[1] Leahy J.G, Colwell R.R. Microbial degradation of hydrocarbons in the environment. Microbiological Reviews. 1990;54.
[2] Mohammed N, Allayla R.I, Nakhla G.F, Farooq S, Husain T. State-of-the-art review of bioremediation studies. Journal of Environmental Science and Health. 1996;A31:1547.
[3] Horel A, Schiewer S. Investigation of the physical and chemical parameters affecting biodegradation of diesel and synthetic diesel fuel contaminating Alaskan soils. Cold Regions Science and Technology. 2009;58:113.
[4] Jorgensen K.S. In situ bioremediation. Advances in Applied Microbiology. 2007;61.
[5] Ahn J.H, Kim M.S, Kim M.C, Lim J.S, Lee G.T, et al. Analysis of bacterial diversity and community structure in forest soils contaminated with fuel hydrocarbons. Journal of Microbiology and Biotechnology. 2006;16:704.
[6] Benka-Coker M.O, Ekundayo J.A. Applicability of evaluating the ability of microbes isolated from an oil spill site to degrade oil. Environmental Monitoring and Assessment. 1997;45:259.
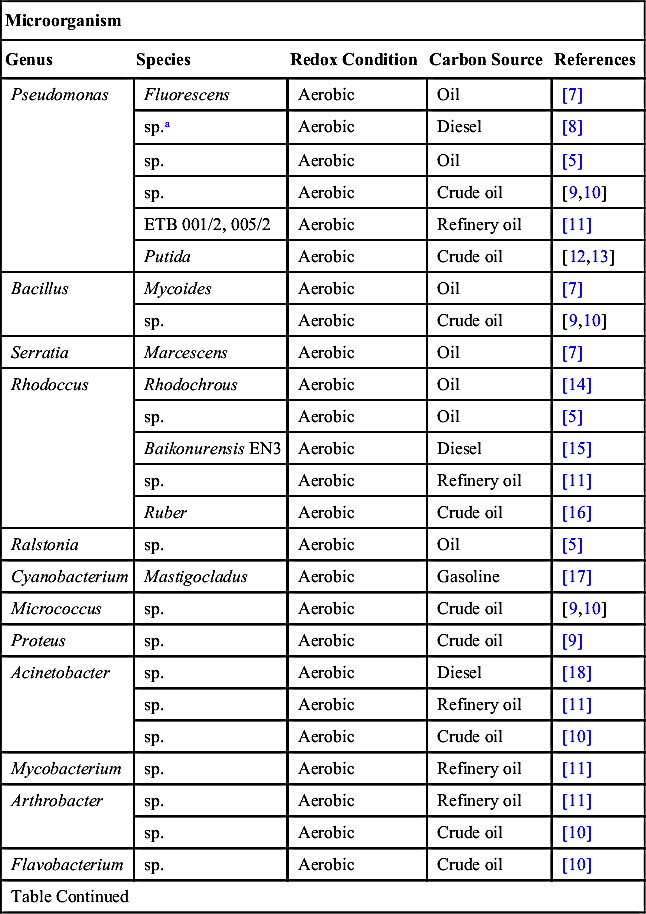
[7] Akoachere J.T.K, Akenji T.N, Yongabi F.N, Nkwelang G, Ndip R.N. Lubricating oil-degrading bacteria in soils from filling stations and auto- mechanic workshops in Buea, Cameroon: occurrence and characteristics of isolates. African Journal of Biotechnology. 2008;7.
[8] Biswas D, Dutta S, Saha R. Effect of Pseudomonas sp. on diesel and other petroleum fractions. Journal of the Indian Chemical Society. 2006;83:1185.
[9] Ijah U.J.J, Antai S.P. The potential use of chicken-drop micro-organisms for oil spill remediation. The Environmentalist. 2003;23:89.
[10] Banat I.M, Rahman K.S.M, Thahira-Rahman J. Bioremediation of hydrocarbon pollution using biosurfactant producing oil degrading bacteria. In: Brebbia C.A, ed. Oil and hydrocarbon spills III. 2002.
[11] Ellis B, Balba M.T, Theile P. Bioremediation of oil contaminated land. Environmental Technology. 1990;11:443.
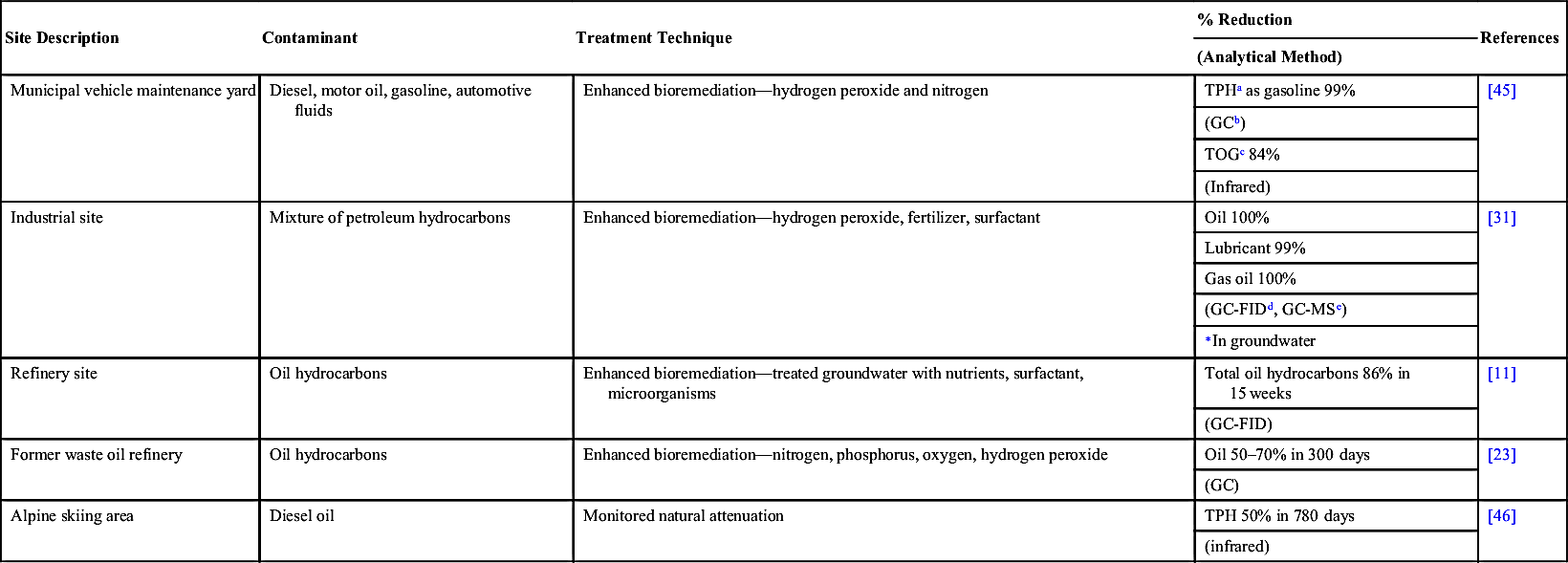
[12] Nwachukwu S.U. Bioremediation of sterile agricultural soils polluted with crude petroleum by application of the soil bacterium, Pseudomonas putida, with inorganic nutrient supplementations. Current Microbiology. 2001;42.
[13] Rughavan P.U.M, Vivekanandan M. Bioremediation of oil-spilled sites through seeding of naturally adapted Pseudomonas putida. International Biodeterioration & Biodegradation. 1999;4:29.
[14] Rema S, Pandian M.R, Sudhakaran S, Devi R. Petroleum hydrocarbon degradation by native isolate Rhodococcus. Asian Journal of Microbiology, Biotechnology and Environmental Sciences. 2007;9:973.
[15] Lee M, Kim M.K, Singleton I, Goodfellow M, Lee S.T. Enhanced biodegradation of diesel oil by a newly identified Rhodococcus baikonurensis EN3 in the presence of mycolic acid. Journal of Applied Microbiology. 2006;100. .
[16] Christofi N, Ivshina I.B, Kuyukina M.S, Philp J.C. Biological treatment of crude oil contaminated soil in Russia. In: Lerner D.N, Walton N.R.G, eds. Contaminated land and groundwater: future directions. 1998.
[17] Banerjee M, Shrivastava S. Sensitivity of cyanobacterium Mastigocladus to petrol and its role in bioremediation. Journal of Industrial Pollution Control. 2005;21:77.
[18] Gallego J.L, Loredo J, Llamas J.F, Vazquez F, Sanchez J. Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation. 2001;12.
[19] Hestbjerg H, Willumsen P.A, Christensen M, Andersen O, Jacobsen C.S. Field scale bioremediation of tar contaminated soil with commercial mushroom Pleurotus ostreatus refuse. In: Brebbia C.A, ed. Oil and hydrocarbon spills III. 2002.
[20] Yateem A, Balba M.T, Al-Awadhi N. White rot fungi and their role in remediating oil-contaminated soil. Environment International. 1998;24:181.
[21] Palittapongampim M, Pokethitiyook P, Upathm E.S, Tangbanluekal L. Biodegradation of crude oil by soil microorganisms in the tropic. Biodegradation. 1998;9:83.
[22] Schaefer M, Peterson S.O, Filser J. Effects of Lumbricus terrestris, Allolobophora chlorotica and Eisenia fetida on microbial community dynamics in oil-contaminated soil. Soil Biology and Biochemistry. 2005;37:2065.
[23] Dott W, Feidieker D, Steiof M, Becker P.M, Kämpfer P. Comparison of ex situ and in situ techniques for bioremediation of hydrocarbon-polluted soils. International Biodeterioration & Biodegradation. 1995;35.
[24] Foght J. Anaerobic biodegradation of aromatic hydrocarbons: pathways and prospects. Journal of Molecular Microbiology and Biotechnology. 2008;15.
[25] Widdel F, Rabus R. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Current Opinion in Biotechnology. 2001;12.
[26] Ulrich A.C, Tappenden K, Armstrong J, Biggar K.W. Effect of cold temperature on the rate of natural attenuation of benzene, toluene, ethylbenzene, and the three isomers of xylene (BTEX). Canadian Geotechnical Journal. 2010;47.
[27] Aislabie J, Saul D.J, Foght J.M. Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles. 2006;10.
[28] Margesin R, Schinner F. Biodegradation and bioremediation of hydrocarbons in extreme environments. Applied Microbiology and Biotechnology. 2001;56.
[29] Chang B.V, Shiung L.C, Yuan S.Y. Anaerobic biodegradation of polycyclic aromatic hydrocarbon in soil. Chemosphere. 2002;48.
[30] Ulrich A.C, Guigard S.E, Foght J.M, Semple K.M, Pooley K, et al. Effect of salt on aerobic biodegradation of petroleum hydrocarbons in contaminated groundwater. Biodegradation. 2009;20.
[31] Menendez-Vega D, Gallego J.L.R, Pelaez A.I, Fernandez de Cordoba G, Moreno J, et al. Engineered in situ bioremediation of soil and groundwater polluted with weathered hydrocarbons. European Journal of Soil Biology. 2007;43.
[32] US EPA. How to evaluate alternative cleanup technologies for underground storage tank sites: a guide for corrective action plan reviewers. United States Environmental Protection Agency; 2004.
[33] Agarry S.E, Aremu M.O, Aworanti O.A. Kinetic modelling and half-life study on bioremediation of soil co-contaminated with lubricating motor oil and lead using different bioremediation strategies. Soil and Sediment Contamination. 2013;22:800.
[34] Kauppi S, Sinkkonen A, Romantschuk M. Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: comparison of biostimulation and bioaugmentation. International Biodeterioration & Biodegradation. 2011;65. .
[35] Walworth J.L, Woolard C.R, Harris K.C. Nutrient amendments for contaminated peri-glacial soils: use of cod bone meal as a controlled release nutrient source. Cold Regions Science and Technology. 2003;37.
[36] Walworth J.L, Woolard C.R, Braddock J.F, Reynolds C.M, Battelle Mem I. The role of soil nitrogen concentration in bioremediation. Battelle Press; 1997.
[37] Boopathy R. Anaerobic biodegradation of no. 2 diesel fuel in soil: a soil column study. Bioresource Technology. 2004;94.
[38] Gogoi B.K, Dutta N.N, Goswami P, Krishna Mohan T.R. A case study of bioremediation of petroleum-hydrocarbon contaminated soil at a crude oil spill site. Advances in Environmental Research. 2003;7:767.
[39] Brown R.A, Crosbie J.R. Oxygen sources for bioremediation. In: Flathman P.E, Jerger D.E, Exner J.H, eds. Bioremediation: field experience. 1994.
[40] Hunkeler D, Meckenstock R.U, Lollar B.S, Schmidt T.S, Wilson J.T. A guide for assessing biodegradation and source identification of organic ground water contaminants using Compound Specific Isotope Analysis (CSIA) EPA 600/R-08/148. 2008.
[41] Aleer S, Adetutu E, Makadia T, Patil S, Ball A. Harnessing the hydrocarbon-degrading potential of contaminated soils for the bioremediation of waste engine oil. Water, Air, and Soil Pollution. 2011;218.
[42] Couto M.N, Monteiro E, Vasconcelos M.T. Mesocosm trials of bioremediation of contaminated soil of a petroleum refinery: comparison of natural attenuation, biostimulation and bioaugmentation. Environmental Science and Pollution Research International. 2010;17.
[43] Wiedemeier T.H, Haas P.E. Designing monitoring programs to effectively evaluate the performance of natural attenuation. Ground Water Monitoring and Remediation. 2002;22.
[44] Van Deuren J, Lloyd T, Chhetry S, Liou R, Peck J. Remediation technologies screening matrix and reference guide. Federal Remediation Technologies Roundtable; 2002.
[45] Nelson C.H, Hicks R.J, Andrews S.D. An integrated approach for in situ bioremediation of petroleum hydrocarbon-contaminated soil and groundwater. In: Flathman P.E, Jerger D.E, Exner J.H, eds. Bioremediation: field experience. 1994.
[46] Margesin R, Schinner F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Applied and Environmental Microbiology. 2001;67.
[47] Balba M.T, Al-Daher R, Al-Awadhi N, Chino H, Tsuji H. Bioremediation of oil-contaminated desert soil: the Kuwaiti experience. Environment International. 1998;24.
[48] Al-Daher R, Al-Awadhi N, El-Nawawy A. Bioremediation of damaged desert environment using the windrow soil pile system in Kuwait. Environment International. 1998;24.
[49] Bleckmann C.A, Oxley M.E, Wilson E.J, Hayes K.W, Hercyk N.L. Land treatment of produced oily sand: field results. Waste Management and Research. 1997;15.
[50] Eszenyiova A, Polakovicova G, Bilska V, Rajnohova H. Biological clean-up of hydrocarbon pollution. PTQ. 2000;133(Autumn).
[51] Guerin T.F. Long-term performance of a land treatment facility for the bioremediation of non-volatile oily wastes. Resource, Conservation and Recycling. 2000;28.
[52] Dibble J.T, Bartha R. Effect of environmental parameters on the biodegradation of oil sludge. Applied and Environmental Microbiology. 1979;37.
[53] Chagas-Spinelli A.C, Kato M.T, de Lima E.S, Gavazza S. Bioremediation of a tropical clay soil contaminated with diesel oil. Journal of Environmental Management. 2012;113.
[54] Coulon F, Al Awadi M, Cowie W, Mardlin D, Pollard S, et al. When is a soil remediated? Comparison of biopiled and windrowed soils contaminated with bunker-fuel in a full-scale trial. Environmental Pollution. 2010;158. .
[55] Pometto A.L, Oulman C.S, DiSpirito A.A, Johnson K.E, Baranow S. Potential of agricultural by-products in the bioremediation of fuel spills. Journal of Industrial Microbiology and Biotechnology. 1998;20:369.
[56] Saez-Navarrete C, Gelmi C.A, Reyes-Bozo L, Godoy-Faundez A. An exploratory study of peat and sawdust as enhancers in the (bio)degradation of n-dodecane. Biodegradation. 2008;19.
[57] Rivera-Espinoza Y, Dendooven L. Dynamics of carbon and nitrogen in a mixture of polycyclic aromatic hydrocarbons contaminated soil amended with organic residues. Environmental Technology. 2007;28.
[58] Martienssen M, Schirmer M. Use of surfactants to improve the biological degradation of petroleum hydrocarbons in a field site study. Environmental Technology. 2007;28.
[59] Uzoigwe C, Burgess J.G, Ennis C.J, Rahman P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Frontiers in Microbiology. 2015;6.
[60] Calvo C, Manzanera M, Silva-Castro G.A, Uad I, Gonzalez-Lopez J. Application of bioemulsifiers in soil oil bioremediation processes. Future prospects. Science of Total Environment. 2009;407.
[61] Singh P, Saini H. Exploitation of agro-industrial wastes to produce low-cost microbial surfactants. In: Brar S.K, Dhillon G.S, Soccol C.R, eds. Biotransformation of waste biomass into high value biochemicals. vol. 18. 2014.
[62] Martins V, Kalil S, Costa J. In situ bioremediation using biosurfactant produced by solid state fermentation. World Journal of Microbiology and Biotechnology. 2009;25.
[63] Lai C.C, Huang Y.C, Wei Y.H, Chang J.S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. Journal of Hazardous Materials. 2009;167.
[64] Jeong S.W, Jeong J, Kim J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. Journal of Hazardous Materials. 2015;286.
[65] Ma J, Yan G, Ma W, Cheng C, Wang Q, et al. Isolation and characterization of oil-degradaing microorganisms for bench-scale evaluations of autochthonous bioaugmentation for soil remediation. Water, Air, and Soil Pollution. 2015;226:272.
[66] Łebkowska M, Zborowska E, Karwowska E, Miaśkiewicz-Pęska E, Muszyński A, et al. Bioremediation of soil polluted with fuels by sequential multiple injection of native microorganisms: field-scale processes in Poland. Ecological Engineering. 2011;37.
[67] Makadia T.H, Adetutu E.M, Simons K.L, Jardine D, Sheppard P.J, et al. Re-use of remediated soils for the bioremediation of waste oil sludge. Journal of Environmental Management. 2011;92.
[68] Ilarionov S.A, Kalachnikova I.G, Sergeev V.A. Rehabilitation of oil-polluted soils using products obtained in the process of organic wastes bioconversion. In: Brebbia C.A, ed. Oil and hydrocarbon spills III: modelling, analysis, and control. 2002.
[69] Moon Y, Yim U.H, Kim H.S, Kim Y.J, Shin W.S, et al. Toxicity and bioaccumulation of petroleum mixtures with alkyl PAHs in earthworms. Human and Ecological Risk Assessment. 2013;19.
[70] Khan S, Afzal M, Iqbal S, Khan Q.M. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere. 2013;90.
[71] Yavari S, Malakahmad A, Sapari N. A review on phytoremediation of crude oil spills. Water, Air, and Soil Pollution. 2015;226.
[72] Kirkpatrick W.D, White P.M, Wolf D.C, Thoma G.J, Reynolds C.M. Petroleum-degrading microbial numbers in rhizosphere and non-rhizosphere crude oil-contaminated soil. International Journal of Phytoremediation. 2008;10. .
[73] Khan S, Afzal M, Iqbal S, Mirza M.S, Khan Q.M. Inoculum pretreatment affects bacterial survival, activity and catabolic gene expression during phytoremediation of diesel contaminated soil. Chemosphere. 2013;91.
[74] Ribeiro H, Almeida C.M, Magalhaes C, Bordalo A.A, Mucha A.P. Salt marsh sediment characteristics as key regulators on the efficiency of hydrocarbons bioremediation by Juncus maritimus rhizospheric bacterial community. Environmental Science and Pollution Research International. 2015;22.
[75] Gomez F, Sartaj M. Field scale ex-situ bioremediation of petroleum contaminated soil under cold climate conditions. International Biodeterioration & Biodegradation. 2013;85.
[76] Grace Liu P.-W, Chang T.C, Whang L.-M, Kao C.-H, Pan P.-T, et al. Bioremediation of petroleum hydrocarbon contaminated soil: effects of strategies and microbial community shift. International Biodeterioration & Biodegradation. 2011;65.
[77] Agarry S.E, Latinwo G.K. Biodegradation of diesel oil in soil and its enhancement by application of bioventing and amendment with brewery waste effluents as biostimulation-bioaugmentation agents. Journal of Ecological Engineering. 2015;16:82.
[78] Llado S, Solanas A.M, de Lapuente J, Borras M, Vinas M. A diversified approach to evaluate biostimulation and bioaugmentation strategies for heavy-oil-contaminated soil. The Science of the Total Environment. 2012:435–436.
[79] Pontes J, Mucha A.P, Santos H, Reis I, Bordalo A, et al. Potential of bioremediation for buried oil removal in beaches after an oil spill. Marine Pollution Bulletin. 2013;76.
[80] Suja F, Rahim F, Taha M.R, Hambali N, Rizal Razali M, et al. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. International Biodeterioration & Biodegradation. 2014;90.
[81] Lin T.C, Pan P.T, Young C.C, Chang J.S, Chang T.C, et al. Evaluation of the optimal strategy for ex situ bioremediation of diesel oil-contaminated soil. Environmental Science and Pollution Research International. 2011;18.
[82] Sun G.D, Xu Y, Jin J.H, Zhong Z.P, Liu Y, et al. Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. Journal of Hazardous Materials. 2012:233–234.
[83] Shouse B. The Gulf War's aftermath: Kuwait unveils plan to treat festering desert wound. Science. 2001;293.
[84] Al-Attar M.H. An integrated approach for overcoming the adverse environmental impacts inflicted on Kuwait during the Iraqi occupation. European Centre Pollution Research; 1996.
[85] Yateem A. Rhizoremediation of oil-contaminated sites: a perspective on the Gulf War environmental catastrophe on the State of Kuwait. Environmental Science and Pollution Research International. 2013;20.
[86] Obuekwe C.O, Badrudeen A.M, Al-Saleh E, Mulder J.L. Growth and hydrocarbon degradation by three desert fungi under conditions of simultaneous temperature and salt stress. International Biodeterioration & Biodegradation. 2005;56.
[87] Radwan S.S, Al-Awadhi H, Sorkhoh N.A, El-Nemr I.M. Rhizospheric hydrocarbon-utilizing microorganisms as potential contributors to phytoremediation for the oily Kuwaiti desert. Microbiological Research. 1998;153.
[88] Radwan S.S, Al-Awadhi H, El-Nemr I.M. Cropping as a phytoremediation practice for oily desert soil with reference to crop safety as food. International Journal of Phytoremediation. 2000;2. .
[89] Mahmoud H.M, Suleman P, Sorkhoh N.A, Salamah S, Radwan S.S. The potential of established turf cover for cleaning oily desert soil using rhizosphere technology. International Journal of Phytoremediation. 2011;13.
[90] Radwan S.S, Sorkhoh N.A, Fardoun F, Al-Hasan R.H. Soil management enhancing hydrocarbon biodegradation in the polluted Kuwaiti desert. Applied Microbiology and Biotechnology. 1995;44.
[91] Radwan S.S, Sorkhoh N.A, ElNemr I.M, ElDesouky A.F. A feasibility study on seeding as a bioremediation practice for the oily Kuwaiti desert. Journal of Applied Microbiology. 1997;83.
[92] Cho B.H, Chino H, Tsuji H, Kunito T, Nagaoka K, et al. Laboratory-scale bioremediation of oil-contaminated soil of Kuwait with soil amendment materials. Chemosphere. 1997;35.
[93] Al-Awadhi N, Al-Daher R, El-Nawawy A, Balba M.T. Bioremediation of oil-contaminated soil in Kuwait 1. Landfarming to remediate oil-contaminated soil. Journal of Soil Contamination. 1996;5.
[94] Radwan S, Sorkhoh N, El-Nemr I. Oil biodegradation around roots. Nature. 1995;376.
[95] Al-Awadhi H, Al-Mailem D, Dashti N, Khanafer M, Radwan S. Indigenous hydrocarbon-utilizing bacterioflora in oil-polluted habitats in Kuwait, two decades after the greatest man-made oil spill. Archives of Microbiology. 2012;194.
[96] Al-Zalzaleh H, Ghulam S. Effect of bioremediated soil on growth of different plant species. Kuwait Journal of Science and Engineering. 2004;31.