Ion beam synthesis
Ion beam synthesis is a powerful method for creating phases with new chemical constituents and specific structures in the surface layers of materials. As example, the ion beam can be used to make metal nanoparticles in the surface layer of a polymer. The mobility of the implanted species in the polymer matrix can be increased significantly if the implantation is performed into a liquid polymer matrix with low viscosity. The influence of viscosity on diffusion processes in the implanted matrix leads to differences in the shape and size of the metal particles. Highly ordered carbonized nanomembranes can be synthesized using ion implantation. The thickness of the PS-PVP-PS co-polymer template is in range of 20–100 nm, and the lateral dimensions of the structures are in the range 10–30 nm. A free-standing carbon film with highly ordered holes can be peeled off the substrate after ion implantation. The excellent preservation of the nanostructure’s order makes it possible to observe a nanoscale Moiré effect.
Keywords
ion beam synthesis; nanoparticle; viscos liquid; nanotemplate; carbon membrane
Ion beam synthesis is a powerful method for creating phases with new chemical constituents and specific structures in the surface layers of materials. Ion beam synthesis differs from ion beam modification in its range of implanted fluences. The ion modification technique is used in the 1013–1016 ions/cm2 fluence range. Such fluences are enough for the modification of materials by rearrangement of atomic bonding in the modified surface layer; however, the concentration of implanted ions is not high enough to create new phases with a significant content of implanted atoms. If the fluence is higher than 1016 ions/cm2, the implanted ions (atoms, clusters) can form structures with new elemental constituents in the target. Ion modification, together with deposition processes, is widely used for the synthesis of structures with altered elemental composition in semiconductors and metals [1]. Ion beam synthesis in polymer materials could also be used for the creation of such new phases. This approach is particularly useful to achieve structures that cannot be formed by other methods.
For example, an ion beam can be used to make metal nanoparticles in the surface layer of a polymer [2]. The metal particles are created by implantation of metal ions into the polymer target. The energy used for implantation is typically in the range of 25–150 keV, providing an ion penetration depth of 20–200 nm in the polymer target for heavy ions like Fe and Co. After ion penetration, the metal atom loses its kinetic energy by collisions and stops in a sub-surface region where it is surrounded by polymer organic macromolecules. If the metal species have low affinity with species in the polymer macromolecules, then the implanted metal atoms aggregate forming metal clusters (or particles) within the polymer matrix.
The process of particle formation occurs in a number of stages as the ion fluence increases: at low fluence the implanted atoms are dispersed; when the number density of implanted atoms reaches a critical concentration, the density of atoms in the polymer matrix becomes high enough for nucleation; then the nucleation of the implanted atoms produces small clusters (particles). These clusters agglomerate to form larger particles as the fluence is further increased, and can form a continuous film at very high fluence. Usually, the fluence used for metal particle synthesis is in the range of 1016–5×1017 ions/cm2. At fluences lower than 1016 ions/cm2, the implanted ions are separated by a large amount of polymer, and diffusion of the atoms is not sufficient for nucleation. For example, at such fluences, iron atoms implanted into PMMA with an energy of 40 keV have about 5–10 monomer PMMA units between them. With a fluence increase up to 1016 ions/cm2, the density of implanted atoms is enough to form metal clusters. However, the synthesized particles are rather small, with a diameter of 1–2 nm, and the distance between particles is too long for aggregation to occur. With a fluence increase, the diameter of the particles increases to 20–30 nm, and at fluences higher than 1017 ions/cm2 overlap of metal clusters is observed. At fluences higher than 1017 ions/cm2 a continuous metal film is formed on the polymer surface.
The process of particle formation depends on the mobility of implanted ions in the polymer matrix. With higher mobility, the implanted atoms can diffuse longer distances from their initial stopping site. Therefore, the implanted atoms can form clusters at lower concentrations in a flexible polymer matrix as compared to in a solid polymer matrix.
As discussed previously, the implanted ions simultaneously cause structural transformations in the surface polymer layer. At high fluences, carbonization is typically observed in implanted polymers. Such carbon structures impede mobility, and the diffusion of atoms in the implanted region of the polymer becomes slower with increasing fluence. Additionally, active carbon atoms with dangling bonds may form new bonds with implanting ions, further preventing their movement. In the case of carbide forming metals, the formation of chemical bonds soon after implantation with carbon in the surface layer prevents the subsequent diffusion of the metal atoms and, therefore, the formation of metal clusters.
The mobility of the implanted species in the polymer matrix can be increased significantly if the implantation is performed into a liquid polymer matrix with low viscosity [3–5]. Such implantations with high fluence were carried out with Fe, Co, and Ag ions into uncured silicon rubber and epoxy resin matrixes that were in a viscous state during ion beam implantation.
For implantation, the liquid polymer matrix is placed in a vacuum chamber and implanted by energetic ions with high fluence. Two methods are used, depending on the polymer matrix state. In the first method, the polymer matrix remains liquid during whole implantation process. The viscosity of the matrix and, thus, the diffusion rate of the implanted atoms depend only on the cross-linking ability of the polymer under radiation. After implantation ceases, the diffusion processes in the implanted layer continue. In the second method, the matrix contains two active components, which react to form solid polymer. The implantation starts when the matrix is in a viscous state. With time, the matrix viscosity increases due to the curing reaction, until the matrix completely solidifies. When the curing reaction is sufficiently slow, ion beam implantation occurs in the polymer matrix at essentially constant viscosity, and the formation of the metal particles can be described in terms of the hydrodynamic behavior of the metal clusters. After implantation and curing, the polymer matrix fixes the metal clusters and the implanted polymer target becomes stable.
The influence of viscosity on diffusion processes in the implanted matrix leads to differences in the shape and size of the metal particles [4]. At the start of the process, when the matrix has low viscosity, the implanting atoms have high mobility and the metal phase can easily move in the liquid matrix to form large particles. In such a case, the shape of the particles can vary from needles to wormlike particles, with long distances between particles. As the viscosity increases, the distance between particles decreases, and the atoms do not have sufficient freedom to move long distances. However, the local mobility that arises in the region of the collision cascade due to energy transfer from the implanting atoms determines the shape of the particles: spherical or ellipsoidal particles are formed depending on the conditions of implantation. In some cases, the formation of particles with a highly ordered crystalline structure can be achieved.
A surface layer of embedded metal particles, synthesized in this way, can be used in a variety of applications. For example, embedded particles with high magnetization can be used in magnetic storage devices. The optimal parameters for ion beam implantation depend on the viscous state of the polymer matrix. A three-fold increase in magnetization was achieved for films implanted in the liquid matrix, as compared to films implanted in the solid matrix [5].
Another application is a nonlinear optical device based on metal implanted polymers. Such a polymer device has gradient in refractive index that varies with depth from the surface, which can be used to transform optical signals in integrated electro-optical elements. The combination of the graded refractive index with the surface plasmon resonance effect in the metal particles is potentially useful for optical sensors integrated into nonlinear optical elements.
In light of these promising applications, the ion beam synthesis of metal inclusions in liquid polymer matrices warrants follow-up investigation and development, which may in turn bring to light new physical effects and further applications.
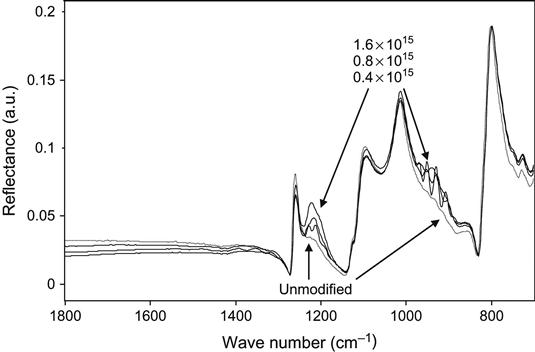
High-fluence ion implantation with gas ions can also be used to generate new phases in the implanted surface layers of polymers. The final structure of the implanted target depends on the initial atomic composition of the polymer and on the nature of the implanting ions. For example, ion implantation of high-energy ions into silicone rubber [6,7] generates a mixture of silicon oxide and silicon carbide in the modified layer below the surface of the rubber. We have observed the appearance of a silicon carbide phase after ion implantation with argon ions by the appearance a Si![]() C band [8] in the infrared spectrum of the silicone rubber (Figure 8.1). Ion implantation can, therefore, be used as a method of synthesizing inorganic layers at the polymer surface.
C band [8] in the infrared spectrum of the silicone rubber (Figure 8.1). Ion implantation can, therefore, be used as a method of synthesizing inorganic layers at the polymer surface.
The simplest case is ion implantation into hydrocarbon polymers, which generates a completely carbonized layer at the surface of the polymer or any other substrate onto which the polymer may be deposited. If the thickness of the polymer layer is low enough and the penetration depth of implanting ions is high enough, a completely carbonized film can be created from the organic polymer layer.
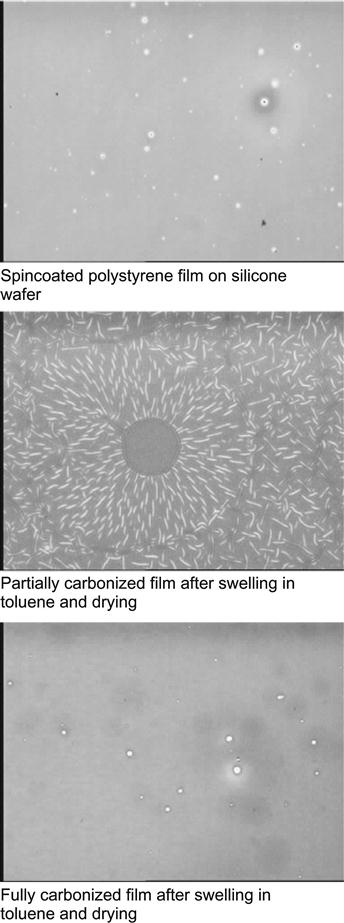
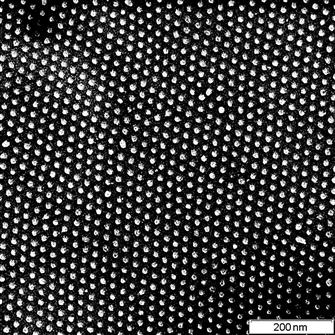
For example, a polystyrene thin film with a thickness of 100 nm was spincoated onto a silicon wafer and subsequently ion implanted [9,10]. The topology of the polystyrene film is identical to the silicon wafer (Figure 8.2). Ion beam implantation was carried out with nitrogen and argon ions at high energy of 20 keV with a high fluence of 1016 ions/cm2. After ion beam implantation, the topology of the surface remains conformal to the silicon wafer, and the roughness parameter RMS shows insignificant change (from 0.246 for spincoated polystyrene to 0.247 nm for modified polystyrene). The FTIR spectrum of the polystyrene film shows a decrease of the polystyrene line intensities with fluence (Figure 8.3). The decrease occurs due to etching of the polystyrene film by the ion beam and due to a structural transformation of the polystyrene into a carbon film. Etching and densification decreases the thickness of polystyrene film. Etching with low energy of ions (from a plasma discharge) is routinely used in the microelectronics industry to remove polymeric photoresists. However, in the case of impacts by energetic ions, the etching process occurs with a high rate only at low ion fluences, when the film still has a structure close to that of the initially spincoated polymer and the penetrating ions cause dehydration and sputtering in the polymer film. At high ion fluence, the etching process slows down greatly due to the high degree of carbonization of the film. The strong carbonization of the polystyrene under the ion beam does not permit complete removal by etching of the film. For example, a 100 nm polystyrene film on silicon and gold substrates becomes a 40–60 nm completely carbonized film after high-fluence ion implantation (for fluence in the range 1016–5×1016 ions/cm2).
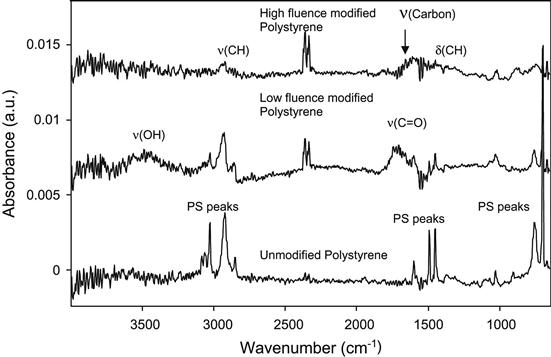
The structural transformations in polystyrene films can be observed in FTIR transmission spectra. After ion beam implantation, the lines attributed to vibrations of aromatic groups in the polystyrene macromolecules disappear completely. In highly implanted films, only about 10–15% of the hydrocarbons remain, as calculated from the vibrations of ![]() CH
CH![]() and
and ![]() CH2
CH2![]() groups. The FTIR spectra of films modified with high fluence also indicate the presence of a carbon structure according to the presence of lines in the 1600 cm−1 region. As measured by ellipsometry, the refractive index of the ion-implanted polystyrene film in the 500 nm wavelength region increases from 1.6 to 2.1–2.3, which is comparable to the refractive indices of carbon materials like graphite, diamond, or their mixtures.
groups. The FTIR spectra of films modified with high fluence also indicate the presence of a carbon structure according to the presence of lines in the 1600 cm−1 region. As measured by ellipsometry, the refractive index of the ion-implanted polystyrene film in the 500 nm wavelength region increases from 1.6 to 2.1–2.3, which is comparable to the refractive indices of carbon materials like graphite, diamond, or their mixtures.
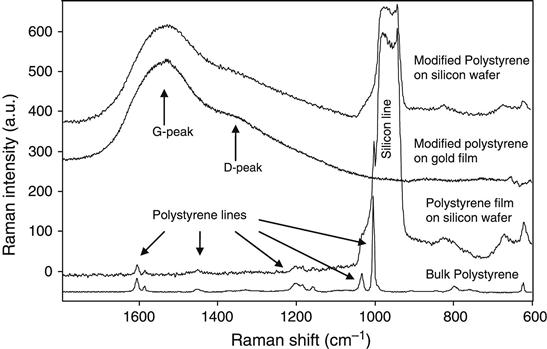
Micro-Raman spectroscopy also observes the completely carbonized layer on the top of the silicon wafer after ion implantation into a spincoated polystyrene film (Figure 8.4). The spectra of unmodified polystyrene (both bulk and spincoated on silicon wafers) show narrow lines attributed to the vibrational modes of the polystyrene macromolecules. After ion beam implantation, the Raman spectra of the modified polystyrene layer show lines, known in the literature as G- and D-peaks, associated with the vibrational modes of carbon structures. A detailed discussion of these peaks is presented in Chapter 4. The presence of these peaks indicates the presence of an amorphous carbon phase with sp2 and sp3 hybridized carbon atoms. Once completely transformed to this structure, the film becomes completely insoluble in any organic solvent and stable at high temperatures (Figure 8.2).
Therefore, after the PIII treatment, the polystyrene film was completely transformed into a carbon coating on the silicon wafer. After carbonization, the film can be removed from the silicon wafer by exposure to alkaline or acidic media that dissolve the silicon oxide under the carbon layer. The film is sufficiently durable in the free state to be placed on a TEM grid or on a wide-pore membrane. For example, carbon films with mm2 area and thickness down to 10–30 nm can be synthesized this way.
Ion implantation, therefore, provides a suitable method for the synthesis of inorganic coatings and membranes when, for example, the polymer film cannot be annealed to form pyrolytic carbon. Ion beam synthesis can also be used for polymers, which decompose when exposed to high temperatures (e.g., PMMA and PLA) and hence the pyrolytic method cannot be applied. Ion beam implantation with high-fluence transforms these kinds of polymers into a pure carbon film.
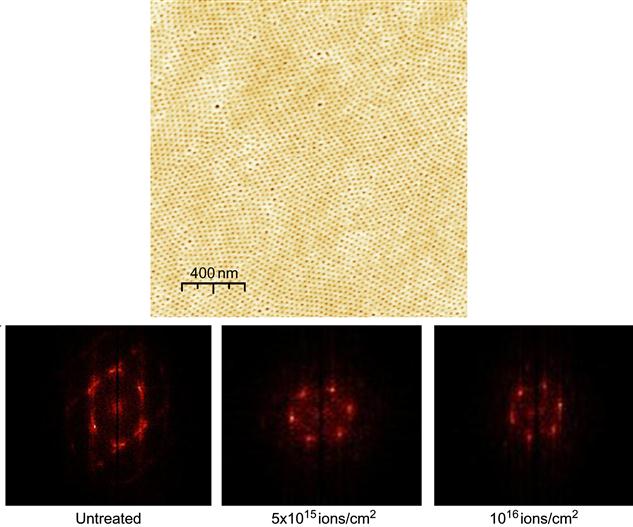
Ion beams can also be used for the synthesis of laterally nanostructured films, which are created from precursor nanoscale films on a substrate. Ion beam synthesis starting from laterally structured polymer films can play a significant role in the synthesis of complex shaped inorganic nanostructures. For example, highly ordered carbonized nanomembranes can be synthesized using ion implantation. Incompatibilities due to unfavorable interfacial energies between different blocks of macromolecules have been used to drive the formation of well-organized self-assembled periodic structures [11–13]. The periodicities of the structures are regulated by molecular mass, ratio, and interfacial compatibility of the copolymer blocks. Typically, the thickness of the template is in range of 20–100 nm, and the lateral dimensions of the structures are in the range 10–30 nm. For example, the block-copolymer PS-PVP-HABA (polystyrene-block-poly(4-vinyl pyridine) with a low molecular mass additive 2-(4-hydroxy-benzeneazo)benzoic acid) was spun on a silicon wafer and the film was annealed to produce a highly ordered self-assembled hexagonal structure (Figure 8.5). Then, the HABA component was washed out and the rest of the film was treated by 20 keV nitrogen ions. At high fluence, the film was completely carbonized: the FTIR spectra do not show any residual vibrations of PS and PVP fragments; micro-Raman spectra show G- and D-peaks associated with nanographite structures; and ellipsometry determines a high refractive index corresponding to graphitic/diamond-like structures. Despite an expected mixing of the target structure under ion implantation, the nanostructure of the film is preserved. The excellent preservation of the nanostructure’s order made it possible to observe a nanoscale Moiré effect [11]. A free-standing carbon film with highly ordered holes could be peeled off the substrate (Figure 8.6). This type of nanostructured film can be used for masking of deposition, quantum devices, filtering, and many other applications.
We believe that many different applications can be found for dewetted polymer and organic structures treated by high-fluence ion implantation. This field of ion beam synthesis for polymers has not yet been well investigated and developed, despite its bright prospects.
