this implies a round plan of action isn't centered simply around offering an item, yet it incorporates a move in contemplating incentive, presenting an entire scope of various plans of action to be utilized. This involves both the motivators and advantages offered to clients for bringing back utilized items and an adjustment in income streams, including installments for a round item or administration, or installments for conveyed accessibility, utilization, or execution identified with the item‐based administration advertised. These better approaches for working together expect organizations to make an alluring plan of action for lenders, and agents to change the way they see the dangers and openings related with these models (Lewandowski 2016).
9.4 CE Indicators in Relation to Eco‐Innovation
Nowadays, one of the most significant challenges in environmental management across the world is ensuring that our activities conform to the principles of sustainable development (Allen and Shonnard 2012; Clift and Allwood 2011; Das 2005; Das et al. 2001). Approaches to sustainable development have focused on “top‐down” quantitative indicators based on scientific expertise and have a tendency to measure progress at global, national, or regional levels (Thomé et al. 2016). In recent years, the strategy of adopting a CE has gained increasing currency as a concept for the pursuit of global sustainability (Staniškis 2012). The most important benefit in moving to a more CE‐based approach is the possibility of retaining the added value in products for as long as possible (Smol et al. 2015), extracting their maximum value and eliminating waste. CE‐based systems keep resources within the economy. When a product has reached the end of its life, products can be efficiently reused again and again and hence create further value (COM, No. 398 2014). One of the factors determining the possibility of moving toward a CE is the implementation of innovation technologies, with a particular emphasis on eco‐innovation. Despite the fact that these two issues are linked, a uniform methodology designed to compare the degree to which economies have moved toward a CE, which also includes eco‐innovation, has not currently been established.
9.4.1 Development of the Concept of the CE
The CE is a relatively new concept, although the idea behind the CE has existed for a long time (Murray et al. 2015). “The better a real factory makes use of its waste, the closer it gets to its ideal, the bigger is the profit” (Lancaster 2002). The CE model is actually the opposite of a leaner one and is based on closed loops like a biological life cycle. It was not widely debated in the academic and scientific literature on sustainability, but it has become more popular with recent research (Pitt and Heinemeyer 2015; Stahel 2015).
In the twenty‐first century the “preventive approach” has been replaced with the “restorative approach” both in Europe and across the whole world. The way of thinking in the twenty‐first century has started to be more global, holistic, and systematic. Society and government have begun to introduce one more element – “restore” – into the “reduce‐recycle‐reuse concept.” The CE model is based on concepts such as “cradle‐to cradle™,” where industry, by being waste‐free, operates with no impact upon the environment (McDonough and Braungart 2002).
As for the development of the CE approach at the nongovernmental level, the experience of the United Kingdom should be mentioned. A leading follower of the CE in the United Kingdom is the Ellen Macarthur Foundation, an NGO which has produced three reports on the concept (Ellen MacArthur Foundation 2012, 2013, 2014). The reports examine the potential of the CE as a new concept for development. The Foundation is very active and for the time being they already have obtained support for global innovation.
At the same time, existing CE approaches are valuable and have a tendency to develop further. They are strongly focused on resource efficient production. This can be proved by the main principles of the concept presented in the analytical report “Towards a Circular Economy: Business Rationale for an Accelerated Transition” (Ellen MacArthur Foundation 2015; Ellen MacArthur Foundation 2016; Smol et al. 2017):
- Principle 1: Preserve and enhance natural capital by controlling finite stocks and balancing renewable resource flows.
- Principle 2: Optimize resource yields by circulating products, components, and materials at the highest utility at all times in both technical and biological cycles.
- Principle 3: Foster system effectiveness by revealing and designing out negative externalities.
It can be observed that an innovative approach is in fact needed for each aspect of the CE concept. All strategic EU documents on the CE and the reports presented above see innovation as the heart of any transition to a CE. It is also obvious that a special role should be given to eco‐innovations and that they should be key drivers, because the CE concept is all about economic growth, creating jobs, and at the same time reducing environmental impacts, including carbon emissions (Smol et al. 2015, 2017).
9.5 Process Intensification and Integration Potential in Manufacturing
Process intensification (PI) aims to dramatically improve manufacturing processes through the application of novel process systems and equipment. The novel approaches can be used to overcome bottlenecks, such as those imposed by thermodynamics, or to combine processing phenomena into fewer processing units with a concurrent reduction of capital and operation and maintenance costs and energy, water and materials intensity. PI approach goes beyond the incremental improvements achieved through optimizing existing equipment and process systems and achieves step changes in energy and materials efficiency, total life‐cycle cost reduction, and environmental impact by minimizing wastes at the sources via various hierarchy pollution prevention techniques (Bielenberg and Bryner 2018).
9.5.1 What Is PI?
PI is not well defined like many multidimensional concepts. Although PI may be best explained through examples, here are a few potential definitions to consider when thinking about PI (Bielenberg and Bryner 2018).
Well‐known experts in PI Stankiewicz and Moulijin (2000), both at Delft University of Technology, defined PI as the development of innovative apparatuses and technologies that bring dramatic improvements in chemical and allied manufacturing and processing, substantially reducing equipment volume, energy consumption, or waste minimization, less environmental and health impacts, and ultimately yields to cheaper, safer, sustainable technologies. They added four guiding principles to that definition (EFCE 2015):
- Maximize the effectiveness of intramolecular and intermolecular events
- Provide all molecules the same process experience
- Optimize driving forces at all scales and maximize the specific surface areas to which they apply
- Maximize synergistic effects from partial processes.
PI targets dramatic improvements in manufacturing and processing by rethinking existing operation schemes into ones that are both more precise and efficient than existing operations. There are a series of technologies that enable equipment sizes to be radically reduced. PI and microreaction technology, as well as reports of experimental results in the use of novel PI systems, including the static mixers, high‐gravity (HiGee) technology, cyclic and reactive distillation, compact high specific surface heat exchanger, multifunctional reactors, microchannel reaction systems, microengineering, microtechnology, the catalytic plate reactor, and a chemical microsystem for pervaporation. Such technologies enable to plant sizes to be correspondingly reduced. The very low inventories have environmental benefits and there are also claimed cost benefits (Reay et al. 2013). An incidental benefit is that the processes may be economic at a smaller scale (mostly batch processes), and that partly contributes to economic sustainability.
The European Roadmap on Process Intensification describes PI as providing “radically innovative principles (paradigm shift) in process and equipment design, which can benefit (often with more than a factor of two) process and chain efficiency, capital and operating expenses, quality, wastes, process safety, process integration and more” (EFCE 2015).
Reay et al. (2013) describe PI as a “chemical and process design approach that leads to substantially smaller, cleaner, safer, and more energy‐efficient process technology.” The common thread among these definitions is a focus on new schemes and equipment that create improved processes by combining, controlling, and/or enhancing the chemistry and transport phenomena in a chemical process.
A classic example of PI equipment is the static mixers. Although there are many different designs for static mixers, the basic concepts are the same. Stationary mixing elements placed in the path of fluid flow create locally highly mixed channels for the fluid to move through. Homogeneous mixing occurs quickly, with no external energy input other than that associated with the small pressure drop, at typically low capital costs. Static mixers can be incorporated into other unit operations (e.g. reactors) to enable the combination of processes and can be tailored to match mixing scales and times to optimize overall process efficiency. For example, static mixers can be placed in a tubular reactor for a two‐phase reaction system – creating a high level of mixing while maintaining a largely plug‐flow profile (typically found at a much smaller scale) at the larger reactor scale. Such an approach could offer many advantages over the alternative of operating a large continuous stirred‐tank reactor to maintain high levels of mixing.
There are many other examples of PI equipment, including microchannel reactors, spinning‐disc reactors, centrifugal contractors, and dividing‐wall column (Figure 9.11) to name a few. Each of these relies on a novel driving force (e.g. rotation) or nonstandard configuration (e.g. microchannels) to enable increased control over mixing, reaction, and heat, mass, and momentum transfer to bring about step changes in the reduction of energy consumption and capital costs.
The dividing‐wall column (Figure 9.11) is one form of process intensification that enables the separation of a three‐phase system in a single distillation tower. The internal wall splits the column into two halves. The three‐phase system is pumped into one side of the column and is reflected by the wall. The lightest component drops to the bottom and is withdrawn. The intermediate component is initially entrained in both streams; the intermediate component that flows upward subsequently separates and falls down on the opposite side of the wall, while the component that is entrained in the heavy component separates and flows up the back side of the column, where the entire intermediate stream is recovered through a side port.
9.5.2 Case Study 9.5: Elimination of Dioxin and Furans by Alternative Chemical PI
In early 1988, a study of effluents from bleached pulp mills showed significant levels of dioxins and furans. As a result of these findings, the industry implemented a series of process changes including (i) eliminating the use of certain defoamers which contained dioxin and furan precursors, (ii) decreasing the use of chlorine as bleaching chemical, and (iii) increasing the use of chlorine dioxide for pulp bleaching. Between 1988 and 1996, there was a very significant reduction in effluent 2,3,7,8‐tetrachloro dibenzo‐p‐dioxin (TCDD) and 2,3,7,8‐tetrachloro dibenzo‐p‐furan (TCDF) concentrations. For example, in 1988 40% of the mill effluent samples contained less than 10 parts per quadrillion (ppq) of 2,3,7,8‐TCDD. In 1990, 70% of the samples contained less than or equal to 10 ppq of 2,3,7,8‐TCDD. By 1996, all but two mill effluent samples showed 2,3,7,8‐TCDD levels below 10 ppq. This record exemplifies how pollution prevention leading to Zero Discharge of dangerous environmental contaminants can be achieved at the source through process changes and substituting and intensifying chemical processes. Figure 9.12 shows a steady reduction of dioxin/furan formation to below detection level by substituting elemental chlorine to chlorine dioxide as a bleaching agent (Das and Jain 2001; NCASI 1993).
9.5.3 Mini‐Case Study 9.2: Multi‐Pollutants Capture and Recovery of SOx, NOx, and Mercury in Coal‐Fired Power Plant
9.5.3.1 Advanced Multi‐Pollutant Control: Intensified Regenerative Activated Coke Technology
Regenerative activated coke technology (ReACT™) is an advanced intensified multi‐pollutants technology that achieves simultaneous capture of SOx, NOx, and mercury in one vessel. The process was first developed in Germany, and it was subsequently advanced and commercialized by J‐Power EnTech in Japan where ReACT™ has been implemented at three large‐scale coal‐fired power plants and at several steel mills, petrochemicals, refineries, and waste incinerator facilities, as a high‐efficiency emission control system.

Figure 9.11 Dividing‐wall column for three‐phase separation in distillation tower.
Source: From Bielenberg and Bryner (2018).
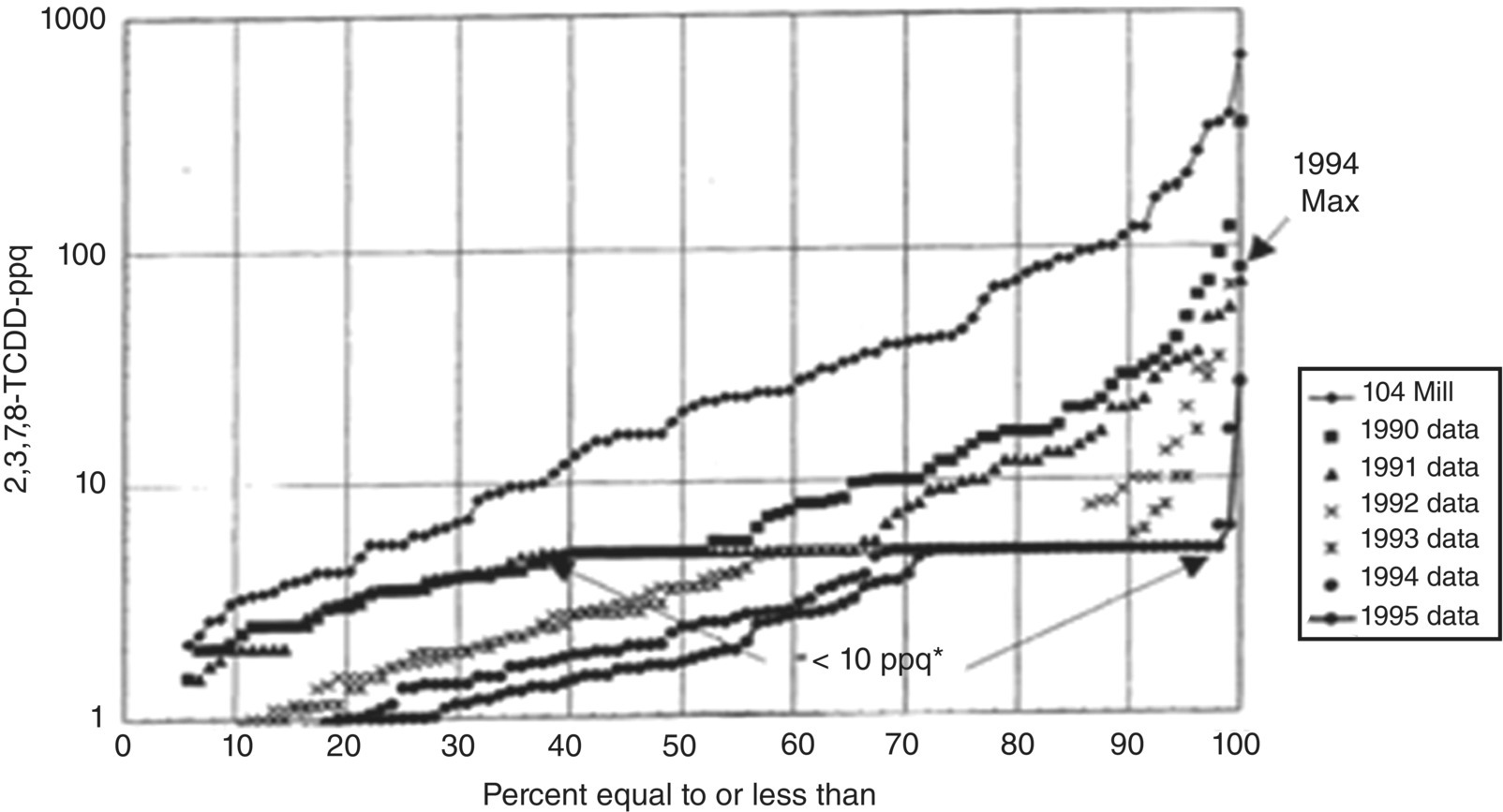
Figure 9.12 Elimination of dioxin/furans formation in bleaching pulp by intensifying process and substituting chemicals.
Source: From Das (2005).
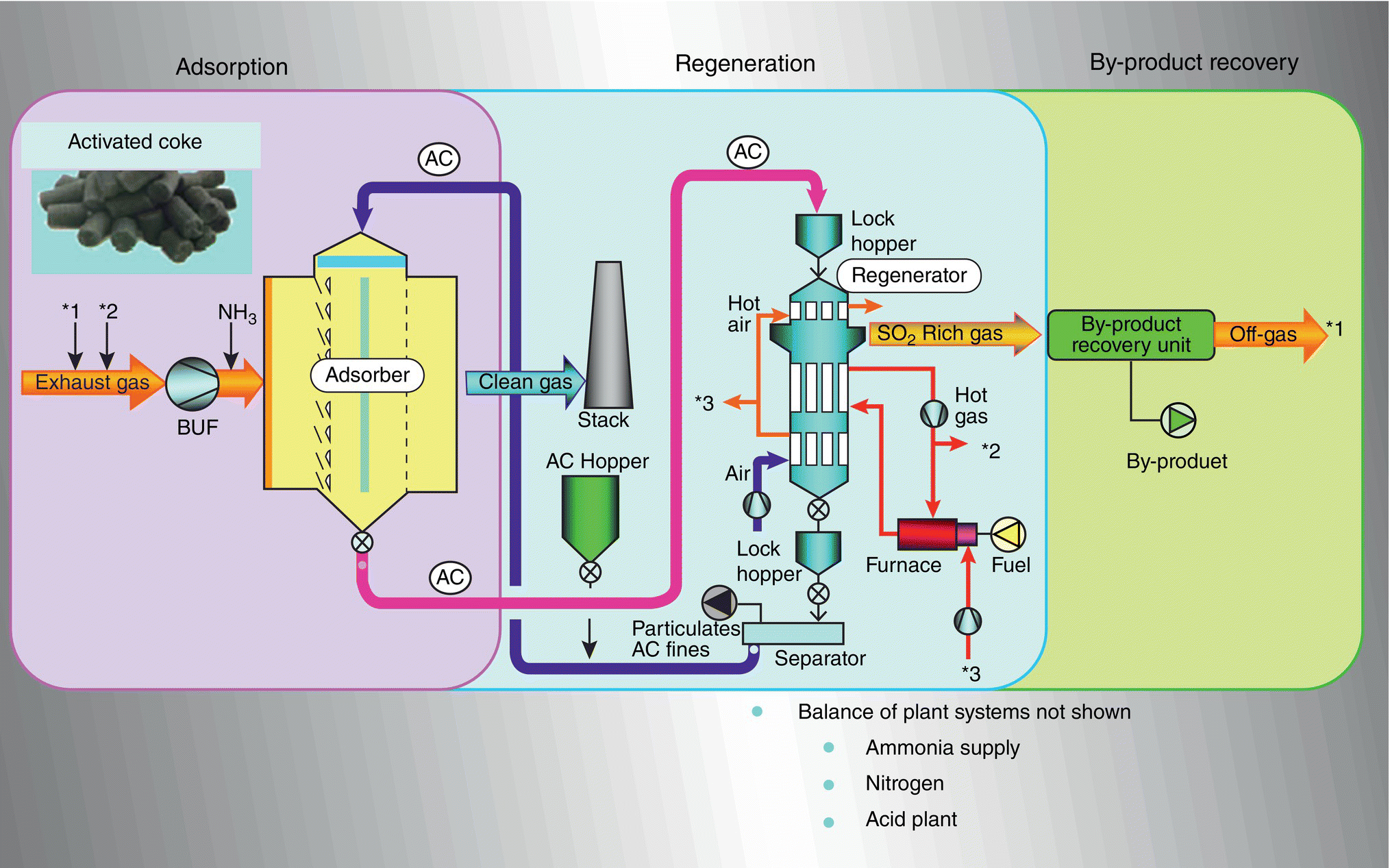
Figure 9.13 Schematic diagram of three‐stage ReACT™ process.
Source: From Reynolds (2018).
Figure 9.13 shows the three‐stage ReACT™ process, including adsorption by activated coke (AC) followed by regeneration of SOx and mercury, and then by‐product recovery of sulfur‐rich gas converting into a marketable sulfuric acid.
ReACT™ technology is based on adsorption of SO2 and NOx on AC in a moving bed, with subsequent regeneration of the coke and production of saleable by‐product sulfuric acid. This technology is a completely dry scrubbing event that does not evaporate any water into the flue‐gas stream, can produce extremely low level of SO2 emissions, and can also provide additional control for NOx, PM, and Hg as co‐benefits. The moving bed dry scrubber operates downstream of a primary particulate control device, which for many plants is traditional ESP technology. For special cases where extremely low levels of particulate are permitted, a downstream polishing ESP can also be applied.
A mix of custom and traditional material handling equipment transports the AC to and from the absorber and regenerator. Careful material handling and the robust nature of the AC allow the coke to be recycled with minimal makeup requirements.
Simultaneous capture of SO2, NOx, and Hg is accomplished in one vessel along with a further reduction in particulate emissions. High removal efficiencies include up to 99.9% SO2, 50–80% NOx, and above 90% Hg (including elemental). Particulate is also reduced by about 50%. Advantages of this technology include high‐emission reduction efficiencies, cost‐effectiveness, low power consumption, minimum installation space, minimal water requirements (dry process), no gas reheating, and low maintenance requirements. The AC process involves three steps: (i) adsorption, (ii) regeneration, and (iii) by‐product recovery (Figure 9.13).
In the absorber, the flue gas passes through a bed of AC moving slowly downward at a constant flow rate. The adsorber is a single stage, reflecting an improvement over previous designs, which had a two‐stage adsorber. Removal of SO2, NOx, Hg, and particulates is taking place in one step. As the AC is saturated with pollutants, it is conveyed through a bucket elevator to the regenerator. The regenerator operates at temperatures in the 399–499 °C range. Simultaneously, sulfuric acid or ammonium salts in the AC are decomposed to N2, SO2, SO3, and H2O. Hg is retained in the AC and removed at some period of time, depending on the Hg concentrations at the inlet of ReACT™.
After cooling, the regenerated AC passes through a separator (vibrating screen), which separates particulates and small particles of AC, from the larger AC, which is returned to the adsorber. The AC removed from the separator can be returned to the boiler for burning or sold and used in industrial applications, such as dioxin adsorption – SO2‐rich gas from the generator is converted to a saleable product, such as sulfuric acid, in the by‐product recovery facility (Figure 9.14).
- Adsorption and reaction on AC: SO2 and SO3 are adsorbed at high efficiency on the AC surfaces as sulfuric acid, ammonium sulfate, and ammonium bisulfate.
- The catalytic effect of AC also leads to reduction reactions to reduce NOx in the presence of ammonia. The NOx control activity is completed in the adsorber as follows:
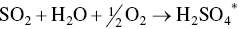
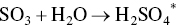
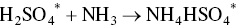 (* denotes adsorbed species)
(* denotes adsorbed species)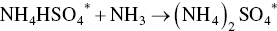
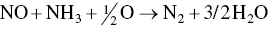
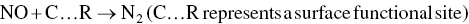
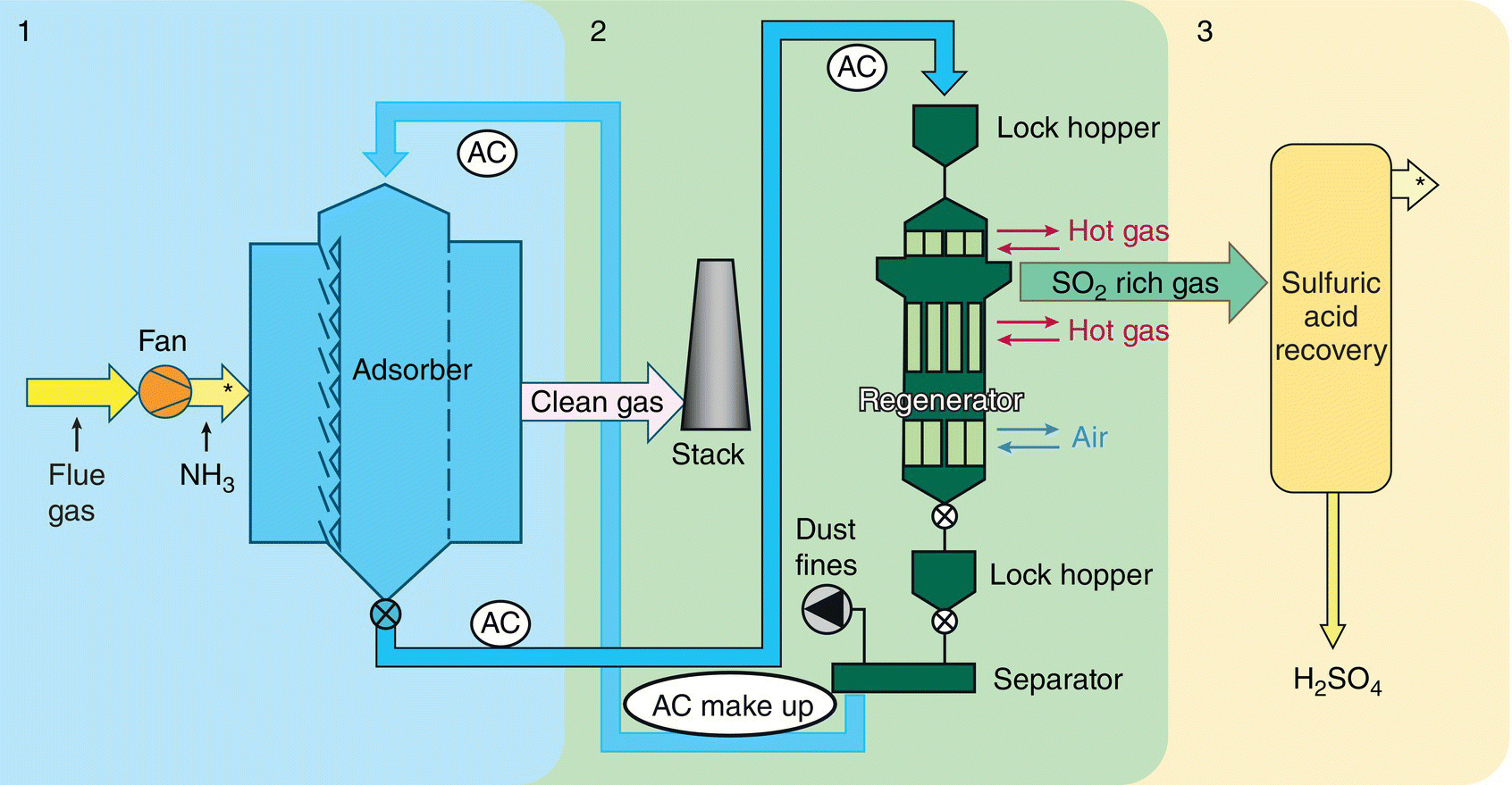
Figure 9.14 SOx recovery and converting it to H2SO4 in acid plant.
Source: From Reynolds (2018).
9.5.3.2 AC and Regenerated Coke
The AC is a carbonaceous material produced by steam activation (at ~900 °C). It has high mechanical strength against abrasion and crushing. Its surface area is 150–300 m2/g, less than the conventional activated carbon, but not much higher than the metallurgical coal. AC comes in tablet or almond‐type shape and is generally much larger than the activated carbon. AC is easier to produce and less expensive than activated carbon. Figure 9.15 shows the internal model of AC and its pore structure. Figure 9.16 shows mercury vapor removal efficiency versus time.
9.5.3.3 Thermal Regeneration of AC
At the temperatures in the regenerator heating section adsorbed sulfuric acid, ammonium sulfate, and ammonium bisulfate are decomposed to SO2. AC pellets enter via lock hoppers and is in gravity counter‐flow against desorbed gases and N2 carrier gas. Sulfur‐rich gas containing SO2, CO2, N2, and H2O exits the regenerator to an acid plant as follows:
(*denotes adsorbed species; non‐isothermal desorption kinetics reach peak rates at 300 °C and completion by 450 °C).
9.5.3.4 Performance and Benefits of ReACTTM
Table 9.3 presents a typical performance at J‐Power EnTech 2x600MW ISOGO coal‐fired power plant in Japan (Reynolds, personal communication). The benefits of the advanced multi‐pollutants control by the ReACT™ can be summarized as follows:
- By‐product revenue – Sulfuric acid is the world's number one commodity chemical with a market value of $50 200/T. Instead of producing disposal gypsum or fly ash/gypsum waste, ReACT™ produces saleable by‐product.
- Avoided disposal costs – For every ton of SO2 controlled in conventional flue‐gas desulfurization (FGD), about three tons of solid waste is generated. More if fly ash is part of the FGD waste stream.
- Near zero water use – ReACT™ uses minimal water, in significant contrast to FGD systems. For a 500 MW plant, a WFGD (wet flue gas desulfurization) system would require 275 000 000 gal/year while ReACT™ would use near zero.
Figure 9.15 Internal model of AC – pore structure.

Figure 9.16 Mercury vapor removal efficiency (%) versus time.
Table 9.3 Typical performance of advanced multi‐pollutant removal.
Pollutants Emissions permit Operating results Efficiency (%) Inlet concentration Outlet concentration SOx 10 ppm
(0.025 lb/MMbtu)>98 <410 ppm
<0.85 lb/MMbtu<1 ppm
<0.002 lb/MMBtuNOx 13 ppm
(0.02 lb/MMbtu)10–50 <20 ppm
0.03 lb/MMbtu<7 ppm
<0.011 lb/MMbtuParticulate 5 mg/Nm3
(0.004 lb/MMbtu)>95
(>99.9 w/ESPs)<100 mg/Nm3
<0.1 lb/MMbtu<3 mg/Nm3
<0.002 lb/MMbtu
(w/backend ESP)Hg –– >90 2.5 μg/Nm3 <0.25 μg/Nm3 - Minimal plant modifications – ReACT™ can flow to the existing stacks with no change in liner materials. ReACT™ is located downstream of existing equipment and does not necessitate modifications to upstream equipment.
- NOxperformance options – ReACT™ systems may be designed for a range of NOx reduction options – from co‐benefit levels of 30% through alternative designs reaching 80%.
9.6 Manufacturing Process Integration
In response to the staggering environmental and energy problems associated with manufacturing facilities, the chemical and allied process industries have dedicated much attention and many resources to mitigating the detrimental impact on the environment, conserving resources, and reducing the intensity of energy usage. These efforts have gradually shifted from a unit‐based approach to a systems‐level paradigm. The past decade has seen significant industrial and academic efforts devoted to the development of holistic process design methodologies that target energy conservation and waste reduction from a system's perspective. Implicit in the holistic approach, however, is the need to realize that changes in a unit or a stream often propagate throughout the process and can have significant effects on the operability and profitability of the process. Furthermore, the various process objectives (e.g. technical, economic, environmental, and safety) must be integrated and reconciled. These challenges call for the development and application of a systematic approach that transcends the specific circumstances of a process and views the environmental, energy, and resource‐conservation problems from a holistic perspective. This approach is called process integration (Parthasarathy and Dunn 2005; Smith 2016). It emphasizes the unity of the process and it is broadly divisible into the categories of mass integration and energy integration. Parthasarathy and Dunn (2005) provide an energy and heat exchange networks with a mini‐case‐study on heat exchange network, mass integration is briefly introduced, and the concept of mass integration is explored in detail.
9.6.1 Process Integration Technique Has Few Possible Applications
- A holistic approach to process design which emphasizes the unity of the process and considers the interactions between different unit operations from the outset, rather than optimizing them separately. This can also be called integrated process design or process synthesis (Smith 2016) describe the approach well. An important first step is often product design which develops the specification for the product to fulfill its required purpose.
- Pinch analysis, a technique for designing a process to minimize energy consumption and maximize heat recovery, also known as heat integration, energy integration, or pinch technology. The technique calculates thermodynamically attainable energy targets for a given process and identifies how to achieve them. A key insight is the pinch temperature, which is the most constrained point in the process. A detailed explanation of the techniques is given by Parthasarathy and Dunn (2005). This definition reflects the fact that the first major success for process integration was the thermal pinch analysis addressing energy problems. Other pinch analyses were developed for several applications such as mass‐exchange networks, water minimization, and material recycle. A very successful extension was “Hydrogen Pinch,” which was applied to refinery hydrogen management. This allowed refiners to minimize the capital and operating costs of hydrogen supply to meet ever stricter environmental regulations and also increase hydrotreater yields (Hallale 2001).
In the context of chemical engineering, process integration can be defined as a holistic approach to process design and optimization, which exploits the interactions between different units in order to employ resources effectively and minimize costs. Process integration is not limited to the design of new plants, but it also covers retrofit design (e.g. new units to be installed in an old plant) and the operation of existing systems. Hallale (2001) explains that with process integration, industries are making more money from their raw materials and capital assets while becoming cleaner and more sustainable.
The main advantage of process integration is to consider a system as a whole (i.e. integrated or holistic approach) in order to improve their design and/or operation. In contrast, an analytical approach would attempt to improve or optimize process units separately without necessarily taking advantage of potential interactions among them.
For instance, by using process integration techniques it might be possible to identify that a process can use the heat rejected by another unit and reduce the overall energy consumption, even if the units are not running at optimum conditions on their own. Such an opportunity would be missed with an analytical approach, as it would seek to optimize each unit, and thereafter it wouldn't be possible to reuse the heat internally.
Typically, process integration techniques are employed at the beginning of a project (e.g. a new plant or the improvement of an existing one) to screen out promising options to optimize the design and/or operation of a process plant. Also it is often employed, in conjunction with simulation and mathematical optimization tools to identify opportunities in order to better integrate a system (new or existing) and reduce capital and/or operating costs.
Most process integration techniques employ Pinch analysis or Pinch tools to evaluate several processes as a whole system. Therefore, strictly speaking, both concepts are not the same, even if in certain contexts they are used interchangeably. The review by Hallale (2001) explains that in the future, several trends are to be expected in the field. In the future, it seems probable that the boundary between targets and design will be blurred and that these will be based on more structural information regarding the process network. Second, it is likely that we will see a much wider range of applications of process integration. There is still much work to be carried out in the area of separation, not only in complex distillation systems but also in mixed types of separation systems. This includes processes involving solids, such as flotation and crystallization. The use of process integration techniques for reactor design has seen rapid progress, but it is still in its early stages. Third, a new generation of software tools is expected. The emergence of commercial software for process integration is fundamental to its wider application in process design.
9.7 New Sustainable Chemicals and Energy from Black Liquor Gasification Using Process Integration and Intensification
In the areas of chemicals and allied products and energy process engineering, it is of particular interest to design processes with low‐energy requirements (energy intensive) (preferably renewable materials, e.g. biomass) and the use of minimum raw materials or use of a by‐product as feedstock (materials intensive) and minimum number of equipment (i.e. lean manufacturing). This is the basis from which the area of PI arises. The main objective of PI is to propose production and manufacturing processes in plants as small as possible and with low total energy, materials, water, and other resources. To reduce the size of plants, the scope is reducing the size of equipment or the number of operations in the processes, or using multifunction within the process is must. Since energy requirements of industrial processes are usually satisfied with steam, for which production is necessary to burn some kind of fuel (usually fossil fuels), by reducing energy requirements a direct reduction on environmental impact (e.g. greenhouse gases, oxides of carbon, nitrogen and sulfur, and particulate matter). In this extended case study, black liquor, a by‐product of wood‐pulping process, is utilized to make chemicals and biofuels, in particular methanol, dimethyl ether, and syngas. Different feedstock, use of by‐product and alternatives and principles of PI are commented (Gómez‐Castro et al. 2016).
9.7.1 Introduction
The US pulp and paper industry is the largest producer and user of biomass energy in the United States today, nearly all derived from sustainably grown trees. Renewable resources used at pulp mills include bark, wood wastes, and black liquor, the lignin‐rich by‐product of cellulose‐fiber extraction.
With substantial renewable energy resources at its immediate disposal and with potentially much more extensive resources available in the long term, the US pulp and paper industry has the potential to contribute significantly to addressing climate change and US energy security concerns, while also improving its global competitiveness. A key requirement for achieving these goals is the commercialization of breakthrough technologies, especially gasification, to enable the clean and efficient conversion of biomass to useful energy forms, including electricity and transportation fuels.
Gasification technology enables low‐quality solid fuels like biomass to be converted with low pollution into a fuel gas (synthesis gas or “syngas”) consisting largely of hydrogen (H2) and carbon monoxide (CO). Syngas can be burned cleanly and efficiently in a gas turbine to generate electricity. It can be passed over appropriate catalysts to synthesize clean liquid transportation fuels or chemicals. It can also be converted efficiently into pure H2 fuel.
While most pulp and paper manufacturing facilities in the United States today do not export electricity and none export transportation fuels, their established infrastructure for collecting and processing biomass resources provides a strong foundation for future gasification‐based “biorefineries” that might produce a variety of renewable fuels, electricity, and chemicals in conjunction with pulp and paper products (Figure 9.17) (Perlack et al. 2005).
If the biomass resources from which energy carriers are produced at such biorefineries are sustainably grown and harvested, there would be few net lifecycle emissions of CO2 associated with biorefineries and their products. To the extent that the biorefinery products replace fuels or chemicals that would otherwise have come from fossil fuels, there would be net reductions in CO2 emissions from the energy system as a whole. The reductions would be even more significant if by‐product CO2 generated at biorefineries were to be captured and sent for long‐term underground storage (Larson et al. 2006b). Carbon capture and storage with fossil fuels is of wide interest today. Several large‐scale CO2 storage projects (storing >1 million T/Y of CO2) are operating and more are under development worldwide to demonstrate feasibility (USDOE 2006).
Coupled with the potential to address national energy security and global warming concerns is the looming need in the US pulp and paper industry for major capital investments to replace the aging fleet of Tomlinson recovery boilers used today to recover energy and pulping chemicals from black liquor. The majority of Tomlinson boilers operating in the United States were built beginning in the late 1960s through the 1970s. With serviceable lifetimes of 30–40 years, the Tomlinson fleet began undergoing a wave of life‐extension rebuilds in the mid‐1980s. Within the next 10–20 years, rebuilt boilers will be approaching the age at which they will need to be replaced, the capital investment for which at a typical mill will be between $100 and $200 million. A similar situation exists in the European pulp industry. This situation provides an unusual window of economic opportunity for introducing black liquor gasifiers as replacements for Tomlinson boilers. Concerted efforts are ongoing in the United States and Sweden to develop commercial black liquor gasification technologies (Ekbom et al. 2005; Larson et al. 2006a; Swedish Forest Agency 2008).

Figure 9.17 “Biorefinery” concept based on pulp and paper manufacturing facility (kraft).
Source: From Perlack et al. (2005).
9.7.2 Black Liquor Gasification (BLG): Introduction
Black liquor is a biomass feedstock with unique properties suitable for gasification (Ǻdahl et al. 2004; Andersson and Harvey 2004; Bajpai, 2008, 2013; Berglin et al. 2002; Dahlquist et al. 2009; Grigoray 2009; Marklund 2006; Salomonsson, 2013; Sricharoenchaikul 2009, 2001; Waldner and Vogel 2005; Wikipedia 2016). First of all, it is available at existing industrial sites in large quantities. Second it is liquid. This makes it possibly to easily feed it by pumping into the pressurized gasifier. The liquid state also makes the black liquor easy to atomize into a fine mist that reacts very fast in the gasifier. Third, the gasification of black liquor char is more rapid than for any other feedstock as the inherently high sodium and potassium content of black liquor acts as a catalyst. These properties make it possible to apply the high temperature, entrained flow gasification principle to black liquor. This type of gasification process provides many advantages over alternative gasification technologies. It is very rapid, single‐stage gasification process with low reactor volume, and low emissions (including particulate matters and odorous TRS gases) and low power consumption.
9.7.2.1 Black Liquor Properties
The black liquor is an aqueous solution of lignin residues, hemicellulose, and the inorganic chemicals used in the process. The black liquor is sent through a series of evaporators to increase the solid content in order to make it suitable for combustion. In the evaporators, the BLS content is increased from about 15 wt% to about 70–75 wt%. The concentrated black liquor that goes into the recovery boiler consists of about 30% water, 30% valuable inorganic cooking chemicals, and 40% lignin and other organic matter separated from the wood. The higher heating value is about 14 000 kJ/kg solids. Typically, a large pulp‐mill will produce about 200 T/day of black liquor corresponding to about 300 MWh (Bajpai 2014).
The organic matter in the black liquor is made up of water/alkali soluble degradation components from the wood. Lignin is degraded to shorter fragments with sulfur content at 1–2% and sodium content at about 6% of the dry solids. Cellulose and hemicellulose is degraded to aliphatic carboxylic acid soaps and hemicellulose fragments. The extractives gives tall oil soap and crude turpentine. The soaps contain about 20% sodium. Table 9.4 presents some properties of black liquor.
9.7.2.2 BLG Technologies
BLG may be performed either at low temperatures or at high temperature based on whether the process is conducted above or below the melting temperature range (650–850 °C) of the spent pulping chemicals (Sricharoenchaikul 2001).
- Low‐temperature gasification – Low‐temperature gasifier operates at 600–850 °C, below the melting point of inorganics, thus avoiding smelt–water explosions.
- High‐temperature gasification – High‐temperature gasification units generally operate in the 900–1000 °C range and produce a molten smelt.
In low‐temperature gasification, the alkali salts in the condensed phase remain as solid products, while molten salts are produced in high‐temperature gasification. Low‐temperature gasification is advantageous over high‐temperature gasification because gasification at low temperature yields improved sodium and sulfur separation. Additionally, low‐temperature gasification requires fewer constraints for materials of construction because of the solid product. However, syngas of low‐temperature gasification may contain larger amounts of tars, which can contaminate gas cleanup operations in addition to contaminating gas turbines upstream of the gasifier. These contamination problems can result in a loss of fuel product from the gasifier (Patrick and Siedel 2003; Sricharoenchaikul 2001).
Several companies have performed trials to develop a commercially feasible process for BLG. History of BLG development is very well described by Whitty and Baxter (2001) and Whitty and Verrill (2004). In the search for alternative ways to recovering the cooking chemicals, gasification techniques have been thoroughly examined several times. In total, more than 20 different technologies have been investigated over the years (Swedish Energy Agency 2008). The most interesting attempts of accomplishing low‐temperature black liquor gasification (LTBLG) and high‐temperature black liquor gasification (HTBLG) processes are presented in Table 9.5. Only two technologies are currently being commercially pursued: the MTCI (Manufacturing and Technology Conversion International Company, low temperature) and Chemrec (high temperature) technologies. A pressurized, oxygen‐blown, HTBLG technology, shown in Figure 9.18, is being developed by Chemrec, a Swedish company (Chemrec 2006; Whitty 2006; Whitty and Nilsson 2001). Table 9.6 gives the difference between these two technologies.
Table 9.4 Properties of black liquor.
| Density (50% solids) at 93 °C | 1.26 g/cm3 |
| Viscosity (50% solids at 121 °C) Viscosity (70% solids at 125 °C) |
5.0 and 100.0 cP |
| Specific heat (40% solids) | 2.0 KJ/(kg·K) |
| Heating value (35% lignin and 70% solids) | ~14 000 kJ/kg |
Table 9.5 Black liquor gasification processes.
Source: From Whitty and Verrill (2004), Grigoray (2009), Naqvi et al. (2010), and Swedish Energy Agency (2008).
| SCA‐Billerud process |
| Copeland process |
| Weyerhaeuser's “dry pyrolysis” process |
| St. Regis hydropyrolysis process |
| The Texaco coking process |
| VIT circulating fluidized gasification process |
| Babcock & Wilcox's bubbling fluidized bed gasification process |
| NSP or Ny Sodahus process |
| DARS process (Direct Alkali Recovery System) |
| ABB circulating fluidized bed gasification process |
| MTCI/TRI's fluidized bed steam reforming process |
| Kellogg, Brown, and Root's spouting and transport fluidized bed gasifiers |
| Paprican's AST process |
| University of California's “pyrolysis, gasification, combustion” process |
| The Champion–Rockwell molten salt gasification process |
| The SKF plasma black liquor gasifier |
| Ahlstrom's suspension gasifier |
| Tampella's entrained flow gasifier |
| Noell's entrained flow gasifier |
| Champion–Rockwell molten salt gasification process |
| Chemrec entrained flow gasification process |
| Catalytic hydrothermal gasification of black liquor |

Figure 9.18 Pressurized, oxygen‐blown, HTBLG technology being developed by Chemrec, a Swedish Company.
Source: From Chemrec (2006), Whitty (2006) and Whitty and Nilsson (2001).
Table 9.6 Difference between LTBLG and HTBLG.
Source: From Swedish Energy Agency (2008).
| Property | LT (TRI) | HT (Chemrec) |
| Heating | Indirectly (syngas) | Directly (black liquor) |
| Chemical recovered | Solid phase | Smelt |
| Sulfur split (gas/smelt) | 90/10 | 50/50 |
| Syngas composition | High H2 concentration | Moderate H2 concentration |
| Syngas energy content | High | Low |
9.8 Chemical Recovery and Power/Steam Cogeneration at Pulp and Paper Mills
9.8.1 The Pulp and Paper Industry
The pulp and paper industry is one of the largest industries in the world. It is also an important source of employment in many countries. A sustainably managed pulp and paper industry can bring several benefits to the local economy and people particularly in rural areas. The industry is dominated by North American, Northern European, and East Asian countries like Japan. Latin America and Australasia also have significant pulp and paper industries. Over the next few years, it is expected that both India and China will become the key in the industry's growth.
Pulp and paper are primarily made out of wood fibers originating from natural forests or pulpwood plantations. Recycled fiber and other fiber sources such as agricultural residue are also utilized and recycled fiber is becoming more commonly used in pulp and papermaking. There are two significant pulping technologies available that differ greatly in terms of process, i.e. mechanical and chemical pulping. Approximately 30% of the total pulp production in European Union is from mechanical pulping, while the rest is produced by means of chemical pulping (Das 2005; Das and Houtman 2004; Swedish Forest Agency 2008). North America has major pulp and paper industry, about 21% of the total pulp produced is from mechanical pulping and rest is produced chemically.
A pulp mill that produces bleached kraft pulp generates 1.7–1.8 T of black liquor (measured as dry content) per ton of pulp. Black liquor, thus, represents a potential energy source of 250–500 MW/mill. As modern kraft pulp mills have a surplus of energy, they could become key suppliers of renewable fuels in the future energy systems. Today, black liquor is the most important source of energy from biomass. It is, thus, of great interest to convert the primary energy in the black liquor to an energy carrier of high value. Table 9.7 gives the relative annual production of some major fuels (Farmer and Sinquefield 2009). A key advantage of black liquor compared to biofuels and fossil fuels is that it is already at the mill; the handling infrastructure already exists and there are no collection and transport costs.
Table 9.7 Annual production of selected fuels.
Source: From Farmer and Sinquefield (2009).
| Production | Million T/Y |
| Black liquor solids | 200 |
| Paper and board | 180 |
| Crude oil | 4000 |
| Hard coal | 500 |
Worldwide, the pulp and paper industry currently processes about 170 million T of black liquor (measured as dry solids) per year, with a total energy content of about 2 EJ, making black liquor a very significant biomass source (IEA Bioenergy Report 2009). In comparison with other potential biomass sources for chemical production, black liquor has the great advantage that it is already partially processed and exists in a pumpable, liquid form. Using black liquor as a raw material for liquid or gaseous biofuel production in a biorefinery has many advantages: biomass logistics are extremely simplified as the raw material for fuel production is handled within the boundaries of pulp and paper plant; the process is easily pressurized, which increases fuel production efficiency; due to the processing of wood to pulp, the produced syngas has a low methane content, which optimizes fuel yield; pulp mill economics become less sensitive to pulp prices when diversified with another product; and gasification capital cost is shared between recovery of inorganic chemicals, steam production, and syngas production. Overall, if the global production of black liquor was to be used for transportation biofuel production, then this would correspond to about 48 million T of methanol, compared with current world production from fossil fuels of about 32 million T, a significant impact.
9.8.2 Black Liquor Gasification Combined Cycle Power/Recovery
Gasifying black liquor enables it to be used as fuel in a gas turbine combined cycle, a much more efficient electricity generating option than the Tomlinson boiler steam turbine technology. In a typical “mill scale” black liquor gasification combined cycle (BLGCC), shown in Figure 9.19, the black liquor is gasified, and the syngas product is cooled, cleaned, stripped of H2S (using a Selexol® unit), and then burned in the gas turbine. The turbine exhaust passes through a “duct burner” to the heat recovery steam generator (HRSG), where steam is raised to drive a steam turbine. A small amount of natural gas is burned in the duct burner to enable production of the requisite process steam needed to run the mill. Steam is extracted at two different pressures from the steam turbine. The HRSG steam production is augmented by steam delivered from hog fuel boilers assumed to be pre‐existing at the reference mill. One design constraint in the BLGCC analysis was the size of the existing hog fuel boilers, which limited the available steam delivered from these boilers (This limitation is the reason a small quantity of natural gas is used in the duct burner (Black Liquor, Wikipedia, 2016).
9.8.3 Biorefinery
Figure 9.20 presents a schematic of the dimethyl ether (DME) biorefinery. In the DME biorefinery, the black liquor gasifier, supplied with oxygen from an air separation unit, provides all of the synthesis gas to a liquid‐phase DME reactor. Unconverted synthesis gas is separated from product DME and 97% of it is recycled to the synthesis reactor to increase DME production. The 3% purge stream taken from the recycle loop prevents excessive buildup of inert gases. The purge gas is sent to the hog fuel boiler, where it burns with an amount of wood residues selected such that the amount of steam generated is sufficient to meet all of the mill's process steam needs. Heat recovered from the black liquor and syngas processing areas are integrated into the boiler to increase steam production and minimize the amount of woody residues needed. The steam is expanded through a back‐pressure turbine to generate some electricity which goes toward meeting the mill's process electricity needs. To fully meet the mill's electricity need, some electricity must be imported from the grid. Because black liquor is being converted primarily into liquid fuel and not electricity, the amount of electricity imported is larger than with a conventional Tomlinson system (ETC 2011; Larson et al. 2006a).

Figure 9.19 Simplified schematic representative “mill‐scale” BLGCC (BLGCC).
Source: From Larson et al. (2006a).
Figure 9.20 Schematic of biorefinery of DME. Key features include recycling of unconverted syngas to increase DME production and use of steam power.
Source: From Larson et al. (2006a).
Figure 9.21 Flow diagram of power/recovery system in pulp mill BLGCC.
Source: From Naqvi et al. (2010).
Figure 9.21 gives a simplified representation of the basic design of the BLGCC system (Naqvi et al. 2010). The electricity output will be increased especially with pressurized gasification and a gas turbine–based cogeneration system. A number of studies were performed to estimate potential electricity generation in the pulp industry by implementing a BLGCC technology (Eriksson and Harvey 2004; KAM Report 2003; Larson et al. 2003).
Hydrogen production from gasified black liquor has also been studied. Andersson and Harvey (2004) evaluated energy and net CO2 emissions consequences of integrated hydrogen production from BLG in a chemical pulp mill. The base capacity of the pulp mill was 2000 ADt/day of pulp production. Hydrogen production at the reference KAM pulp mill required import of 210 000 T of biomass for steam production and 0.43 TWh/year of electricity. If black liquor from all pulp mills in Sweden were gasified to produce hydrogen, the estimated import of biomass was 4.9 and 2.3 TWh/year of electricity. The results showed a large potential of hydrogen production, i.e. 188 T/day of hydrogen fuel which was equivalent to 261 MW [lower heating value (LHV), 120 MJ/kg] (KAM Report 2003).
Larson et al. (2006a, b) made a “well‐to‐wheels” (WTW) comparative environmental assessment of BLG for liquid fuel production with BLGCC electricity production at kraft pulp mills in the United States. The introduction of BLGCC systems in the United States had potential to displace up to 35 million T net CO2, 16 000 T net SO2, and 100 000 T net NOx within 25 years (Naqvi et al. 2010).
9.8.4 Liquid Fuels Synthesis
The conversion of clean synthesis gas into a liquid fuel involves passing the syngas over a catalyst that promotes the desired synthesis reactions and then refining the raw product to obtain the final desired liquid fuel. Two basic designs for commercial synthesis reactors have been developed: gas‐phase (or fixed‐bed) and liquid‐phase (or slurry‐bed). Fixed‐bed reactors have a long commercial history, but liquid phase reactors have been gaining popularity in commercial applications because of attractive performance attributes and lower cost. Liquid phase reactors are now commercially offered for Fischer–Tropsch liquids (FTL), methanol, and DME synthesis. Liquid phase reactors for mixed‐alcohol synthesis are still under development.
Fixed‐bed and liquid‐phase reactor designs differ primarily in their handling of reactor temperature control. Synthesis reactions are exothermic, such that the reactor temperature increases as the reactions proceed if no heat is removed. Higher temperatures promote faster reactions, but maximum (equilibrium) conversion is favored by lower temperatures. Also, catalysts are deactivated when overheated. Thus, the temperature rise in a synthesis reactor must be controlled. In commercial practice, a reactor‐operating temperature of 250–280 °C for methanol, DME, or FTL synthesis balances kinetic, equilibrium, and catalyst activity considerations. For mixed‐alcohols synthesis, which is not yet a commercially established technology, higher reaction temperatures (300–400 °C) have been indicated with catalysts identified to date (Aden et al. 2005; Air Products and Chemicals, Inc. 2001).
A gas‐phase reactor incorporates the flow of syngas over a fixed‐bed of catalyst pellets. With this design it is difficult to maintain isothermal conditions by direct heat exchange (due to low gas‐phase heat transfer coefficients). To limit temperature rise, the synthesis reactions are typically staged, with cooling between reactor stages. Also, by limiting the initial concentration of CO entering the reactor (to 10–15 vol%) the extent of the exothermic reactions can be controlled. Control of the CO fraction is achieved in practice by maintaining a sufficiently high recycle of unconverted H2‐rich syngas back to the reactor.
In a liquid‐phase reactor, syngas is bubbled through an inert mineral oil containing powdered catalyst in suspension (Figure 9.22). Much higher heat release rates (i.e. extents of reaction) can be accommodated without excessive temperature rise as compared to a gas‐phase reactor because of more effective reactor cooling by boiler tubes immersed in the fluid. The vigorous mixing, intimate gas‐catalyst contact, and uniform temperature distribution enable a high conversion of feed gas to liquids in a relatively small reactor volume. Conversion by liquid‐phase FT synthesis is especially high. A single‐pass fractional conversion of CO of about 80% can be achieved (Bechtel Group, Inc. 1990), compared to less than 40% for conversion with traditional fixed‐bed FT reactors. For the FT reactor conditions it is assumed in simulations, the single‐pass CO conversion is about 65%.
9.8.5 Dimethyl Ether
Single‐step DME synthesis reactors typically utilize a mix of two catalysts, one promoting the synthesis of methanol from syngas (CO + 2H2 → CH3OH) and one promoting the dehydration of the methanol to DME (2CH3OH → CH3OCH3 + H2O). Both liquid‐phase and fixed‐bed reactors are offered commercially. Leading developers of liquid‐phase DME synthesis reactors are DME Development, Inc., a Japanese consortium of nine companies led by NKK and Nippon Sanso (Adachi et al. 2000; Air Products and Chemicals 2001; Brown et al. 1991; Fujimoto et al. 1995; Ohno 2006).
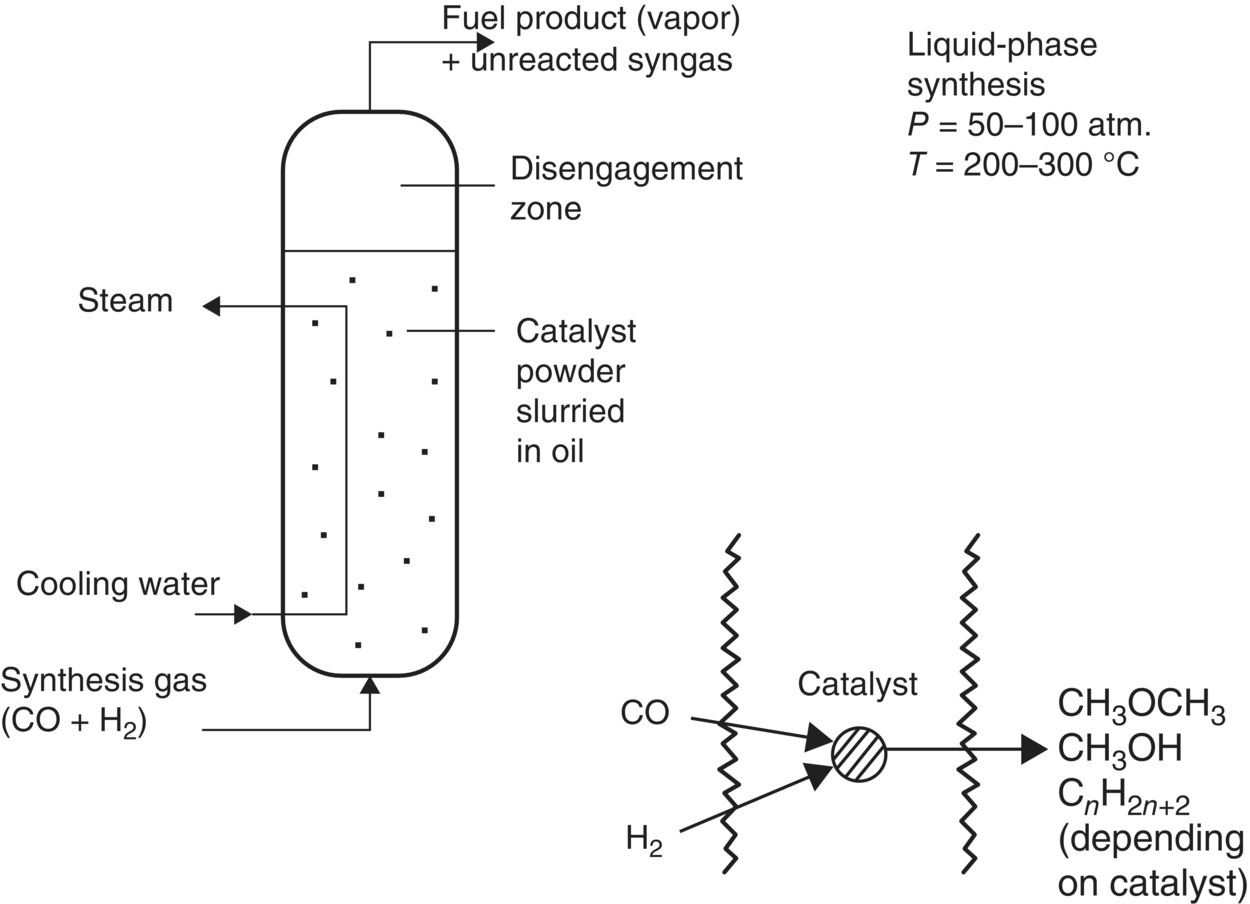
Figure 9.22 Schematic of liquid‐phase synthesis reactor.
Source: From Larson et al. (2006a).
The DME reactor design of Air Products is derived from its liquid‐phase methanol (LPMEOH) synthesis process developed in the 1980s. A commercial‐scale LPMEOH demonstration plant (250 T/day methanol capacity) has been operating since 1997 with gas produced by the Eastman Chemical Company's coal gasification facility in Kingsport, Tennessee (Eastman Chemical and Air Products and Chemicals 2003). The construction of this facility was preceded by extensive testing in a 10 tpd process development unit (PDU) in LaPorte, Texas. The PDU was operated in 1999 to generate test data on direct DME synthesis (Air Products and Chemicals 1993, 2001, 2002; Weyerhaeuser Company 2000).
9.8.6 Pressurized Chemrec BLG
In 2010, a black liquor gasification DME and motor fuel (MF) demonstration plant was inaugurated in Piteå in Sweden (Landälv et al. 2010). Figure 9.23 shows a schematic of the Piteå DME and MF production plant (Landälv et al. 2010; Larson et al. 2006a). When used as a fuel in a truck diesel engine, BioDME gives an equally high‐efficiency rating and a lower noise level compared with a conventional engine. Compared with diesel, BioDME generates an impressive lower carbon dioxide emission (CO2). In addition, its combustion produces very low emissions of particulate matters (PM) and nitrogen oxides (NOx). This makes BioDME an ideal fuel for diesel engines. DME is a gas at ambient conditions but is liquefied at low pressure, just five bars and is handled as a liquid during distribution and use. It is simple to handle, similar to propane. DME can be produced from natural gas and also from various types of biomass. When it is produced from biomass, it is known as BioDME (Larson et al. 2006a; Salomonsson 2011).
The BioDME project aims to demonstrate production of environmentally optimized synthetic biofuel from lignocellulosic biomass at an industrial scale (Landävl 2010; Lindblom 2012; Salomonsson 2013). The project involves a consortium of Chemrec, Haldor Topsøe, Volvo, Preem, Total, Delphi, and ETC. The project is supported by the Swedish Energy Agency and the EU's Seventh Framework Program. The output of this demonstration is DME produced from black liquor through the production of clean syngas and a final fuel synthesis step. The overall possible chemicals that can be produced from the syngas are hydrogen, methanol, DME, FT fuels, ethanol, and methyl tertiary butyl ether (Tampier et al. 2004).
9.8.7 Catalytic Hydrothermal Gasification of Black Liquor
The process is also known as supercritical water oxidation. This is a novel technology to produce methane‐rich synthesis gas as an alternative to replace the conventional recovery system (Naqvi et al. 2010). Heat demand for bringing water to supercritical conditions is less than that for evaporating at subcritical pressure. This phenomena leads to better energy savings as compared to conventional recovery system (Sricharoenchaikul 2009; Vogel et al. 2005). High‐water content in the black liquor – nearly 80% under supercritical condition – would increase gasification reactions that lead to high organics conversion to the synthesis gas (Calzavara et al. 2005; Williams and Onwudili 2006). This phenomenon results in direct introduction of black liquor to hydrothermal gasifier, removing energy demanding evaporation unit in conventional process, thus decreasing the overall stream demand of the pulp mill. The multi‐effect evaporation unit represents nearly 37% of total heat demand of the pulp mill and can be removed in this technique. Unlike other gasification processes, black liquor from the digestion unit is directly introduced to catalytic hydrothermal gasifier at supercritical water conditions (600 °C, 300 bar). The catalytic hydrothermal gasification process involves three steps:
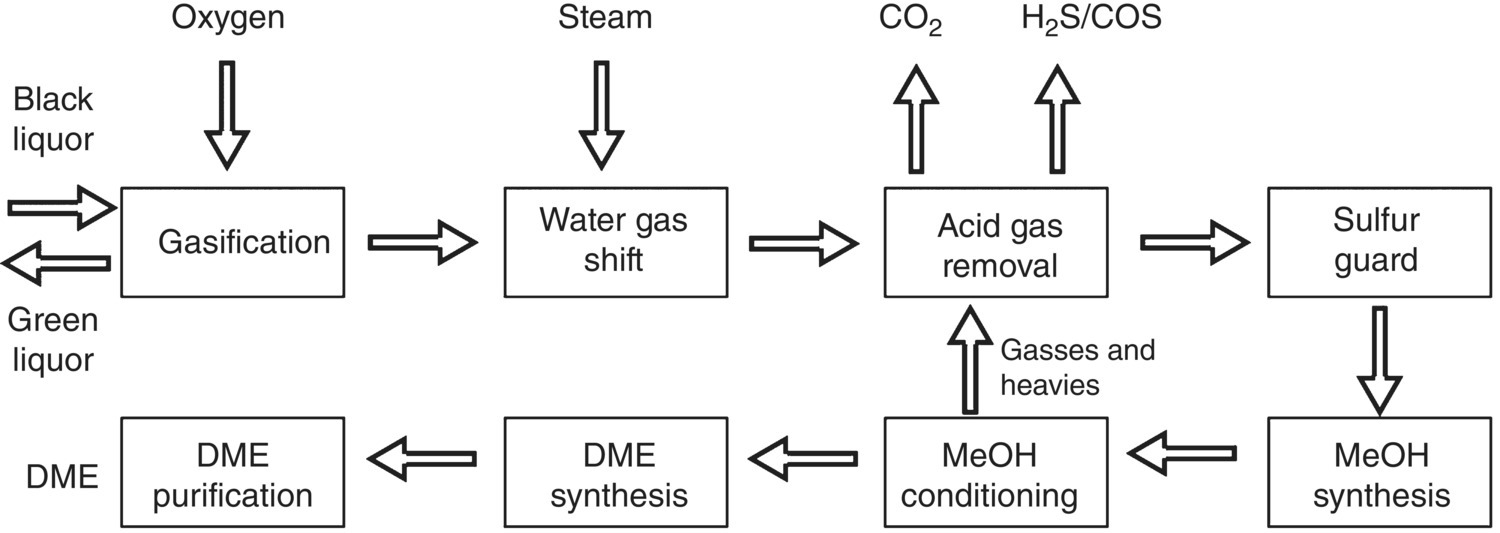
Figure 9.23 Schematic of the Pitéa DME production plant.
Source: Adapted from Landälv et al. (2010) and Larson et al. (2006a).
- Heat up phase (decomposition unit): During this phase, large molecules in black liquor hydrolyze to form alcohols due to the presence of lignin. Cellulose present in lignin decomposes rapidly in water at about 250 °C.
- Salt separation: In salt separator, inorganic salts present in black liquor precipitate and are returned to the pulp mill.
- Catalytic reactor: This is the actual methane synthesis unit where smaller organic molecules, such as carboxylic acid, alcohols, and aldehydes, are converted to CH4, CO2, H2, and CO.
Carboxylic acid is converted to methane, carbon dioxide, and hydrogen. The tar formation is avoided due to supercritical conditions and presence of catalyst.
Sricharoenchaikul (2009) studied the feasibility of supercritical water oxidation technique to convert black liquor to value‐added fuel products and recovery of pulping chemicals. The experiments were conducted in a quartz capillary heated in a fluidized bed reactor based on important operating parameters which were pressure, temperature, feed concentration, and reaction time. The investigator found that pressure between 220 and 400 bar had insignificant influence on the syngas and carbon conversion. But increasing temperature and residence time between 375–650 °C and 5–120 seconds resulted in high‐carbon conversion, greater synthesis gas production, and energy efficiency.
9.8.8 Fischer–Tropsch Liquids
Commercial Fischer–Tropsch catalysts include iron‐ and cobalt‐based materials. Cobalt catalysts produce a large heavy‐wax fraction that can be easily and with high selectivity refined into desired lighter products by subsequent hydrocracking (breaking up of the large hydrocarbon molecules into desired final products in a hydrogen‐rich environment). Hydrocracking of the large straight‐chain hydrocarbons formed by FT synthesis can be done under much less severe temperature conditions (350–400 °C for cracking to C5–C18 range hydrocarbons) than is required for hydrocracking of aromatic molecules found in conventional petrochemical refining. Iron‐based catalysts produce a broader product mix that requires a greater level of refining than with cobalt catalysts. Also, unlike cobalt catalysts, iron catalysts promote water‐gas shift activity (CO + H2O → H2 + CO2), making them well suited for use with syngas characterized by H2/CO ratios below the stoichiometric value of 2.2 for FT synthesis.
9.8.9 Mixed Alcohols
Compared with FTL or DME synthesis, the technology for synthesis of methyl‐, ethyl‐, and propanol‐mixed alcohols (MA) is considerably less commercially advanced, and there is sparse published literature on which to base detailed reactor performance estimates. Catalysts that have been examined in the past can be divided into four categories (Liu et al. 1997): ruthenium‐based catalysts, modified methanol catalysts, modified Fischer–Tropsch catalysts, and molybdenum sulfide–based catalysts. Among these, the MoS2‐based catalysts (originally discovered by researchers at Dow and Union Carbide in the 1980s) have received considerable recent attention due to their high tolerance for sulfur‐contaminated syngas, their water–gas shift activity, and their high activity and selectivity for linear alcohols. Selectivity is an especially important characteristic because if all possible chemical reactions between CO and H2 are allowed to compete without constraints, reactions other than those for synthesis of higher alcohols will thermodynamically out‐compete reactions for synthesis of higher alcohols. In particular, the formation of Fischer–Tropsch hydrocarbons (α‐olefins and n‐paraffins) from CO and H2 is thermodynamically favored over the formation of higher alcohols. For this reason, to maximize performance of alcohol synthesis catalysts, high selectivity is an essential feature.
The simplified reactions scheme adopted for the higher alcohols are as follows:
9.8.10 “WTW” Environmental Impact of Black Liquor Gasification
In addition to energy aspects of the biorefinery systems discussed earlier, we have also examined environmental attributes. Water effluents, air emissions, and solid wastes are all of potential concern. In assessing the impact that biorefinery systems would have on these effluents relative to levels found with Tomlinson power/recovery systems, one may consider changes both in direct effluents and in effluents associated with the displacement of grid electricity generation and conventional petroleum‐based motor fuels. In particular, to effectively estimate the full environmental impacts of biorefineries, the current analysis involves estimating the emissions impacts from resource extraction to end use. This so‐called WTW analysis is a common approach for making meaningful comparisons between different alternative and conventional fuels. This approach is necessary because of the different upstream production and conversion processes, different downstream vehicle/engine types for different fuels and significant differences in fuel properties and combustion characteristics.
Biofuels which are produced from BLG process excel in terms of WTW carbon dioxide emission reduction and energy efficiency. Also, synthetic diesel and DME produced from forest harvest residues over the BLG route both showed among the highest WTW GHG reduction and energy efficiency. The total available black liquor volume in the United States with the conversion efficiency of this process is equivalent to approximately 5 billion gal/year as ethanol. The renewable fuels standard calls for 16 billion gal of cellulosic biofuels by 2022. Therefore, this route can give a significant contribution to meeting this target.
The pulp and paper companies in the United States today are meeting severe competition from low‐cost producers overseas and from alternative solutions in both packaging and printed media. Mill operators and their investors now option to transform mills into biorefineries that use this fuels‐from‐the‐forest process. This transformation completely changes a pulp mill's competitive position by adding 30–50% of profitable revenue with the typical 25–40% internal rate of return. It also makes needed reinvestment possible by replacing aged recovery boilers with high‐maintenance costs and low performance. The fuel plant investment can also be used to provide additional recovery capacity, allowing for higher pulp production in many cases. Mills producing only 500 T of BLS per day are viable as fuels‐from‐the‐forest biorefineries are using this method. Most mills are significantly larger. Such a biorefinery mill would produce upward of 8 million gal a year of green motor fuel calculated as gasoline equivalents at the minimum capacity size. In a mill investing in second‐generation biofuels technology, jobs are not only preserved but also additional jobs are created, mainly for extraction of biomass from the forest as well as to operate and maintain the biofuels plant. Other economics and social benefits are also significant such as possible tax benefits and air emissions reductions (Bajpai 2014).
Typical capital investment for a biorefinery project that uses fuels from the forest is $200–400 million, depending on plant size and the cost to interconnect to the mill. The BLG industry is vigorously pursuing federal and state grants and loan guarantees to ramp up this technology as soon as possible to large‐scale commercial capacity. As a source of ultra‐clean, renewable motor fuels, the BLG route that transform pulp and paper mills into refineries is standing up to a critical scrutiny as a viable and practical way of producing alternative, renewable energy, while making good use of the land and being gentle to the environment (Bajpai 2014).
Forest biorefinery utilizing gasification in a BLGCC configuration rather than a Tomlinson boiler is predicted to produce significantly fewer pollutant emissions due to the intrinsic characteristics of the BLGCC technology. Syngas cleanup conditioning removes a considerable amount of contaminants and gas‐turbine combustion is more efficient and complete than boiler combustion. There could also be reductions in pollutant emissions and hazardous wastes resulting from cleaner production of chemicals and fuels that are now manufactured using fossil energy resources. In addition, it is generally accepted that production of power, fuels, chemicals, and other products from biomass resources creates a net zero generation of carbon dioxide, as plants are renewable carbon sinks. A key component of the forest biorefinery concepts is sustainable forestry. The forest biorefinery concept utilizes advanced technologies to convert sustainable woody biomass to electricity and other valuable products and would support the sustainable management of forest lands (Farmer 2005). In addition, the forest biorefinery offers a productive value‐added use for renewable resources such as wood thinning and forestry residues and also urban waste (Mabee et al. 2005; Miller et al. 2005).
BLG whether conducted at high or low temperatures is still superior to the current recovery boiler combustion technology (Bajpai 2008, 2012). The thermal efficiency of gasifiers is estimated to be 74% compared to 64% in modern recovery boilers, and the integrated gasification and combined cycle (IGCC) power plant could potentially generate same amount of fuel (Dance 2005; Farmer and Sinquefield 2003). While the electrical production ratio of conventional recovery boiler power plants is 0.025–0.10 MWe/MWt, the IGCC power plant can produce an estimated 0.20–0.22 MWe/MWt (Farmer and Sinquefield 2003; Sricharoenchaikul 2001). This increase in electrical efficiency is significant enough to make pulp and paper mills potential exporters of renewable electric power. Alternatively, pulp mills could become manufacturers of bio‐based products by becoming biorefineries. Additionally, the new technology could potentially save more than 100 trillion Btu of energy consumption annually, and within 25 years of implementation, it could save up to 360 trillion Btu/year of fossil fuel energy (Larson et al. 2003). The new technology also offers the benefits of improved pulp yields if alternative pulping chemistries are included and reductions in solid waste discharges. Also, the process is inherently safer because the gasifier does not contain a bed of char smelt unlike in recovery boilers, which reduces the risk of deadly smelt–water explosions (Argonne National Laboratory 2006; Sricharoenchaikul 2001).
9.8.11 Water and Solid Waste
Water quality, temperature, and consumption are all potential concerns with biorefineries. Over time, as demand rises for limited freshwater supplies, these issues are likely to only become more important. Briefly, the issues are as follows:
- Water quantity: Any water savings results in a direct financial benefit to a mill and also addresses growing concerns over the availability of freshwater for other purposes (e.g. agriculture, human consumption).
- Water quality: It is of major concern for rare and endangered species, recreation, and for its effects on other users downstream (e.g. municipalities).
- Thermal discharge: The temperature of the cooling water discharge is also of concern for its effect on flora and fauna.
Depending on the configuration (amount of fuels and electricity produced), the biorefinery will have different effects on water quantity and thermal discharge at a mill, but overall the conversion to biorefineries is not expected to significantly impact water quality, especially when considering the impacts on displaced grid power (Larson et al. 2003). Also, IGCC power plant will reduce cooling water and make‐up water discharges locally at the mill, and because the efficient gasifiers will cause grid power reductions, substantial reductions in cooling water requirement at power plant will also occur (Larson et al. 2003). Overall, the implementation of IGCC power plant will cause net savings in cooling water requirements and net reductions in wastewater discharges. An additional benefit is also the avoided water usage in conventional fuel production, which has not been quantified. Moreover, the consequences of spills from petroleum and petroleum product transportation are also reduced. Also, some of the biofuels, namely DME and MA, pose much lower risks of groundwater contamination in the event of a fuel leak or spill (e.g. at refueling stations). FT liquids, since they contain very low aromatics, should also pose a lower risk than conventional diesel and gasoline.
Solid waste issues relate to the quantity and toxicity of any solids that must be disposed of. In this regard, biorefineries are not expected to result in significant changes at the mill, in part because the solids produced (mainly ash from biomass) are not problematic to deal with. There will be the need to periodically replace catalysts and guard beds, such as zinc oxide (for H2S) and activated carbon (for other trace contaminants). Nevertheless, as with water usage, the impacts of displaced grid power (particularly for the coal component of that grid power) and conventional transportation fuel use would likely result in important reductions of solid waste generation (Larson et al. 2003).
9.8.12 Mill‐Related Air Emissions
The most significant effluent differences between biorefineries and Tomlinson systems are expected to be in air emissions. This is particularly expected to be the case in a WTW context. As discussed below, air emissions were estimated in detail for both the biorefineries and the Tomlinson power/recovery systems. For comparison, the BLGCC case from our earlier study is also shown, with the updated assumptions consistent with the current analysis (e.g. grid power emissions). Actual air emissions data are available for modern Tomlinson systems. Since emissions data do not exist for BLGCC or biorefinery systems, estimates were made starting with data for coal IGCC and natural‐gas combined cycle power systems and adjusting appropriately. Note that relative to the BLGCC configuration, sources of air emissions in a biorefinery are expected to be similar, namely the power island. The production of the biofuel itself does not lead to significant additional sources of air emissions at the biorefinery.
The air emissions analysis presented below is not intended to serve as a complete lifecycle analysis of biorefinery emissions. Rather the estimates provide indicative results of the potential impacts of biorefinery options relative to “business as usual” in the pulp and paper industry. For example, upstream emissions for grid power (i.e. fuel production and transportation to the power plant) are not included, but these are relatively small compared to the power plant emissions themselves and to the total emissions from conventional motor fuel chains. To the extent that most of the biorefinery configurations result in more displaced grid power than the Tomlinson case, the emissions benefits estimated in this study can be considered conservative because they do not also factor in emissions reductions related to fuel supply for power plants.
Air emissions fall into three basic categories: criteria pollutants, hazardous air pollutants (HAPs), and GHGs. This study includes quantitative estimates for the criteria pollutants: sulfur dioxide (SO2), nitrogen oxides (NOx), carbon monoxide (CO), volatile organic compounds (VOCs), PM, and total reduced sulfur (TRS). Estimates are also made for carbon dioxide (CO2), the major greenhouse gas. HAPs and other emissions issues are discussed qualitatively.
9.8.13 Tomlinson Boiler Air Emissions
Modern Tomlinson boilers are characterized by emissions of criteria pollutants that are similar overall to grid power (some are higher, like CO and PM, while others are lower, like SO2 and NOx). The most significant pollutants, in terms of both environmental impacts and relative emissions rates from Tomlinson boilers, are NOx and particulates. While many furnaces already have particulate controls in place, there is no effective form of NOx after treatment. Furnace rebuilds and replacements trigger the New Source Review (NSR) process, which generally results in process modifications being made to reduce TRS emissions. Installation of more efficient particulate control is also common following a NSR, and generally modern furnaces have better design and controls than older ones, which results in lower overall emissions. Table 9.8 presents a qualitative analysis of relative environmental impact of different mill‐level emissions, together with relative emission rates for controlled and uncontrolled Tomlinson furnaces and with biorefinery technology (Argonne National Laboratory 2006; USEPA 2002).
9.8.14 Economic Development Opportunities
Biorefineries could have important economic development benefits, stemming from the enhancement of the competitiveness of the pulp/paper industry. The financial analysis illustrated the potential for attractive financial returns and significantly increased cash flows relative to Tomlinson systems. The related economic development benefits include preserving and growing employment in the industry and potentially adding to rural and semi‐rural employment by creating increased demand for raw materials for paper production and biomass supply and, in the longer term, energy and other products derived from biomass. On a national scale these impacts are likely to be modest, but in certain regions or states (especially southeast, upper mid‐west), the impacts could be very significant. However, if biorefineries help catalyze a new, larger, bio‐energy industry, the economic impacts would be more substantial at the national level as well.
A kraft mill that adopts BLG exclusively to displace the boiler and to produce more electricity can be profitable (Larson et al. 2003, 2006a). But at present, a much developed biorefinery with a diverse product range adds many new options that may become economically attractive if BLG technology can solve a few technical challenges (Bajpai 2008). Current BLG technology has excited new interest in biorefinery and has the ability to leverage BLG into a wide portfolio of products, retrofitted to different types of mill, including medium‐sized mills facing shutdown. The Chemrec development plant in Piteå, Sweden, is the most developed BLG model with strong industry momentum. Volvo Technology Transfer AB and affiliates new hold a majority share. The biorefinery aims to produce pulp (and paper) and to use the BLG syngas to produce DME fuel. DME is clean‐burning and has advantage over diesel. Chemrec has successfully implemented a DME demonstration in Piteå. The Chemrec 2020 plan anticipates five large and three small BLGDME and BLGMF plants. Economic projections are attractive. This is largely due to return from DME (Ekbom et al. 2003, 2005; Connor 2007).
Table 9.8 Qualitative indication of relative environmental impact of different mill‐level emissions, together with relative emission rates for controlled and uncontrolled Tomlinson furnaces and with biorefinery technology.
Source: From Larson et al. (2006b).
| Pollutant/discharge | Relative environmental impact of pollutant | Relative emissions rates from Tomlinson furnace (uncontrolled) | Relative emissions rates with control on Tomlinson | Relative emission rates expected with biorefineries |
| SO2 | H | L | L (not required) | VL |
| NOx | H | M | Md | VL |
| CO | L | M (can be highly variable) | Md | VL |
| VOC | H | L | Ld | VL |
| PMb | H | Hc | L–M | VL |
| CH4 | L–M | L | Ld | VL |
| HAPs | M–H | Lc | Lc | VL |
| TRSe | L | L | Ld | VL |
| Waste Waterf | M–H | L | L | VL–L |
| Solidsa | L | L | L | L |
VL, very low; L, low; M, moderate; H, high.
a General importance, not specifically for the P&P industry.
b PM, particulate matter. Of greatest concern with PM emissions are fine particulates smaller than 10 and 2.5 μm in diameter (PM10 and PM2.5 respectively).
c Current MACTII rules are expected to result in about a 10% reduction of HAPs and a modest reduction in PM.
d Not generally practiced other than by maintaining good combustion efficiency.
e Total reduced sulfur.
f For power systems, the issue is mainly one of the cooling water (quantity and discharge temperature).
If biorefineries were to penetrate slowly rather than rapidly into the market, the cumulative (25‐year) energy savings would be roughly 2–12 quads less. Assuming a rough average fossil fuel price range of $5–10/MMBtu (which corresponds to $29–58/barrel of crude oil or 1.7–3.4 ¢/kWh of electricity), the corresponding added energy costs would be $10–120 billion over this period (Larson et al. 2006a, b).
For certain emissions, it is also possible to estimate a market value since there are existing cap‐and‐trade markets. At $625/T (the recent price for SO2 allowances), and assuming prices remain at this level in real terms, SO2 reductions have a cumulative value of up to $301 million over the 25‐year period following commercial introduction of biorefineries. (In some of the configurations, the net SO2 benefit is negative because of the large decreases expected in grid power SO2 emissions discussed earlier.) NOx, if valued at $2100/T over the same period, has a market value as high as of $1.5–2.6 billion in the Aggressive market penetration scenario. If a system for trading CO2 is put in place, the CO2 value could be as high as $37 billion in the Aggressive market penetration scenario at a price of $25/MT of CO2. While it will likely be difficult for biorefineries to capture all of these additional revenue streams, these estimates provide an indication of the value to the nation of emissions reductions that biorefineries could enable. Thus, in addition to energy costs savings, the value of lost SO2, NOx, and CO2 emissions reductions due to slower market penetration could also be in billions of dollars (Larson et al. 2006b).
9.8.15 Cost‐Benefit Analysis
A cost‐benefit analysis of BLG‐based bio‐refining in the kraft pulp and paper mill was studied by Larson et al. (2006a) and Consonni et al. (2009). The study assumed that BLG systems are technologically comparable with Tomlinson recovery boilers. Hence, BLGCC or bio‐refinery systems can be integrated within kraft pulp mill replacing the conventional recovery technology.
The reference mill with a base capacity of 2458 T BLS/day was an integrated pulp mill producing uncoated free sheet paper from a 65/35 mix of hardwood and softwood. DME production from LTBLG was compared to HTBLG with identical black liquor input. DME energy flow of 137.4 MW was estimated in the LTBLG integration and 168 MW for the HTBLG case. The power boiler consumed larger fuel (unconverted syngas and wood residues) in the LTBLG case than in the HTBLG case but resulted in 64% more back‐pressure steam turbine electricity production. From the preliminary calculations, it appeared that the LTBLG in DME pulp mill bio‐refinery integration would produce 15–20% less DME than in the HTBLG bio‐refinery, with both configurations requiring electricity import. In addition, the LTBLG case required about double biomass import than the HTBLG, and fuel oil import for the lime kiln was about 25% higher. Table 9.9 compares potential electricity or fuel production from various studies.
9.9 Conclusions
One may consider a modern pulp and paper mill as a first‐generation forest biorefinery, with steam, power, and other products being produced alongside the wide range of paper products we normally associate with the industry. Black liquor and biomass gasification are key technology platforms for realizing the forest biorefinery of the future. Current research and development has shown that gasification‐based pulp mill biorefinery technologies, once fully commercialized, offer the potential for attractive investment returns. They also offer the potential for important contributions toward national petroleum savings, emissions reductions, improved energy security, and rural economic development – contributions that could be two times or larger the size of contributions from the existing US corn–ethanol industry. The introduction of BLGCC systems in the United States had potential to displace up to 35 million T net CO2, 16 000 T net SO2, and 100 000 T net NOx within 25 years.
These potential private and public benefits arise, fundamentally, because of the integration of biorefining with pulp and paper production, such that the biorefinery is providing chemical recovery services, process steam, and process electricity in addition to exporting liquid fuels (FTL, DME, MA) and perhaps some electricity. We have analyzed in detail a variety of integrated pulp/paper mill biorefinery designs encompassing a broad range of product slates. An overarching finding is that integration can effectively enable more efficient use of biomass resources for liquid biofuel production compared to nonintegrated biofuel production. Integration also can effectively reduce the capital investment required per unit of biofuel production to levels comparable to investments needed for coal‐to‐liquids facilities that are an order of magnitude or more larger than prospective pulp mill biorefineries. Finally, integration can effectively reduce the cost of producing gasification‐based biofuels to approximately $1/gal of ethanol equivalent, which would make them competitive with the current cost target developed by analysts at the National Renewable Energy Laboratory for ethanol made from lignocellulosic biomass by enzymatic hydrolysis/fermentation processes.
Table 9.9 Biorefinery estimates based on BLG studies, e.g. BLGCC and BLG for biofuel production.
Source: From Naqvi et al. (2010).
| Parameter | BLGCC | BLG for biofuel production | |||||||
| Reference | Larson et al. (2003) | Eriksson and Harvey (2004) | Ekbom et al. (2003) | Andersson and Harvey (2004) | Larson et al. (2006b) | Naqvi et al. (2010) | |||
| Product | Electricity | Electricity | MeOH | DME | H2 | DME | FTL | MA | CH4 |
| Pulp production (ADt/day) | 1600 | 2000 | 2000 | 2000 | 2000 | 1600 | 1600 | 1600 | 1000 |
| BLS flow (tDS/day) | 2724 | 3420 | 3420 | 3420 | 3420 | 2724 | 2724 | 2724 | 1700 |
| BLS flow (MW) | 350.7 | 487 | 487 | 487 | 487 | 350 | 350 | 350 | 243.5 |
| Biomass import (MW) | 27.1 | 21.3 | 129 | 125 | 123.5 | 77.4 | 102 | 89.2 | 107 |
| Electricity import/export (+/−) (MW) | 15.2 | 86.5 | 45.9 | −48.7 | −56.7 | 99.6 | 12.4 | 8.2 | 1.1 |
| Fuel production (MW) | — | — | 272 | 275 | 261 | 168 | 112 | 60 | 240.2 |
Fuel values are based on LHV.
9.9.1 Summary
- The forest biorefinery offers a business strategy that potential forestry companies are seriously considering for improving the overall financial performance of the sector. However, there are considerable technology and business risks related to its implementation. These risks can be mitigated to a great extent by using systematic product and process design tools for analyzing biorefinery strategies.
- Industry leaders, investors, policy‐makers, and others are now beginning to better understand the vital role to be played by biorefineries as we move from a fossil fuel‐based energy economy toward a bio‐based one (Connor 2007).
- When properly located and operated, the potential of an integrated forest biorefinery is believed to be huge: a very attractive and synergistic business and economic opportunities for both co‐located pulp and paper mill and for the biorefinery itself.
- Biorefineries are a key pathway to our biofuture, displacing fossil fuels and supplying clean, renewable, and carbon neutral energy. Biorefineries fit very well at pulp and paper mills because of their inherent ability to gather and process biomass and create energy from biomass.
- BLG for liquid fuel production with BLGCC electricity production at kraft mills has potential to significantly reduce air emissions, including CO2, SO2, NOx, CO, VOCs, PM, and TRS.
- Forest biorefinery has significant impact on the society, our economy, environment, and sustainable forestry and pulp and paper industry.
Problems
- 9.1 Which of the following are steps needed to achieve zero waste?
- Some specialized or specific waste minimization process and hazardous waste designation technology.
- Old technologies are intensified with integration processes.
- New products are created using current products
- 9.2 Why can all wastes reach a zero waste “grave”?
- 9.3 Biorefineries have current and future speculative production of renewable fuels, electricity, and chemicals in conjunction with pulp and paper mills.
- Where do national energy security and global warming concerns enter this picture?
- Black liquor gasification processes and properties parallel in value, and liquid fuels synthesis have potential to save fossil fuel energy and reduce solid waste discharges. What steps are used in black liquor gasification and liquid fuel synthesis?
- What five changes can you anticipate for the near future in use of biorefineries?
- 9.4 Define industrial ecology (IE). Give your perspective about the IE pertaining to industrial environmental management.
- 9.5 What is an “eco‐industrial park”? Give examples with a case study.
- 9.6 Define “cradle to cradle.”
- 9.7 Describe circular economy.
- 9.8 What are process intensification and process integration in manufacturing? Describe the differences between the two approaches.
- 9.9 Describe how (a) process redesign and (b) process modifications could achieve greater pollution prevention for chemical production industries.
- 9.10 Describe the water and energy nexus in industrial sector. Describe how climate change could affect water supply and electricity use.
- 9.11 Highlight deinking process in recovery fiber from recycled fiber. A benefit/cost analysis of the process.
References
- Adachi, Y., Komoto, M., Watanabe, I. et al. (2000). Effective Utilization of Remote Coal Through Dimethyl Ether Synthesis. Fuel 79: 229–234.
- Ǻdahl, A., Harvey, S., and Berntson, T. (2004). Process industry energy retrofits: the importance of emission baselines for greenhouse gas reduction. Energy Policy 32: 1375–1388.
- Aden, A., Spath, P., and Atherton, B. (2005). The potential of thermochemical ethanol via mixed alcohols production. Milestone completion report, National Renewable Energy Laboratory, Golden, CO, 31 October.
- AGU, American Geophysical Union (2012). Water‐Energy Nexus: Solutions to Meet a Growing Demand. Washington, DC: AGU.
- Air Products and Chemicals, Inc. (1993). Synthesis of Dimethyl Ether and Alternative Fuels in the Liquid Phase from Coal‐Derived Synthesis Gas, Report DOE/PC/89865‐T8. Pittsburgh, PA: US Department of Energy, Pittsburgh Energy Technology Center.
- Air Products and Chemicals, Inc. (2001). Liquid Phase Dimethyl Ether Demonstration in the LaPorte Alternative Fuels Development Unit. Prepared for US Department of Energy by APCI, Allentown, Pennsylvania, January.
- Air Products and Chemicals, Inc. (2002). Market Outlook for Dimethyl Ether (DME). Prepared for US Department of Energy by APCI, Allentown, Pennsylvania, April.
- Allen, D.T. and Rosselot, K.S. (1997). Pollution Prevention for Chemical Processes. Hoboken, NJ: Wiley.
- Allen, D.T. and Shonnard, D.R. (2012). Sustainable Engineering: Concepts, Design, and Case Studies. Hoboken, NJ: Wiley.
- Allenby, B. (2006). The ontologies of industrial ecology. Progress in Industrial Ecology: An International Journal 3 (1/2): 28–40.
- Andersson, E. and Harvey, S. (2004). Pulp‐mill integrated bio‐refineries: a framework for assessing net CO2 emission consequences. Proceedings, AIChE 2004 Fall Annual Meeting, Austin, TX (7–12 November 2004), 203–208.
- Argonne National Laboratory (2006). Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) Model, version 1.7 (beta), released January 18.
- Ashton, W. (2009). The structure, function, and evolution of a regional industrial ecosystem. Journal of Industrial Ecology 13(2): 228–246.
- Bajpai, P. (2008). Chemical Recovery in Pulp and Paper Making, 166 pp. Leatherhead: PIRA International.
- Bajpai, P. (2012). Biotechnology in Pulp and Paper Processing. New York, NY: Springer‐Verlag.
- Bajpai, P. (2013). Biorefinery in the Pulp and Paper Industry, 114 pp. London: Elsevier Inc.
- Bajpai, P. (2014). Black Liquor Gasification, 92 pp. London: Elsevier Inc.
- Bechtel Group, Inc. (1990). Slurry Reactor Design Studies. Slurry vs. Fixed‐Bed Reactors for Fischer‐Tropsch and Methanol: Final Report, US Department of Energy Project No. DE‐AC22‐89PC89867. Pittsburgh: Pittsburgh Energy Technology Center.
- Benyus, J. (2003). Biomimicry: Innovation Inspired by Nature. New York: HarperCollins.
- Berglin, N., Lindblom, M., and Ekbom, T. (2002). Efficient production of methanol from biomass via black liquor gasification. TAPPI Engineering Conference, San Diego, CA (7–11 September 2002).
- Bielenberg, J. and Bryner, M. (2018). Realize the potential of process intensification. Chemical Engineering Progress 114 (3): 41–45.
- Blok, K., Hoogzaad, J., Ramkumar, S. et al. (2018). Implementing Circular Economy Globally Makes Paris Targets Achievable. Circle Economy. Circle Economy, Ecofys (accessed 12 April 2018).
- Brown, D.M., Bhatt, B.L., Hsiung, T.H. et al. (1991). Novel technology for the synthesis of dimethyl ether from syngas. Catalysis Today 8: 279–304.
- Calzavara, Y., Joussot‐Dubien, C., Boissonnnet, G., and Sarrade, S. (2005). Evaluation of biomass gasification in supercritical water process for hydrogen production. Energy Conversion and Management 46: 615–631.
- CFR‐40 (1999) Protection of Environment.
- Chemrec (2006). Chemrec pressurized BLG (black liquor gasification): status and future plans. Paper presented at the 7th International Colloquium on Black Liquor Combustion and Gasification, Jyväskylä, Finland (2 August 2006).
- Chertow, M.R. (2000). Industrial symbiosis: literature and taxonomy. Annual Review of Energy and the Environment 25: 313–337.
- Clark, C.E. and Veil, J.A. (2009). Produced Water Volumes and Management Practices in the United States. Argonne, IL: Argonne National Laboratory.
- Clift, R. and Allwood, J. (2011). Rethinking the Economy. The Chemical Engineer, March.
- Commission of European Communities (COM) (2014). Towards a Circular Economy: A Zero Waste Program for Europe, Communication No. 398. Brussels: Commission of European Communities.
- Connor, E. (2007). The integrated forest biorefinery: the pathway to our bio‐future. Proceedings of the International Chemical Recovery Conference: Efficiency and Energy Management, Quebec City, QC (29 May to 1 June 2007), pp. 323–327.
- Consonni, S., Katofsky, R., and Larson, E. (2009). A gasification‐based bio‐refinery for the pulp and paper industry. Chemical Engineering Research and Design 87 (9): 1293–1317.
- Dahlquist, E., Avelin, A., and Yan, J. (2009). Black liquor gasification in a CFB gasifier: system solutions. Proceedings of the First International Conference on Applied Energy (ICAE09), Hong Kong (5–7 January 2009).
- Dance, M. (2005). Hydroxide formation and carbon species distribution during high‐temperature kraft black liquor gasification. MSc thesis. Georgia Institute of Technology.
- Das, T.K. (2005). Process pollution prevention in pulp and paper industry. In: Toward Zero Discharge: Innovative Methodology and Technologies for Process Pollution Prevention. Hoboken, NJ: Wiley‐InterScience.
- Das, T.K. (2017). Optimum, sustainable and integrated water management. In: Sustainable Water Management Technologies, vol. I (ed. D. Chen), 397–414. Boca Raton, FL: CRC Press.
- Das, T.K. and Cabezas, H. (2018). Tools and concepts for environmental sustainability in the food‐energy‐water nexus: chemical engineering perspective. Environmental Progress and Sustainable Energy 37 (1): 73–81.
- Das, T.K. and Houtman, C.J. (2004). Evaluating chemical‐, mechanical‐, and bio‐pulping processes and its sustainability characterization using life‐cycle assessment. Environmental Progress and Sustainable Energy 23 (4): 347–357.
- Das, T.K. and Jain, A.K. (2001). Pollution prevention advances in pulp and paper processing. Environmental Progress 20 (2): 87–92.
- Das, T. K., Sharma, M. P., and Butner, S. (2001). The concept of an Eco‐Industrial Park: a solution to industrial ecology. Paper presented at the Topical Conference: Industrial Ecology and Zero‐Discharge Manufacturing, American Institute of Chemical Engineers Spring Meeting, Paper No. 18c, Houston, TX (April 2001).
- Eastman Chemical and Air Products and Chemicals (2003). Project Data on Eastman Chemical Company's Chemical‐from‐Coal Complex in Kingsport, TN. Prepared for US Department of Energy (under cooperative agreement DE‐FC22‐92PC90543).
- Ebara Corporation, Tokyo, Japan (2010). Progress on Our Zero Emission Challenge. Summary of Annual Reports.
- Eco‐Innovation Observatory (EIO) (2012). Methodological Report. Eco‐Innovation Observatory. Funded by the European Commission, DG, Environment, Brussels.
- Ekbom, T., Berglin, N., and Logdberg, S. (2005). Black liquor gasification with motor fuel production – BLGMF‐II: a techno‐economic feasibility study on catalytic Fischer‐Tropsch synthesis diesel production in comparison with methanol and DME as transport fuels. P21384‐1, Nykomb Synergestics AB, Stockholm.
- Ekbom, T., Lindblom, M., Berglin, N., and Ahlvik, P. (2003). Technical and commercial feasibility study of black liquor gasification with methanol/DME production as motor fuels for automotive uses: BLGMF. Report for Contract No. 4.1030/Z/01‐087/2001.
- Ellen Macarthur Foundation (2012). Towards the Circular Economy, Economic and Business Rationale for a Circular Economy, vol. 1. Cowes: Ellen Macarthur Foundation.
- Ellen Macarthur Foundation (2013). Towards the Circular Economy, Opportunities for the consumer goods sector, vol. 2. Cowes: Ellen Macarthur Foundation.
- Ellen Macarthur Foundation (2014). Towards the Circular Economy, Accelerating the Scale‐Up Across Global Supply Chains, vol. 3. Cowes: Ellen Macarthur Foundation.
- Ellen Macarthur Foundation (2015). Towards a Circular Economy: Business Rationale for an Accelerated Transition. Ellen Macarthur Foundation.
- Ellen Macarthur Foundation (2016). The New Plastics Economy: Rethinking the Future of Plastic.
- Ellen Macarthur Foundation, SUN, McKinsey and Co (2015). Growth Within: Circular Economy Vision for a Competitive Europe. London: McKinsey Center for Business and Environment.
- EPRI, Electric Power Research Institute (2011). Water Use for Electricity Generation and Other Sectors: Recent Changes (1985–2005) and Future Projections (2005–2030). Palo Alto, CA: EPRI.
- Eriksson, H. and Harvey, S. (2004). Black liquor gasification: consequences for both industry and society. Energy 29: 581–612.
- ETC, (2011). Gasification of Black Liquor. Popular Science Report from the BLG II Program 2007–2010.
- European Federation Chemical Engineering (EFCE) (2015). European Roadmap on Process Intensification.
- Farmer, M.C. (2005). The adaptable integrated biorefinery for existing pulp mills. Presentation at TAPPI Engineering, Pulping, and Environmental Conference, Philadelphia, PA (28–31 August 2005).
- Farmer, M.C. and Sinquefield, S. (2003). An External Benefits Study of Black Liquor Gasification. Final Report. Atlanta: Georgia Institute of Technology, June 15.
- Farmer, M.C. and Sinquefield, S. (2009). Black Liquor Gasification: Paths and Obstacles to Commercialization. Surrey, UK: Pira Industry Insight.
- Frosch, R. and Gallopoulos, N. (1989). Strategies for manufacturing. Scientific American (September 1989), p. 144–152.
- Fujimoto, K., Shikada, T., and Yamaoka, Y. (1995). Method of producing dimethyl ether. US Patent 5,466,720, 14 November 1995.
- Geissdoerfer, M., Savaget, P., Bocken, N., and Hultink, E.J. (2017). The circular economy: a new sustainability paradigm? Journal of Cleaner Production 143: 757–768.
- Gómez‐Castro, F.I., Cano‐Rodriguez, I.C., and Gamińo‐Arroyo, Z. (2016). Process intensification in the production of liquid biofuels: strategies to minimize environmental impact. In: Process Intensification in Chemical Engineering: Design Optimization and Control (eds. J.D. Segovia‐Hernandez and A. Bonilla‐Petriciolet). Cham: Springer.
- Grigoray, O. (2009). Gasification of black liquor as a way to increase power production at kraft pulp mills. Master thesis. Lappeenrantea University of Technology Faculty of Technology Degree Program of Chemical Technology.
- Hallale, N. (2001). Burning bright: tends in process integration. Chemical Engineering Progress 97 (7): 30–41.
- Hansen, J. and Sato, M. (2004). Greenhouse gas growth rates. Proceedings of the National Academy of Sciences 101 (46): 16109–16114.
- Hein, A.M., Jankovic, M., Farel, R., and Yannou, B. (2015). A conceptual framework for Eco‐Industrial Parks. Proceedings of the ASME 2015 International Design Engineering Technical Conferences & Computers and Information in Engineering Conference IDETC/CIE, Boston, MA (2–5 August 2015).
- Intergovernmental Panel on Climate Change (IPCC) (2007). Climate Change 2007: The Physical Science Basis Summary for Policymakers. Geneva: U.N. Environment Program and World Meteorological Organization.
- International Energy Agency (2009). Black liquor gasification summary and conclusions from the IEA Bioenergy ExCo54 Workshop. Bioenergy Report.
- Jensen, P.D. (2011). Reinterpreting industrial ecology. Journal of Industrial Ecology 15(5): 680–692.
- KAM Final Report (2003). Eco‐cyclic pulp mill: KAM project. Report no. A100, Stockholm, Sweden: STFI. www.stfi‐packforsk.se
- Lancaster, M. (2002). Principles of sustainable and green chemistry. In: Handbook of Green Chemistry and Technology (eds. J. Clark and D. Manqueary). Oxford: Blackwell Science.
- Landälv, I., Granberg, F., and Nelving, H. (2010). Production of di methyl ether from kraft black liquor for use in heavy duty trucks. Proceedings of International Chemical Recovery Conference, Williamsburg, VA (29 March 29 to 1 April), vol. 2, p. 179–186.
- Landävl, I. (2010). Woods to wheel: Chemrec's BioDME demonstration plant at the Smurfit Kappa mill. Pulp Paper Int.
- Larson, E.D., Consonni, S., and Katofsky, R.E. (2003). A Cost‐Benefit Assessment of Biomass Gasification Power Generation in the Pulp and Paper Industry, Final Report. Princeton, NJ: Princeton Environmental Institute.
- Larson, E.D., Consonni, S., Katofsky, R.E. et al. (2006a). A Cost‐Benefit Assessment of Biomass Gasification Power Generation in the Pulp and Paper Industry, Final Report. Princeton, NJ: Princeton Environmental Institute.
- Larson, E.D., Williams, R.H., and Jin, H. (2006b). Electricity and fuels from biomass with carbon capture and storage. Proceedings of the 8th International Conference on Greenhouse Gas Control Technologies, Trondheim, Norway (19–22 June 2006).
- Lewandowski, M. (2016). Designing the business models for circular economy: towards the conceptual framework. Sustainability 8(1): 43.
- Lienig, J. and Bruemmer, H. (2017). Recycling requirements and design for environmental compliance. In: Fundamentals of Electronic Systems Design, 193–218. New York: Springer.
- Lindblom, M. (2012). Chemrec, history and future plants Chemrec sales presentation to ETF. Nenet http://www.nenet.se/.../Chemrec_biodme_motor_fuels_from_the_forest_matsli... (accessed 27 February 2019).
- Liu, Z., Li, X., Close, M.R. et al. (1997). Screening of alkali‐promoted vapor‐phase‐synthesized molybdenum sulfide catalysts for the production of alcohols from synthesis gas. Industrial and Engineering Chemistry Research 36: 3085–3093.
- Lowe, E. and Warren, J. (1996). The Source of Value: An Executive Briefing and Sourcebook on Industrial Ecology. Washington, DC: Pacific Northwest National Laboratory.
- Lowe, E.A. (1995). The Eco‐Industrial Park: a business environment for a sustainable future. Paper presented at Designing, Financing and Building the Industrial Park of the Future Workshop, San Diego, CA.
- Lowe, E.A. (2001). Eco‐industrial Park Handbook for Asian Developing Countries. A Report to Asian Development Bank. Environment Department, Indigo Development, Oakland, CA.
- Mabee, W.E., Gregg, D.J., and Saddler, J.N. (2005). Assessing the emerging biorefinery sector in Canada. Applied Biochemistry and Biotechnology 121–124: 765–777.
- Marklund, M. (2006). Pressurized entrained‐flow high temperature black liquor gasification CFD based reactor scale‐up method and spray burner characterization. PhD thesis. Lulea&c.dotab; University of Technology.
- McDonough, W. and Braungart, M. (2002). Cradle to Cradle: Remaking the Way We Make Things. North Point Press.
- Miller, M., Justiniano, M., and McQueen, S. (2005). Energy and Environmental Profile of the U.S. Pulp and Paper Industry. Columbia, MD: Energetics Incorporated.
- Milne, G. and Readon, C. (2005). Embodied energy. In: Your Home Technical Manual: Australia's Guide to Environmentally Sustainable Homes. Adelaide, Australia: Commonwealth of Australia.
- Murray, A., Skene, K., and Haynes, K. (2015). The circular economy: an interdisciplinary exploration of the concept and application in a global contex. Journal of Business Ethics 127(3): 525–536.
- Naqvi, M., Yan, J., and Dahlquist, E. (2010). Black liquor gasification integrated in pulp and paper mill: a critical review. Bioresource and Technology 101: 8001–8015.
- NCASI (1993). Summary of Data Reflective of Pulp and Paper Industry Progress in Reducing the TCDD/TCDF Content of Effluents, Pulps, and Wastewater Treatment Sludges. New York: NCASI, June.
- NREL (2011). A Review of Operational Water Consumption and Withdrawal Factors for Electricity‐Generating Technologies, TP‐6A20‐50900. Golden, CO: NREL.
- Ohno, Y. (2006). The role of DME in the World: a perspective. Proceedings of the Second International DME Conference, London (15–17 May 2006).
- Parthasarathy, G. and Dunn, R. (2005). Minimization of environmental discharge through process integration. In: Toward Zero Discharge: Innovative Methodology and Technologies for Process Pollution Prevention (ed. T.K. Das), 597–645. Hoboken, NJ: Wiley‐InterScience.
- Patrick, K. and Siedel, B. (2003). Gasification edges closer to commercial reality with three new N.A. mills startups. PaperAge 119 (Part 7): 30–33.
- Pearce, D.W. and Turner, R.K. (1989). Economics of Natural Resources and the Environment. Baltimore MD: Johns Hopkins University Press.
- Pearce, J.M. (2008). Industrial symbiosis for very large scale photovoltaic manufacturing. Renewable Energy 33: 1101–1108.
- Perlack, R.D., Wright, L.L., Turhallow, A. et al. (2005). Biomass as Feedstocks for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion‐Ton Annual Supply, ORNL/TM‐2005/66 for USDA and USDOE, April. Oak Ridge, TN: Oak Ridge National Laboratory.
- Pitt, J. and Heinemeyer, C. (2015). Introducing ideas of a circular economy. In Environment, Ethics and Cultures: Design and Technology Education's Contribution to Sustainable Global Futures. London, UK: University of London. ScienceDirect.com.
- Prentice, I.C., Farquhar, G.D., Fasham, M.J. et al. (2001). The carbon cycle and atmospheric carbon dioxide. In: Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (eds. J. Houghton et al.). Cambridge: Cambridge University Press.
- Reay, D., Ramshaw, C., and Harvey, A. (2013). Process Intensification: Engineering for Efficiency, Sustainability and Flexibility, 2e. Oxford: Butterworth‐Heinemann.
- Richards, D.J., Allenby, B.R., and Frosch, R.A. (1994). The greening of industrial ecosystems: overview and perspective. In: The Greening of Industrial Ecosystems (eds. B.R. Allenby and D.J. Richards), 1–19. Washington, DC: National Academy Press.
- Salomonsson, P. (2011). BIOMED project. Proceedings of the Seventh Asian DME Conference, Nigata, Japan (November 2011).
- Salomonsson, P. (2013). Final report of the European BioDME project. Proceedings of the Fifth International DME Conference, Ann Arbor, MI (18 April 2013).
- Smith, R. (2016). Chemical Process Design and Integration, 2e. Hoboken, NJ: Wiley.
- Smol, M., Kulczycka, J., and Avdiushchenko, A. (2017). Circular economy indicators in relation to eco‐innovation in European regions. Clean Technologies and Environmental Policy 19(3): 669–678.
- Smol, M., Kulczycka, J., Henclic, K. et al. (2015). The possible use of sewage sludge ash (SSA) in the construction industry as a way towards a circular economy. Journal of Cleaner Production 95: 45–54.
- Sricharoenchaikul, V. (2001). Fate of carbon containing compounds from gasification of kraft black liquor with subsequent catalytic conditioning of condensable organics. PhD dissertation. Georgia Institute of Technology.
- Sricharoenchaikul, V. (2009). Assessment of black liquor gasification in supercritical water. Bioresource Technology 100: 638–643.
- Stahel, W.R. (2015). The Business Angle of a Circular Economy: Higher Competitiveness, Higher Resource Security and Material Efficiency. Stockholm: EMF.
- Staniškis, J.K. (2012). Sustainable consumption and production: how to make it possible. Clean Technologies and Environmental Policy 14(6): 1015–1022.
- Stankiewicz, A.I. and Moulijn, J.A. (2000). Process intensification: transforming chemical engineering. Chemical Engineering Progress 96(1): 22–34.
- Swedish Energy Agency (2008). System Aspects of Black Liquor Gasification: A Review of Existing Reports. Eskilstuna: Swedish Energy Agency ISSN 1403‐1892.
- Swedish Forest Agency (2008). Forestry Statistics. Jönköping, Sweden: Swedish Forest Agency.
- Tampier, M., Smith, D., Bibeau, E., and Beauchemin, P.A. (2004). Identifying Environmentally Preferable Uses for Biomass Resources. Stage I Report: Identification of Feedstocks‐to‐Product Threads. Prepared for Natural Resources Canada, Commission for Environmental Co‐operation, and National Research Council of Canada. Envirochem Services Inc., Vancouver, BC.
- Thomé, A.M., Scavarda, A., Ceryno, P.S., and Remmen, A. (2016). Sustainable new product development: a longitudinal review. Clean Technologies and Environmental Policy 18(7): 2195–2208.
- USDOE (2006). Proceeding of the 5th Annual Conference on Carbon Capture and Sequestration, Alexandria, VA (8–11 May 2006) and Proceedings of the 8th International Conference on Greenhouse Gas Control Technologies, Trondheim, Norway (19–22 June 2006).
- USDOE (2013a). Effects of Climate Change on Federal Hydropower: Report to Congress. Washington, DC: DOE. http://www1.eere.energy.gov/water/pdfs/hydro_climate_change_report.pdf (accessed 10 April 2018).
- USDOE (2013b). U.S. Energy Sector Vulnerabilities to Climate Change and Extreme Weather. Washington, DC: DOE. http://energy.gov/sites/prod/files/2013/07/f2/20130716‐Energy%20Sector%20Vulnerabilities%20Report.pdf (accessed 28 February 2019).
- USDOE (2014a). The Water‐Energy Nexus: Challenges and Opportunities. USDOE, Pacific Northwest National Laboratory.
- USDOE (2014b). Energy Demands on Water Resources. USDOE Report to Congress on the Interdependency of Energy and Water.
- USEPA (2002). AP‐42, Compilation of Air Pollutant Emission Factors, Section 1.6: Wood Residue Combustion in Boilers. Research Triangle Park, NC: U.S. Environmental Protection Agency, March.
- Van der Ryn, S. and Cowan, S. (1996). Ecological Design. Washington, DC: Island Press.
- Vogel, F., DiNaro, J., Marrone, P. et al. (2005). Critical review of kinetic data for the oxidation of methanol in supercritical water. Journal of Supercritical Fluids 34: 249–286.
- Waldner, M. and Vogel, F. (2005). Renewable production of methane from woody biomass by catalytic hydrothermal gasification. Industrial and Engineering Chemistry Research 44 (13): 4543–4551.
- Weetman, C. (2016). A Circular Economy Handbook for Business and Supply Chains: Repair, Remake, Redesign, Rethink. London: Kogan Page.
- Weyerhaeuser Company (2000). Biomass Gasification Combined Cycle. Final Report Under Contract DE‐FC36‐96GO10173 to U.S. Department of Energy, Federal Way, WA, 160 pages.
- Whitty, K. (2006). Conversion of black liquor in a fluidized bed steam reformer. Paper presented at the 7th International Colloquium on Black Liquor Combustion and Gasification, Jyvaskyla, Finland (2 August 2006).
- Whitty, K. and Baxter, L. (2001). State of the art in black liquor gasification technology. Proceedings of the Joint International Combustion Symposium, Kauai, Hawaii (9–12 September 2001).
- Whitty, K. and Nilsson, A. (2001). Experience from a high temperature, pressurized black liquor gasification pilot plant. Proceedings of the Tappi Chemical International Recovery Conference, Whistler, BC (11–14 June 2001). Atlanta: Tappi Press, pp. 655–662.
- Whitty, K. and Verrill, C.L. (2004). A historical look at the development of alternative black liquor recovery technologies and the evaluation of black liquor gasifier designs. Proceedings of the International Chemical Recovery Conference, Charleston, SC (6–10 June 2004).
- Wikipedia (2016). Black liquor, 2 February 2016. https://en.wikipedia.org/wiki/Black_liquor (accessed 6 August 2019).
- Williams, P. and Onwudili, J. (2006). Subcritical and supercritical water gasification of cellulose, starch, glucose, and biomass waste. Energy Fuels 20: 1259–1265.
- Zhong (2018). Tightening the loop on the circular economy: coupled distributed recycling and manufacturing with recyclebot and RepRap 3‐D printing. Resources, Conservation and Recycling 128: 48–58.
