Inks
This chapter discusses the components of a packaging ink, its vehicle and its pigment. The vehicle serves as a solid chemical matrix in which the colored pigment is suspended. The vehicle must have the ability to keep the pigment suspended in the ink’s fluid and cured forms. A pigment represents a complex organic chemical that differentially absorbs and transmits wave lengths of light to give the impression of color to an observer. Packaging inks require a curing process of some sort to transform the ink from its fluid state to its solid, cured state. Traditionally, this has been accomplished by drying a volatile solvent or water from the ink and allowing the vehicle to cross-link in solid form into a stable layer. Energy-cured inks use ultraviolet light or electron beam energy to cross-link small vehicle molecules into larger immobile ones.
Keywords
Aqueous emulsion; electron beam; energy-cured; ink vehicle; nitrocellulose (NC) bases; nitrocellulose; pigment; pigment constitution number; pigment generic name; ultraviolet; van der Waals force
Printed images represent a critical part of packages that both market the packaged product and convey important information about it. The physical demands placed on the ink used to print the images and texts are as strenuous as those expected of the rest of the package material. In addition, the message must remain clear and legible throughout a package’s useful life. Ink pigments, the colorants, and inks’ “vehicle,” the matrix holding pigment in the packaging comprise its two major components. Special additives may be included for special performance features, such as coefficient of friction. Some means must be provided to cure the ink, change it from a fluid to a solid. And once in place, the ink must adhere to one or more substrates and resist environmental and product challenges to its printed form and layer adhesion [1]. The ink chemistry basics presented here provide only a cursory overview of ink technology for flexible packaging. Small amounts of additives (e.g., 1–3%) in an ink can change the fit-for-use performance of a packaging material.
Ink Vehicles
An ink vehicle must cure on a substrate to form a water-clear film layer with good cohesive strength and the ability to “wet out” pigments suspended in them. Liquid inks required for flexographic and gravure ink vehicles frequently use “nitrocellulose” (“NC”). This is formed by “nitrating” cellulose by exposing it to nitric acid or another powerful nitrating agent. Fully nitrated, it serves as a propellant or low-grade explosive. If the cellulose is nitrated to only about 10–12% and dissolved in alcohol, it will dry to form a film layer. Nitrocellulose-based inks find extensive application in inks for films and foils. Nitrocellulose is also a very good pigment-dispersing resin. NC “bases” represent high concentrations of pigment intimately blended in the resin and minimal solvent. These are later diluted to press-ready concentrations using un-pigmented nitrocellulose “extender.”
Other vehicles for liquid inks are thermoplastic, low-molecular-weight polymers, produced from a wide range of feedstocks. The major source of feedstocks is various petrochemicals with average molecular weight of resins below 2000.
Polyamide vehicle resins soluble in ethanol, n-propanol, isopropanol are used for surface-print polyolefin film inks. These resins are compatible with alcohol-soluble nitrocellulose. This provides faster drying and the ability to use economical NC bases.
Recent solvent-based ink developments include polyurethane-based, flexographic and rotogravure printing ink system designed for use on multiple flexible packaging lamination structures and substrates. These provide high bond strengths (~500 gm/inch width) for extrusion and adhesive laminations on multiple substrates, but may not be compatible with NC bases.
Styrene acrylic aqueous emulsions serve paper printing needs and some film printing markets for which solvent-based printing inks are not an option. Their ability to wet out and adhere to plastic film surfaces limits plastic printing applications elsewhere.
Seldom are ink systems for flexible packaging applications formulated with a single vehicle resin. Vehicle design provides significant competitive advantages for ink formulators, and much ink development work progresses on a pragmatic empirical basis.
Ink Pigments
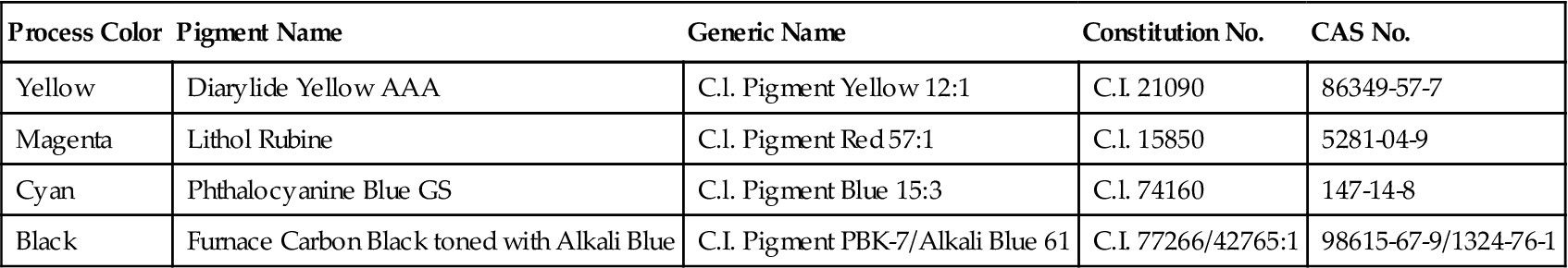
The colorants in flexible packaging inks are much more standardized than vehicles. Although common names may vary with location, a reasonable uniform global pigments identification system identifies pigments by “Generic Name” and “Constitution Number.” Such pigments are well-defined (although not always totally disclosed) organic chemicals and they also have a unique Chemical Abstracts System (CAS) Registry Number. Table 26.1 summarizes these designations for the four process colors.
Table 26.1
Pigments for Four-Color CMYK Inks
| Process Color | Pigment Name | Generic Name | Constitution No. | CAS No. |
| Yellow | Diarylide Yellow AAA | C.l. Pigment Yellow 12:1 | C.I. 21090 | 86349-57-7 |
| Magenta | Lithol Rubine | C.l. Pigment Red 57:1 | C.l. 15850 | 5281-04-9 |
| Cyan | Phthalocyanine Blue GS | C.l. Pigment Blue 15:3 | C.l. 74160 | 147-14-8 |
| Black | Furnace Carbon Black toned with Alkali Blue | C.I. Pigment PBK-7/Alkali Blue 61 | C.I. 77266/42765:1 | 98615-67-9/1324-76-1 |
Pigment chemistry is very complex. The absorption of color provided by a given pigment results from the pigment’s pattern of chemical bonds.1 Different chemical bonds arranged in a chemical molecule absorb the energy of specific waves lengths of light (i.e., “subtract” color). Some 13,000 Generic Names are maintained in the global index [2]. Each of these represents a distinct chemical formula, a distinct color value, and its own production process. The quest for plentiful and affordable synthetic dyes and pigments provided much of the motivation for chemical discovery in the nineteenth century.2
Because of the sensitive dependence of color value on the molecular structure of pigments, any change to the molecule changes the color perceived when viewing it. Chemical stressors, such as ultraviolet light and environmental pH and temperature, can change color values, sometimes irreversibly.
Ink Curing
Ink printed on an impermeable substrate takes the form of a very thin (0.04–0.8 milil 1–20 µ) film of cured vehicle with its embedded pigment.3 This “film-forming” ability represents a major criterion for ink vehicles. Such a film, formed from a fluid with solids dissolved or suspended in it, has physical properties just as any extruded thermoplastic film. The properties depend on the effects of the liquid solvent that is evaporated from the ink and on the presence of various other additives in the fluid.
Film forming may result from molecules of the vehicle simply arranging themselves into relatively cohesive patterns as a result of the chemical forces (hydrogen bonds and van der Waals forces) involved in interlayer adhesion (Chapters 28 and 29). Such films redissolve in solvents and lack resistance to similar chemicals that may challenge them while a package is in use. Heat resistance may also be minimal, both with respect to adhesive and cohesive strength and color stability.
Other vehicles (e.g., polyurethanes) form films in which cross-linked chemical bonds bind molecules together. Applications requiring heat and chemical resistance require these more durable inks.
Traditionally fluid inks rely on evaporating the solvent (organic or water) that holds the vehicle in solution or suspension and allowing the remaining solid materials to form films as their chemistry dictates.4 “Energy-cured” inks involve 100% solids (vehicle plus pigment) formulation in which external energy (UV light or electron beam energy) causes chemical bonds between vehicle molecules to form the ink film. These cure using the mechanisms described for energy-cured adhesives (Chapter 28). Formulations include a mix of small reactive molecules (making them sufficiently low viscosity for ink transfer) and larger molecules (reducing the amount of energy needed to cure the ink). “Dark” pigments (in the sense of absorbing UV energy) can impair UV curing by absorbing curing energy before it reaches the lowest layers of the ink coating.
Ink Selection
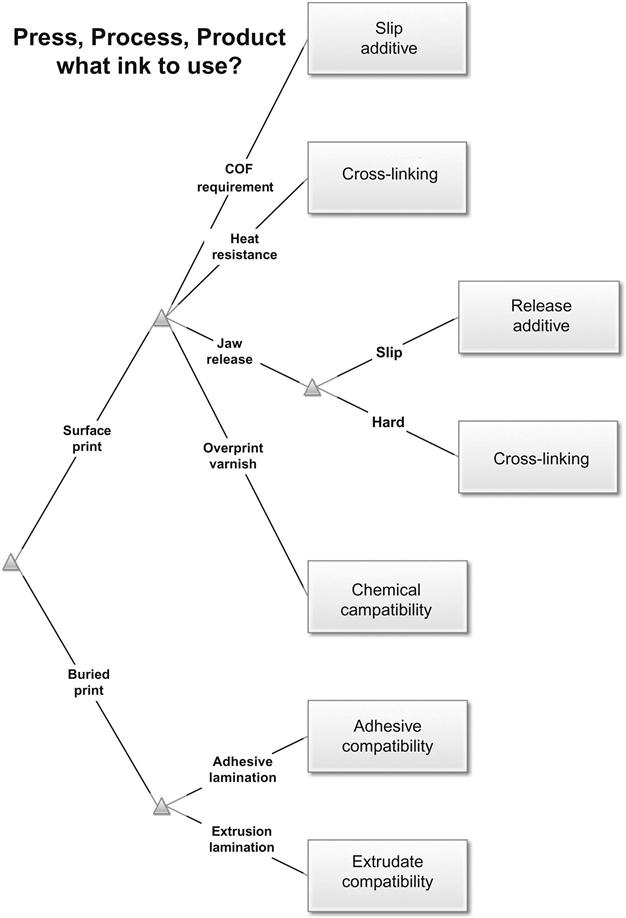
Choosing the set of inks that matches a converter’s equipment, processes, and product mix may well be the most critical purchase decision faced by the organization. Often only experience serves to anticipate interactions between an ink and the surrounding chemistry of the packaging structure (e.g., chemical forces changing color values, ink additives migrating through adjacent layers or offsetting to unprinted surfaces in roll form). Figure 26.1 suggests a few initial considerations for choosing an ink with its associated additives and functionality for a particular process and product. When the need is recognized, a small amount of the proper additive (e.g., 1–3%) may satisfy the need, but often more than one technique is available to satisfy the need. In the figure “jaw release” for surface-printed webs refers to the need to have a printed area contacted by hot sealing jaws on packaging machinery release from the jaws after a heat sealing cycle after its heat, pressure and length of time tends to soften and attach to the ink film. The solution may be to add a release additive that will free the ink from the jaw after pressure is relieved, or an ink film hardener that will reduce the ink’s tendency to attach to the jaw. Scuff resistance of a surface-printed ink can be addressed with similar tactics: additives to make the ink slide without disruption or to harden the ink to withstand greater forces. The choice often depends on other concurrent requirements (e.g., coefficient of friction or gloss).