Recent Studies on the Effects of Oil
Abstract
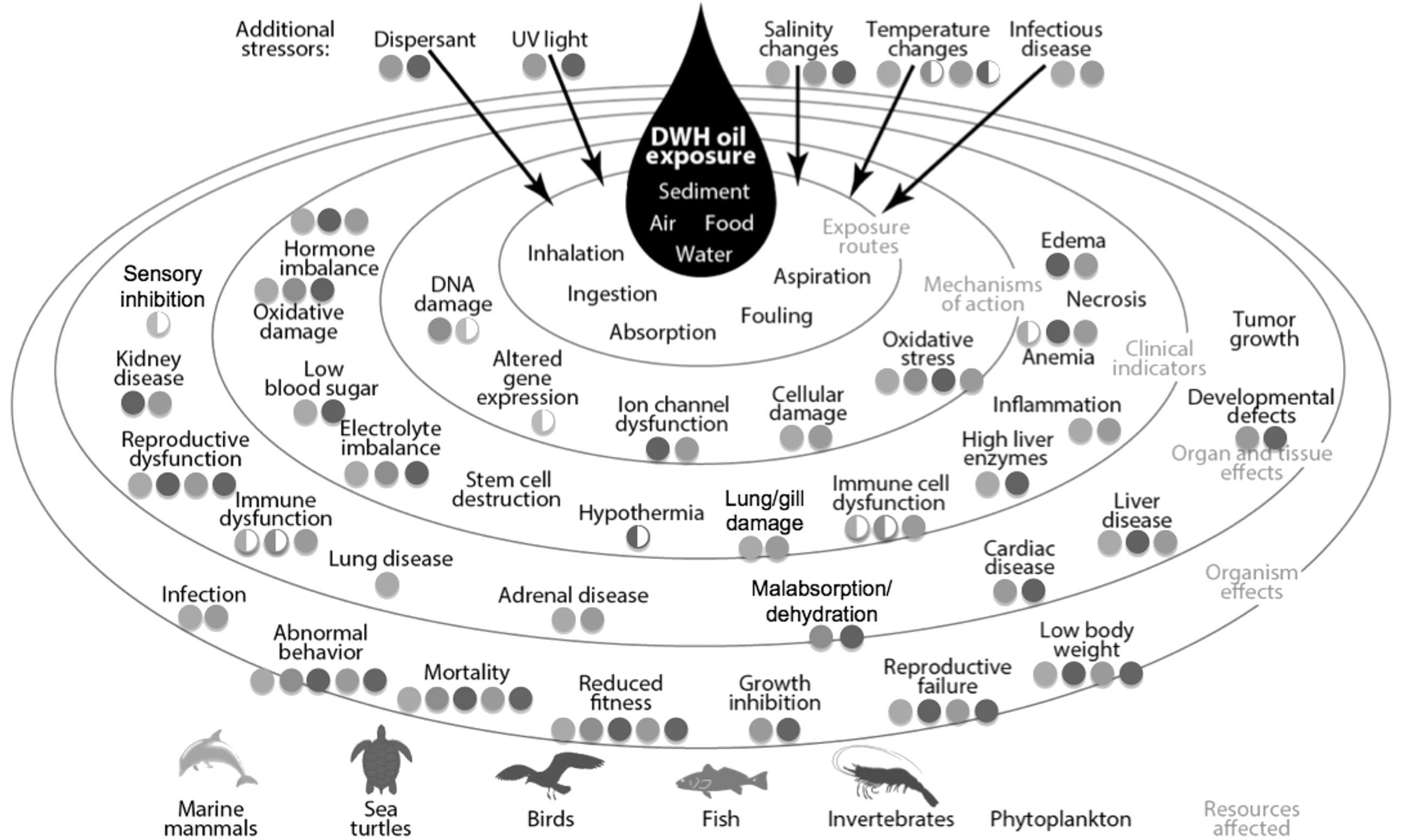
The Deepwater Horizon oil spill in the Gulf of Mexico has stimulated a surge in new research into the effects of oil. Although this new work has largely focused on species, oils, and conditions common to the Gulf of Mexico, many of the lessons can be reasonably extrapolated and applied to other situations and exposure scenarios. A synthesis of the large body of impact studies performed under the Natural Resource Damage Assessment has suggested that many of the effects determined across many levels of biological organization are conserved and common to many different taxa.
Because it is not possible to be comprehensive about the effects of oil in a single book chapter, the discussion here will take advantage of new insights emerging from recent and ongoing work related to the Deepwater Horizon incident. While the chapter will focus on the state of knowledge for only three groups of animals (fish, marine mammals, and sea turtles), the background and science for these taxa provide an excellent overview of research and forensic approaches to oil effects, as well as the status of what we currently understand. The chapter opens with a review of the history of oil effects research to provide context for this complicated topic.
With new research results continuing to be reported in the literature, we can reasonably expect our understanding of oil effects to progress and expand in the near future. For spill responders, the challenge will be to remain informed and translate the new and improved science into oil spill response guidance.
Keywords
20.1. Introduction
20.2. Some Historical Background
Of recent years pollution by oil has assumed grave proportions. Oil has fouled beaches and thus destroyed the amenities of many seaside resorts, floating oil-films have been the cause of fires in harbours and innumerable birds have perished miserably through the clogging of their wings and beaks.
The oil floated upon the waves; we could see it washing up here on the shore. At first we could not think what it was—it made the water look black; but soon we learnt from the smell—in fact, we were almost driven away by the smell. They say if it had caught fire it would have cleared the islands, it would have been like a sea of fire, and the smoke would have suffocated all the islanders. As it was, many of the rabbits and birds on Annet were killed by the oil, and lay dead upon the shore.
It is by direct toxicity, however, that petroleum pollution produces the most severe damage. Naphtha-based substances are toxic to all animal life. This effect can be observed in a simple experiment: If fish are placed in water laced with naphtha, they die sooner than fish in similar water without naphtha. Different kinds of oil have different degrees of toxicity, but (raw petroleum) is the most toxic…Some initially innocuous products of petroleum such as lubricating oil and machine oil acquire greater toxicity under the oxidizing influence of sunlight and oxygen in the presence of water.
Translation from the original German courtesy of R. Childs.
The natural phenomena responsible for the disappearance of surface films (of oil) are…vaguely understood, and little is known about the eventual fate of oil thrown into the sea. From the biological point of view the disappearance of the oil from the surface does not mean that the pollutant has been eliminated…its effects may still persist when no traces of oil are visible on the surface.
If there is any quantity of oil on them, they cannot fly, and it is stated that they cannot swim or dive either; they must die of starvation. It is also stated that the oil sometimes penetrates into the down under the feathers next the skin, thereby displacing the air in this down which normally forms a most efficient thermal insulation against cold, and that the temperature of a bird covered with oil may fall many degrees below the normal; they may therefore die of cold.
A bird once oiled is doomed to death…
The general balance of evidence seems to me to point to little, if any, damage with ordinary amounts of petroleum, except occasionally to beds of shellfish between tidemarks. It has been shown that some kinds of fuel oil, in a layer permanently on top of a tank containing fish, will kill the fish in a course of time; also that if water is well shaken up with fuel oil, it may dissolve traces of substances which are toxic to fish. But these experiments refer to a proportion of oil to water which is very much greater than that prevailing on shores subject to occasional pollution by oil.

20.3. Oil and Fish: A Paradigm Shift
The measured field exposures to…hydrocarbons from untreated and chemically dispersed crude oils are much lower than those observed to kill a wide range of organisms in laboratory bioassays…Thus, oil spills and chemical dispersion of crude oil slicks are unlikely to have an adverse impact on larval, juvenile or adult organisms in the water column.
… the low concentrations of oil in the water under the slicks were presumed to be too low to be acutely toxic, because the concentrations were substantially below the acutely toxic levels reported in the literature of the 1970s and 1980s. For example, laboratory toxicity tests with WSF (water-soluble fractions of oil dominated by 1- to 2-ringed aromatic hydrocarbons) had 96-h LC50 of WSF for pink salmon ranging from 1500 to 8000 ppb (parts per billion), depending on life stage, salinity, and type of exposure…After the spill, environmental samples had PAH concentrations well below those concentrations found to be acutely toxic during 28-day exposures of most marine organisms to water-soluble fractions…Evidence of acute toxicity from herring larvae field studies (abnormalities) occurred…and it was realized that the composition of the PAH after the spill was substantially different than the WSFs reported in the literature.
The early life-history studies we conducted…demonstrated that the problems that might have been produced by the oil spill, in fact, did not occur…Whether these data are interpreted to mean that pink salmon were not affected by oil, or alternatively, pink salmon must not have been exposed to biologically meaningful concentrations of oil, the result is the same. There was no detectable effect of the Exxon Valdez oil spill on the (Prince William Sound) wildstock pink salmon population.
In some instances, these studies agree, but in several cases, especially the areas involving effects on various life stages, similar studies, but with different experimental designs, have led to differing findings and conclusions. Studies have examined both short- and long-term effects, and disagree on the extent of the damage, and even on the existence of damage to pink salmon. Several studies examine the same issue but were seldom conducted identically. Consequently, most of the research effort is more (complementary) and open to differences in interpretation.

20.4. Fish Studies Post–Deepwater Horizon
The current state of the science is now focused on linkages across biological scales, from channel blockade and the disruption of excitation–contraction coupling in individual heart muscle cells to heart development, circulatory and respiratory performance, delayed mortality of individual fish, and associated consequences for the abundance and dynamics of wild populations. For forage fish such as Pacific herring, the biological scaling will eventually extend to communities and ecosystems.

20.5. Marine Mammals and Oil: Early Studies

Overall, the current population level data for sea otters in PWS are consistent with the EVOSTC definition of recovery for sea otters from the long-term injury incurred in the wake of the 1989 oil spill. The support for this is based primarily on demographic data, including (1) a return to estimated pre-spill abundance of sea otters at northern Knight Island, a heavily oiled area within PWS, and (2) a return to pre-spill mortality patterns based on ages-at-death. Gene transcription rates in 2012 were similar in sea otters from oiled, moderately-oiled and unoiled areas, suggesting abatement of exposure effects in 2012. However, because 2012 gene transcription rates generally were low for sea otters from all areas relative to 2008, these observations cannot be fully interpreted without data from a wider panel of genes. This slight uncertainty with respect to the data from the biochemical indicator is outweighed by the strength of the data for the demographic indicators. The return to pre-spill numbers and mortality patterns suggests a gradual dissipation of exposure to lingering oil over the past two decades, to the point where continuing exposure is no longer of biological significance to the PWS sea otter population.
20.6. The Exxon Valdez and Killer Whales: Circumstantial but Compelling


20.7. Dolphins and the Deepwater Horizon: “Uncommon Disease Conditions”


20.8. Oil and Sea Turtles: Known Risk but Little Information