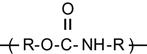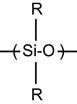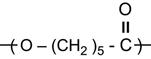Interactions of energetic ions with polymers: chemical picture
A brief review of the chemistry and physics of polymers is presented. The polymers are characterised by molecular mass, strong intramolecular interactions between atoms in the macromolecule, and weak intermolecular interactions between neighboring macromolecules, glass transition temperature and crystallinity. Polymers can also contain stabilizers, antioxidants, vulcanizing agents, plasticizers, fillers, and other functional additives that affect their properties and responses to ion implantation. In general, under the influence of ion irradiation, macromolecules are fragmented and radicals created. Radicals are very reactive and cause a number of chemical reactions to occur in the polymer including reactions with atmospheric oxygen and nitrogen. Ion implantation generates particular kinds of radicals with high local concentration. The result of radical reactions is the formation of double bonds, conjugated aromatic structures, as well as oxygen and nitrogen containing groups. The kinetics of the radical reactions can be described by a number of different reaction rates corresponding to various local structures with time constants extending up to years.
Keywords
polymer; macromolecule; radical; unsaturated group; kinetics of reaction
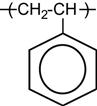
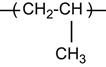
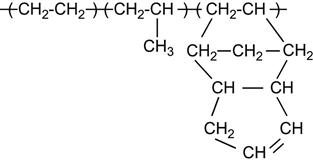
A polymer is a material composed of long macromolecules with high molecular mass. The macromolecules are chains or networks of chemically bonded monomers. The monomer is the regularly repeated unit in the polymer chain. The properties of the polymer depend on the type of monomer. An example of the simplest macromolecule is the saturated hydrocarbon macromolecule. A well-known representative of this type of polymer is polyethylene (PE). The PE macromolecule contains a simple monomer unit—CH2![]() CH2—which repeats thousands or millions of times in the polymer chain. There are more complex saturated hydrocarbons, such as:
CH2—which repeats thousands or millions of times in the polymer chain. There are more complex saturated hydrocarbons, such as:
These are homopolymers because they contain only one kind of monomer in the macromolecule. There are also heteropolymers, which consists of two and more different monomer units. For example,
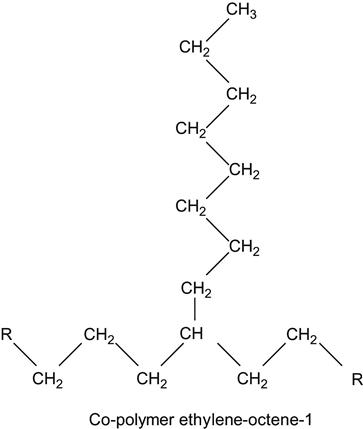
which contains two kinds of monomers: ethylene unit and octane-1 unit.
If its macromolecules contain unsaturated double or triple carbon-carbon bonds, the polymer is called an unsaturated hydrocarbon polymer, for example:
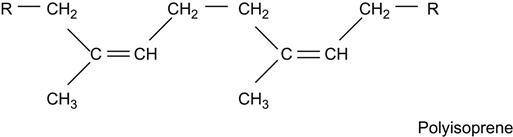
Polymer macromolecules can contain heteroatoms (oxygen, nitrogen, phosphorus, and others) in side chains:
Heteroatoms may also occur in the backbone chain, for example:
A special class of polymers are halogen-containing polymers such as:
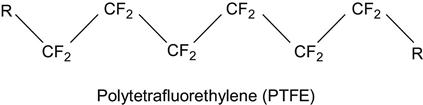
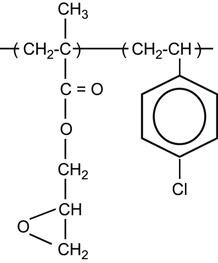
Table 3.1 contains a number of polymers made from various monomers that were investigated after ion implantation.
Table 3.1
Types of polymers with references to works involving their treatment with ion implantation
| Polymer name | Monomer unit | References |
| PE in particular: | ||
|
|
[1–13 vol. 69, 14–59] | |
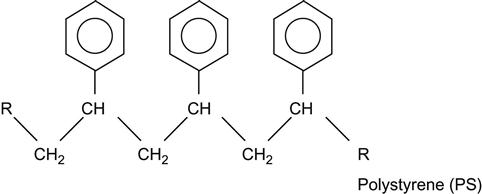
| Polystyrene (PS) and copolymers |
|
[1,2,7,60–96] |
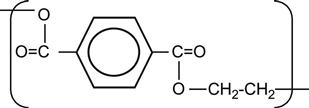
| Polyethylenterephtalate (PET, Mylar) |
|
[6,12,97–122] |
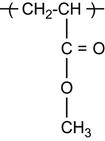
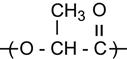
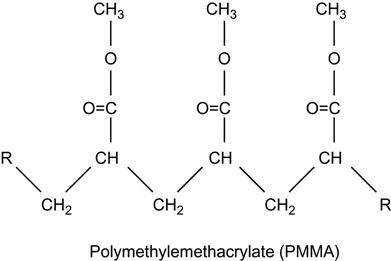
| Polymethylmethacrylate (PMMA) and copolymers |
|
[1,2,26,60,62,75,81,123–136] |
| Polytetrafluorethylene (PTFE, Teflon) and related copolymers |
|
[2,75,137–164] |
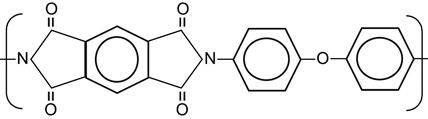
| Polyimide (PI, Kapton) |
|
[1,13,125,165–181] |
| Polyurethane (PU) |
|
[68,182–194] |
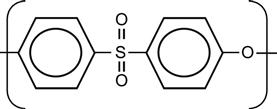
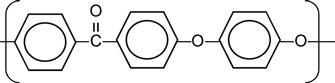
| Polyethersulfone (PSU, PES) |
|
[12,71,100,165,170,173,195,196] |
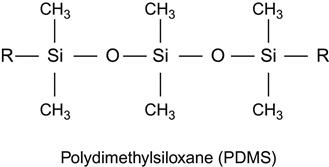
| Silicone rubber, polysiloxane, PDMS |
|
[102,103,184,197–204] |
| Polyvinyl chloride (PVC) |
|
[60,71,205,206] |
| Polyvinyl fluoride (PVDF) |
|
[12] |
| Polyethylene glycol (PEG) |
|
[207,208] |
| Polyamide (PA) |
|
[4,12,58,209–212] |
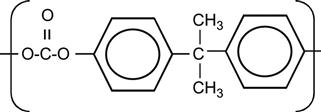
| Polycarbonate (PC) |
|
[75,100,213–226] |
| Polypropylene (PP) |
|
[6,12,26,227–231] |
| Polycaprolactone (PCL) |
|
[98,207] |
| Polyvinylpyridine (PVP) |
|
[195] |
| Polyvinyl acetate and copolymers (PVA) |
|
[12,60,184] |
| Poly glycidil methacrylate-co-3-chlorostyrene |
|
[60] |
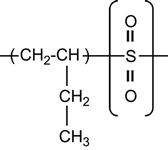
| Poly (butane-1-sulfone) |
|
[60] |
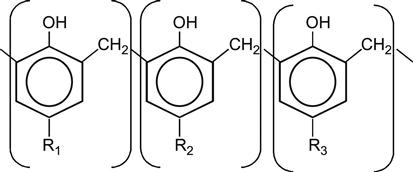
| Novolac |
|
[60] |
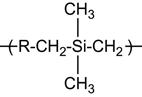
| Polycarbosilane (PCS) |
|
[165] |
| Polyphenylquinoxaline (PPQ) |
|
[1] |
| Poly-p-chlorstyrene |
|
[125] |
| Polyoxymethylene |
|
[3] |
| Cellulose |
|
[4] |
| Polyphenylvinylene |
|
[7] |
| Poly (L-lactic acid) and copolymers (PLLA, PLA, PLGA) |
|
[232–236] |
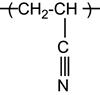
| Polyacrylonitrile and copolymers |
|
[77] |
| Poly(aryl ether ether ketone) (PEEK) |
|
[237–246] |
| Polyacetylene |
|
[247] |
| Ethylene–propylene rubber (EPDM) |
|
[12] |
| Poly(ethylene-tetrafluorethylene) (ETFE) |
|
[12] |
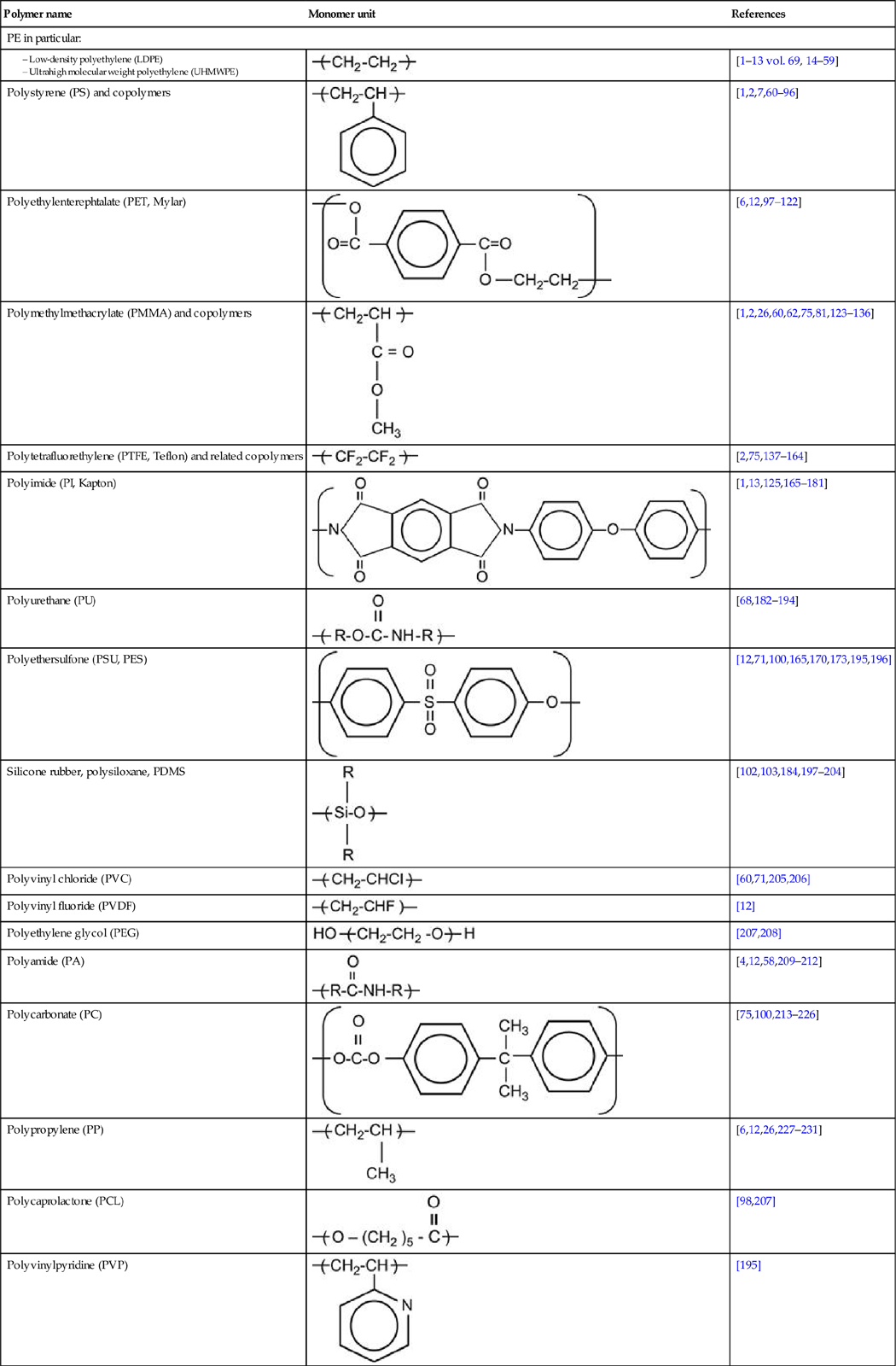
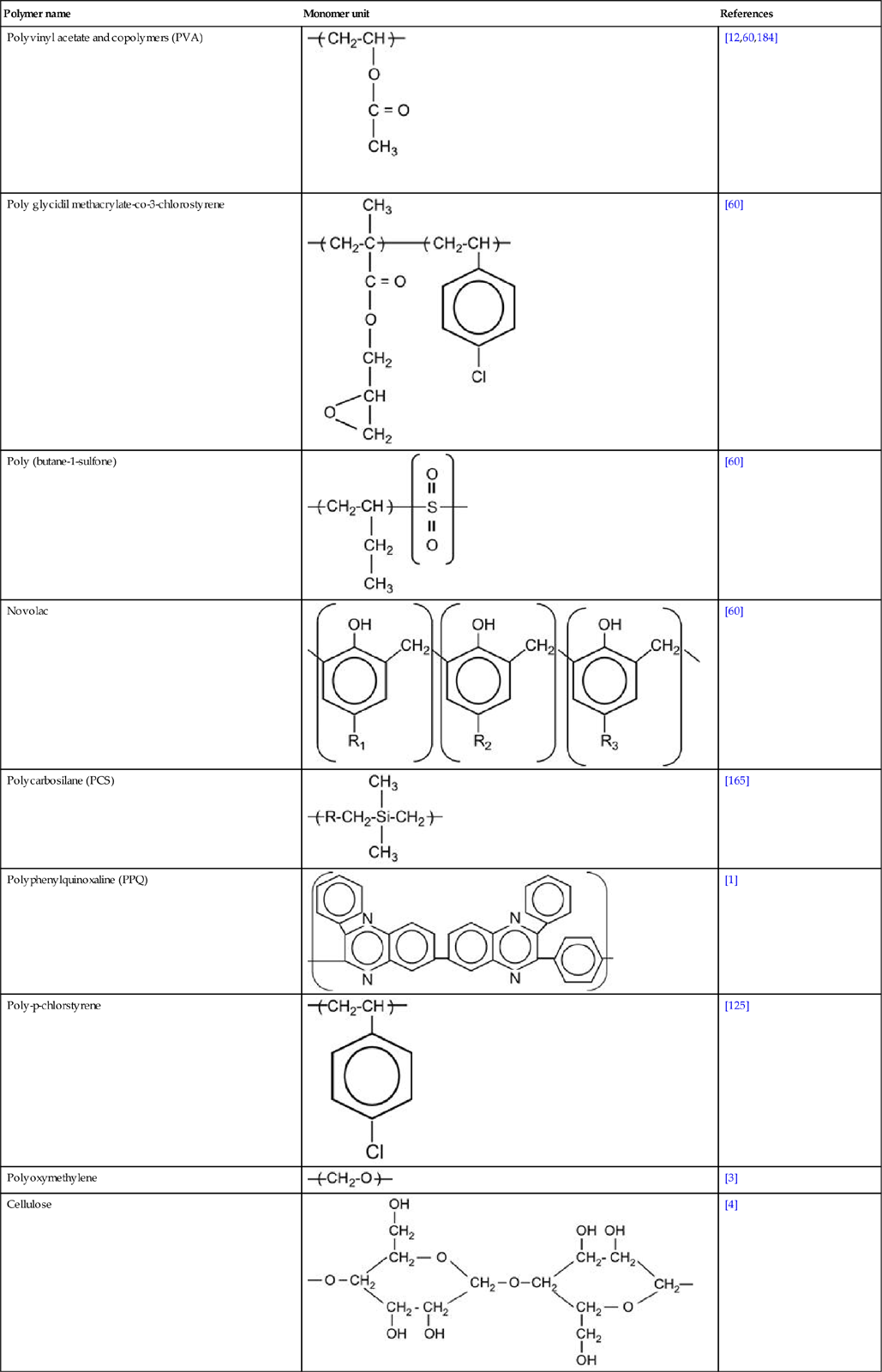
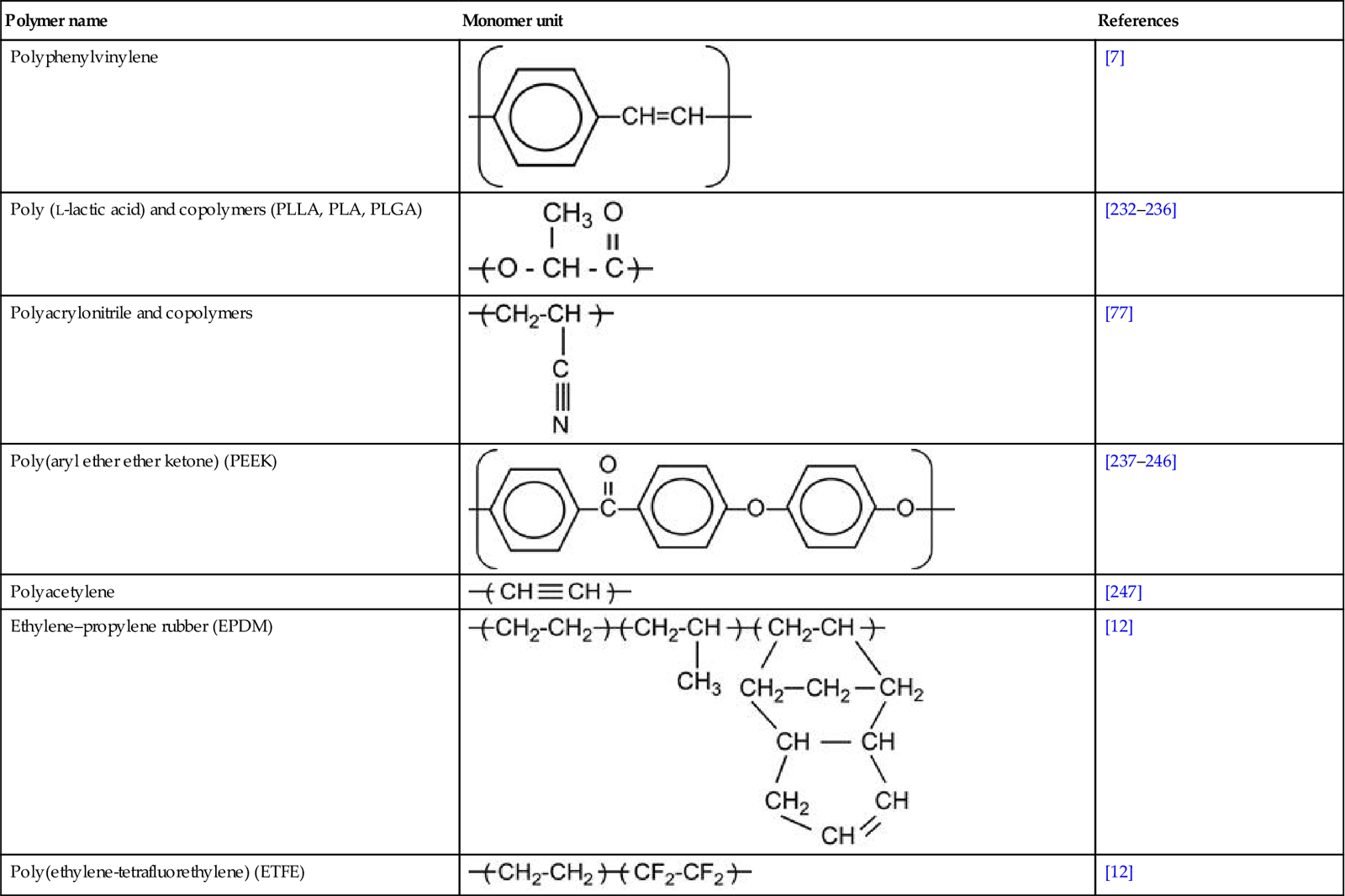
Macromolecules contain hundreds and thousands of monomers. The mass (or number of monomers, or length) of the macromolecule is characterized by a molecular mass distribution function and average molecular mass (MMW). Usually, the MMW of a polymer is in the range of 104–106. Polymers with MMW in a range of 300–3000, known as oligomers, are viscous liquids.
Polymers are characterized by strong intramolecular interactions between atoms in the macromolecule, and weak intermolecular interactions between neighboring macromolecules. The intramolecular interactions in the macromolecule are usually provided by covalent bonds. In some kinds of polymers, intramolecular interactions are provided by ionic and donor–acceptor bonds. Polymer macromolecules have relatively high mobility at room temperature; they can change conformation and macromolecules can diffuse between neighbors. The macromolecular mobility is characterized by the glass-transition temperature (Tg), at which the polymer transforms from a solid (glass state) to a viscous liquid.
Polymer macromolecules can form structures like clews or globules. Due to disordering of the long chains, polymers have mostly an amorphous structure. Some polymers have partly ordered structures and some have crystalline structures. For example, PE has amorphous and crystalline fractions. The ratio of these fractions depends on the degree of structural defects in the macromolecules. The crystalline fraction in LDPE is about 40% and in UHMWPE, it is about 85–90%.
Industrial polymers contain additives: stabilizers, antioxidants, vulcanizing agents, plasticizers, fillers, and other functional additives (sometimes up to 20–30 components) to provide the required properties for their practical exploitation. Polymers can contain uncontrolled additives, such as minor products of the synthesis reactions, oxidation, and pollutants from the production processes. Polymer surfaces can contain additional components, such as lubricants used in rolling processes, antisticking powder, or even fat traces from fingers after being touched. For example, after ion beam implantation with high fluence, fingerprints are observed as carbon traces on Teflon surfaces. Such contaminants must be removed from polymer surfaces before modification. If complete removal is impossible, then the effect of the contaminants must be taken into account during analysis after ion beam implantation.
Polymer surfaces can be cleaned with solvents. The choice of solvent should be based on knowledge of compatibility of the polymer and solvent. If the polymer is immersed in solvent, the solvent will penetrate into the surface layer of the polymer during the cleaning process. Therefore, after cleaning, ion implantation will modify the mixture of polymer and solvent, and this may significantly alter the structure of the modified surface layer. Immersion in solvent can also bring low-molecular-weight components from deep in the bulk of the polymer up to the surface layer, and thus further modify the composition of surface layer prior to ion implantation. Additional effects of the solvent and other low-molecular-weight components of the polymer occur when they evaporate as volatiles in the vacuum chamber and contaminate the plasma. Such effects of small molecule evaporation into the plasma and incoming ion beam are observed, particularly for highly plasticized rubbers, which usually contain 30–40% low-molecular-weight plasticizers. Careful and consistent preparation of the polymer surface and thorough knowledge of contaminations in the polymer bulk and surface regions are a must to ensure desired and consistent results of the ion implantation treatment.
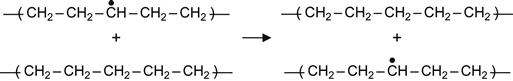
Polymer macromolecules are stable in the absence of radiation. Under radiation (for example, UV light or ion beam), some bonds are broken and macromolecules are transformed into free radicals. Such free radicals are very active and will cause a number of chemical reactions to occur in the polymer. Here, for example, we review free radical reactions in PE, which has been extensively investigated after irradiation [248–250]. Two kinds of initial radicals can be generated in PE macromolecules: a radical that appears at the end of a chain after a C![]() C bond break which splits the chain in two and a radical appearing within the macromolecule after a C
C bond break which splits the chain in two and a radical appearing within the macromolecule after a C![]() H bond break. The following reactions involving these two kinds of free radicals can occur:
H bond break. The following reactions involving these two kinds of free radicals can occur:
![]() (3.1)
(3.1)
 (3.2)
(3.2)
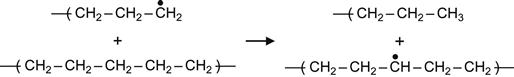 (3.3)
(3.3)
Reactions (3.1), (3.2), and (3.3) are interpreted as a transfer of the free radical (due to H atom swapping) along a macromolecule or from the excited macromolecule to a neighboring virgin macromolecule. Reactions (3.2) and (3.3) are the most sensitive to the intermolecular environment and are favored by strong intermolecular interactions between two neighbor macromolecules. As a consequence of these reactions, the radical can move deep into the polymer, reaching regions that were not directly affected by irradiation. Such reactions can, therefore, increase the extent of a modified surface layer to depths well beyond the ion penetration depth.
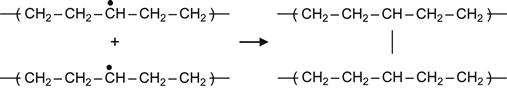 (3.4)
(3.4)
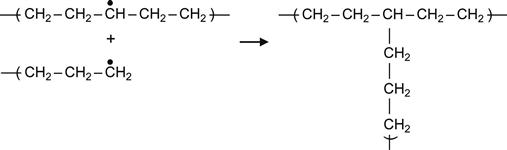 (3.5)
(3.5)
Reactions (3.4) and (3.5), which involve two proximate radicals, lead to the appearance of cross-links between macromolecules. These result in cross-links between central regions of two neighboring macromolecules or T-junction, where the end of one macromolecule links to the center of another macromolecule.
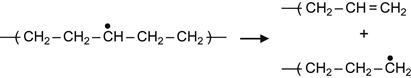 (3.6)
(3.6)
Due to reaction (3.6), the backbone of a macromolecule breaks. As the result, a double bond is formed between carbon atoms and the free radical remains active, generating further structural transformations.
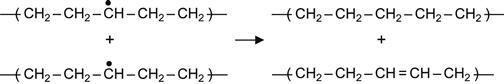 (3.7)
(3.7)
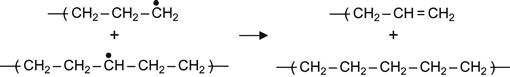 (3.8)
(3.8)
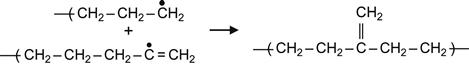 (3.9)
(3.9)
Reactions (3.7) and (3.9) occur in the presence of neighboring macromolecules, with free radicals that take hydrogen from adjacent macromolecules. As a result, a carbon-carbon double bond appears in the macromolecule. For the PE macromolecule, three kinds of double bonds can occur, resulting in: vinylene groups as a result of reaction (3.7), vinyl groups as a result of reaction (3.8), and vinylidene group as a result of reaction (3.9).
Although these processes of free radical transformation depend on neighboring macromolecules, phonon excitation, and the electronic states of the macromolecule fragments, they are mostly spontaneous and their evolution is best described by probability functions.
In other polymers, the free radical reactions are even more complicated. For example, the free radicals can generate depolymerization reactions. The result of depolymerization reactions is a decomposition of the macromolecules into a number of separate monomer molecules. A classic example of depolymerization reactions is the decomposition of butyl rubber into isobutylene monomers:
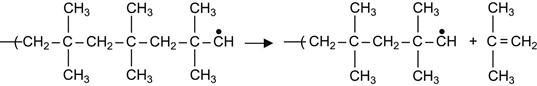 (3.10)
(3.10)
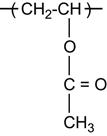
For some polymers, the radicals can initiate reactions that result in the spontaneous release of gaseous products. These can be gaseous monomers or other molecules formed as products of free radical reactions. For example, free radical reactions in a carbonyl-containing polymer (for example, PMMA) cause the release of CO gas:
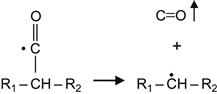 (3.11)
(3.11)
All polymers can be divided into two classes: polymers where scission (depolymerization) reactions dominate, causing the polymer to depolymerize with increasing ion irradiation; and polymers where cross-linking reactions dominate, causing the polymers to become more cross-linked with increasing ion irradiation. Examples of cross-linking polymers are PE and PS; examples of scission polymers are polyisobutylene and PMMA.
In contrast to UV light, γ-irradiation, and X-rays, ion implantation generates particular kinds of radicals, which appear as a result of atoms and ions recoiling in the structure. Direct collisions of high-energy ions with macromolecules transfer substantial energy to individual atoms or fragments of the macromolecules, which then leave their position and move with high speed away from the mother macromolecule. After a number of collisions that involve disintegration of fragments into individual atoms and the loss of kinetic energy, the atoms stop. At this time, the atoms have dangling bonds (or unpaired electrons) and are located between adjacent polymer macromolecules. For example, if PE is ion implanted, carbon atoms with four free valence electrons each and hydrogen atoms with one free valence electron appear at a range of distances from the initial track of the penetrating ion. These recoiled atoms are extremely active radicals, and they cause specific chemical reactions.
The hydrogen atoms can bond to macromolecules with dangling bonds, or to an unsaturated carbon-carbon bond or to another hydrogen atom, resulting in the formation of a hydrogen molecule that can diffuse out of and leave the structure.
Recoiled carbon atoms penetrating into hydrocarbon polymers can react with the virgin macromolecules according to the following scheme:
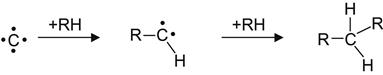 (3.12)
(3.12)
for example, in the case of PE macromolecules to produce a cross-link:
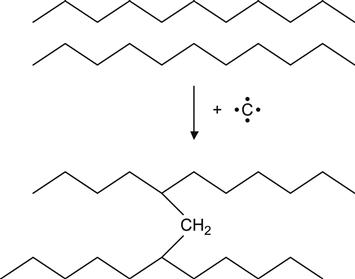 (3.13)
(3.13)
Such reactions dominate at low-ion fluences when the concentration of carbon recoil atoms is low and the concentration of unchanged virgin macromolecules is high. With increasing ion fluence, the volume density of carbon recoil atoms increases and the recoiled carbon atoms have increased probability of meeting radicals and forming bonds with them.
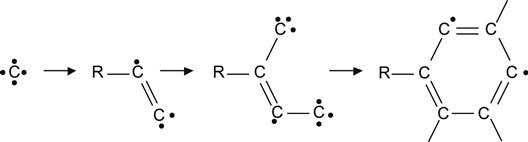 (3.14)
(3.14)
Thus, high fluences of ion implantation generate carbon clusters with irregular structures, containing unsaturated double and triple bonds, aromatic five- and six-membered rings, and conjugated structures, as depicted in (3.14) and (3.15).
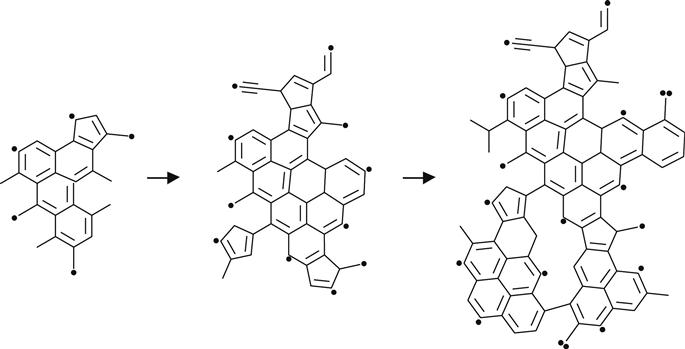 (3.15)
(3.15)
For high-fluence ion implantation, the modified layer no longer contains polymer macromolecules. This structure is amorphous carbon with short fragments of initial macromolecules. The size of the conjugated structures grows with increasing fluence. This is a length of polyene structures—(C![]() C)n—and a number of conjugated aromatic structures, as for example:
C)n—and a number of conjugated aromatic structures, as for example:
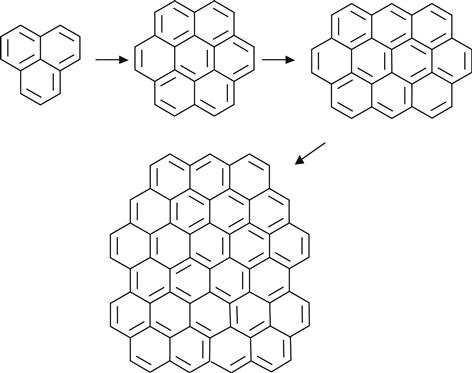 (3.16)
(3.16)
At high fluence, nanoscale pi-conjugated carbon clusters dominate in the modified polymer surface layer. Subsequent ion implantation occurs into the carbon structures. At this fluence, the ion implantation into polymer is finished and an ion beam implantation into carbon structure is started.
The presence of radicals means that the structure and chemistry of the modified layer continues to evolve long after the completion of ion implantation. The free radicals have been observed to survive for a long time after the treatment process has ceased. Despite a rapid rate of reactions immediately after ion implantation, the presence and activity of radicals can be observed for days to many months or even years after the treatment. The modified layer continues to restructure itself over these time scales. If a modified polymer remains in high vacuum (for example, in an ion implanter chamber or in a free-space environment) after ion beam implantation, the free radical reactions that continue to take place do not significantly change the structure of modified layer. Typically however, the modified polymer is taken out of the vacuum and used in air or in another reactive environments. Due to the presence of reactive species in particular oxygen, oxidation reactions occur and significantly change the structure of polymer when stored in air. The reactions of radicals with air oxygen proceed as follows:
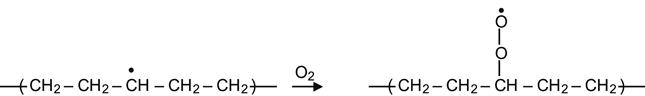 (3.17)
(3.17)
resulting in the formation of a peroxide radical. Such reactions in the surface layer begin immediately after contact of the modified polymer with air. Reactions in deeper layers are limited by diffusion of oxygen into the modified layer. Peroxide radical groups are very active and they can react with alkyl radicals to produce peroxide cross-links:
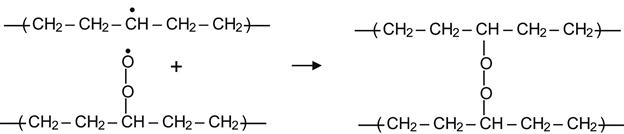 (3.18)
(3.18)
The peroxide radical can also react with neighboring hydrocarbon molecules, taking up hydrogen to form a hydroperoxide group:
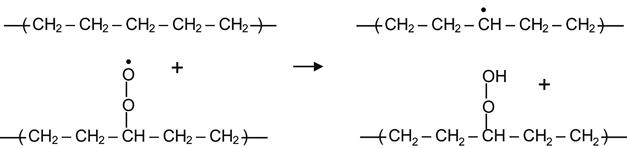 (3.19)
(3.19)
The peroxide radical can also take hydrogen from a neighboring group on the same molecule, resulting in the formation of a hydrocarbon radical. For example, in the context of a PE macromolecule, this reaction can occur in following way:
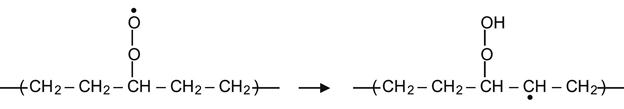 (3.20)
(3.20)
The peroxide group is more stable than free radicals and it can exist in the modified layer of the polymer for hours and even days. With time, the peroxide group can degrade according to the following schemes:
ROOH→R*+*OOH (3.21)
ROOH→RO*+*OH (3.22)
ROOH→ROO*+*H (3.23)
or react with free radicals
ROOH+RO*→ROO*+ROH (3.24)
ROOH+R*→ROO*+RH (3.25)
Reactions (3.21), (3.23), and (3.25) are reversible and do not give new products. Reaction (3.24) produces stable hydroxyl groups. Reaction (3.22) produces an alkoxyl radical that is unstable and can be transformed into various stable groups according to:
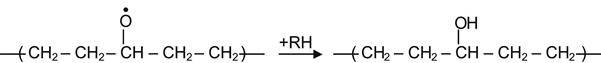 (3.26)
(3.26)
yielding a hydroxyl group,
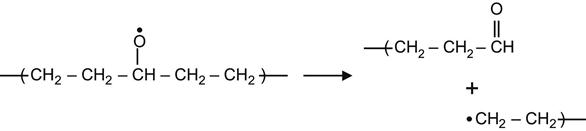 (3.27)
(3.27)
yielding an aldehyde group,
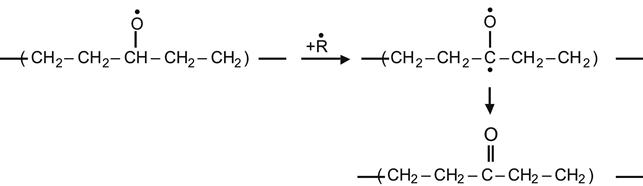 (3.28)
(3.28)
yielding a ketone group,
 (3.29)
(3.29)
yielding an ether group.
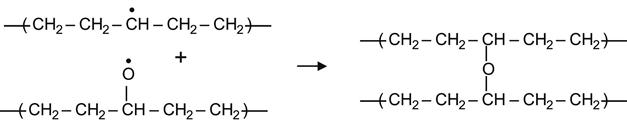
Hydrogen transfer between two interacting alkoxyl radicals can also yield a ketone and a hydroxyl group by a reaction of disproportionation.
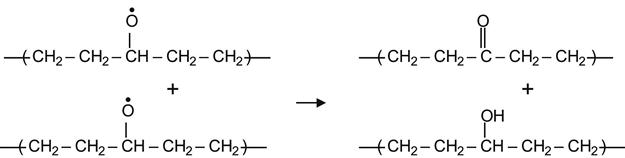 (3.30)
(3.30)
The aldehyde group can react with an oxidizing agent and form a carboxyl group.
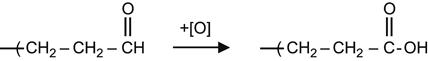 (3.31)
(3.31)
Such reactions proceed both in the modified layer and also in the deeper layer, in which free radicals move according to reactions of free radical transfer.
The reactions of ion-modified polymers with atmospheric nitrogen are not as well known and typically ignored. When there is a low concentration of radicals in the polymer after low-energy irradiation, the probability of reaction within nitrogen molecule is low due to the stability of the N![]() N triple bond. However, for high-ion irradiation fluences, when the concentration of multiple radicals is high, the following reactions can be expected to occur with implanted nitrogen atoms:
N triple bond. However, for high-ion irradiation fluences, when the concentration of multiple radicals is high, the following reactions can be expected to occur with implanted nitrogen atoms:
![]() (3.32)
(3.32)
![]() (3.33)
(3.33)
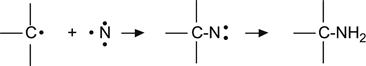 (3.34)
(3.34)
and with atmospheric nitrogen
![]() (3.35)
(3.35)
![]() (3.36)
(3.36)
Pyridine and pyrrole ring structures also form at fluences when the polymer macromolecules are highly damaged:
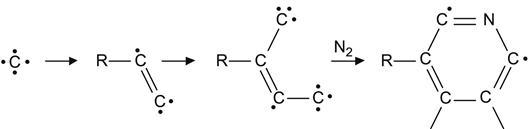 (3.37)
(3.37)
At high-ion implantation fluence, the oxidation and nitration of the highly carbonized subsurface layer also occurs. In the first stage of radical-induced transformation in the modified layer, clusters of condensed aromatic rings are formed. Radicals survive predominantly on the edges of these clusters. Therefore, reactions with atmospheric oxygen and nitrogen proceed mostly on the edges of the condensed aromatic structures. An example of showing how oxygen- and nitrogen-containing groups are incorporated with the carbon cluster can be depicted as follows:
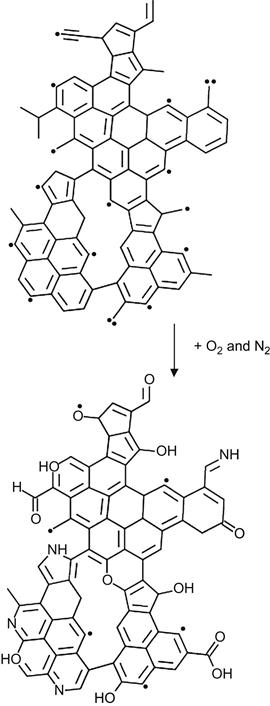 (3.38)
(3.38)
The modified surface is not only active to atmospheric oxygen and nitrogen. The radicals are active to a wide number of substances with different kinds of reactive groups. One example is the alkene group, which is reacts with radicals according to the following reaction:
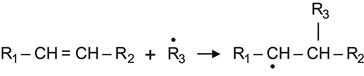 (3.39)
(3.39)
This reaction can be exploited to graft any functional compounds to the surface of the ion-modified polymer. For example, acrylic substances can react can be grafted through the following radical reaction:
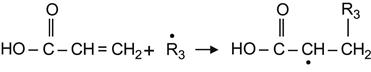 (3.40)
(3.40)
finally forming a polyacrylic acid layer on the modified polymer:
 (3.41)
(3.41)
Alternatively, an amine layer can be grafted through reactions with allylamine:
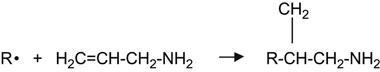 (3.42)
(3.42)
After such reactions with active substances, the modified surface acquires a new chemical activity that can be used for the attachment of further components. For example, the ion-implanted surface reacted with acrylic acid becomes active to bases as follows:
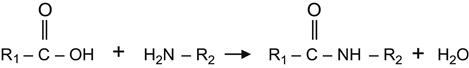 (3.43)
(3.43)
where R2 could be the remainder of a linker molecule, polyamine glue, or a protein molecule. Further useful examples showing experimental data of chemically grafted substances will be considered in subsequent chapters.
The chemical reactions in polymer after ion implantation that we have considered above have multiple pathways. The propagation paths, rates, and products of the reactions are difficult to predict and the paths they follow depend strongly on the type of polymer implanted, the ion beam or plasma parameters, and the postmodification exposure to reactive species of the polymer. The reactions of radicals begin immediately after ion penetration into the polymer. Kinetics of the reactions are difficult to measure and investigate because of the high rates of the reactions, the dependence of the reactions on distance from the surface, and the wide range of possible reaction paths and products, as well as the influence of environmental reactive species. However, some common principles of the reactions can be elucidated by applying our basic knowledge of free radical reactions in polymers and organic substances. Such reactions and their products are experimentally observed in polymers after γ-irradiation, high-energy electron and ion beam irradiations, X-ray irradiation and irradiation with UV light. The rate of these reactions depends on the mobility of the radicals, the macromolecules, and their activity. Fundamentally, the reaction kinetics are described by the first-order reaction equation:
(Eq. 3.1)
where ![]() is the concentration of the ith radical, [RjH] is the concentration of the jth unreacted macromolecular fragment, and k is the rate of the reaction, which depends on the temperature (T) according to the Arrhenius law:
is the concentration of the ith radical, [RjH] is the concentration of the jth unreacted macromolecular fragment, and k is the rate of the reaction, which depends on the temperature (T) according to the Arrhenius law:
(Eq. 3.2)
The rates and paths of the reactions depend on the local structure of the polymer (crystalline or amorphous phases), on the structure of neighboring molecules, and on the conformation of the macromolecules. The kinetics of the reactions could be described by a number of different reaction rates corresponding to different local structures. Reaction kinetics of this kind is called polychronos kinetics. The theory of polychronos kinetics is based on a distribution function, f(E), of active free radicals with activation energy, E [249], where the concentration of the active radicals at time t can be expressed as the integral over different ensembles of radicals with different activation energies of their radical reactions:
(Eq. 3.3)
where G[k(E),t] is a kinetic equation for ensembles with activation energies, E, in the range from Emin to Emax, and n0 is the initial concentration of the radicals.
Experimentally, the kinetics of the radical reactions is observed as a step-by-step unfreezing of radicals with temperature elevation (Figure 3.1). The kinetic curve of the integral number density of radicals with time after irradiation can be described by a number of exponential functions with a number of preexponential coefficients. This character of free radical reactions is observed for a wide number of polymers, radical reactions, and methods of free radical generation. Ion beam implantation is not an exceptional case.
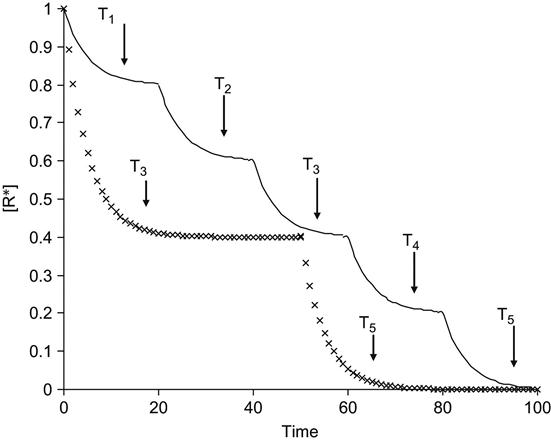
However, ion implantation is exceptional with respect to the high concentration of active free radicals created simultaneously at high local temperature in the small volume of the collision cascade around each ion track. Calculated phonon temperatures in the track region of the penetrating ion can reach 104 K, which is incredibly high compared to any characteristic temperature for a polymer. Therefore, immediately after ion propagation, the free radical reactions proceed in a limited volume and at extremely high temperatures.
The kinetics of reactions is sensitive to the local temperature, which depends on the ion-induced temperature spike, ion current density, temperature of the sample holder, thermal conductivity of the sample, and sample holder. Even in a low-current density regime in the absence of extrinsic sample heating, the temperature near the ion track is high and affects the kinetics of the reactions because they take place at temperatures much higher than the average temperature of the polymer. If the ion current is dense enough and a second ion penetrates the region of the polymer close to the first ion track, the temperature spikes from both ions overlap. Therefore, the radical reactions in this region of the polymer proceed at an even higher temperature. This effect changes the path and rates of radical reactions in the case of ion implantation in a high-current density regime even if the average temperature of the sample does not increase. Experimentally, this effect is observed by comparison of continuous and pulsed ion beam implantation regimes with equal average current densities. In the case of the pulsed irradiation, ions come with higher density during the pulse than in the continuous regime. Therefore, the products of free radical reactions created in continuous and pulse regimes are expected to be different, despite the fact that the average temperature of the polymer is the same.
Despite the high rates of the radical reactions, a residual concentration of free radicals remains in many irradiated polymers for years after ion implantation. For example, an estimation of the radical concentration in PS after nitrogen ion implantation shows, that 1 of 70,000 initially generated unpaired electrons remains after the first hour. The decay of these residual radicals is slow and they remain in irradiated PE, PS, and PTFE for years. This is due to the long lifetime of some kinds of free radicals and the relatively stable middle products of free radical reactions, as well as trapped free radicals at the edges of aromatic structures, where the presence of the conjugated aromatic structure with π-electrons stabilizes the unpaired electrons.
After ion implantation, reactive species in the environment can react with residual radicals in the polymer surface layer. The kinetics equation for these reactions includes the concentration of the reactive species. For example, the reactions with atmospheric oxygen depend on the concentration of oxygen at the polymer surface:
(Eq. 3.4)
where ![]() is the concentration of the ith residual free radical,
is the concentration of the ith residual free radical, ![]() is the concentration of the product of oxidation, and [O2] is the concentration of oxygen in the polymer layer.
is the concentration of the product of oxidation, and [O2] is the concentration of oxygen in the polymer layer.
Even if the concentration of oxygen is low (such as in the case of residual oxygen in a vacuum vessel), oxygen-containing groups can appear in the irradiated polymer after sufficiently long storage times in the vacuum system, despite a lack of direct contact with the atmosphere after ion beam implantation.
The same effect is observed in the presence of other residual gases in the vacuum chamber (oil from vacuum pumps, grease, or products of polymer degradation). The polymer surface becomes very active after ion implantation, and residual gases or vapors at very low concentrations can react with the radicals in the modified surface layer.