APPENDIX G
Capturing Photons with Photographic Film
The discovery of photography in the early 19th century was essentially the discovery that certain silver salts, e.g. silver chloride (AgCl), silver bromide (AgBr) and silver iodide (AgI), are affected by light and can be processed to amplify the effect and make it permanent. These silver salts, known as silver halides, form crystals that visibly darken when exposed to bright light; the process continues so that in the presence of light the crystals get darker and darker. This darkening effect is neither very sensitive nor stable and is not very useful for recording images. The enabling discovery for photography was that chemical processing, after exposure, can convert some of the exposed crystals into grains of metallic silver and also stop the darkening process. Indeed, there was a mystery. Even a low level of exposure does something to the crystals so that they can be “developed” later to obtain a silver grain record of the exposure. After exposure, the halide crystals contain a latent image which lasts until the crystals are developed.
Today, after many decades of experimentation, technological advances, and (more recently) scientific understanding, we have black and white and color films that provide stable, high-resolution images. However, they are still based on latent images stored in silver halide crystals. Modern film consists of a sturdy base about 100 µm to 200 µm thick, usually cellulose acetate, which serves to support multiple layers of emulsion held together with gelatin. For example, Fujicolor Provia 100 color reversal film contains more than a dozen layers, starting with a protective layer on top and ending with an antihalation layer to prevent reflection of light back into the emulsion. The thickness of the emulsion ranges from about 10 µm to 20 µm.
Since the photon capture step in film is always the same, it is easier to begin the discussion with black and white film as illustrated in Figure G.1. The silver halide crystals are roughly one micron in diameter and are randomly distributed.
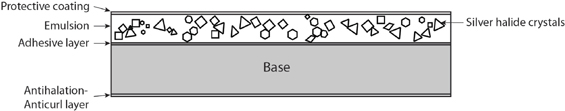
FIGURE G.1 Monochrome (black and white) film. The thickness is not to scale.
In the commonly used silver bromide crystals, there is a lattice of silver ions (Ag+) and bromide ions (Br-), as shown in Figure G.2. An energetic photon can knock an electron out of a Br-ion, and the electron has some probability of being captured by a lattice defect (shallow trap). If a mobile silver ion happens to be present in the same trap or to reach the trap while the electron is still there, it will be reduced to a silver atom. This is not sufficient to contribute to the latent image, because a single trapped atom is not stable. However, the newly minted silver atom will continue to hop from trap to trap, and it may encounter one or more other atoms to form a stable microcluster. The important point is that a certain level of exposure produces a proportionate number of stable microclusters of silver atoms in different silver bromide crystals.
The microclusters of silver atoms are called sensitivity centers or development centers, and they constitute the latent image. This latent image can be “developed out” by applying a developer solution with a reduction potential sufficient to reduce only those crystals containing microclusters to silver particles. In chemical terminology, reduction means adding an electron so that the silver ion (+1) is converted into metallic silver (0). There are usually two additional steps in film processing. First, there is an acid “stop” bath (usually acetic acid) to terminate development and then a “fixer” or “hypo” solution (usually sodium thiosulfate) to dissolve and wash away “unsensitized” crystals while leaving the silver particles (grains) in place. In this way a few silver atoms in a cluster can act as a trigger for the conversion of billions of silver ions to metallic silver. The silver particles remain in the emulsion and constitute a negative image, since it is darker where there was more exposure. In going from latent image to silver image we effectively have a powerful photon multiplier.
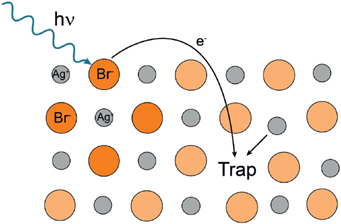
FIGURE G.2 Photo excitation of silver bromide crystal.
Silver halides are primarily sensitive to blue and UV radiation. Modern panchromatic film is possible because of sensitizers that are added to the emulsion. The sensitizers are dye molecules that are designed to absorb a certain color of light and to transfer electrons to a crystal grain. Sensitizers also make possible the construction of color film that can produce color negatives and positives (color reversal film). Color perception and representation are discussed in detail in Chapter 15. Here it suffices to say that the “colors” of light can be represented with combinations of red (R), green (G), and blue (B) light in an additive process. The three colors that are necessary for use as pigments, or in any other subtractive process, are cyan, magenta, and yellow. This means that color film must start with the capture of red, blue, and green light and end up after development with cyan, magenta, and yellow dyes for positive or reversal film and the corresponding negative colors for print film.
Figure G.3 presents a simplified picture of the emulsion layers in a film such as Fujifilm Provia. Each of the light sensitive layers contains silver halide crystals along with appropriate sensitizers and dye-coupler molecules. All of the layers are sensitive to blue light, so the first layer is assigned to blue and is followed by a yellow filter to absorb the remaining blue light. The second and third layers are assigned to green and red light, respectively, and contain the required sensitizers. When the film is exposed to light, the light produces latent images in the three layers with concentrations of sensitized sites that are proportional to the exposure by the three colors of light. Thus the light is a stimulus that is represented in the film by latent images for R, G, and B colors.
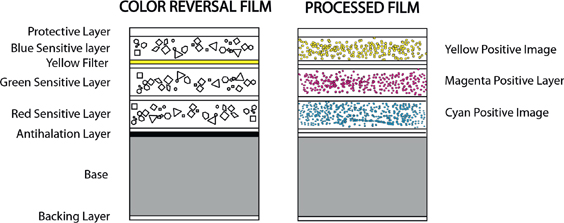
FIGURE G.3 Simplified cross-section of color reversal film.
The development process consists of the reduction of silver ions in sensitized crystals to metallic silver and the oxidization of the developer molecules, i.e. the removal of electrons. The oxidized developer molecules are then free to react with dye-coupler molecules to produce the required dyes in each layer. Films with this design are very practical now, but initially there was much difficulty keeping the dyes in their proper layers. For that reason Kodachrome, the first successful subtraction-process film, was quite different. In order to avoid the wandering dyes, Kodachrome was designed without any coupling-dyes in the film! It was essentially a multilayer black and white film with appropriate sensitizers, and the dyes were all added during processing. The result was an extremely complicated processing procedure with 28 steps and requiring large, specialized equipment and highly trained technicians. In addition to providing outstanding color and fine grain, the Kodachrome system had two major advantages. First, all the couplers were removed during processing, thereby improving stability; and second the layers in the emulsion were much thinner than those required to hold dye couplers in competitive products, thus improving resolution.
Other successful schemes for manipulating dyes in color images were based on bleaching dyes already in place. A good example is Cibachrome (now Ilfochrome), that was engineered by Ciga-Geigy Corp. for color prints. In this material, stable azo dyes are incorporated into the light sensitive layers. The development process selectively destroys dye molecules in the exposed areas. This results in vivid colors and archival prints. Cibachrome/Ilfochrome prints are among the best archival color prints that can be obtained.
The evolution of color photography extended over about a hundred years. There was a flurry of activity in the early 20th century motivated largely by the movie industry, and by the 1940s a number of practical color films were available to photographers. In 1947 Polaroid Corp. introduced the instant camera with self-developing film, and in 1963 the color instant camera was announced. This was perhaps the most sophisticated application of organic chemistry at the time. It also demanded reliable and accurate mechanical designs to ensure smooth movement of the print and delivery of developer as soon as an exposure was made. This heroic achievement served its purpose for decades, but now has been swept away by the digital revolution. The Polaroid Corporation ceased to exist (except in name) in 2001, and other film companies are regularly announcing cancellations of products and developing services.
Historical note (Holography, music, and color photography): Early attempts to make color photographs were all based on the additive color process in which black and white negatives of the same scene were obtained through red, blue, and green filters. Positive images were made from the negatives and then projected through the corresponding color filters to recreate the color scene. This method, first demonstrated by James Clerk Maxwell (1861), continued to be used with a variety of ingenious variations well into the 20th century. The positive color method, at its best, was used by Sergey Prokudin-Gorsky to document the Russian Empire, 1905–1915. A single plate version, in which the color filters took the form of colored grains of starch, was known as Autochrome Lumière. It produced a type of color pointillism that is still popular with collectors.
Pure color without dyes: In 1908 Gabriel Lippmann received the Nobel Prize in physics for color photography! Furthermore, he obtained true colors without any dyes. Lippmann was an important physicist and inventor, and he held positions as Professor of Experimental and Theoretical Physics at the Faculty of Sciences Laboratory in Paris and Director of the Research Laboratory (later part of the Sorbonne). He was a giant in his time but is relatively unknown today.
So what did Lippmann do to impress the Nobel committee? He proposed and demonstrated a way to encode color information into photographic plates so that colored images could be observed through the selective reflection of light. This is the same effect that gives rise to the iridescent color of hummingbirds through the diffraction of light. Diffraction was explained in Appendix B and illustrated in Figure B.4. Lippmann’s idea was sophisticated and simple and almost impossible to realize with the materials available to him. He proposed an arrangement consisting of a plate with a thin, fine grained panchromatic photographic emulsion in contact with a mercury mirror as illustrated in Figure G.4. Light would be directed through the plate and emulsion, and would be reflected back by the mercury surface. The phase of the reflected light is shifted by 180°, and the incoming and outgoing beams interfere to give nodes and loops (bright spots) separated by the wavelength of the light divided by two. If we take red light from a He-Ne laser with λ = 633 nm as an example, we immediately see Lippmann’s dilemma. The separation of the layers (lamina) in the latent image is 316 nm, and can only be recorded with extremely fine grain film. If blue light is added, a set of layers with smaller separation is produced; and so on as other colors are added.
Lippmann devoted considerable time to producing fine-grain, high-resolution film and optimizing developer solutions, and he was able to obtain color images in spite of the absence of true panchromatic film. This feat can now be reproduced in an undergraduate laboratory by using commercial holographic plates with a resolution of more than 1000 lines/mm.
Lippmann’s method of interference color photography was not practical for photographers, but his ideas have found new life in laser-based optical technology. Dennis Gabor was fascinated by Lippmann’s ideas and set about making interference patterns in 3D space. The result was the hologram, another Nobel Prize-winning invention. Reflection-type holograms can be made by exposing an emulsion to laser light scattered from an object and simultaneously to light directly from the laser at a different angle. The reflecting planes in the developed film are similar to Lippmann’s color photographs, and the reflection hologram is known as a Lippmann hologram. Also, Lippmann-Bragg volume holograms are used as filters in optical instruments, and the concept has been exploited for storing information in standing-wave memory devices where layers of information are recorded at different depths in an optically sensitive material. Gabriel Lippmann provides another example of an inventor who was ahead of his time. Fortunately, Lippmann was acknowledged and celebrated during his lifetime for a number of contributions.
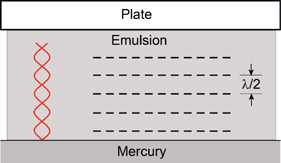
FIGURE G.4 Lippmann color photography illustrated with a single color of light.
Modern color film: Color film as we know it is based on the subtractive process. In the developed film light is filtered through yellow, cyan, and magenta layers before it reaches the eye, as illustrated in Figure G.3. A surprisingly complete description of this method with the correct order of sensitized layers and the concept of coupler dyes was described by Rudolph Fischer in 1911. Fischer was prescient, but the time was not right for the realization of his dream. His sensitizers and dyes diffused between layers, and he could not obtain satisfactory color photographs. It would be almost 30 years before the technical problems could be overcome, and transparency film could be manufactured with dyes that would stay put.
The magnificent (three-color) Kodachrome film for movies and 35 mm stills was brought to the market by Kodak in 1936, and was not retired until 2009. This product was the brainchild of brilliant amateurs. Leopold Godowsky, Jr., and Leopold Mannes, who shared interest in music and photography, met as boys in New York City. They recognized the mediocre quality of so-called color movies before 1920, and they decided to set up a laboratory to experiment with color photography. They also worked as musicians, a vocation that would continue throughout their lives. Godowsky studied violin and chemistry and physics at UCLA and later mathematics at Columbia. Meanwhile, Mannes studied physics and musicology at Harvard.
Fortunately, Godowsky and Mannes were able to continue their collaboration by mail, and in 1922 they established a real laboratory in New York. Soon they were on the track that would lead to Kodachrome, and at the same time they were becoming successful musicians. Through a chance encounter with Lewis Strauss, who was a junior associate with Kuhn, Loeb and Co., they were able to obtain financial backing. Their work was so impressive that in 1930 they were invited to move to Rochester to work in the well-equipped Kodak Laboratories. The result of this collaboration was the development of a highly successful color-subtractive film that proved to have amazing archival properties, and their coworkers quipped that Kodachrome was created by “God and Man.” In 2005 Godowsky and Mannes were inducted into the National Inventors Hall of Fame.
Further Reading
Matsugasaki, S. (1991). Lippmann Color Photography. Journal of Optics, 22(6), pp. 267–273.
Peres, M. R., Editor (2007). FOCAL Encyclopedia of Photography, 4th Ed. Burlington, MA: Focal Press.
Rosenblum, N. A. (1997). World History of Photography, 3rd Ed. New York: Abbeville Press Publishing.
Schwall, R. E., and Zimmerman, P. D. (1970). Lippmann Color Photography for the Undergraduate Laboratory. American Journal of Physiology, 38(11), pp. 1345–1349.
Williamson, S. J., and Cummins, H. Z. (1983). Light and Color in Nature and Art. New York: Wiley and Sons.
