Enzymatic Hydrolysis of Pretreated Biomass
Abstract
The key technologies in biochemical biomass conversion are how to degrade complex polysaccharides in the biomass polymer into monosaccharides efficiently and convert monosaccharides with special functions into biobased products. Compared with physical–chemical treatment methods, enzymatic hydrolysis method holds many advantages, such as mild conditions, no toxic degradation products, high sugar yield, low equipment cost, and high specificity. It plays an irreplaceable role in biological conversion of biomass. The previous section has carried out a detailed discussion on the physical structure and chemical characteristics of biomass resources. It lays the foundation for further enzyme conversion and how to choose an effective pretreatment method to eliminate the degradation barrier, and expose enzyme loci. The enzymes adopted/applied in the biomass conversion will be discussed in detail in this chapter. Furthermore, it will also be illustrated how to build and exploit the enzyme platform to realize the efficient biomass conversion.
Keywords
4.1. Reviews on the Enzymes Participating in the Biomass Degradation Process
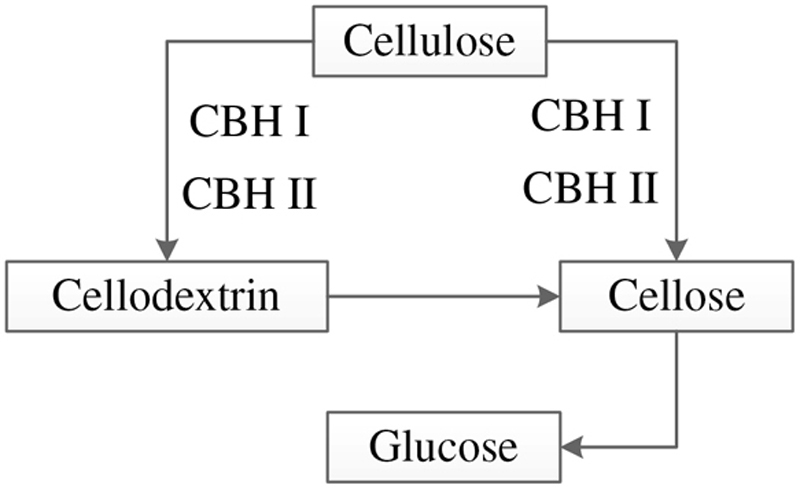
4.1.1. Cellulase
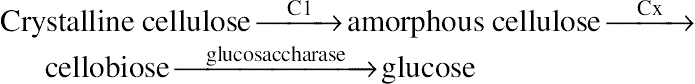

4.1.2. Hemicellulase
4.1.2.1. Depolymerase
4.1.2.1.1. Xylanase
4.1.2.1.2. Mannanase
4.1.2.1.3. β-Glucanase
4.1.2.1.4. Xyloglucanase
4.1.2.2. Debranching Enzyme
4.1.2.2.1. α-Glucuronidase
4.1.2.2.2. α-Arabinosidase
4.1.2.2.3. α-d-Galactosidase (Pei, Yemin, & Azure, 2003a)
4.1.2.2.4. Acetyl Xylan Esterase
4.1.2.2.5. Ferulic Acid Esterase
4.1.2.3. Hemicellulase for Different Biomass Feedstocks
4.1.3. Lignin-Degradating Enzymes
4.1.3.1. Lignin Peroxidase and Manganese Peroxidase
4.1.3.2. Laccase (LaC)
4.1.4. Cellulase Cofactors
4.2. The Setup of Biomass Invertase Platform
4.2.1. The Enzyme Production
4.2.1.1. Preparation of Cellulase Production
4.2.1.2. Hemicellulase Production
4.2.1.3. Preparation of Lignin-Degrading Enzyme
4.2.1.3.1. Liquid Fermentation
4.2.1.3.2. Solid-state Fermentation
4.2.1.4. Cutinase Production
4.2.2. Chemical Modification Enzymes
4.2.2.1. Chemically Modified Cellulases
4.2.2.2. Chemical Modification of Laccase
4.2.3. Enzyme Engineering
4.2.3.1. Gene Cloning
4.2.3.2. Site-directed Mutagenesis of the Enzyme Molecule
4.2.3.3. Enzyme-directed Evolution
4.2.3.4. Fusion Enzyme
4.3. Enzymes Conversion Platform for Biomass
4.3.1. Enzyme Synergy
4.3.2. Multicomponent Enzyme System
4.3.3. Fiber Bodies
4.3.4. Optimization of the CBH–EG–BG System
4.3.5. Design of the Multienzyme Complex
Table 4.1
Compound Enzymes and Their Enzymatic Hydrolysis Effects for Different Substrates
| Substrate | Abf II (%) | Abf III (%) | β-xyl | Xyl III (%) | Hydrolysis yield (mg.g DM) | |
| Arabinose | Xylose | |||||
| Water-soluble Arab xylan in wheat | 20 | 20 | 40 | 20 | 343.7 | 548.7 |
| Water-insoluble Arab xylan in wheat | 25 | 25 | 25 | 25 | 162.5 | 286.5 |
| Vinasse | 10 | 40 | 50 | 51.47 | 91.58 | |

4.3.6. Enzyme Reactor
Table 4.2
Introduction of Enzyme Reactors
| Type | Operation mode | The main characteristics | |
| Homogeneous enzyme reactor | Agitator tank | Batch, batch | Using blender mixing |
| Ultrafiltration membrane reactor | Batch, batch and continuous | Membrane allows only low molecular compounds and enzyme pass through, suitable for large molecules substrates | |
| Immobilized enzyme reactor | The drum | Partial, half batch and continuous | Immobilized enzyme suspended in the solution |
| Fixed bed/packed bed | Continuous | ||
| Fluidized bed | Batch and continuous | ||
| Membrane reactor | Continuous | ||
| Bubbling tower | Partial, half batch and continuous | Suitable for the reactions with gas |


4.4. Economic Analysis of Cellulase Production
4.4.1. Relations Between Particularity of Cellulose and Production Costs
4.4.2. Relationship Between Process Indicators and Production Costs
4.4.2.1. Relationship Between Production Costs and the Fermentation Period
4.4.2.2. The Relationship Between Fermentation Yield and Cost
Table 4.3
List of Fermentation Yield and Cost Relationships
| Project | Name | The unit price | Consumption/t | Consumption/t | A total of 480 tons |
| The raw material | The raw material | 500 | 5.17 | 4.4807 | 165,432 |
| Wheat bran | 1,000 | 1.293 | 1.1206 | 82,752 | |
| Yeast extract | 1,500 | 0.012 | 0.0104 | 1,152 | |
| Lignocellulose | 2,800 | 0.24 | 0.208 | 43,008 | |
| Peptone | 2,500 | 0.072 | 0.0624 | 11,520 | |
| MgSO4 · 7H2O | 5,000 | 0.0072 | 0.0062 | 2,400 | |
| KH2PO4 | 8,000 | 0.096 | 0.0832 | 49,440 | |
| CaCl2 | 1,250 | 0.0072 | 0.00624 | 960 | |
| Tween-80 | 15,000 | 0.176 | 0.152533 | 169,440 | |
| Ammonium sulfate | 1,200 | 1.573 | 1.363267 | 120,960 | |
| Bubble enemy | 700 | 6.667 | 5.778067 | 299,040 |

4.4.2.3. The Relationship Between Enzyme Activity and Production Costs
4.4.2.4. The Relationship Between the Filtration Method and the Production Cost
4.4.3. The Major Policy to Reduce Cellulose (or Conversion) Enzyme Economic Costs
Table 4.4
The Main Strategy to Reduce the Cellulase Cost and the Corresponding Effects
| Source of enzyme gene | Expression host | Enzyme type | Enzyme activity |
| Thermonospora YX | E. coli | Endoglucanase | 5.8 μmol/min/mg × 103 |
| B. subtilisDR | E. coli | Endoglucanae | 0.82 U/mL |
| Xylella fastidiosa | E. coli | Endoglucanase | 2.39 μKat |
| Azoarcussp. strain BH72 | E. coli | Exoglucanase | 30 U/mg of protein |
| Ruminococcus flavefaciens | E. coli | Endoglucanase | 19.4 μg/min/mg |
| Bacillus sp. strain KSM-64 | B. subtilis | Endoglucanase | 21,700 U/L |
| Thermomonospora fusca | S. lividans | Endoglucanase | 10 U/mL |
| A. tubingensis | K. lactis | Endoglucanase | — |
| Cryptococcus sp. S-2 | P. pastoris | Endoglucanase | 4.36 U/mg of protein |
| T. reesei | P. pastoris | Exoglucanase-7 | — |
| T. reesei | P. pastoris | Exoglucanase-1 | — |
| T. reesei QM9414 | S. cerevisiae | Endoglucanase | — |
| T. reesei QM9414 | S. pombe | Endoglucanase | — |
| T. reesei | S. pombe | Endoglucanase-2 | Exoglucanase-3.81 U/mg of protein |
| T. reesei | Y. lipolytica | Endoglucanase-1 | Endoglucanase-0.1 U/mg of protein |
| A. fumigatus Z5 | P. pastoris | β-Glucosidase | 101.775.2 U/mg of protein |
| Candida wickerhamii | S. cerevisiae | β-Gluocosidase | — |
| P. chrysosporium | P. pastoris | β-Glucosidase | 52 U/mg of protein |
| Commercial mixture | Supplier | Reported enzyme activities (U/mL unless otherwise specified |
| Celluclast 1.5 L FG | Novozyme | 65 FPU, 12 β-glucosidase, 660 xylanase (bwx) 60.7 FPU, 6.5 β-glucosidase |
| Novozyme 188 | Novozyme | 8.5 FPU, 665 β-glucosidase, 123 xylanase (osx), 29.3 α-arabinofuranosidase, 16.6 β-xylosidase, 116 α-galactosidase, 0.6 feruroyl esterase (U/mg) 1 endoglucanase, 0.35 exoglucanase, 14.75β-glucosidase, 10 β-xylanase, 0.22 β-xylosidase, 0.09 α-arabinofuranosidase 0.1 FPU, 661 β-glucosidase |
| Spezyme CP | Genencor | 58.2 FPU/ml, 128 β-glucosidase, 2622 xylanase(osx), 22.6 α-arabinofuranosidase, 7.3 β-xylosidase, 0.39 α-galactosidase (U/mg) 1.4 FPU, 21.8 CMCase, 0.09 exoglucanase, 1.82 β-glucosidase, 15 β-xylanase, 0.56 β-xylosidase, 0.38 α-arabinofuranosidase, 55.2 FPU, 15.4 β-glucosidase |
| Multifect (xylanase) | Genencor | 0.77 FPU, 35.9 β-glucosidase, 25,203 xylanase (osx), 9.44 α-arabinofuranosidase, 22.6 β-xylosidase, 2.39 α-galactosidase, 1.3p-coumaroyl esterase (U/mg) 6.3 endoglucanase, 0.46 exoglucanase, 3.3 β-glucosidase, 209 β-xylanase, 4.9 β-xylosidase, 3.21 α-arabinofuranosidase |
| Accelerase 1000 | Genencor | 93 FPU, 7.3 CMCase, 1632 β-glucosidase, 849-xylanase 67.3 FPU, 84.2β-glucosidase |
| Primafast Luna CL | Genencor | Endoglucanases, no exoglucanase |
| GC220 | Genencor | 92.8 FPU, 99.7 β-glucosidase, 2782 xylanase 9(osx), 3.06 α-arabinofuranosidase, 7.3 β-xylosidase, 3.9 α-galactosidase |
| Shearzyme | Novozyme | 27 FPU, 5.0 β-glucosidase, 2293 xylanase (bwx) |
| NS50013 | Novozyme | 63 FPU, 8 β-glucosidase, 1117 xylanase (bwx) |


