Chih-Ming Ho, Dean Ho, and Dan Garcia
The solid-state microelectronics industry started in the late 1940s and has been the main driving force of the U.S. economy in the past few decades. In the 1980s, microelectromechanical systems (MEMS) technology demonstrated the capability of fabricating mechanical devices at a scale far smaller than those made of traditional machining processes. Fan, Tai, and Muller (1988) developed a micromotor that was on the order of 100μm by using the MEMS fabrication process, which was derived from the integrated circuit (IC) industry.
Further reduction of the scale into the nanoscale has enabled the fusion between nanotechnology and biology. By working on the same scale as that of macro functional molecules, such as DNA and proteins, nanotechnology emerges as an opportunity to further build upon earlier technologies. An example is the nanomotor developed by Soong et al. (2000), which grew from the amalgamation of the mitochondrial ATPase and a nanofabricated metal rod. The progression of time has produced machines of diminishing size, leading the engineering sciences into progressively smaller dimensions. This movement has facilitated the expansion of the field of nanotechnology to explore and develop this new scientific frontier.
Technological innovations such as nanotechnology aim to enrich human life. However, nanotechnology presents a unique technical challenge in that a disparity of nine orders of magnitude separates the length scales of a human (a meter) and a nanometer. Ultimately, this goal needs to be achieved through the development of a definitive pathway that fuses existing technology with future technology to link the nanoscale with human life.
An extremely intelligent, complex, and adaptive system, the human body is managed by natural processes driven by molecules, such as DNA and proteins, that are on the order of a nanometer. The challenges in exploring the governing mechanisms across a wide span of length scales is best depicted by P. W. Anderson in a paper published in Science (1972): “At each level of complexity entirely new properties appear, and the understanding of the new behaviors requires research which I think is as fundamental in its nature as any other.” For example, a cell might fuse the genetic information contained in the DNA with nanoscale sensors and actuators to result in perhaps one of the most efficient and autonomous micron-scale “factories.”
The richness of the science that spans a difference of three orders of magnitude in length scale, from the nanoscale realm to the macroscale realm, significantly exceeds our comprehension. An imperative task will be to address the question of how we will span the length scales of these nanoscale capabilities in a way that will eventually enable us to enrich human lives. These basic processes that occur at the molecular level have opened a world where the integration of individual components can eventually derive higher-order functionalities, or emergent properties. This leads us toward a compelling approach that fuses biotechnology, nanotechnology, and information science, which will enrich the development of revolutionary, application-specific technologies.
To drive the commercialization of nanotechnology, we need to further our understanding of the nanosciences. During the past decades, our understanding of nanoscale molecules and their functionalities has been significantly furthered by distinguished scientists in biology, physics, and chemistry. Nanotechnology, on the other hand, is still in its infancy, but it is the key to realizing a new industry. The establishment of the National Nanotechnology Initiative (NNI) has further increased the momentum of progress in these areas.
For this realization to occur, three goals must be achieved. First, core technologies must be developed to enable us to visualize, pick, place, manipulate, and characterize nanoparticles with a high degree of precision. Second, we will need to establish manufacturing technologies to enable systematic integration toward larger length scales with the higher information content embedded within the composite structures. The final step toward establishment of a nanotechnology industry will result from the capabilities displayed by emergent behavior at the nanoscale for increasing functionalities.
Instead of providing a comprehensive review, this chapter uses examples based primarily on our experience to illustrate the challenges and the potential associated with the impacting field of bio-nano-information fusion.
Perhaps one of the most beneficial tools afforded by the process of realizing the full potential of nanotechnology will be the ability to visualize nanoscalemolecular activity in real time. Continually advancing imaging methods have pushed the boundaries of nanoscale resolution.
Fluorescence microscopy greatly enhances the visualization of micro-and nanometer particles in biological specimens. For example, confocal microscopy augments the fluorescent labeling of everything from individual proteins to whole cells by offering several benefits over traditional fluorescent microscopy. Of particular note is that confocal microscopy reduces background fluorescence by using a filter that blocks out fluorescence located outside the focal point of the lens. Because it contains multiple excitation sources, confocal microscopy provides the further benefit of the simultaneous use and visualization of multiple fluorescent dyes. This allows, for example, the visualization of multiple neuronal cell attributes, as illustrated in Figure 17-1.

Figure 17-1. Ten-day-old mouse cerebellar neuronal culture labeled with neuronal marker beta III-tubulin and a marker for de novo DNA methyltransferase (Feng, Chang, Li, and Fan 2005).
Atomic force microscopy (AFM) has evolved as a valuable method for evaluating surface topography down to the nanoscale, largely because of its incorporation of a flexible cantilever containing a nano-sized tip. Recently, AFM has been used to study nano-sized pores contained in cell membranes that facilitate the exchange of solutes and nutrients between their cytoplasm and their environment. For example, within its outer membrane the bacterium Escherichia coli contains channels called porins that open and close in response to changes in pH and transmembrane voltage. To study the conformational changes of the porins under the influence of pH and voltage, Muller and Engel (1999) employed atomic force microscopy. Upon varying the pH or voltage across the membrane, AFM confirmed conformational changes in the porin, showing a transition from a columnar structure to one consisting of a nano-sized hole, as shown in Figure 17-2.

Figure 17-2. High-resolution AFM images of ion gradient and pH-dependent conformation changes of porin OmpF: (A) closed OmpF porin channel; (B) open OmpF porin channel (Müller et al. 1999).
Most recently, using materials that can amplify near field optical waves to visualize subjects far below the diffraction limit (Fang et al. 2003; Pendry 2001) will open a new territory for advancing nanotechnologies. The continued enhancement of the nanoscale visualization methods will enable discoveries of complex processes, including receptor-ligand interactions, DNA translocation across cellular membranes, and beyond. Such a capability will revolutionize our ability to directly observe reactions upon which the functionality of future nanotechnological devices will be based.
The exploitation of macro-functional molecules will inherently involve the application of their natural characteristics. Taking advantage of those interesting properties of nanoscale particles will require the successful manipulation of their position and properties. For example, a self-assembling biological system could be employed in a bottom-up fabrication scheme. The fusion of biology with nanotechnology will not only result in technological innovation but will also encourage future research into natural biological nanosystems.
Current technology harnesses the ability to deform individual molecules such as proteins or DNA. DNA is a nanoscale, long-chain macromolecule and behaves like a mass-spring system. Placing a DNA molecule inside a viscous flow stretches the DNA molecule from its coiled position at equilibrium by applying stress across the length of the molecule. To stretch DNA molecules, Wong et al. (2003) designed microfluidic channels with two buffer streams that converge to sandwich a middle stream containing the DNA solution, effectively extending the DNA molecule, as shown in Figure 17-3. Such a method proves advantageous in that the DNA extensional rates depend upon the flow rate of the outer buffer streams, thereby minimizing the effect of the flow from the centerline on the DNA. Furthermore, the low mixing among the streams enabled independent DNA control.

Figure 17-3. Relaxation of a DNA molecule after stretching is shown here in 2.5-second increments (Wong et al. 2003).
A precursor to the use of nanoparticles for practical, beneficial purposes will be the ability to characterize their properties with respect to composition, structure, and so on. Biomolecular characterization—with respect, for example, to proteins—has provided key information about three-dimensional structure as well as mechanistic behavior. Information gleaned from these studies serves as the foundation for current development of devices based purely on biomolecular function (Ho et al. 2004). Toward the realization of improving the human condition through nanotechnology, a full-scale characterization of DNA will elicit a broader understanding of the body of embedded information that governs this condition.
The mapping of the human genetic code as part of the Human Genome Project took ten years. Building on this foundation and developing a technology to quickly sequence an individual’s DNA hold enormous potential for health maintenance and drug development. With specific respect to the interrogation of composition and structure, one pioneering methodology has been the use of nanoscale pores to characterize DNA molecules. For example, Meller et al. (2000) have monitored electrical conductance through an α-hemolysin channel from Staphylococcus aureus to yield important information regarding nucleotide composition and the effects of temperature on DNA transport.
Current results can discern transport events involving adenine polymers (poly dA100) as well as cytosine polymers (poly dC100) (Figure 17-4). These studies enable investigation of several characteristics of the analyzed molecule, from chain length to its exact composition and structure.

Figure 17-4. Translocation events of both poly(dA)100 and poly(dC)100. Differentiation between dA and dC translocation was determined using tD provided in μs10 (Meller et al. 2000).
The use of membrane proteins to examine DNA strands possessed certain limitations, including the range of conditions in which characterizations could be performed, because the α-hemolysin and lipid membranes possessed their own ranges of optimal conditions for preserved activity.
To build upon the groundwork that was established by protein- and lipid-based characterization, Chen et al. (2004) used Si3N4-based nanopores to serve as solid-state characterization systems. Not confined to the same set of limitations observed with a protein-based setup, the solid-state nanopores were able to successfully detect translocation, or movement, of DNA through the pore while possessing the versatility of varied pore diameters to decrease the chances of blockages to DNA translocation and so on. Furthermore, solid-state nanopores will withstand broader ranges of pH, temperature, and pressure, as well as voltages that would normally impair the protein/lipid assembly, thereby allowing the testing of a broader class of molecules in a wider range of environments.
Atomic force microscopy initially evolved as a valuable method for the evaluation of surface topography. Recently, AFM has been frequently used as a tool for the characterization and manipulation of nanoscale particles. For example, the field of AFM lithography is used to transfer nanoscale patterns onto photoresist, a UV or chemically reactive polymer commonly used with microfabrication. The unparalleled resolution achieved using AFM-based techniques makes it an ideal tool for nanolithography.
Furthermore, AFM has also been applied in studies involving biomolecules, such as membrane and motor proteins. Possessing the capabilities for intimate exploration of these biological systems, atomic force microscopy has been used for protein-folding measurements, as was done by Rief et al.(1997) with titin immunoglobulin domains. Understanding protein-folding mechanisms is important because it underlies the use of protein engineering, protein-based device engineering, and even potential applications in using the characterization of a person’s protein-folding properties as a means of health monitoring.
The use of atomic force microscopy is a prime example that demonstrates how existing technologies have been applied to the understanding of a requisite component of future nanotechnological systems.
The human benefit of advances in nanotechnology will stem from the transition from handling single nanoparticles to realizing large-scale manufacturing capabilities. Spanning from the nanoscale to the macroscale is a necessary process in the transition of the nanosciences into industry. Artificial fabrication technologies are usually considered top-down, meaning that they involve manipulations at the macroscale, such as cutting or etching bulk materials. Nature, on the other hand, relies on bottom-up fabrication, involving molecule-by-molecule assembly to create a predesigned subject. Thus, the goal of artificial bottom-up fabrication involves the directed and orderly integration of molecules at the nanoscale to form a macroscale device.
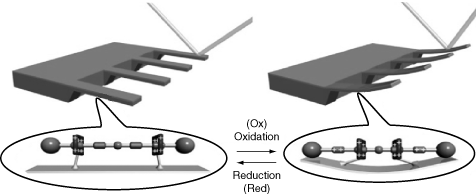
An example of bottom-up fabrication is the self-assembling monolayer (SAM) used by chemists (Ulman 1991, 1995), such as the attachment of nano-sized motor molecules to cantilever beams to form actuators. Rotaxane, a motor molecule shown in Figure 17-5, has two separate recognition sites, each associated with a different energy potential. Placing the rotaxane molecule in an oxidant oxidizes one site, thereby raising the energy level associated with the site (Collier 1999). As a consequence, the energy profile of the rotaxane molecule is altered so that the ring prefers association with the other recognition site, thereby causing the ring to move. Applying a reductant will return the energy profile to its original state, forcing the ring back to its original position. In this way, a molecular motor is created. This is an example of how to use artificial self-assembly to integrate atoms into a molecular system.

Figure 17-5. Graphical representation of a rotaxane molecule. Alternating oxidation and reduction of the molecule results in movement of the ring structures (Collier et al. 1999).
The rotaxane molecule was further modified to create a molecule containing two rings and four recognition sites. Anchoring the two rings to a gold surface creates a molecular muscle that, when placed in an oxidant, causes the two rings to move to the center of the molecule and bend the beam, a movement that can be detected by a laser beam. An array of molecular muscles attached to cantilever beams was created, as shown in Figure 17-6. Alternating the application of oxidant and reductant causes the beam to bend upward and return to its original position. This experiment illustrates how we combine the top-down and the bottom-up fabrication techniques to form an integrated micromechanical system from the nanoscale integrated system.
A human cell serves as a culmination of what nature has taken millions of years to evolve: an autonomously responsive system of sensors and actuators that operates based on commands from an embedded and distributed intelligence. It is a self-regulating, self-governing unit, with its nucleus serving as its central information processor, and hence it represents a system based on the fusion of a number of factors. The transfer and transduction of information using various signal pathways found in cells serve to process and apportion this information to induce a concerted action. For example, a chemical signal results in a sensory response that elicits mechanical movement or actuation from the cytoskeletal network—all characteristic behavior in chemotaxis, or chemical-induced cellular movement.
As a composite system, a cell exemplifies the concept of emergence, where inputs result in coordinated feedback. The example of a neutrophil hunting down and encircling the Staphylococcus aureus bacterium (Figure 17-7) demonstrates concerted, self-determining behavior. The bacterium emits a chemical gradient that is sensed by a cell. The cell, which is able to follow a complex path of the chemical, moves toward the bacterium and eventually surrounds the bacterium to envelope, or phagocytoses, it. In this process, the chemical is sensed by the chemical sensors inside the neutrophil and is processed by the signal pathways. Eventually, the neutrophil acts as a whole to capture the bacterium.

Figure 17-7. A neutrophil is observed chasing a Staphylococcus aureus bacterium. The bacterium emits a chemical that is in turn sensed by the neutrophil, which then coordinates an autonomous actuation directed toward the phagocytosis of the bacterium (Rogers et al. 1950).
A key element of manufacturing large-scale molecular systems will be the derivation of emergence, or true mimicry, toward applications in energy production, nanoscale medicine, and so on. Continued progress in nanotechnological development will result in promising approaches whereby the input of stimuli (such as light, reactive chemicals, and so on) induces systemic behavior not previously present to produce emergent behavior.
Showing particular promise are micrometer-sized photonic crystals of porous silicon presented by Link and Sailor (2003). The optical properties of these crystals change upon the absorption of chemicals, the fabrication of which is illustrated in Figure 17-8.
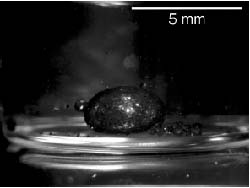
Figure 17-8. Surrounding of dichloromethane drop by self-orienting, self-assembling “smart dust” particles. This is accomplished through hydrophobic-hydrophobic interaction between the dichloromethane and the hydrophobic porous silicon (Link et al. 2003).
The “smart dust” particles are composed of two sides: a green, “water-fearing” (hydrophobic) side, and a red, “water-loving” (hydrophilic) side. Furthermore, because of their amphiphilic nature, the smart dust particles will orient themselves spontaneously at a water surface to form a monolayer so that the hydrophilic side faces the water and the hydrophobic side faces the air.
If porous silicon particles are incubated in water with a drop of dicholoromethane solvent, the smart dust particles will self-assemble and orient themselves around the drop of dichloromethane so that the hydrophilic side (red) faces the water and the hydrophobic side faces the solvent drop, as shown in Figure 17-8. The individual particles aggregate together to form a large, macroscopic collection that emerges as a result of the particle self-assembly. As demonstrated by Link and Sailor, the smart dust particles prove useful in the detection of a chemical such as dichloromethane. The modification of the particles with recognition elements may add further use to the particles by facilitating the detection and isolation of pathogenic organisms in food or water.
Beyond the practical application of this work to detection and similar uses, a compelling approach to deriving an intrinsic, higher-order behavior from the system has been established. This was accomplished through the addition of a new condition to the solution, represented by the drop of dichloromethane.
We are currently at a burgeoning stage with respect to the development of emergent behavior in artificial systems, and continued efforts will seek to embed increased quantities of information in artificial systems to derive even more complex higher-order functionality, such as usable energy or other coordinated activity. The push toward true fusion will inevitably arrive at the achievement of true mimicry. This will be an essential precursor to realizing the human benefit of nanotechnology.
This work has outlined the requisite strategies for realizing the ultimate goal of nanotechnology: to benefit the human condition. Fundamental studies in the nanosciences have provided the building blocks on which nanotechnology will drive the creation of novel devices with applications in energetics, electronics, materials, medicine, and beyond. These systems will address the differences between being integration- or fusion-based by possessing embedded intelligence through a series of nanoscale sensors and actuators. The example of coordinated smart dust activity provides a promising demonstration of basic emergent behavior. Future work will seek to dramatically increase the amounts of information contained within fabricated systems to provide progressively advanced outputs in response to various impulses. For example, by inputting a specific stimulus (such as sunlight) into these devices, an autonomous reactivity (peptide-driven energy transduction) will ultimately produce a usable output (electricity).
Bridging the elements of nanotechnology with beneficial larger-scale systems will parallel current and continued advancements in methodologies with which nanotechnology will develop. These will include the handling abilities to enable us to see and manipulate nanoparticles with the goal of characterizing them. Manufacturing strategies will transition single-molecule studies toward large-scale fabrication of systems that possess increasing information content. Finally, the realization of systems based on the fusion of biology, nanotechnology, and informatics will result in truly emergent, or biomimetic, systems that combine sensors and actuators that respond to specific stimuli.
Although the nanotechnology industry is in a nascent stage, rapid advancements and a streamlined road map of progress ensure that the future is quite promising. The realization of this industry’s potential will have revolutionary and compelling impacts upon humankind. For example, the fruition of rapid DNA screening and diagnosis modalities will open the gateway to designing custom therapeutics tailored to individuals based on their genetic makeup. An achievement of this magnitude would serve dual roles. First, nanotechnology would be cemented as the visionary industry for the next millennium. Second, the true benefits for humankind enabled by the maturation of this technology will have been realized.
However, to reach this point in the road map, we must address in depth several key areas previously outlined. Through the use of emerging technologies and methodologies for discovery—such as the use of superlens material for direct imagery of nanoscale processes or the enhancements of single-molecule manipulation abilities—we will achieve an unprecedented, more complex level of control of biological processes. This, in turn, will give us a deeper understanding of how these biomolecules and their respective activities contribute to a global functionality (such as the systemic performance of the human body) to create an emergent behavior in nature. In this way, nanotechnology will then be poised to reproduce this behavior to combat disease, to produce advanced energy sources, and to support other advancements that will redefine the way we live.
The authors would like to gratefully acknowledge The Institute for Cell Mimetic Space Exploration (CMISE, a NASA URETI), the National Institutes of Health (NIH), the National Science Foundation (NSF), and the Defense Advanced Research Projects Agency (DARPA) for the support of this work.