Barrier Resins
This chapter presents the ability of oxygen barrier resins, polyvinylidene chloride (PVDC), ethylene vinyl alcohol (EVOH), and nylon (PA), to retard the movement of oxygen across layer made of them when different oxygen partial pressures exist on either side of the layer. Polyolefins, especially highly crystalline ones, have a similar ability to retard the movement of water vapor when the partial pressure differences represent relative humidity. The process is quantitatively described at the molecular level and can be quantified in relative terms for flexible packaging materials. Similar molecular interactions are recognized in such effects as slip additive migration, flavor scalping, and extractables from packaging into packaged foods. Coextrusion of barrier resins with olefin polymer and copolymers provides an effective and economical means of incorporating this functionality into a packaging material.
Keywords
Barrier kinetics; coextrusion; diffusion coefficient; ethylene vinyl alcohol (EVOH); Fick’s first law; nylon (PA); permeability; permeant; permeation coefficient; polyvinylidene chloride (PVDC); solubility coefficient; solute
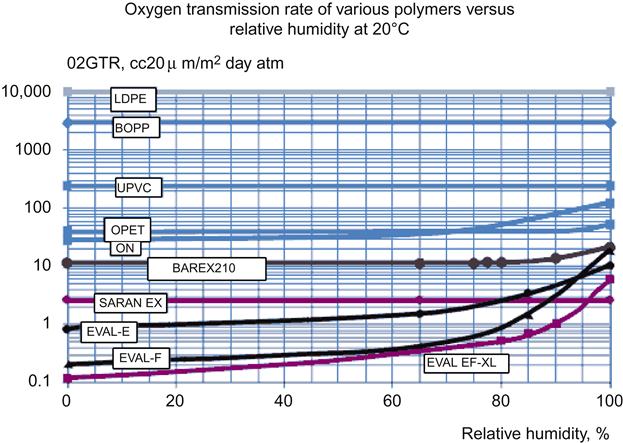
“Barrier resin” is a relative term in the flexible packaging industry, but it typically includes polyvinylidene chloride (PVDC), ethylene vinyl alcohol (EVOH), and nylon (PA) resins.1 In this regard, oxygen transmission rate (OTR) is the permeability of concern. Polyolefins (particularly biaxially oriented polypropylene) provide low water vapor transmission rates (WVTRs). Figure 25.1 summarizes general barrier properties for these barrier resins.
Figure 25.1 uses a logarithmic scale on the vertical axis for the respective OTR and WVTR values. It indicates that the OTR performance of EVOH varies with relative humidity (RH). The OTR of both PA and EVOH depend on temperature and RH. The effect in use is minimal under most ambient conditions, but becomes limiting when in-package processing conditions stress the polymers (Figure 25.2).
Barrier Kinetics
Understanding polymer barrier properties requires considering polymer permeability. Permeability is itself a well-understood property of small molecules as they move around in matter with free spaces at least as large as those molecules. The gasses, vapors, and volatiles (taste and aroma chemicals) of concern in packaging applications are the small molecules (see Table 25.1). Although polymers are themselves typically large molecules (having molecular mass of tens of thousands), they are not typically very dense (see Table 25.2). Aluminum and glass are two to three times denser than polymers. All molecules move because of their kinetic energy (at temperatures above absolute zero). The higher a molecule’s temperature, the higher is its speed. Importantly, that movement is random unless the matter is subject to a particular attractive or repulsive force, such as an electric field.
Table 25.1
Permeating Molecules Relative Size
| Molecule | Chemical Formula | Molecular Mass* |
| Gas & vapor permeants | ||
| oxygen | O2 | 32 |
| Water vapor | H2O | 18 |
| carbon dioxide | CO2 | 44 |
| nitrogen | N2 | 28 |
| Flavor/aroma chemistry | ||
| limonene | C10H16 | 136 |
| octane | C8H18 | 114 |
| capsaicin | C18H27NO3 | 305 |
| cinnamaldehyde | C9H8O | 132 |

*Mass used here as a substitute for volume
Table 25.2
| Packaging Material | Density (gm/cm3) |
| aluminum | 2.7 |
| glass | 2.4-2.8 |
| Water | 1.0 |
| polyethylene terephthalate (crystalline) | 1.5 |
| polyethylene terephthalate (amorphous) | 1.3 |
| nylon-6 (crystalline) | 1.2 |
| nylon-6 (amorphous) | 1.1 |
| High density polyethylene | 0.95 |
| polypropylene (crystalline) | 0.95 |
| medium density polyethylene | 0.93 |
| low density polyethylene | 0.92 |
| polypropylene (amorphous) | 0.85 |
On average, that random motion can appear to be directional—not random—if areas with different concentrations of mobile molecules are connected. By definition in the high-concentration area, more molecules per unit volume are present and moving. If molecules from both high- and low-concentration areas move with the same random paths, a greater number of molecules from the high-concentration area will move themselves to the low-concentration area than vice versa. Given enough time, the concentrations of the two areas will be the same. The result is a mass transfer of molecules from one area to the other, and an equalization of molecules per unit area in the two regions.
These very general observations about motion at the molecular level serve as the basis for a more quantitative discussion of the permeability of gasses, vapors, and volatiles through polymers. Hansen (1998) provides the basis for the following explanation [1].
For a generic kind of molecule (“solute”) in a polymer, its net movement to one side of the polymer or the other (the solute as a “permeant”) depends on (1) an intrinsic polymer property, D (its “Diffusion Coefficient”), (2) L the distance from one side to the other, and (3) the difference in concentration of the molecule from one side to the other (C1 and C2, respectively). This mathematical relationship is called “Fick’s first law.” If time enough is allowed to reach the point at which the net transfer, Q, is constant, Fick’s first law is expressed as:
(25.1)
In the more general case, for any time before the net transfer is constant and for any position “x” (from point 0 to point L), Fick’s law expresses transfer, q, as2:
(25.2)
Together with mass movement (assuming δc/δx ≠ 0), molecular motion results in pressure, p.3 With the introduction of another constant, S (“solubility coefficient”), Henry’s law holds that:
(25.3)
Substituting for c in Eq. 25.2 gives4:
(25.4)
Rearranging terms suggests a new term, P5
(25.5)
The relationship P=DS (Eq. 25.5) describes the movement of gas and vapor molecules through polymers with three fundamental factors6:
1. P: “Permeation coefficient” (mass transfer, q, over distance (x1−x2) with pressure (p2−p1));
2. D: “Diffusion coefficient” (the speed at which permeant passes through the polymer);
3. S: “Solubility coefficient” (the amount of permeant that can be dissolved in the polymer).
Table 25.3
Units of Measure for Gas Transport in Polymers
| Symbol | Factor | Units (SI) |
| P | Permeation Coefficient | gm/cm·s |
| R | Resistance | cm·s/gm |
| D | Diffusion Coefficient | cm2/s |
| S | Solubility Coefficient | gm/cm3 |
| q | rate of mass transfer | gm/cm2s |
| Q | rate of mass transfer (Steady state) | gm/cm2s |
| c | concentration | gm/cm3 |
| x | length | cm |
| l | Overall layer thickness | cm |
| c1 | concentration Side 1 | gm/cm3 |
| c2 | concentration Side 2 | gm/cm3 |
| p1 | vapor partial pressure Side 1 | (dimensionless) |
| p2 | vapor partial pressure Side 2 | (dimensionless) |
| R1 | surface resistance Side 1 | cm·s/gm |
| R2 | surface resistance Side 2 | cm·s/gm |
| RTOT | å resistances | cm·s/gm |
The three factors in Eq. 25.5 have the benefit of being reasonably tangible notions which describe interactions among solids, liquids, and gases in other contexts (e.g., carbon dioxide gas dissolved in carbonated beverages, small solid particulates in smoke diffusing through the air; moisture permeating through textiles). Those can be discussed as theoretical and mathematical relationships beyond the scope of this book, but a few comments here help to anticipate barrier behaviors in plastic packaging applications. The concepts add to an understanding of other observations in plastic packaging applications (e.g., “blooming” of slip additives, flavor scalping, extractables in packaged food).
The diffusion of a solute in a polymer depends on characteristics of both. The size, shape, and polarity of the solute are critical, as are the structure and mobility of polymer chains. Polymer chains arranged in crystalline form provide less free volume for solute diffusion. Some solutes in a polymer can embed themselves between the chains of polymers, pushing them apart (increasing the “free volume”), and significantly lower the glass transition temperature (TG—see Chapter 22) of the plastic and make it softer.7
Similarly, the solubility of a solute in a polymer depends on the size, shape, and polarity characteristics of solute. Additionally, hydrogen bonding and related van der Waals forces in the polymer can attract-hold or repel solutes. Solubility has surface dependencies as well as volume considerations. Localized surface effects (e.g., stagnant air, a layer of condensed moisture or other coating, convection) may decrease the probability that a given solute molecule will enter the polymer and begin permeating at a polymer specific rate of P=DS.
The inverse of a permeation coefficient, represented as R “Resistance,” begins to agree with the familiar notion of barrier. R, by inverting Eq. 25.5, equals:
(25.6)
Solving for q with a unit distance (i.e., x1−x2=1), R (just as the usual notion of barrier) is seen to be inversely related to mass transfer, so that q equals “driving force” divided by resistance:
(25.7)
Equation 25.7 suggests that mass flow through a multilayer film may behave in a manner analogous to the flow of an electric current through a circuit of resistors connected in parallel (cf. Ohm’s law). Two implied assumptions must be addressed: (1) normalizing Eq. 25.6 to a unit distance must be readjusted for actual thicknesses of various film layers and (2) the diving force through each layer i (pi2−pi1) must be known at best a mathematical challenge and at worst an experimental impossibility.
Neither adjustment is needed if (see Eq. 25.6) 1/P is substituted for R values and the thickness, li, for each layer i is substituted for (xi1−xi2). Additionally each surface of the composite film may present “surface resistance” (R1 and R2) equal to or greater than 0 for any permeating solute. The total resistance, RTOT, equals two surface resistance values as “R,” plus the thickness of each of “n” layers in an n-layered material, divided by its layer permeability:
(25.8)
With these adjustments for layer thicknesses and the internal permeant vapor pressure at each interface, total mass transport for a multilayered film (see Eq. 25.7) can be expressed as:
(25.9)
Equation 25.9 stresses that mass transport of a gas or vapor through a multilayered film depends on both the total resistance of the film to molecular movement and the existing pressure differential of the permeant.
Polyvinylidene Chloride
PVDC (also known by its trade name Saran®) has long served as an important role as a flexible packaging material. The material used typically represents a copolymer consisting of percent vinylidene chloride and vinyl chloride, or methyl methacrylate. Its hydrophilic nature allows its formulation into water- and solvent-based coatings. Its polarity favors high crystallinity, providing a dense moisture barrier microstructure. The high chlorine content of the polymer attracts (and slows) any oxygen molecules attempting to diffuse through it.
The material itself is stable, safe to handle, and not environmentally harmful under normal circumstances. PVDV resin presents significant handling challenges for extruding. If the resin reaches high temperatures 392°F−200°C, it will degrade, evolving hydrogen chloride gas (hydrochloric acid) at concentrations that may cause eye, skin, and respiratory irritation and/or injury. Such degradation limits its use in extrusion equipment that is not especially designed and fabricated for PVDV use. Hydrogen chloride gas evolving from PVDC-containing packaging incinerated in energy-from-waste facilities has also discouraged its use in the industry.
Ethylene Vinyl Alcohol
Polyvinyl alcohol (PVA) is not prepared by polymerization of the corresponding monomer, vinyl alcohol. Such monomers are unstable in the presence of acetaldehyde. PVA is prepared instead by first polymerizing vinyl acetate, and the resulting polyvinylacetate is converted to the PVA.8 Typically supplied as beads or aqueous solutions, PVA itself enjoys great demand in papermaking, textiles, and a variety of coatings. Ethylene vinyl acetate (EVA), having greater than 50% vinyl acetate content, can similarly be converted to EVOH. EVOH copolymer is defined by its mole % ethylene content: lower ethylene content grades have higher barrier properties; higher ethylene content grades have lower extrusion temperatures.
EVOH resins used as a barrier layer within rigid and flexible plastic packaging have become a global standard for polymeric oxygen barrier.9 The sensitivity of its barrier performance to RH (Figure 25.2) often requires unique design approaches, but helps illustrate some of the nuance of barrier kinetics. Moisture readily dissolves in EVOH resin (just as liquid water and alcohols form intimate mixtures).10 The dissolved moisture becomes intimately bound within EVOH crystals, the result of “hydrogen bonds” as the hydrogen atom of the hydroxyl group attracts the oxygen molecule. This causes general disruption of the crystalline structure and more free space within the polymer for oxygen to permeate. The effect is termed plasticizing (i.e., increasing the fluidity of a polymer).
When packaging aqueous liquids (e.g., condiments, juices) a desiccant can be compounded in a polyolefin layer between the product and EVOH layer to absorb water vapor migrating through the polyolefin [2]. High temperature accelerates and increases the amount of water vapor dissolving in the EVOH. The effect on polymer structure and barrier performance is reversible to some degree as ambient temperature and RH decrease. Foods in EVOH barrier packaging can be heat sterilized (called “retort” processed) if sufficient design considerations and postprocess controls are available.
Nylon
Nylon actually represents a large family of resins comprised of two different monomers, a diacid and a diamine, generically called “polyamides.” Typically, the number of carbon atoms in the two monomers defines a type of nylon. Nylon 6,6 (the most common polyamide used in flexible packaging) consists of the polymer made from adipic acid [HOOC(CH2)4COOH] and hexamethylene diamine (H2N(CH2)6NH2). The carboxyl groups (COOH) and the amine groups (NH2) of adjacent polymer chains in solid nylon attract one another to form crystals. Much as in EVOH crystals, these areas of molecular interaction will attract and hold water vapor. Nylon can absorb so much water vapor that drying the resin with hot air before extrusion is common. The plasticizing effect of moisture impairs oxygen barrier of nylon in flexible packaging uses.
Coextrusion
Coextruded flexible films with cores of barrier resins have addressed some of the limitations posed by handling difficulties of PVDC and moisture sensitivity of EVOH and nylon. In the former case, “encapsulation” of PVDC by polyolefins separates corrosion-sensitive parts of extrusion equipment from hot hydrochloric acid. The acrylate copolymers provide adequate adhesion to both core and skin layers to deliver a film with structural integrity. In the latter case, the good water vapor barrier of polyethylene and polypropylene can sufficiently isolate EVOH and/or nylon layers from moisture to maintain effective oxygen barrier.11 To this end, a diverse array of specialty tie resins has been developed to provide interlayer adhesion (Chapter 24).
Barrier resins can reinforce frail oxygen barrier of other films. Metallized oriented polypropylene films provide good oxygen barrier if the vapor-deposited coating is not cracked or crazed (the plain film, with no coating, has poor oxygen barrier). Coextruding the polypropylene with an adhesive layer to an EVOH layer allows the oriented film to provide robust and high oxygen barrier performance in spite of handling during filling and distribution [3].