Adhesives
This chapter describes the chemical composition of traditional flexible packaging adhesives. These materials have the demanding challenge to adhere strongly to two different substrates and provide enough cohesive strength that the multi-layered lamination is able to integrate the desired properties of its individual components. The chemistry of adhesives is typically rather complex in order to deliver all of this functionality. Their curing process usually involves chemical reactions that bond one type of chemical to another forming a nonreversible solid. The usual type of this adhesive is based on urethane chemistry that is able to form polymeric materials from monomers at atmospheric pressure and near room temperatures. Alternate chemistries use acrylics for aqueous emulsion. Energy-cured adhesive formations are also available to the industry.
Keywords
Acrylates; contact angle; curing; electron beam; photoinitiator; polyurethane; surface wet-out; ultraviolet
The adhesive laminating process (Chapter 4) involves adhering two surfaces to one another with a layer of a third substance that (1) provides high adhesion to both surfaces and (2) has internal strength (cohesiveness) greater than either interface. Both the adhesive and cohesive strength of a suitable flexible packaging material must resist any expected thermal, chemical, or physical exposure that would negate those strengths over the life of the packaged product.
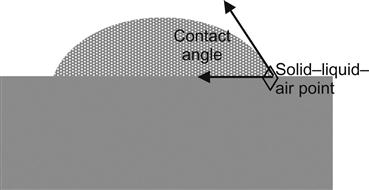
The adhesion to other surfaces requires that the adhesive “wet out” that surface. If complete wetting does not occur, then liquid of will form a bead, having a contact angle relative to the surface (See Figure 28.1. The value of the angle results from the surface energies of the solid–liquid–air system). The surface is solid and the adhesive must intimately cover the plane of its surface with no air (or other substances) trapped between the solid and the fluid.
Cohesion of the adhesive layer itself results from “curing” the adhesive. The curing mechanism differs for different adhesive types but often involves a chemical reaction [1].1 The reaction may require multiple chemical “parts” (details follow) and may originate a gaseous by-product that must escape the structure. Adhesives, coatings, and inks often involve chemical reactions. As a result, regulatory control over flexible packaging adhesives, particularly for food packaging, is considerable. The converter as well as the supplier of these materials must comply with these both in formulating the materials and using them.
Adhesion by a coated adhesive to the second substrate requires that the coating still has a fluid nature, either because it has not yet begun to cure, or the partial curing that has occurred still allows the adhesive surface to soften when heated and conform to the other surface under pressure.
Polyurethane Adhesives
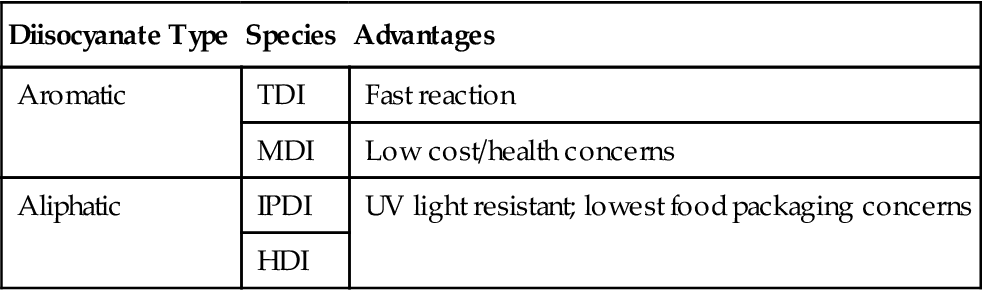
A urethane is formed by the reaction of the hydroxyl (i.e., “-OH” or alcohol functionality) of a “polyol” (two or more hydroxyl functions) with a suitable isocyanate chemical. If that chemical is a “diisocyanate” it reacts with another hydroxyl group on another polyol, lengthening a polymer chain and increasing molecular weight of the “polyurethane.” Specific polyol and diisocyanate chemistry result in different adhesive tendencies, environmental resistance, and health implications (Tables 28.1 and 28.2) [2].
Table 28.1
Influence of Polyol Choice on Polyurethane Adhesive Performance
| Polyol Used | Advantages | |
| Toluene diisocyanate (TDI) | Moisture resistance | Flexible at low temperatures |
| Microbial resistance | Soft/rubbery feel | |
| Diphenylmethane 4,4′ diisocyanate (MDI) | Chemical resistance | Brittle at low temperatures |
| Better metal adhesion | Design options: crystallinity-based | |
| Isophorone diisocyanate (IPDI) | Balance hardness and low temperature flexibility | |
| Hexamethylene diisocyanate (HDI)a | Greater tensile properties |

aUsed as modifier with polyether/polyester polyols.
Table 28.2
Influence of Diisocyanate on Polyurethane Adhesive Performance
| Diisocyanate Type | Species | Advantages |
| Aromatic | TDI | Fast reaction |
| MDI | Low cost/health concerns | |
| Aliphatic | IPDI | UV light resistant; lowest food packaging concerns |
| HDI |
In any specific adhesive formulation, the polyol can be low- to high-molecular weight of singular or mixed materials, polyurethane “prepolymer,” hydroxyl terminated, or mixtures of the two. These are reacted with low- to medium-molecular weight diisocyanate prepolymer. They cross-link to form the urethane link and the final cured adhesive. This would be called a two-part adhesive system.
There is always the potential that water will react with the isocyanate as a result of humidity in the air and water in solvents.2 Though in this case the goal is to create polyurethane adhesive, A reaction can occur between the diisocyanate and water to form an amine, considered carcinogenic. When the adhesive is fully cured, the amine is completely reacted and the laminate is safe to use. (If the laminate is brought into contact with food before curing is complete, the amine formed may migrate into the packaged product) [3]. The actual chemistry of adhesive reactions is complex and a converter must wait sufficient time to assure the reaction of the amine into the cured adhesive before the food is packed in a laminated material.
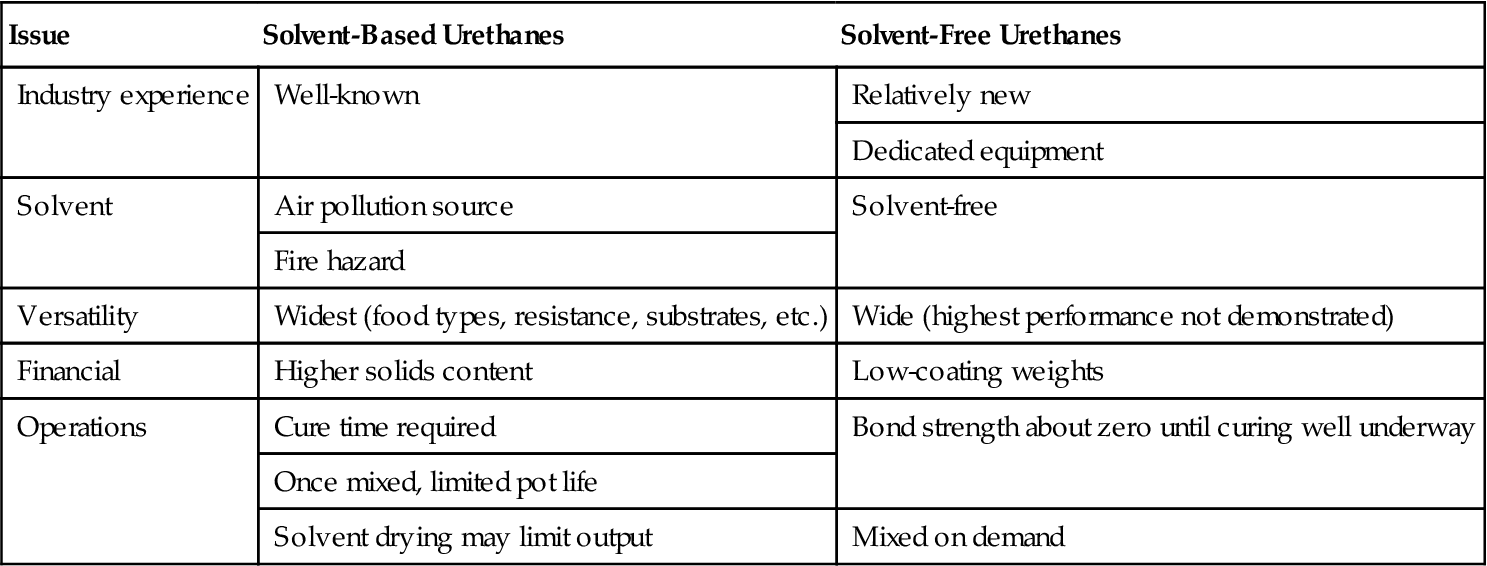
The breadth of urethane chemistry provides the flexible packaging industry with a wide range of adhesive choices. The historical solvent-based grades are now giving way to solvent-free (100% solids) versions because of their financial and pollution control advantages (Table 28.3).
Table 28.3
Polyurethane Adhesives: Solvent and Solvent-Free
| Issue | Solvent-Based Urethanes | Solvent-Free Urethanes |
| Industry experience | Well-known | Relatively new |
| Dedicated equipment | ||
| Solvent | Air pollution source | Solvent-free |
| Fire hazard | ||
| Versatility | Widest (food types, resistance, substrates, etc.) | Wide (highest performance not demonstrated) |
| Financial | Higher solids content | Low-coating weights |
| Operations | Cure time required | Bond strength about zero until curing well underway |
| Once mixed, limited pot life | ||
| Solvent drying may limit output | Mixed on demand |
(Adapted from Jopko, 2004)
Acrylic-Based Adhesives
Acrylic is a general term for polymers made from acrylic acid. Acrylic esters (“acrylates”) such as methyl, ethyl, butyl and 2-ethylhexyl acrylates, copolymers, and various blends of are used to formulate acrylic adhesives.
The acrylic-based polymers usually have mid- to high-molecular weights and are emulsified in water. This produces very small particles dispersed in water and allows high percent solids at very low viscosity. Many of these adhesives are used as one-part with either no cross-linking or some self-cross-linking. In essence, they remain pressure sensitive and rely on their inherent molecular weight for the properties of adhesion, tack, and intrinsic bond strength and shear resistance.
For higher performance, a suitable cross-linker can be used to create a two-part system. There are water-dispersible isocyanates that can form acrylic urethane polymers when cured. This allows the performance and benefits of both the acrylic and the urethane chemistry. Another approach is to use amine–epoxy cross-linking for the cure. The acrylic polymer is terminated either in amine or epoxy, and the opposite prepolymer is used for the cure. Many other cross-linking materials are available for acrylic-based adhesives, but few have status in the food laws.
Acrylic chemistry is naturally crystal clear and relatively low cost. One-part acrylic adhesives provide limited bond strength, and chemical and heat resistance. Two-part systems are somewhat better but are not able to perform in demanding chemical and thermal environments.
Energy-Cured Adhesives
This type of adhesive cross-links acrylics and polyurethanes with ultraviolet light or electron beam energy (streams of electrons). UV cross-linking relies on “photoinitiator” chemicals that release “free radical” species (an atom, molecule, or ion that has unpaired electrons). Free radicals provide energy to cross-link adhesive prepolymers and to in-turn release more free radicals to continue the process. Electron beam energy cross-links the chemicals directly.
Photoinitiators are small molecules and if not completely reacted in the curing process remain mobile in the adhesive matrix from which they can migrate into food. Electron beam energy averts the photoinitiator requirement, leaving less concern about chemical contamination.
Energy-cured adhesives systems are not in widespread use in the industry, even though the process eliminates the need to wait for adhesives to cure.
Proper selection of flexible packaging adhesives challenges the industry regularly. New chemical options, new regulatory limitations, new product/package interactions, long product shelf life requirements all make finding the optimum adhesive solution difficult. Table 28.4 provides directional guidance, but any choice must be carefully designed to meet all fit-for-use requirements.
Table 28.4
Summary of Available Adhesive Chemistry
| Adhesive Type | Snack Low Demand | General Purpose | Chemical Resistance | Hot Fill | Boil-Cook-in Retort | |
| Medium | High | |||||
| Solvent PU | OK | OK | OK | OK | OK | OK |
| 100% solid PU | OK | OK | OK | (OK) | OK | (OK) |
| Acrylic | OK | OK | (OK) | NO | OK | NO |
| Energy cure | (OK) | (OK) | (OK) | NO | (OK) | NO |

(OK), careful testing/experience required.
(Adapted from Jopko, 2004)