Chapter 5
Role of Biofuel Processing in Creating Global Warming
5.1 Introduction
The global hydrological cycle is integral to the climate system. It has become almost universally acceptable that fossil fuels – totally natural energy source – are inherently unsustainable. Islam et al., (2010) show the unsustainability of fossil fuels comes from the fact the refining process is unsustainable and not from the fossil fuel. Ironically, when it comes to biofuels, no concern is raised.
The use of vegetable oils as alternative fuels has been around for 100 years when the inventor of the diesel engine Rudolph Diesel first tested peanut oil, in his compression ignition engine (Say, 1993). He said, “The use of vegetable oils for engine fuels may seem insignificant today. But such oils may in course of time be as important as petroleum and the coal tar products of the present time” (Say, 1993). However, this technology could not survive against the petroleum technology that produced petroleum products and now accounts for vast majority of world’s energy consumption.
As we have seen in Chapter 3, biofuels would be inherently unsustainable just because of the chemical fertilizer and pesticide (and genetic alteration, whenever applicable) used. In discussing this aspect of sustainability, Chhetri and Islam (2008) presented a true green bio-diesel model. They showed how biodiesel can be produced from vegetable oil as well as animal fat. The focus of their work was the processing part of biodiesel. Of course, it is conceivable, as we have seen in the previous chapter, to have unsustainability arising from the source itself. For instance, any use of antibiotic, hormone for animals and chemical fertilizer and pesticide for vegetable would render them unsustainable and the resulting CO2 will not be absorbed within the ecosystem.
The existing bio-diesel production process is neither completely “green” nor renewable because it utilizes fossil fuels, mainly natural gas, as an input for methanol production. The conventional bio-diesel production process entails the use of fossil fuels, such as methane, as an input to methanol. It has been reported that up to 35% of the total primary energy requirement for bio-diesel production comes from fossil fuels (Carraretto et al., 2004). Methanol makes up about 10% of the feedstock input, and since most methanols are currently produced from natural gas, bio-diesel is not completely renewable (Gerpen et al., 2004). The catalysts and chemicals currently used for bio-diesel production are highly caustic and toxic. The synthetic catalysts used for the transesterification process are sulfuric acid, sodium hydroxide, and potassium hydroxide, which are all highly toxic and corrosive chemicals. The pathway for conventional bio-diesel production and petrodiesel production follows a similar path (Figure 5.1).
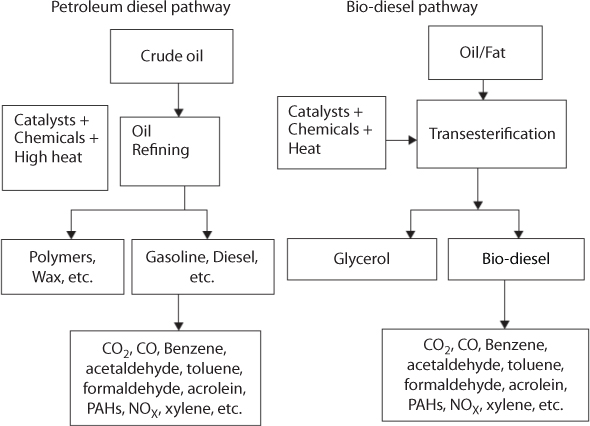
Figure 5.1 Pathway of mineral diesel and conventional bio-diesel (Chhetri and Islam 2008).
It turns out, both fuels have similar pollutants in their emissions, such as benzene, acetaldehyde, toluene, formaldehyde, acrolein, PAHs, and xylene (Huang et al., 2016). However, bio-diesel has fewer pollutants in quantity than petrodiesel. In this chapter, the refining process involved in biofuel as well as petroleum fuel are investigated in order to assess the role played in generating GHG.
Vegetable oils occupy a prominent position in the development of alternative fuels although, there have been many problems associated with using it directly in diesel engine (especially in direct injection engine). These problems are associated with large triglyceride molecules and its higher molecular mass and avoided by modifying the engine less or more according to the conditions of use and the oil involved. The modified engines built by Elsbett in Germany and Malaysia and Diesel Morten und Geraetebau GmbH (DMS) in Germany and the USA show a good performance when fuelled with vegetable oils of different composition and grades (Srivastava and Prasad, 2000).
5.2 The Process of Biodiesel Manufacturing
In producing biofuel, the main process involves transforming a natural oil into refined products. For instance, Chemically biodiesel, which is chemically a monoalkyl esters of long chain fatty acids, is derived from oil and/or fat through a process called transesterification (Meher et al., 2006). In this process, oil or fat reacts with a monohydric alcohol in the presence of a catalyst. The process of transesterification is affected by the mode of reaction condition, molar ratio of alcohol to oil, type of alcohol, type and amount of catalysts, reaction time and temperature and purity of reactants. Each component will bring in ingredients that can render the product unsustainable (Chhetri and Islam, 2008).
The plant oils usually contain free fatty acids, phospholipids, sterols, water, odourants and other impurities. Because of these, the oil cannot be used as fuel directly, particularly because the combustion engines are designed for refined fluids only. To overcome these problems the oil requires slight chemical modification mainly transesterification, pyrolysis and emulsification. Among these, the transesterification is the key and most important step in the production of the cleaner and environmentally safe fuel from vegetable oils. Vegetable oils as such cannot be used directly in a combustion chamber. Difficulties with that include (Meher et al., 2006): (1) Coking and trumpet formation on the injectors to such an extent that fuel atomization does not occur properly or even prevented as a result of plugged orifices, (2) Carbon deposits, (3) Oil ring sticking, (4) Thickening or gelling of the lubricating oil as a result of contamination by vegetable oils, and (5) Lubricating problems. In addition, vegetable oils and especially animal fats have 10–17 times higher viscosity than diesel fuel, meaning pumping it would take extraordinary power for any engine. Finally, these raw materials have lower volatilities that causes the formation of deposits in engines due to incomplete combustion and incorrect vaporization characteristics.
Biodiesel has been considered as a “green fuel,” however, its production processes results in a high environmental impact, because it is based on homogeneous alkaline catalysts (i.e., KOH, NaOH, or NaOCH3), which although are inexpensive, provoke serious environmental drawbacks (Maeda et al., 2011). Firstly, a large amount of effluents and residues are generated during the products neutralization. In addition, homogeneous alkaline catalysts are corrosive and are nonrecyclable, that comprises the main disadvantags of its use in the biodiesel production. Most importantly, each of these components continue to remain with the product and pollute the environment, resulting in CO2 that cannot be assimilated back in the environment.
Transesterification is the main process that turns vegetable oils into various components as shown in Figure 5.2 (Chhetri et al., 2008). The nature of a catalyst employed during the transesterification reaction is crucial in converting triglycerides to biodiesel. As a result different catalysts have being explored for converting triglycerides to biodiesel fuel. The catalysts usually employed to catalyze transesterification reaction are homogeneous catalysts and heterogeneous catalysts. Conventionally, homogeneous alkaline catalysts such as NaOH, KOH, CH3ONa, and CH3OK are more often used in producing biodiesel (Sharma et al., 2008). The catalytic performance of these catalysts and their ability to perform under moderate conditions has led to their choice (Hideki et al., 2001). Among these homogeneous alkaline catalysts, CH3ONa is most active, providing biodiesel yield above 98 wt% in short reaction time (30 min) (Helwani et al., 2009; Demirbas, 2009).
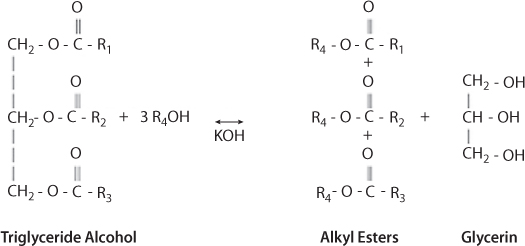
Figure 5.2 Biodiesel production (Sharma et al., 2008).
Transesterification of triglycerides produce fatty acid alkyl esters and glycerol. The glycerol layer settles down at the bottom of the reaction vessel. Diglycerides and monoglycerides are the intermediates in this process. The mechanism of transesterification is described in Figure 5.3. The step wise reactions are perceived to be reversible and a little excess of alcohol is used to shift the equilibrium towards the formation of esters. In presence of excess alcohol, the foreword reaction is pseudo-first order and the reverse reaction is found to be second order. It was also observed that transesterification is faster when catalyzed by alkali (Freedman et al., 1986). The mechanism of alkali-catalyzed transesterification is described in Figure 5.4. The first step involves the attack of the alkoxide ion to the carbonyl carbon of the triglyceride molecule, which results in the formation of a tetrahedral intermediate. The reaction of this intermediate with an alcohol produces the alkoxide ion in the second step. In the last step the rearrangement of the tetrahedral intermediate gives rise to an ester and a diglyceride (Ma and Hanna, 1999).
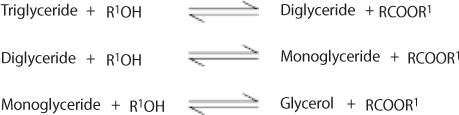
Figure 5.3 General equation for transesterification of triglycerides (Ma and Hanna, 1999).
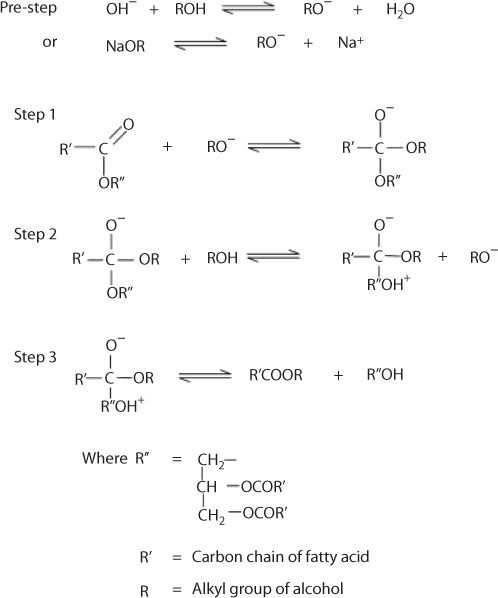
Figure 5.4 Mechanism of base catalyzed transesterification (Ma and Hanna, 1999).
Transesterification can be catalyzed by a Brownsted acid, preferably by sulfonic and sulfuric acids. These catalysts give very high yields in alkyl esters but these reactions are slow, requiring typically temperature above 100 C and more than 3 hr to complete the conversion (Schuchardt et al., 1998). The mechanism of acid catalyzed transesterification of vegetable oil (for a monoglyceride) is shown in Figure 5.4. However, it can be extended to di- and tri-glycerides. The protonation of carbonyl group of the ester leads to the carbocation, which after a nucleophilic attack of the alcohol produces a tetrahedral intermediate. This intermediate eliminates glycerol to form a new ester and to regenerate the catalyst. The methanolysis of soybean oil in the presence of 1% H2SO4 with an alcohol/oil molar ratio 30:1 was studied. At a reaction temperature of 65 8C the conversion was observed to be completed in 20 hr, while butanolysis at 117 C and ethanolysis at 78 C using the same quantities of catalyst and alcohol, take 3 and 18 hr, respectively (Freedman et al., 1986).
5.2.1 Variables Affecting Transesterification Reaction
The process of transesterification is affected by various factors depending upon the reaction condition used. The important process variables affecting the rate of reaction and the yield and purity of fatty esters from the transesterification process include (Sayed, 2006):
- Temperature,
- Nature of catalyst,
- Molar ratio of alcohol to vegetable oil,
- Type of alcohol,
- Type of oil,
- Quality of the oil, such as moisture and free fatty acid content,
- Glycerol separation.
This discussion is carried out below.
5.2.1.1 Effect of Free Fatty Acid and Moisture
The production of biodiesel from raw materials with high content of free fatty acid (FFA) requires the use of homogeneous acid catalysts, which affect the reaction rate. Usually, a two-stage process is performed, where the first step (i.e., FFA esterification) is acid-catalyzed and the second step (i.e., triglycerides transesterification) is base-catalyzed. Both of these reactions do take place under mild reaction conditions (60 C). An option is converting both FFA and TG into biodiesel in a one-stage process. However, these processes require higher temperatures (up to 130 C) and stronger mineral acids such as H2SO4 as a catalyst. Actually, the use of homogeneous Brønsted acid catalysts also have similar problems to those of the alkaline catalysts, i.e., large effluents generation and salts from the neutralization steps, high reactor corrosion, and non-reuse of the catalysts.
The FFA and moisture content are key parameters for determining the viability of the vegetable oil transesterification process. To carry the base catalyzed reaction to completion, an FFA concentration lower than 3% is needed. The higher the acidity of the oil, smaller is the conversion efficiency. Both excess as well as insufficient amount of catalyst may cause soap formation (Dorado et al., 2002). Ma et al., (1998) studied the transesterification of beef tallow catalyzed by NaOH in presence of free fatty acids and water. Without adding FFA and water, the apparent yield of beef tallow methyl esters (BTME) was highest. When 0.6% of FFA was added, the apparent yield of BTME reached the lowest, less than 5%, with any level of water added. When 0.9% of water was added, without addition of FFA, the apparent yield was about 17%. If the low qualities of beef tallow or vegetable oil with high FFA are used to make biodiesel fuel, they must be refined by saponification using NaOH solution to remove free fatty acids. This saponification adds non-organic components that become imbedded to the produced diesel. Alternately, an acid catalyzed process can also be used for esterification of these free fatty acids. The addition of more sodium hydroxide catalyst compensates for higher acidity, but the resulting soap causes an increase in viscosity or formation of gels that interferes in the reaction as well as with separation of glycerol (Freedman et al., 1984). When the reaction conditions do not meet the above requirements, ester yields are significantly reduced. The methoxide and hydroxide of sodium or potassium should be maintained in anhydrous state. Prolonged contact with air will diminish the effectiveness of these catalysts through interaction with moisture and carbon dioxide. However, any time these catalysts come in contact with the atmosphere, the resulting CO2 is irreversibly polluted and becomes unacceptable to the ecosystem (Khan and Islam, 2012). Most of the biodiesel is currently made from edible oils by using methanol and alkaline catalyst. As such edible oil itself is polluted if chemical fertilizer or pesticide is used during the cultivation process. To-date no scientific study has identified this source of unsustainability However, there are large amounts of low cost oils and fats that could be converted to biodiesel. In order to deal with the problems with processing these low cost oils and fats is that they often contain large amounts of free fatty acids that cannot be converted to biodiesel using alkaline catalyst, a two-step esterification process is required for these feed stocks. Initially, the FFA of these can be converted to fatty acid methyl esters by an acid catalyzed pretreatment and in the second step transesterification is completed by using alkaline catalyst to complete the reaction (Canakci and Gerpen, 2001). Initial process development was performed with a synthetic mixture containing 20% and 40% free fatty acid prepared by using palmitic acid. Process parameters such as molar ratio of alcohol to oil, type of alcohol, amount of acid catalyst, reaction time, free fatty acid level were investigated to determine the best strategy for converting the free fatty acids to usable esters. The work showed that the acid level of the high free fatty acids feed stocks could be reduced to less than 1% with a two-step pretreatment reaction. The reaction mixture was allowed to settle between steps so that the water containing phase could be removed. The two-step pretreatment reaction was demonstrated with actual feedstocks, including yellow grease with 12% free fatty acid and brown grease with 33% free fatty acids. After reducing the acid levels of these feedstocks to less than 1%, the transesterification reaction was completed with an alkaline catalyst to produce fuel grade biodiesel.
Turck (2002) received a patent that investigated the negative influence of base catalyzed transesterification of triglycerides containing substantial amount of free fatty acid. This invention relates to a method for producing fatty acid esters of monovalent alkyl alcohols by base-catalyzed transesterification of fatty acid esters of polyvalent alcohols. In his method, oils, which contain free fatty acids and phosphatides, are used in addition to fatty acid esters of polyvalent alcohols. The method consists of several stages. The fatty acids contained in the starting material are treated with a base mixture of glycerine and a catalyst. This mixture is produced as a polar phase in the following transesterification stages and can be separated from the reaction mixture, using phase separation. The invention also relates to the use of esters produced by this method as diesel fuels. As a rule, the fraction of free catalyst from the basic glycerin phase deriving from transesterification is also sufficient for raw materials presenting a high fraction of free fatty acids. The basic glycerin phase has a glycerin level of 20% to 99%, preferably 40% to 60%. The catalyst level ranges between 1% and 30%, preferably 5% and 10%. The fraction of the transesterification alcohol ranges between 5% and 40%, preferably 15% and 25%. Whenever this will be necessary, the basic glycerin phase can be supplemented also by the addition of a solid alkaline catalyst or an alcohol/catalyst mixture. Mixing glycerins of different qualities, from industrial to pharmaceutical qualities, and an alkaline catalyst with or without alcohols directly can also produce the agent used for glycerin extraction. All catalysts used for transesterification reactions, metal hydroxides and/or alcoholates particularly of metals from the main groups I to III of the periodic table, preferably NaOH, KOH and sodium alcoholates such as sodium ethylate, come into question as alkaline catalysts.
Free fatty acids react with the basic catalyst added for the reaction and give rise to soap, as a result of which, one part of the catalyst is neutralized and is therefore no longer available for transesterification. These high FFA content oils/fats are processed with an immiscible basic glycerol phase so as to neutralize the free fatty acids and cause them to pass over into the glycerol phase by means of monovalent alcohols. The triglycerides are subjected to transesterification, using a base as catalyst, to form fatty acid alkyl esters, characterized in that after its separation, the basic glycerol phase produced during transesterification of the triglycerides is used for processing the oils/fats for removal of free fatty acids. The minimum amount of catalyst required for this process was calculated, relative to 1000 g of the oil to be processed, as a function of the acid value and the mean molar mass of the oil/fat.
5.2.1.2 Catalyst Type and Concentration
Transesterification reactions can be catalyzed either using homogeneous catalysts (acid or base) or heterogeneous catalysts (acid, base, or enzyme). The use of homogeneous alkaline catalysts provides higher reaction rate and conversion than the acid catalysts for the transesterification of triglycerides to biodiesel. Catalysts used for the transesterification of triglycerides are classified as alkali, acid, enzyme or heterogeneous catalysts, among which alkali catalysts like sodium hydroxide, sodium methoxide, potassium hydroxide, potassium methoxide are more effective (Ma and Hanna, 1999). Homogeneous alkali-catalyzed transesterification reaction is about 4000 times faster than the homogeneous acid-catalyzed reaction. The alkali (sodium and potassium hydroxides) catalysts are more popular and most preferred in the commercial production of biodiesel for their low cost and availability. The application of heterogeneous catalysts such as solid metal oxide in the production of biodiesel to circumvent the problems of downstream purification is faced with elevated reaction temperatures and cost (Qiu et al., 2011). If the oil has high free fatty acid content and more water, acid catalyzed transesterification is suitable. The reaction mixture obtained from this acid post-esterification can then be added to the transesterification mixture by the end of the transesterification reaction. In such an approach one should note, however, that the acid catalyst neutralizes one part of the basic catalyst required for the transesterification. After completion of the pre-processing step the triglyceride contained in the material can be directly mixed with the base for carrying out the step of transesterification of the fatty acid esters of polyvalent alcohols to produce fatty acid esters of monovalent alkyl alcohols.
The acids can be sulfuric acid, phosphoric acid, hydrochloric acid or organic sulfonic acid. Ma et al., (1998) studied methanolysis of beef tallow with catalysts NaOH and NaOMe. Comparing the two catalysts, NaOH was significantly better than NaOMe. They further discovered that the catalysts NaOH and NaOMe reached their maximum activity at 0.3% and 0.5% w/w of the beef tallow, respectively. Sodium methoxide causes formation of several by-products mainly sodium salts, which are to be treated as waste. In addition, Ahn et al., (1995) pointed out that high quality oil is required with this catalyst. This finding is different from previous previous findings of Freedman et al., (1984), in which ester conversion at the 6:1 molar ratio of alcohol/oil for 1% NaOH and 0.5% NaOMe were almost the same after 60 min. Part of the difference may be attributed to the differences in the reaction system used. As a catalyst in the process of alkaline methanolysis, mostly sodium hydroxide or potassium hydroxide have been used, both in concentration from 0.4 to 2% w/w of oil. Refined and crude oils with 1% either sodium hydroxide or potassium hydroxide catalyst resulted successful conversion. Methanolysis of soybean oil with the catalyst 1% potassium hydroxide has given the best yields and viscosities of the esters (Tomasevic et al., 2003). Attempts have been made to use basic alkaline-earth metal compounds in the transesterification of rapeseed oil for production of fatty acid methyl esters. The reaction proceeds if methoxide ions are present in the reaction medium. The alkaline-earth metal hydroxides, alkoxides and oxides catalyzed reaction proceeds slowly as the reaction mixture constitutes a three-phase system oil–methanol-catalyst, which inhibits the reaction (Gryglewicz, 1999). The catalytic activity of magnesium oxide, calcium hydroxide, calcium oxide, calcium methoxide, barium hydroxide, and for comparison, sodium hydroxide during the transesterification of rapeseed oil was investigated. Sodium hydroxide exhibited the highest catalytic activity in this process. The degree to which the substrates were reacted reached 85% after 30 min of the process and 95% after 1.5 hr, which represented a close value to the equilibrium. Barium hydroxide was slightly less active with a conversion of 75% after 30 min. Calcium methoxide was medially active. The degree to which the substrates were reacted was 55% after 30 min. Eighty percent after 1 hr and state of reaction equilibrium (93%) was reached after 2.5 hr. The rate of reaction was slowest when catalyzed by CaO. Magnesium oxide and calcium hydroxide showed no catalytic activity in rapeseed oil methanolysis. Acid catalyzed transesterification was studied with waste vegetable oil. The reaction was conducted at four different catalyst concentrations, 0.5, 1.0, 1.5 and 2.25 M HCl in presence of 100% excess alcohol and the result was compared with 2.25 M H2SO4 and the decrease in viscosity was observed. H2SO4 has superior catalytic activity in the range of 1.5–2.25 M concentration (Mohamad and Ali, 2002).
Although chemical transesterification using an alkaline catalysis process gives high conversion levels of triglycerides to their corresponding methyl esters in short reaction times, the reaction has several drawbacks: it is energy intensive, recovery of glycerol is difficult, the acidic or alkaline catalyst has to be removed from the product, alkaline waste water require treatment, and free fatty acid and water interfere the reaction. Any leftover would be embedded in the final product, causing long-term effects on the environment.
Enzymatic catalysts, such as, lipases are able to effectively catalyze the transesterification of triglycerides in either aqueous or non-aqueous systems, which can overcome the problems mentioned above (Fuduka et al., 2001). In particular, the by-products, glycerol can be easily removed without any complex process, and also that free fatty acids contained in waste oils and fats can be completely converted to alkyl esters. On the other hand, in general the production cost of a lipase catalyst is significantly greater than that of an alkaline one.
Lukić et al., (2009) employed alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil, a high yield of biodiesel was obtained. But the reaction temperature was very high and the best methanol to oil molar ratio was 15:1, so that extensive energy and methanol were wasted. Suppes et al., (2004) reported the use of EST-10 catalyst to obtain conversion of 94.6% at 120 C and 24 hr reaction time, respectively. Kim et al., (2004) prepared a solid superbase of Na/NaOH/γ-Al2O3, which showed almost the same catalytic activity under the optimized reaction conditions as that of the conventional homogeneous NaOH catalyst. But they are quite expensive or complication to prepare, which limited their industrial application. Therefore, homogeneous base catalysts which include sodium hydroxide, potassium hydroxide, their carbonates, and sodium and potassium alkoxides such as NaOCH3, were still favorable to be used.
5.2.1.3 Molar Ratio of Alcohol to Oil and Type of Alcohol
One of the most important variables affecting the yield of ester is the molar ratio of alcohol to triglyceride. The stoichiometric ratio for transesterification requires three moles of alcohol and one mole of triglyceride to yield three moles of fatty acid alkyl esters and one mole of glycerol. However, transesterification is an equilibrium reaction in which a large excess of alcohol is required to drive the reaction to the right. For maximum conversion to the ester, a molar ratio of 6:1 should be used. The molar ratio has no effect on acid, peroxide, saponification and iodine value of methyl esters (Tomasevic et al., 2003). However, the high molar ratio of alcohol to vegetable oil interferes with the separation of glycerin because there is an increase in solubility. When glycerin remains in solution, it helps drive the equilibrium to back to the left, lowering the yield of esters.
The transesterification of Cynara oil with ethanol was studied at molar ratios between 3:1 and 15:1. The ester yield increased as the molar ratio increased up to a value of 12:1. The best results were for molar ratios between 9:1 and 12:1. For molar ratios less than 6:1, the reaction was incomplete. For a molar ratio of 15:1 the separation of glycerin is difficult and the apparent yield of esters decreased because a part of the glycerol remains in the biodiesel phase. Therefore, a molar ratio 9:1 seems to be the most appropriate (Encinar et al., 2002).
Qiu et al., (2011) studied the effect of mole ratio of methanol to oil, reaction temperature, catalyst amount and reaction time on the yield. In order to decrease the operational temperature, a co-solvent (hexane) was added into the reactants and the conversion efficiency of the reaction was improved. The optimal reaction conditions were obtained by this experiment: methanol/oil mole ratio 5.0:1, reaction temperature 55 C, catalyst amount 0.8 wt.% and reaction time 2.0 hr. Under the optimum conditions, approximately 94% yield of methyl esters was reached.
The base catalyzed formation of ethyl ester is difficult compared to the formation of methyl esters. Specifically the formation of stable emulsion during ethanolysis is a problem. Methanol and ethanol are not miscible with triglycerides at ambient temperature, and the reaction mixtures are usually mechanically stirred to enhance mass transfer. During the course of reaction, emulsions usually form. In the case of methanolysis, these emulsions quickly and easily break down to form a lower glycerol rich layer and upper methyl ester rich layer. In ethanolysis, these emulsions are more stable and severely complicate the separation and purification of esters (Zhou et al., 2003). They studied the effects of alcohol/oil molar ratio, base concentration, and temperature on the single-phase base-catalyzed ethanolyses of sunflower and canola oils. The use of tetrahydrofuran as co-solvent, as well as higher than usual alcohol/substrate molar ratios, prevented glycerol separation. This allowed each reaction to reach equilibrium rather than just steady-state conditions. High conversions of oil lowered the concentrations of MG and DG surfactants in the products, and thereby mitigated the formation of emulsions usually associated with ethanolysis reactions. An alcohol/oil molar ratio of 25:1, together with the necessary amount of cosolvent, gave optimal results. At this molar ratio, despite equilibrium being achieved, ethanolysis, unlike methanolysis, did not quite produce biodiesel-standard material, the MG content being approximately 1.5 mass%. For methanolysis and 1-butanolysis, the corresponding values were 0.6 and 2.0 mass%, respectively. The use of 1.4 mass% KOH (equivalent to 1.0 mass% NaOH) led to ethanolysis equilibrium within 6–7 min at 23 °C rather than 15 min when only 1.0 mass% was used. At 60 °C, equilibrium was reached within only 2 min. Soybean and canola oils behaved the same.
The emulsions are caused in part by formation of the intermediates monoglycerides and diglycerides, which have both polar hydroxyl groups and non-polar hydrocarbon chains. These intermediates are strongly surface-active agents. In the process of alcoholysis, the catalyst, either sodium hydroxide or potassium hydroxide is dissolved in polar alcohol phase, in which triglycerides must transfer in order to react. The reaction is initially mass-transfer controlled and does not conform to expected homogeneous kinetics. When the concentrations of these intermediates reach a critical level, emulsions form. The larger non-polar group in ethanol, relative to methanol, is assumed to be the critical factor in stabilizing the emulsions. However, the concentration of mono- and di-glycerides are very low, then the emulsions become unstable. This emphasizes the necessity for the reaction to be as complete as possible, thereby reducing the concentrations of mono- and di-glycerides.
Anastopoulos et al., (2012) studied ethanolysis of four different vegetable oils (sunflower, soybean, cotton seed, and used frying oil), using sodium ethoxide as a catalyst. The ester preparation involved a two-step transesterification reaction, followed by purification. The effects of the mass ratio of catalyst to oil, the molar ratio of ethanol to oil, and the reaction temperature were studied on conversion of sunflower oil to optimize the reaction conditions in both stages. The rest of the vegetable oils were converted to ethyl esters under optimum reaction parameters. Ethyl esters of four different types of vegetable oils were blended with the diesel fuel at 2 %, 5 %, 10 %, and 20 %, on a volume basis. The experimental results showed that the densities and viscosities of the blends increased with the increase of biodiesel concentration in the fuel blend. Cold flow properties were negatively affected as ethyl ester content was increasing. Distillation characteristics and cetane indexes were not significantly altered. These results are promising, and ethyl esters can be seen as a viable fully renewable alternative to petroleum diesel.
The study of Anastopoulos et al., (2012) shows the influence of sodium ethoxide concentration on the evolution of ester yields with time (Figure 5.5). The best results were reached with a concentration of 1.0%. For higher values the yields were lower. This fact, as has it been indicated, seems to be related to the free acidity of the oil. When there is a large free fatty acid content, the addition of more sodium ethoxide, or any other alkaline catalyst, compensates this acidity and avoids catalyst deactivation. The addition of an excessive amount of catalyst, however, gives rise to the formation of an emulsion, which increases the viscosity and leads to the formation of gels. These hinder the glycerol separation and; hence, reduce the apparent ester yield. The result of these two opposing effects is an optimal catalyst concentration that, in this case, is 1.0% CH3CH2ONa. In consequence, further increases in catalyst concentration did not increase the conversion and lead to extra costs because it was necessary to remove it from the reaction medium at the end. These results were qualitatively similar to those obtained by other authors in the ethanolysis of rapeseed oil (Fillieres et al., 1995). In the case of the alkaline catalysis, the literature presents many works relating to these processes (Encinar et al., 1999; Schwad et al., 1987). In each case, the more suitable catalyst depends on the type of oil utilized, and the best-suited concentrations are between 0.5 and 1.0 wt. %.
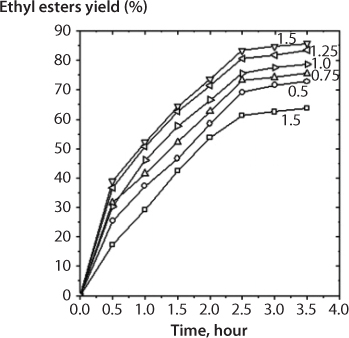
Figure 5.5 First stage transesterification. Effect of the mass ratio of C2H5ONa to oil on ethyl esters yield. Ethanol/oil molar ratio, 12:1; reaction temperature, 80 C (From Anastopoulos et al., 2012).
In order to study the effect of the molar ratio of ethanol to oil on biodiesel yield, Anastopoulos et al., (2012) conducted a series of experiments with ethanol/oil molar ratio ranging between 6:1 and 14:1. For all these tests, a catalyst concentration of 1 % wt/wt, was used. The temperature was fixed at 80 C. Figure 5.6. shows the evolution of esters yield with the reaction time. As can be observed, with a 6:1 molar ratio, the conversion to esters was near 69.4 % wt/wt after 3.5 hr. The esters yield increased as the molar ratio increased, with the best results (85.4 %) being for a molar ratio 12:1. Nevertheless, a later increase of molar ratio to 14:1 did not produce an increase in the yield, since a lower value was obtained (79.7 %). This was because for higher molar ratios, the separation of the glycerol was difficult, since the ethanol excess hinders the decantation by gravity so that the apparent yield of esters decreases since part of the glycerol remains in the biodiesel phase. These results are similar to those obtained by previous researchers, except for the fact there is also a slight recombination of esters and glycerol to monoglycerides since the other report’s concentration keeps increasing during the course of the reaction; in contrast with reactions conducted with low molar ratios. Krisnamgkura and Simamaharnnop (1992) had observed that when glycerol remains in solution it helps to drive the equilibrium back to the left, lowering the esters yield. In consequence, the alcohol/oil molar ratio is one of the most important variables affecting the esters yield, and although the stoichiometric ratio for transesterification requires 3 mol of alcohol and 1 mol of triglyceride, an excess of alcohol is used in practice. Hence, the alcohol molar/oil ratio is a variable that must be always optimized.

Figure 5.6 First stage transesterification. Effect of the molar ratio of ethanol to sunflower oil on ethyl esters yield. C2H5ONa/oil mass ratio, 1.0%; reaction temperature, 80 C (From Anastopoulos et al., 2012).
From one environmental point of view, ethyl esters utilization is also more advantageous than the utilization of methyl esters. According to Makareviciene and Janulis (2003), the results showed that when considering emissions of nitrogen oxides (NOx), carbon monoxide (CO) and smoke density, rapeseed oil ethyl ester had a less negative effect on the environment in comparison with that of rapeseed oil methylester. When fuelled with pure rapeseed oil ethyl ester, HC emissions decreased by 53 %, CO emissions by 7.2% and smoke density by 72.6% as compared to respective emissions when fossil diesel fuel was used. Also carbon dioxide (CO2) emissions, which contribute to the greenhouse effect, decreased by 782.87 g/kWh when rapeseed oil ethyl ester was used instead of fossil diesel fuel. Finally, these authors found that the rapeseed oil ethyl ester was more rapidly biodegradable in a water environment than rapeseed oil methyl ester and especially when blended with fossil diesel fuel. However, the utilization of ethanol also presents inconveniences (Zhou et al., 2003). The base-catalyzed formation of ethyl ester is difficult compared to the formation of methyl esters. Specifically, the formation of a stable emulsion during ethanolysis is a problem. Methanol and ethanol are not miscible with triglycerides at room temperature, and the reaction mixture is usually mechanically stirred to enhance mass transfer. During the course of reaction, emulsions are usually formed. In the case of methanolysis, these emulsions break down quickly and easily to form a lower glycerol rich layer and upper methyl ester rich layer. In ethanolysis, these emulsions are more stable and severely complicate.
5.2.1.4 Effect of Reaction Time and Temperature
Reaction temperature can influence the reaction rate and the ethyl esters yield because the intrinsic rate constants are strong functions of temperature. The conversion rate increases with reaction time. Freedman et al., (1984) transesterified peanut, cottonseed, sunflower and soybean oil under the condition of methanol–oil molar ratio 6:1, 0.5% sodium methoxide catalyst and 60 8C. An approximate yield of 80% was observed after 1 min for soybean and sunflower oils. After 1 hr, the conversion was almost the same for all four oils (93–98%). Ma et al., (1999) studied the effect of reaction time on transesterification of beef tallow with methanol. The reaction was very slow during the first minute due to mixing and dispersion of methanol into beef tallow. From one to 5 min, the reaction proceeds very fast. The production of beef tallow methyl esters reached the maximum value at about 15 min. Transesterification can occur at different temperatures, depending on the oil used. For the transesterification of refined oil with methanol (6:1) and 1% NaOH, the reaction was studied with three different temperatures (Freedman et al., 1984). After 0.1 hr, ester yields were 94, 87% and 64% for 60, 45 and 32 C, respectively. After 1 hr, ester formation was identical for 60 and 45 C runs and only slightly lower for the 32 C run. Temperature clearly influenced the reaction rate and yield of esters.
Anastopoulos et al., (2012) conducted a series of experiments, using an ethanol/sunflower oil molar ratio of 12:1, and a catalyst concentration of 1 % m/m to study the effect of reaction temperatures. The reaction temperature was varied between 35C and 90C. Figure 5.7. shows the effect of the reaction temperature on the biodiesel yield. It indicates that the reaction rate was higher at high temperature than at low temperature. The ethyl esters yield was only 54.6% at 35C after 2.5 hr of reaction, and it reached to 81.4% at 80C at the same reaction period. Therefore, the final ethyl ester concentration was almost reached in 2.5 hr at 80C. After this initial period, there was a second period in which the composition evolved slowly. The yields obtained in the 90C and 80C experiment were very similar, and the one in the 35C run was clearly less.

Figure 5.7 First stage transesterification. Effect of reaction temperature on ethyl esters yield. C2H5ONa/oil mass ratio, 1.0%; ethanol/oil molar ratio, 12:1 (From Anastopoulos et al., 2012).
5.2.1.5 Mixing Intensity
Mixing is very important in the transesterification reaction, as oils or fats are immiscible with sodium hydroxide–methanol solution. Once the two phases are mixed and the reaction is started, stirring is no longer needed. At this point, the surface area between two phases dictates the efficacy of the overall reaction. Initially the effect of mixing on transesterification of beef tallow was studied by Ma et al., (1999). No reaction was observed without mixing and when NaOH–MeOH was added to the melted beef tallow in the reactor while stirring, stirring speed was insignificant. Reaction time was the controlling factor in determining the yield of methyl esters. This suggested that the stirring speeds investigated exceeded the threshold requirement of mixing.
Zheng et al., (2006) studied the reaction kinetics of acid-catalyzed transesterification of waste frying oil in excess methanol to form fatty acid methyl esters (FAME), for possible use as biodiesel. Rate of mixing, feed composition (molar ratio oil:methanol:acid) and temperature were independent variables. A particular series of experiments were dedicated to determining the effect of mixing rate. Three different stirring speeds: 100, 400, and 600 rpm, to investigate the effect of mixing rate. The mixing intensity of an impeller can be expressed by the Reynolds number, NRe
(5.1)
where η is the rotational speed of the impeller, D is the diameter of the impeller, ρ is the fluid density and μ is the fluid viscosity. With the rotational speed of the impeller set at 100, 400, or 600 rpm, the Reynolds numbers are estimated to be in the range 6000–12,000, indicating that the flow in the reactor was turbulent in the above range of impeller speeds. There was no significant difference in the yield of FAME when the rate of mixing was in the turbulent range 100 to 600 rpm. Whereas, the oil:methanol:acid molar ratios and the temperature were the most significant factors affecting the yield of FAME. At 70 C with oil:methanol:acid molar ratios of 1:245:3.8, and at 80 C with oil:methanol:acid molar ratios in the range 1:74:1.9–1:245:3.8, the transesterification was essentially a pseudo-first-order reaction as a result of the large excess of methanol which drove the reaction to completion (99 ± 1% at 4 hr). In the presence of the large excess of methanol, free fatty acids present in the waste oil were very rapidly converted to methyl esters in the first few minutes under the above conditions. Little or no monoglycerides were detected during the course of the reaction, and diglycerides present in the initial waste oil were rapidly converted to FAME. This study essentially reduces the mixing rate into maintaining a turbulent regime, in which the surface area between phases is order of magnitude higher than that in the laminar flow regime.
5.2.1.6 Effect of Using Organic Co-Solvents
The methoxide base catalyzed methanolysis of soybean oil at 40 C (methanol–oil molar ratio 6:1) shows that to form methyl esters proceeds approximately more slowly than butanolysis at 30 C (Meher et al., 2006). This is interpreted to be the result of a two-phase reaction in which methanolysis occurs only in the methanol phase. Low oil concentration in methanol causes the slow reaction rate; a slow dissolving rate of the oil in methanol causes an initiation period. Intermediate mono- and di-glycerides preferentially remain in the methanol, and react further, thus explaining the deviation from second order kinetics. The same explanations apply for hydroxide ion catalyzed methanolysis. In order to conduct the reaction in a single phase, Meher et al., (2006) employed cosolvents, such as, tetrahydrofuran, 1,4- dioxane and diethyl ether were tested. Although, there are other cosolvents, initial study was conducted with tetrahydrofuran. At the 6:1 methanol–oil molar ratio the addition of 1.25 vol of tetrahydrofuran per volume of methanol produces an oil dominant one phase system in which methanolysis speeds up dramatically and occurs as fast as butanolysis. In particular, THF is chosen because its boiling point of 67 8C is only two degrees higher than that of methanol. Using tetrahydrofuran, transesterification of soybean oil was carried out with methanol at different concentrations of sodium hydroxide. The ester contents after 1 min for 1.1, 1.3, 1.4 and 2.0% sodium hydroxide were 82.5, 85, 87% and 96.2%, respectively. Results indicated that the hydroxide concentration could be increased up to 1.3 wt%, resulting in 95% methyl ester after 15 min. (Boocock et al., 1998). Similarly for transesterification of coconut oil using THF/MeOH volume ratio 0.87 with 1% NaOH catalyst, the conversion was 99% in 1 min. A single-phase process for the esterification of a mixture of fatty acids and triglycerides were investigated. The process comprises forming a single-phase solution of fatty acids and triglyceride in an alcohol selected from methanol and ethanol, the ratio of said alcohol to triglyceride being 15:1–35:1. The solution further comprises a co-solvent in an amount to form the single phase. In a first step, an acid catalyst for the esterification of fatty acid is added. After a period of time, the acid catalyst is neutralized and a base catalyst for the transesterification of triglycerides is added. After a further period of time, esters are separated from the solution (Boocock, 1994). An improved process was investigated for methanolysis and ethanolysis of fatty acid glycerides such as those found in naturally occurring fats and oils derived from plant and animals. The processes comprise solubilizing oil or fat in methanol or ethanol by addition of a cosolvent in order to form a one-phase reaction mixture, and adding an esterification catalyst. The processes proceed quickly, usually in less than 20 min, at ambient temperatures, atmospheric pressure and without agitation. The co-solvent increases the rate of reaction by making the oil soluble in methanol, thus increasing contact of the reactants. The lower alkyl fatty acid monoesters produced by the process can be used as biofuels and are suitable as diesel fuel replacements or additives (Boocock, 1996).
Tian et al., (2018) conducted experiments to ascertain the effect of fatty acid methyl esters (FAME) as co-solvents in the system. Adding FAME to the ferric sulfate system greatly improves the reaction yield compared to the no FAME condition (Figure 5.8.). However, the FAME chain length did not seem to have a significant impact on the reaction yield or rate. The addition of FAME with varying alkyl chain lengths – methyl decanoate (C10:0), methyl myristate (C14:0), and methyl palmitate (C16:0) – all demonstrated similar reaction kinetics. The experiment without FAME addition showed negligible reaction over the initial time period. Subsequently, the reaction rate increased substantially, likely due to small amounts of methyl oleate being formed and acting as a co-solvent for its own reaction. Follow-up optimization experiments were conducted with the addition of methyl myristate as an additive to determine the effect of temperature and methyl acetate loading on the system.
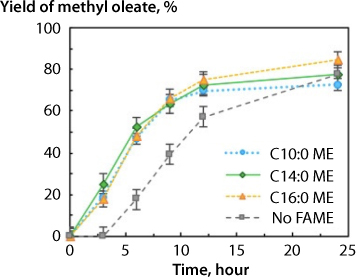
Figure 5.8 Co-solvent effect on reaction rate and product yield at 120 °C 20:1 MAOMR and a mass loading of 7.5% ferric sulfate with FAME additives (C10:0 = methyl decanoate, C14:0 = methyl myristate, C16:0 = methyl palmitate). (From Tian et al., 2018).
The effect of co-solvent loading was also explored in order to determine how FAME addition would impact both the yield and reaction rate in the system (Figure 5.9). All concentrations of FAME (methyl myristate) that were tested had comparable initial reaction rates, however increasing the amount of methyl myristate in the reactors led to an improvement in reaction yield (83% in 12 h). From these results, it is clear that even small amounts of FAME in the reaction lead to improved reaction kinetics.

Figure 5.9 Reaction yield with varying amounts of FAME co-solvent at 120 °C, 20:1 MAOMR, and 7.5% ferric sulphate (from Tian et al., 2018).
5.2.2 Catalysts
Any refining process relies on catalysts. Biodiesel production is no exception. The main process, namely, transesterification reaction, is bolstered with catalysts that are of the broad category of homogeneous and heterogeneous. The most notable catalyst used in producing biodiesel is the homogeneous alkaline catalyst such as NaOH, KOH, CH3ONa and CH3OK. The choice of these catalysts is due to their higher kinetic reaction rates. More importantly, the selection is not based on environmental concerns and there is almost an inverse correlation between the effectiveness of a catalyst and its long-term impact on the environment.
The primary concern that the industry has is the high cost of refined feedstocks and difficulties associated with use of homogeneous alkaline catalysts to transesterify low quality feedstocks for biodiesel production, development of various heterogeneous catalysts are now on the increase. Development of heterogeneous catalyst such as solid and enzymes catalysts could overcome most of the problems associated with homogeneous catalysts. More recently, there has been an increase in the development of heterogeneous catalysts to produce fatty acid methyl esters, because their utilisation in the transesterification reaction greatly simplifies and economises the post-treatment of the products (separation and purification). Besides, the use of heterogeneous catalysts does not produce soaps through free fatty acid neutralisation and triglyceride saponification. However, the heterogeneous catalysed reaction also requires extreme reaction conditions, while the methyl ester yield and the reaction time are still unfavourable compared to the alkali catalysts (Vicente et al., 1998).
Atadashi et al., (2013) critically analyzed the effects of different catalysts used for producing biodiesel. Among these homogeneous alkaline catalysts, CH3ONa is most effective one, with as high as 98 wt% for a short reaction time of 30 min (Helwani et al., 2009). However because of their low price, industrial biodiesel production process mostly employs NaOH and KOH. The process involving these catalysts needs high-quality feedstocks, thus the free fatty acid (FFAs) level of the feedstocks should not exceed 3 wt%, beyond which the reaction will not occur. In addition, water content of the feedstocks is critical, as a result the feedstocks used in alkali-catalyzed transesterification have to anhydrous. Thus, presence of water leads to hydrolysis of oils to FFAs.
Biodiesel production is achieved via different kinds of feedstocks. The nature of feedstock used is dependent on the geographical position and climate of the place. For instance, Europe employs sunflower and rapeseed oils, palm oil predominates in tropical countries, soybean in United States and canola oil in Canada (Cao et al., 2008). Singh and Singh (2010) reported the major feedstocks employed in producing biodiesel are cotton seed, palm oil, sunflower, soybean, canola, rapeseed, and Jatropha curcas. Additionally, Zhang et al., (2003) as well as Chhetri and Islam (2008) remarked that employing feedstocks such as waste frying oils, non-edible oils, and animal fats, as feedstocks could be useful in producing biodiesel. Table 5.1 shows FFAs contents of different vegetable oils. In addition, Table 5.2 presents FFAs levels of most of the feedstocks used to produce biodiesel.
Table 5.1 Values of FFAs content of different vegetable oils (from atadashi et al., 2013).
| Vegetable oils | FFA levels (%) |
| Polanga oil | 22.0 |
| Cottonseed oil | 0.11 |
| Tobacco oil | 35.0 |
| Spent bleaching earth | 24.1 |
| Rubber oil | 17.0 |
| Palm fatty acid distillate (PFAD) | 93 |
| Pongamia pinnata | ≤20 |
| Palm oil | 5.3 |
| Rape seed oil | 2.0 |
| Tall oil | 100 |
| Jatropha oil | 14.0 |
| Tung oil | 9.55 |
| Soybean soapstock | >90 |
| Ponagamia oil | 0.64 |
| Pongamia oil | 2.69 |
| Salvadora oil | 1.76 |
| Moringa oleifera | 2.9 |
| Karanja oil | 2.53 |
| Sorghum bug oil | 10.5 |
| Mahua oil | 21.0 |
| Mahua oil | 19.0 |
| Madhuca indica | 20.0 |
| Zanthoxylum bungeanum | 45.5 |
| Acid oil | 59.3 |
| Karanja oil | 2.53 |
| Trap grease | 50–100% |
| Finished greases | 8.8–25.5% |
| Crude soybean oil | 0.4–0.7% |
| Restaurant waste grease | 0.7–41.8% |
| Waste palm oil | >20% |
| Municipal sludge | Up to 65% |
| Animal fat | 5–30% |
| Trap grease | 75–100% |
| Use cooking oil | 2–7% |
| Waste oil | 46.75% |
Table 5.2 Ffas levels in feedstocks (from atadashi et al., 2013).
| Feedstocks | FFA levels |
| Trap grease | 50–100% |
| Refined vegetable oils | <0.05% |
| Finished greases | 8.8–25.5% |
| Crude soybean oil | 0.4–0.7% |
| Restaurant waste grease | 0.7–41.8% |
| Waste palm oil | >20% |
| Municipal sludge | Up to 65% |
| Animal fat | 5–30% |
| Trap grease | 75–100% |
| Use cooking oil | 2–7% |
| Waste oil | 46.75% |
5.2.2.1 The Effects of Homogeneous Catalyst in Biodiesel Production
There are two types of catalysts for transestenfication: acid catalysts and base catalysts. Sulphuric acid and hydrochloric acid are usually employed for acid catalysis and metal hydroxides and alkoxides for base catalysis (Sayed, 2006). The mechanism of acid-catalyzed transesterification and base-catalyzed transesterification are equivalent to acid and base-catalyzed ester hydrolysis.
Alkali-based catalysts make the transesterification reaction some 4000 times faster than that catalyzed by the same amount of an acid catalyst (Sayed, 2006). Some of the alkaline catalysts used for the transesterification reaction include among others; NaOH, KOH, and sodium methoxide. Other alkaline catalysts include; sodium ethoxide, potassium methoxide, sodium propoxide, sodium butoxide and carbonates, etc. Based on biodiesel yield, CH3ONa or CH3OK are better and more suitable catalyst than NaOH and KOH. Thus, CH3ONa and CH3OK are more suitable due to their ability to dissociate into CH3O− and Na +and CH3O− and K+ respectively. Besides, the catalysts do not form water during transesterification reaction. For these reasone, alkaline catalyst is mostly preferred in commercial production of biodiesel fuel.
The base-catalyzed transesterification of vegetable oils and fats to form alkyl esters occurs at a faster rate than the acid-catalyzed reaction. The base-catalyzed reaction proceeds rapidly at ambient temperature, whereas the acid-catalyzed reaction commonly uses temperatures above 100 C, thus creating additional constraint from both economic and environmental perspectives. This temperature varies depending on the boiling point of the alcohol.
Transesterification of refined oils with less than 0.5 wt% FFAs via chemical catalysts could lead to high-quality biodiesel fuel with better yield within short time of 30–60 min. Figure 5.10 presents the mechanism of base-catalyzed transesterification reaction.
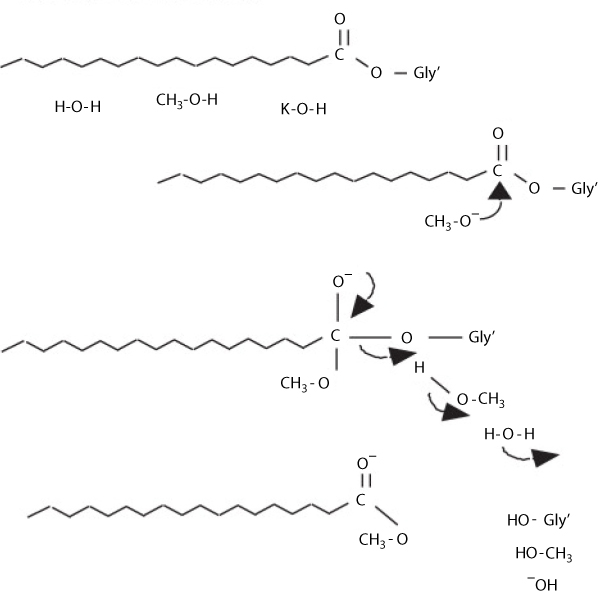
Figure 5.10 Mechanism of base-catalyzed transesterification reaction (from Sayed, 2006).
Vicente et al., (2004) compared different basic catalysts (sodium hydroxide, potassium hydroxide, sodium methoxide and potassium methoxide) to produce biodiesel fuel using sunflower oil. The reactions were conducted at temperature of 65 C, methanol to oil molar ratio of 6:1 and basic catalyst by weight of vegetable oil of 1%. They achieved 85.9 and 91.67 wt% yield of esters for NaOH and KOH and 99.33 and 98.46 wt% yields of esters for CH3ONa and CH3OK, respectively. The authors recorded 98 wt% yields of esters for methoxides after separation and purification steps were completed. Further, less yields losses and negligible ester dissolution in glycerol were observed with methoxides compared to hydroxides.
To evaluate the biodiesel purity, the methyl ester concentration (wt %) in the biodiesel phase was calculated. Conversely, to estimate the biodiesel yield after the reaction and separation stages, the biodiesel weight yield, relative to the initial amount of vegetable oil, was worked out. The results for all the experiments and their repetitions are shown in Table 5.3. The arithmetical averages and standard deviations of the results are also presented in Table 5.3. The standard deviations were very low in all the experiments, indicating a low variation among the repeated experiments.
Table 5.3 Effect of the catalyst on the biodiesel purity and yield (from vincente et al., 2004). Temperature = 65 °C, Methanol:Sunflower oil molar Ratio = 6, Catalyst = 1 Wt%.
| Catalyst | ||||||||
Sodium hydroxide |
Potassium hydroxide |
Sodium methoxide |
Potassium methoxide |
|||||
| Biodiesel purity (wt.%) | 99.70 |
99.71 ± 0.04 |
99.69 |
99.76 ± 0.05 |
99.70 |
99.72 ± 0.03 |
99.40 |
99.52 ± 0.10 |
99.75 |
99.80 |
99.69 |
99.50 |
|||||
99.72 |
99.80 |
99.72 |
99.65 |
|||||
99.65 |
99.74 |
99.75 |
99.53 |
|||||
| Biodiesel yield (wt.%) | 86.33 |
86.71 ± 0.28 |
91.67 |
91.67 ± 0.27 |
99.17 |
99.33 ± 0.36 |
98.33 |
98.46 ± 0.16 |
86.67 |
91.67 |
99.33 |
98.50 |
|||||
87.00 |
91.33 |
99.83 |
98.33 |
|||||
86.71 |
92.00 |
99.00 |
98.67 |
|||||
When the four catalysts were used, methyl ester concentrations were nearly 100 wt.%. According to these results, all the transesterification reactions were completed and, therefore, no difference in biodiesel purity was found after 3 hr of reaction. However, if there are no side reactions, the biodiesel weight yields, relative to the initial amount of vegetable oil, should be nearly 100 wt.%. In this sense, the two possible side reactions are triglyceride saponification or neutralisation of the free fatty acid in the vegetable oil. Both of them produce sodium or potassium soaps and, therefore, decrease the biodiesel yield. In this case, however, the free fatty acid neutralisation could not be substantial since the acid index in the sunflower oil was only 0.45 mg KOH/g. Consequently, triglyceride saponification must be the only possible side reaction. As shown in Table 5.3, high biodiesel yields were obtained by using the sodium or potassium methoxides (99.33 and 98.46 wt.%, respectively), because they only contain the hydroxide group, necessary for saponification, as a low proportion impurity. However, when sodium or potassium hydroxides were utilised as catalysts, biodiesel yields decreased to 86.71 and 91.67 wt.%, respectively. This is due to the presence of the hydroxide group that originated soaps by triglyceride saponification. Owing to their polarity, the soaps dissolved into the glycerol phase during the separation stage after the reaction. In addition, the dissolved soaps increased the methyl ester solubility in the glycerol, an additional cause of yield loss.
Chung (2010) transesterified V. fordii and C. japonica seed oils with methanol using alkaline catalysts (KOH, NaOH, and CH3ONa) to produce biodiesel. The fatty acid methyl ester (FAME) contents in the biodiesel produced from the seed oils were above 96% on KOH catalyst in the reaction. The composition and physicochemical properties were investigated in the raw seed oils and the biodiesel products. It was acceptable for the limit of European biodiesel qualities for BD100. Other qualities such as cetane number, acid value, density, and kinematic viscosity, of the produced biodiesels also matched the biodiesel qualities. The optimum reaction conditions used were: 6:1 molar ratio of methanol to the seed oils, 1 wt% loading amount of catalyst, 65 °C reaction temperature, and reaction time of 3 hr. The biodiesel contents of the C. japonica and V. fordii seed oils under these reaction conditions were 97.7% and 96.1% on KOH catalyst.
Figure 5.11 shows the FAME contents on KOH catalyst with various reaction conditions. The basic reaction conditions were fixed as a 65 C reaction temperature, 1 wt% loading amount of catalyst to the seed oils, a 3 hr reaction time, and a 6:1 molar ratio of methanol to feedstock. The FAME contents of the C. japonica and V. fordii seed oils to the reaction conditions were 97.7% and 96.1% on KOH catalyst, respectively. The high content of FAME was obtained at 65 °C as shown in Figure 5.11 (a). The variation of the FAME contents with different loading amounts of KOH catalyst is shown in Figure 5.11 (b). The high content of FAME exhibited at 1 wt% KOH catalyst loading to the feedstock. While the loading amount of catalyst exceeded more than 1 wt%, the FAME contents decreased reversely.
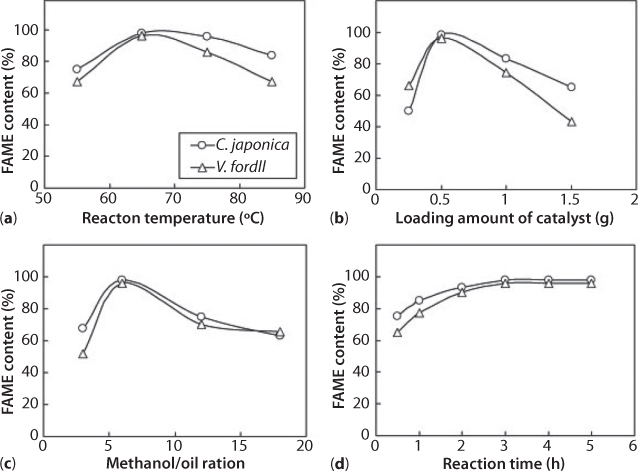
Figure 5.11 FAME content on KOH catalyst with various reaction conditions: (a) FAME content with variation of reaction temperature. (b) FAME content with variation of catalyst loading amount of catalyst. (c) FAME content with different molar ratios of methanol to feedstock. (d) FAME content with reaction time. Other basic reaction conditions were fixed as a 65 °C reaction temperature, a 0.5 g catalyst loading, a 3 hr reaction time, and a 6:1 molar ratio of methanol to feedstock (From Chung, 2010).
The effect of methanol addition was evaluated with FAME contents on KOH catalyst (see Figure 5.11 (c)). When methanol was added according to the stoichiometry of the reaction as 3:1 molar ratio of methanol to triglyceride, the FAME contents were below 75%. The FAME contents of the biodiesels increased significantly above 96% at 6:1 molar ratio of methanol to feedstock. The FAME contents with reaction time are represented in Figure 5.11 (d). The FAME contents reached at equilibrium after 3 hr. The FAME contents exceeded 75% even for 1 hr reaction in the reaction of C. japonica seed oil.
Refaat et al., (2008) reported the use of microwave irradiation in order to enhance the reaction rate. The optimum parametric conditions obtained from the conventional technique were applied using microwave irradiation in order to compare both systems. The results showed that application of radio frequency microwave energy offers a fast, easy route to this valuable biofuel with advantages of enhancing the reaction rate and improving the separation process. Using the microwave system, the vegetable oil was preheated to a desired temperature of 65 C. The mixture of alcohol and catalyst then charged into the flask through the condenser. The power output adjusted to 500 W and under reflux the mixture irradiated via different reaction times of 0.5, 1, 1.5, 2, 2.5, 3 and 6 min. Using the microwave irradiation technique, reaction time was reduced by 97% and the separation time reduced by 94%. The study also showed that there is an optimum reaction time for microwave-enhanced biodiesel production that should be respected. Exceeding the optimum reaction time will lead to deterioration of both biodiesel yield and purity. These results are depicted in Figure 5.12. At reaction times more than 2 min., drastic decreases in biodiesel yields were observed. The most accepted interpretation is that the exceeded time favors the equilibrium in the reverse direction. This is typical of catalytic reactions. In this particular application, attributing the decrease in yield after exceeding the optimum time to cracking followed by oxidizing of the formed fatty acid methyl esters to aldehydes, ketones and lower chained organic fractions could be excluded because the GC results do not show peaks of oxygenated compounds. Also, Saifuddin and Chua (2004) optimized transesterification of used frying oil to ethyl ester using microwave irradiation. They used a microwave oven equipped with non-contact infrared continuous feedback temperature system and magnetic stirrer to heat the oil and the alcohol at 60 C. Twenty five percent (25%) of an exit power of 750 W was used to irradiate the reaction mixture. Instead of studying the role of exposure to microwave, they studied the role of exit power and observed an optimum at 50% of exit power. They used different concentrations of sodium methoxide (0.3 wt% to 0.5 wt%) and achieved maximum conversion (87 wt%) at 0.5 wt%. During transesterification process, both sodium ethoxide and potassium hydroxide provided good conversions. However, due simplicity in products phase separation, sodium ethoxide was viewed as most promising catalyst for producing biodiesel. Besides, microwave-assisted transesterification process dramatically reduced the reaction time from 75 min to 4 min at 60 °C, thus saving great time. Additionally, during transesterification, irradiation times must be controlled and the levels of radiation power should not be too high, to avoid destruction of organic molecules. This particular combination of energy catalyst and mass catalyst has been in place without being recognized as such. For instance, the mechanism involved in pre-heating actually is akin to how microwave itself accelerates the reaction.
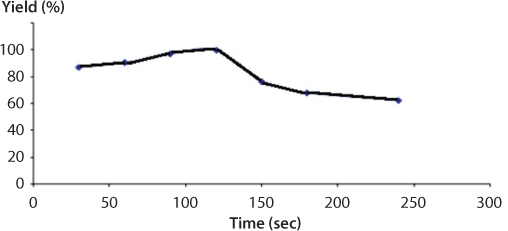
Figure 5.12 Effect of microwave irradiation exposure time on yield (Refaat et al., 2008).
These studies are interesting from a scientific perspective. In this case, an ‘energy’ catalyst is used in place of a ‘mass’ catalyst. The science of catalytic reactions is poorly handled due to the inability of New Science to include intangibles. The use of energy form as a catalyst adds to that difficulty. For instance, anytime the term ‘energy catalyst’ is used, the fact that energy cannot be isolated from mass (there is no energy without mass) is covered up. Along with it, disappears any possibility to connect the product from the source as if energy acted in isolation. Whenever an artificial energy source is used, this is a concern as eventually it leads to conflation of artificial (which is harmful) with natural (which is beneficial). New Science has no way of measuring the effect of such treatment on the pathway followed by the products and that’s why Khan and Islam (2012; 2016) mandated that ‘science of intangibles’ is the only way to determine sustainability of a process.
Moreover, to determine optimum operating parameters, such as catalyst concentration, Ferella et al., (2010) studied transesterification reaction of rapeseed oil for biodiesel production using response surface methodology (RSM). They employed a 500 ml jacketed stirred (at 600 rpm) reactor tank. At optimum conditions of temperature of 50 °C, KOH concentration of 0.6% (w/w), reaction time of 90 min, and 60 methanol to KOH ratio by weight, large amount of triglycerides, diglycerides and monoglycerides were converted into biodiesel. Furthermore, the final concentrations were 0.05% triglycerides, 0.09% diglycerides and 0.36% monoglycerides and the triglyceride conversion was 98–99%.
The most notable acids commonly employed in transesterification reaction include, among others; sulfuric acid, sulfonic acid, hydrochloric acid, organic sulfonic acid, ferric sulfate, etc. Among these acids, hydrochloric acid, sulfonic acid, and sulfuric acid are usually favored as catalysts for the production of biodiesel. Although the catalysts give high yield of biodiesel, the reaction rates are slow. The alcohol to oil molar ratio is the main factor influencing the reaction. Therefore, the addition of excess alcohol speeds up the reaction and favors the formation biodiesel products. The steps involve during acid-catalyzed transesterification are:
- initial protonation of the acid to give an oxonium ion;
- the oxonium ion and an alcohol undergo an exchange reaction to give the intermediate;
- loosening of a proton to produce an ester.
Reversibility of each of the above steps is possible but the equilibrium point of the reaction is displaced in the presence of excess large alcohol, by allowing esterification to advance to completion.
Additionally, Soriano Jr. et al., (2009) studied transesterification of canola oil to produce biodiesel via homogeneous Lewis acid (AlCl3 and ZnCl2) as catalyst. The reaction occurred in a round bottom flask submerged in an oil bath equipped with a reflux condenser, temperature controller and a magnetic stirrer. The authors reported use of variable parameters such as: reaction time (6, 18, 24 hr), methanol to oil molar ratio (6, 12, 24, 42 and 60), reaction temperature (75, 110 C), with tetrahydrofuran (THF) as co-solvent (1:1 methanol to THF by weight in runs with THF), and a catalyst (AlCl3 or ZnCl2). In all the runs, the catalyst amount was kept at 5% based on the weight of oil. The best conditions with AlCl3 were reported to be 24:1 molar ratio at 110 C and 18 hr reaction time with THF as co-solvent provided a conversion of 98%. AlCl3 was far more active compared to ZnCl2 due to its higher acidity. Regardless of molar ratio and reaction time, conversions using AlCl3 had increased with increase in reaction temperature. It was also a function of molar ration. On the other hand, ZnCl2 was only a function of reaction time, independent of molar ratio.
Cardoso et al., (2009) discussed the effects of Lewis acid on the transesterification process in producing biodiesel. The authors have introduced an inexpensive Lewis acid Tin (II) chloride dihydrate (SnCl2·2H2O), and evaluated its potential as catalyst on the ethanolysis of oleic acid of fats and vegetable oils. Tin chloride efficiently promoted the conversion of oleic acid into ethyl oleate in ethanol solution and in soybean oil samples, under mild reaction conditions. The SnCl2 catalyst was shown to be as active as the mineral acid H2SO4. Its use has relevant advantages in comparison to mineral acids catalysts, such as less corrosion of the reactors and as well as avoiding the unnecessary neutralization of products. The effect of the principal parameters of reaction on the yield and rate of ethyl oleate production has been investigated. Kinetic measurements revealed that the esterification of oleic acid catalyzed by SnCl2·2H2O is first-order in relation to both FFAs and catalyst concentration. Experimentally, it was verified that the energy of activation of the esterification reaction of oleic acid catalyzed by SnCl2 was very close those reported for H2SO4. The catalytic tests were carried out in triplicate with a molar ratio fatty acid: catalyst (100:1), reaction time of 2 hr. Using these conditions, the process provided more than 90% biodiesel yield with a high selectivity of more than 93%. The two acidic catalysts have different structures and acid character and certainly, different mechanisms of action. In spite of that, they displayed quite similar activities, as can be confirmed by the attainment of comparable ethyl oleate yields at a given reaction time, as shown in Figure 5.13. Note that reaction yields have increased steadily to a maximum value of 90 %, in approximately 120 min after setting up the reaction. The monitoring of reaction for periods higher than 120 min reveals that the yields of both remained invariable after this time.
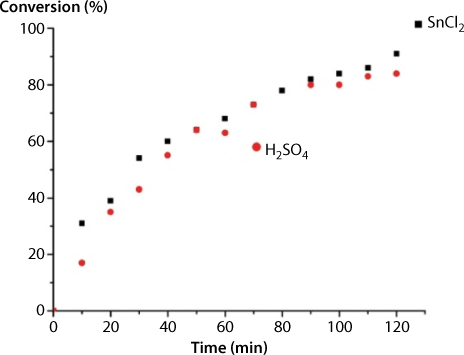
Figure 5.13 Ethanolysis of oleic catalyzed by Brønsted (H2SO4) and Lewis acids (SnCl2) (from Cardoso et al., 2009).
Table 5.4 presents reaction yield as function of the homogeneous catalyst weight. The data obtained shows that production of biodiesel via homogeneous catalyst could yield more than 99%.
Table 5.4 Reaction yield as function of the homogeneous catalyst weight (from cardoso et al., 2009).
| Feedstock | Catalyst | Conc. (wt/v/v) |
Reaction time (h) |
Reaction temp (°C) |
Yield/conv. (w/w%) |
Molar ratios |
| Waste tallow (chicken) | H2SO4 | 1.25 |
24 |
50 |
99.01 ± 0.71 |
1:30 |
| Palm fatty acid | H2SO4 | - |
2 |
70 |
99.6 |
7.2:1 |
| Sunflower oil | KOH | 0.55 |
- |
70 |
96 |
- |
| Jojoba oil-wax | Sodium methoxide | 1 |
4 |
60 |
55 |
7.5:1 |
| Brassica carinata | KOH | - |
- |
- |
98.27 |
- |
| Canola oil | KOH | 0.5 |
0.33 |
25 |
86.1 |
6:1 |
| Jatropha curcas | KOH | 1 |
30 |
92 |
- |
|
| Cottonseed oils | Sodium hydroxide | 0.5 |
1 |
55 |
77 |
- |
| Roselle oil | Potassium hydroxide | 1.5 |
1 |
60 |
99.4 |
8:1 |
| Rubber seed oil(Hevea brasiliensis) | NaOH | 1 |
1 |
60 |
84.46 |
6:1 |
| Mahua oil (Madhuca indica) | NaOH | 1 |
2 |
60 |
92 |
6:1 |
| Mahua oil | H2SO4/KOH | 6:1 |
||||
| Sunflower frying oil | KOH | 1 |
0.5 |
25 |
max |
6:1 |
| Tobacco seed oil | NaOH | 1.5 |
1.5 |
55 |
max |
3:1 |
| Rice bran oil | Sulfuric acid | 2 |
8 |
100 |
98 |
- |
| Used frying oil | KOH | 1 |
2 |
60 |
72.5 |
12:1 |
| Waste cooking oils | KOH | 0.75 |
0.33–2 |
30–50 |
88–90% |
7:1–8:1 |
| Jatropha oil | H4SO4/KOH | 0.25–1.5/0.5 |
2 |
60 |
90–95 |
6:1/9:1 |
| Karanja oil | KOH | 1 |
3 |
65 |
97–98 |
6:1 |
5.2.2.2 Effect of Heterogeneous Catalysts
Recent research on alcoholysis has focused on heterogeneous catalysts (Suppes et al., 2001; Ebiura et al., 2005; Corma et al., 1998; Xie et al., 2006; Suppes et al., 2004). The number of researches in the area of heterogeneous catalysts has increased recently. A great variety of catalysts in catalytic transesterification of vegetable oils have been used. These include zeolites, hydrotalcites, oxides, γ-alumina, etc.
Recently, several researches were conducted on heterogeneous catalysts with the aim of finding solutions to problems caused by using homogeneous catalysts in producing biodiesel. As a result, a good number of heterogeneous catalysts were explored and many of the catalysts have displayed very good catalytic performances (Wang and Yang, 2007).
Heterogeneous catalysts could improve the synthesis methods by eliminating the additional processing costs associated with homogeneous catalysts. However, heterogeneous catalysts have a different appeal: the fact that natural catalysts are the most heterogeneous ones. This aspect has not been considered in conventional analysis, although the key to developing sustainable technologies reside within the use of natural catalysts (Helwani et al., 2009). Helwani et al., (2009) reviewed biodiesel production by transesterification of triglycerides from a catalytic standpoint with the aim of using heterogeneous catalysts and to replace or complement the current homogeneous catalysts with the heterogeneous ones, to incorporate catalysts that are effective for a broader spectrum of reactants that can tolerate higher levels of impurities. Even though they are called impurities, if they are natural they in fact add synergy to the reaction, adding benefits that are not tractable with conventional analysis. They review biodiesel production using heterogeneous solid catalysts, such as metals, anchored metal complexes, solid bases and solid acids.
Some of these catalysts include, among others; oxides, hydrotalcides, zeolites, etc. Currently, majority of heterogeneous catalysts used in producing biodiesel are either oxides of alkali or oxides of alkaline earth metals supported over large surface area (Helwani et al., 2009).
Wang and Yang (2007) investigated the possibility of using nano-MgO to improve the transesterification reaction of soybean oil with supercritical/subcritical methanol. The variables affecting the yield of methyl ester during the transesterification reaction, such as the catalyst content, reaction temperature and the molar ratio of methanol to soybean oil were investigated and compared with those of non-catalyst. When nano-MgO was added from 0.5 wt% to 3 wt%, the transesterification rate increased evidently, while the catalyst content was further enhanced to 5 wt%, little increased in yield. It was observed that increasing the reaction temperature had a favorable influence on methyl ester yield. In addition, for molar ratios of methanol to soybean oil ranging from 6 to 36, the higher molar ratios of methanol to oil was charged, the faster transesterification rate was obtained. When the temperature was increased to 533 K, the transesterification reaction was essentially completed within 10 min with 3 wt% nano-MgO and the methanol/oil molar rate 36:1. Such high reaction rate with nano-MgO was mainly owing to the lower activation energy (75.94 kJ/mol) and the higher stirring.
Figure 5.14 shows the relationship between the reaction time and the catalyst content. It can be affirmed that nano-MgO can evidently accelerate the methyl ester conversion from soybean oil in 523 K and 24.0 MPa even if a little catalyst (0.5 wt%) was added. The transesterification rate was improved obviously as the content of nano-MgO increased from 0.5 to 3 wt%. However, when the catalyst content was further enhanced to 5 wt%, methyl ester yield increased a little. Such phenomena can also be confirmed in their velocity coefficients. The coefficients are almost identical when the nano-MgO content increased from 3 to 5 wt%. Furthermore, the maximal yield fell down slightly if an excess of nano-MgO was put in, it may be because that nano-MgO not only exhibits the higher catalytic activity for the transesterification of triolein with methanol, but also is effective for the glycerolysis of triolein with glycerol. Therefore, 3% nano-MgO is more suitable.

Figure 5.14 Effect of nano-MgO content on methyl ester yield of the transesterification of soybean oil. Reaction temperature 523 K; reaction pressure 24.0 MPa; soybean oil 100 ml; methanol 150 ml; molar ratio of methyl to soybean oil: 36:1; stirring 1000 rpm. (From Wang and Yang, 2007).
Table 5.5 lists different heterogenous catalysts for transesterification of vegetable oils reported in the literature. Most of these catalysts are alkali or alkaline oxides supported over large surface area supports. Similar to their homogeneous counterparts, solid basic catalysts are more active than solid acid catalysts. CaO, used as a solid basic catalyst, possesses many advantages such as long catalyst lifetimes, higher activity and requirement of only mild reaction conditions. The reaction rate, however, was slow in producing biodiesel.
Table 5.5 Different heterogeneous catalysts used for transesterification of vegetable oils.
| Vegetable oil | Catalyst | Ratio MeOH/oil |
Reaction time, h |
Temperature, C |
Conversion % |
| Blended vegetable oil | Mesoporous silica loaded with MgO | 8 |
5 |
220 |
96 |
| Soybean oil | WO3/ZrO2, zirconia–alumina and sulfated tin oxide | 40 |
20 |
200–300 |
90 |
| Soybean oil | Calcined LDH (Li–Al) | 15 |
1–6 |
65 |
71.9 |
| Palm oil | Mg–Al–CO3 (hydrotalcite) | 30 |
6 |
100 |
86.6 |
| Soybean oil | La/zeolite beta | 14.5 |
4 |
160 |
48.9 |
| Soybean oil | MgO MgAl2O4 | 3 |
10 |
65 |
57 |
| Sunflower oil | NaOH/alumina | 6–48 |
1 |
50 |
99 |
| Soybean oil | MgO, ZnO, Al2O3 | 55 |
7 |
70,100,130 |
82 |
| Soybean oil | Cu and Co | 5 |
3 |
70 |
– |
| Sunflower oil | CaO/SBA-14 | 12 |
5 |
160 |
95 |
| Jathropa Curcas oil | CaO | 9 |
2.5 |
70 |
93 |
| VO | Cs-heteropoly acid, SO42–/ZrO2, SO42–/Al2O3, SO42–/SiO2,WO3/ZrO2 | 19.4 |
1 |
75 |
70 |
| Rape oil | Mg–Al HT | 4 |
65 |
90.5 |
|
| Soybean oil | CaO, SrO | 0.5–3 |
65 |
95 |
|
| Soybean oil | ETS-10 | 6 |
24 |
120 |
94.6 |
| Cotton seed oil | Mg–Al–CO3 HT | 6 |
12 |
180–220 |
87 |
Lee and Saka (2010) reported simultaneous esterification and transesterification of waste cooking oil using solid catalyst ZnO–La2O3, which combines acid (ZnO) and base sites (La2O3). Although the process provided high conversion of 96% in 3 hr, but like zirconia, lanthanum is a rare and expensive metal, therefore cost of the catalyst would prohibit it high use in the production of biodiesel. More importantly, these metals are far more toxic to the environment than others in use.
Solid alkaline catalysts such as CaO provide many advantages for instance higher activity, long catalyst life times, and could run under only moderate reaction conditions (Kouzu et al., 2008). Kouzu et al., (2008) carried out transesterification of edible soybean oil with refluxing methanol in the presence of calcium oxide (CaO) – hydroxide (Ca(OH)2), or – carbonate (CaCO3). At 1 hr of reaction time, yield of FAME was 93% for CaO, 12% for Ca(OH)2, and 0% for CaCO3. Under the same reacting condition, sodium hydroxide with the homogeneous catalysis brought about the complete conversion into FAME. Also, CaO was used for the further tests transesterifying waste cooking oil with acid value of 5.1 mg-KOH/g. The yield of FAME was above 99% at 2 hr of reaction time, but a portion of catalyst changed into calcium soap by reacting with free fatty acids included in the oil at initial stage of the transesterification. Owing to the neutralizing reaction of the catalyst, concentration of calcium in FAME increased from 187 ppm to 3065 ppm. By processing waste cooking oil at reflux of methanol in the presence of cation-exchange resin, only the free fatty acids could be converted into FAME. The transesterification of the processed waste cooking oil with acid value of 0.3 mg-KOH/g resulted in the production of FAME including calcium of 565 ppm.
Also, Liu et al., (2008) have produced biodiesel fuel from soybean oil using CaO as a solid base catalyst. The authors reported that use of CaO as a catalyst could provide a numbers of advantages such as: high activity, lengthen catalyst life and moderate condition of reaction. In this study, transesterification of soybean oil to biodiesel using CaO as a solid base catalyst was studied. The reaction mechanism was proposed and the separate effects of the molar ratio of methanol to oil, reaction temperature, mass ratio of catalyst to oil and water content were investigated. The experimental results showed that a 12:1 molar ratio of methanol to oil, addition of 8% CaO catalyst, 65 C reaction temperature and 2.03% water content in methanol gave the best results, and the biodiesel yield exceeded 95% at 3 hr. The catalyst lifetime was longer than that of calcined K2CO3/γ-Al2O3 and KF/γ-Al2O3 catalysts. CaO maintained sustained activity even after being repeatedly used for 20 cycles and the biodiesel yield at 1.5 hr was not affected much in the repeated experiments. Figure 5.15 shows the effects of the mass ratio of catalyst to oil. The results indicate that the biodiesel yield was significantly improved with the increase of CaO. The biodiesel yield reached 90% after 3 hr when the CaO/oil mass ratio was 8%. However, only 55% yield was obtained at a 2% CaO/oil mass ratio. It was also recognized that the catalytic activity was influenced by alkalinity, but the effect of CaO amount on biodiesel yield was slight when the mass ratio of CaO to oil was above 8%. In these conditions, the intensification of mass transfer becomes more important than increasing the amount of catalyst. Even though, this series of tests do not clearly show the existence of an optimum concentration, the function of yield vs time reaches saturation after 8% concentration.

Figure 5.15 Effect of mass ratio of CaO to oil on biodiesel yield. Methanol/oil molar ratio: 6:1; reaction temperature: 65 °C; water content: 2.03% (from Liu et al., 2008).
Lim et al., (2009) noted that transesterification reaction involving CaO needs longer reaction time. But the benefits gained from the process such as elimination of neutralization process, less waste generation and prospect of catalyst reusability compensates the delay. They conducted parametric study found the optimal conditions to be: methanol/oil mass ratio 0.5:1; catalyst amount 6 wt.%, and reaction temperature of 65 C. The highest purity of 98.6 ± 0.8% was achieved within 2.5 hr. Biodiesel yield under the solid catalyst was quantified as 90.4% as compared to 45.5% and 61.0%, respectively for classical NaOH, and KOH homogeneous catalysts.
Zabeti et al., (2009) talked about environmental impacts due to its low solubility in methanol and can be synthesized from cheap sources like limestone and calcium hydroxide. They discussed one of the ways to minimize the mass transfer limitation for heterogeneous catalysts in liquid phase reactions by using catalyst supports. Supports can provide higher surface area through the existence of pores where metal particles can be anchored. Supports such as: alumina, silica, zinc oxide and zirconium oxide have been used in biodiesel production. They identified the following features of the naturally occurring chemicals.
Alumina: Aluminum oxide exists in forms of porous γ-alumina and ή-alumina and nonporous crystalline α-alumina has been widely used as a support in catalysis processes, such as polymerization, reforming, steam reforming, dehydration and hydrogenation owing to its extremely thermal and mechanical stability, high specific surface area, large pore size and pore volume and. This also includes in the synthesis of biodiesel. Catalytic activity of titanium oxide–zinc oxide supported by alumina (ATZ) and titanium oxide–bismuth oxide supported by alumina (ATB) for transesterification of colza oil with methanol was studied (Belfort et al., 2006). Al2O3/ZrO2/WO3 solid acid catalyst was prepared by co-precipitating method and was investigated in the methanolysis of soybean oil (Furuta et al., 2006). The catalyst was compatible for both esterification and transesterification reaction and under reaction conditions of temperature of 250 C and alcohol/oil molar ratio of 40:1 methyl ester yields of 90% were obtained. This catalyst was also compared to Al2O3/ZrO2; however, the yields were 80%. Xie et al., (2006) evaluated the catalytic efficiency of potassium supported by alumina as a solid base catalyst in the transesterification of soybean oil to biodiesel. The catalyst was prepared by an impregnation method of aqueous solution of potassium nitrate alumina. The results demonstrated that the catalyst calcined at 500 C and loaded by 35 wt.% of KNO3 was the most active. From Hammett indicator method, the basicity was 6.75 mmol/g and a correlation between catalyst performance and basic sites on the surface of catalyst, probably K2O and Al–O–K, was detected. The highest conversion of oil reached 87%, after the reaction time of 8 hr with molar ratio of methanol to soybean oil of 15:1 and 6.5 wt.% of catalyst.
Zinc oxide: Yang and Xie (2007) compared the catalyst performance of alkali earth metals loaded on different catalyst supports for soybean oil conversion to biodiesel and discovered a correlation between loading amount of catalyst precursor on support and the conversion of oil. They also found that the catalyst performance depends upon concentration of basic sites on the surface of the catalyst. Different catalysts were prepared by impregnation method of water solution of an alkali earth metal nitrate with specified concentration and calcined at 600 °C for 5 hr and among them ZnO/Sr (NO3)2 showed the best catalytic activity. In this case the most active catalyst was obtained by loading 2.5 mmol of strontium nitrate on zinc oxide which resulted in basicity of 10.8 mmol/g. After 5 hr of reaction time, at 65 °C, with 12:1 molar ratio of alcohol/oil and 5 wt.% of catalyst content, the maximum conversion achieved was 93.7%. Xie and Huang (2006) also applied potassium supported by zinc oxide as a solid base catalyst and the catalyst was prepared by an impregnation method of aqueous solution of potassium fluoride followed by calcination at 600 °C for 5 hr. Based on Hammett titration method the concentration of basic sites reached 1.47 mmol/g when 15 wt.% of KF was loaded on ZnO. The reaction was performed with alcohol/oil molar ratio of 10:1 and catalyst content of 3 wt.% and after 9 hr of the reaction time 87% of soybean oil was converted. The result is quite similar to that of Al2O3/KNO3 which was previously described (Xie et al., 2006). A mixture of Zn/I2 was used as a heterogeneous catalyst for transesterification of soybean oil with methanol. The metal was treated with distilled water and diluted HCL, and iodine was treated by sublimation. A mixture of 5 wt.% Zn and 2.5 wt.% I2 was applied in the reaction. After 26 hr, by using alcohol/oil molar ratio of 42:1, the conversion of oil reached 86%. The influence of co-solvent on the reaction rate was also investigated by introducing co-solvents such as THF and DMSO into the reaction and a 10% increase in conversion of soybean oil was observed after adding a certain amount of DMSO into the reaction mixture.
A mixture of Zn/I2 was used as a heterogeneous catalyst for transesterification of soybean oil with methanol (Li and Xie, 2006). The metal was treated with distilled water and diluted HCL, and iodine was treated by sublimation. A mixture of 5 wt.% Zn and 2.5 wt.% I2 was applied in the reaction. After 26 hr, by using alcohol/oil molar ratio of 42:1, the conversion of oil reached 86%. The influence of co-solvent on the reaction rate was also investigated by introducing co-solvents such as THF and DMSO into the reaction and a 10% increase in conversion of soybean oil was observed after adding a certain amount of DMSO into the reaction mixture.
Silicate: Catalyst properties of CaO supported on different types of mesoporous silica were studied and results from XRD characterization showed that after impregnation of CaO, the hexagonal structure of SBA-15 remained stable while this structure of MCM-41 and fumed silica was collapsed indicated that only SBA-15 can stabilize calcium oxide species on its surface (Albuquerque et al., 2008). The optimum catalyst was obtained by loading 15 wt.% of calcium oxide on SBA-15 followed by calcination at 900 °C and using this catalyst the maximum conversion of sunflower oil achieved 95% within 5 hr. Transesterification reaction was carried out at 60 C, with 1 wt.% catalyst and methanol to sunflower oil molar ratio of 12. Lin and Radu (2006) patented the application of sulfonic acid supported by mesoporous silica as a strong solid acid for transesterification reaction. The method of catalyst synthesis was relatively complicated, whereby Tetraethoxysilane (TEOS) was used as a precursor of silica. Mesoporous silica was synthesized by cetthyldimethylammonium bromide functionalized group denoted as CDAB, while those prepared by using tri-block Pluronic L64 and Pluronic P123 copolymers denoted as SBA-15.
Zirconium oxide: Zirconia-supported heteropoly and isopoly tungsten acids were applied in biodiesel synthesis from sunflower oil and the result indicated that isopoly tungsten was more active than heteropoly tungsten since it possesses more Bronsted acid sites (Sunita et al., 2007). Oil conversion of 97% was reached after 5 hr of reaction time under reaction conditions of temperature of 200 C with catalyst content of 15 wt.% and alcohol/oil molar ratio 15:1. The catalyst activity of some metal oxides and catalyst supports for the biodiesel production is summarized in Table 5.6. From this table it can be concluded that, generally solid catalysts need a longer reaction time than homogeneous catalysts. Solid acid catalysts either supported or unsupported require higher temperature to give more methyl esters yields, even though solid acid catalysts are active for both transesterification and esterification reactions are able to convert oils with high amount of FFA. All alkali earth metal oxides except MgO produced high concentration of methyl esters at lower temperature of 65 C at a moderate reaction time. Furthermore, from the table it can be observed that alumina was more preferred as a support than other supports for transesterification reaction.
Table 5.6 Summarization of the activity of metal oxides and supported catalysts as heterogeneous catalysts for biodiesel production (from sunita et al., 2007).
| Catalyst | Catalyst characterizations | Catalyst preparation | Feedstock | Operation conditions | Result conversion or yield |
| Nano MgO solid base | Particle size = 60 nm | Not reported. | Soybean oil | P=24 MPa, T = 250 °C, t = 10 min, alcohol/oil = 36:1, catalyst content = 3% | 99% |
| CaO solid base | SSAa =32 m2/g, MPSb = 25–30 nm | CaO powder was calcined at 1000 C. | Sunflower oil | T = 60 °C, t = 100 min, alcohol/oil = 13:1, catalyst content = 3% | 94% |
| CaO solid base | SSA = 56 m2/g | Not reported. | Soybean oil | T = 65 °C, t = 3 hr, alcohol/oil = 12:1, catalyst content = 8% | 95% |
| Ca(C2H3O2)2/SBA-15 solid base T = 60 °C, t = 5 hr, | SSA = 7.4 m2/g, pore volume = 0.019 cm3/g | The catalyst was prepared by an impregnation method of aqueous solution of calcium acetate on the support followed by calcination at 900 C for 4 hr. | Sunflower oil | methanol/oil = 12:1, catalyst content = 1% | 95% |
| Ca(OCH3)two solid base | SSA = 19 m2/g, pore size = 40 nm | Calcium methoxide was synthesized by a direct reaction of calcium and methanol at 65 C for 4 hr. | Soybean oil | T = 65 °C, t = 2 hr, alcohol/oil = 1:1, catalyst content = 2% | 98% |
| SrO solid base | SSA = 1.05 m2/g | SrO was prepared from calcinationof strontium carbonate in amuffle furnace at 1200 C for 5 hr. | Soybean oil | T = 65 °C, t = 30 min, alcohol/oil = 12:1, catalyst content = 3% | 95% |
| ZnO/Sr(NO3)two solidbase | Basicity = 10.8 mmol/g | The aqueous solution of Sr (NO3) two was loaded on ZnO by impregnation method and calcined at 600 C for 5 hr. | Soybean oil | T = 65 °C, t = 5 hr, methanol/oil = 12:1, catalyst content = 5% | 93% |
| ZnO/Ba solid base | Basicity = 14.54 mmol/g. | The catalyst was prepared by an impregnation method using barium nitrate as precursor on ZnO. Dried overnight and calcined at 600 C in air for 5 hr. | Soybean oil | T = 65 °C, t = 1 hr, methanol/oil = 12:1, catalyst content = 6% | 95% |
| ZnO/KF solid base | Basicity = 1.62 mmol/g | The supported catalyst was prepared by impregnation method with an aqueous solution of KF. Afterward was dried at 393 K and calcined at 600 C in air for 5 hr. | Soybean oil | T = 65 °C, t = 9 hr, methanol/oil = 10:1, catalyst content = 3% | 87% |
| Al2O3/KI solid base | Basicit y = 1.57 mmol/g | The catalyst was prepared by an impregnation method from aqueous solution of potassium iodide, dried at 120 °C and activated at 500 C for 3 hr. | Soybean oil | Methanol/oil = 15:1, catalyst content = 2.5%, t = 8 hr Methyl ester | yields = 96% |
| Al2O3/KNO3 solid base | Basicity = 6.67 mmol/g | KNO3 was loaded on alumina by an impregnation method from aqueous solution, dried at 393 K for 16 hr and finally calcined at 500 C for 5 hr. | Soybean oil | Methanol/oil = 15:1, catalyst content = 6.5%, t = 7 hr Methyl ester | 87%. |
| Al2O3/Na/NaOH solid base | SSA = 83.2 m2/g | The catalyst was prepared by mixing of γAl2O3, NaOH and metal sodium in a stainless steel reactor at 320 C. | Soybean oil | T = 60 °C, t = 2 hr, methanol/oil = 9:1, catalyst content = 1 g Methyl ester | 83% |
| VOPO4·2H2O solid acid T | SSA = 2–4 m2/g | Vanadyl phosphate was obtained from the suspension of V2O5 in diluted phosphoric acid. Then activated at 500 C. | Soybean oil | = 150 °C, t = 1 hr, alcohol/oil = 1:1, catalyst content = 2% Methyl ester | 80% |
| ZnO solid acid | Not reported | Pure metal oxide was used. 1-palm kernel oil 2-. | coconut oil | T = 300 °C, t = 1 hr, alcohol/oil = 6:1, catalyst content = 3% 1-methyl ester yields = 86.1% 2- methyl ester | 77.5% |
| ZnO/I2 solid acid | Not reported | The metal was prepared just by some treatment with distilled water and dilute HCl. Iodine was treated by sublimation. | Soybean oil | T = 65 °C, t = 26 hr, methanol/oil = 42:1, catalyst content = 5% Zn and 2.5% I2 | 96% |
| ZrO2/WO3 2– solid acid | Not reported | The isopoly tungstated zirconia was prepared by suspending a known amount of zirconium oxyhydroxide powder in an aqueous solution of ammonium metatungstate, finally calcined at 750 C. | Sunflower oil | T = 200 °C, t = 5 hr, alcohol/oil = 20:1, catalyst content = 15% | 97% |
| ZrO2/SO4 2– solid acid | Not reported | Zirconia powder were immersed in sulfuric acid solution, filtered, dried and calcined at 500 °C for 2 hr. 1-palm kernel oil 2-. | coconut oil | T = 200 °C, t = 1 hr, alcohol/oil = 6:1, catalyst content = 1% 1-methyl ester | 1-methyl ester yields = 90.3% 2- methyl ester yields = 86.3% |
| TiO2/SO4 2- solid acid | SSA = 99.5 m2/g | TiO2·nH2O was prepared from precipitation of TiCl4 using aqueous ammonia. Then immersed to sulfuric acid and finally calcined at 550 °C for 3 hr to give TiO2–SO42−. | Cottonseed oil T = 230 °C, t = 8 hr, alcohol/oil = 12:1, catalyst content = 2% Methyl ester | 90%. | |
| Al2O3/PO4 3– solid acid. | Not reported | Aluminum nitrate hydrated with nine moles of water dissolved in water and 85% orthophosphoric acid was added. The PH was controlled at seven by aqueous solution of ammonia. The final precipitation was filtered out, washed, and dried at 383 K for 12 hr. finally calcined at 400 °C for 3 hr. | Palm kernel oil | T = 200 °C, t = 5 hr, methanol/oil = 5:1, catalyst content = 10 g | Methyl ester yields = 69%. |
| Al2O3/TiO2/ZnO solid acid | SSA = 62 m2/g | The catalyst was prepared by comixing of boehmite, titanium gel and zinc oxide in the presence of nitric acid and water. Calcined at 600 C for 3 hr. | Colza oil | T = 200 °C, t = 8 hr, alcohol/oil = 1:1, atalyst content = 6% | Methyl ester yields = 94% |
| Al2O3/ZrO2/WO3 solid acid | Not reported | The catalyst was prepared from mixture of hydrated zirconia, hydrated alumina and ammonium metatungstate and deionized water. Calcined at 900 C for 1 hr. | Soybean oil | T = 250 °C, t = 20 hr, methanol/oil = 40:1, catalyst content = 4 g | Methyl ester yields = 90% |
| SBA-15-SO3H-P123 solphonic acid supported on mesoporous silica.Solid acid | SSA = 735 m2/g, pore volume = 0.67 cm3/g | Tetraethoxysilane (TEOS) was used as silica source. 3-(mercaptopropyl)-trimethoxysilane was used as mesoporous silica modifier. Pluronic P123 (tri-block copolymer) used as surfactant. A cocondensation method was applied. | Soybean oil | T = 75 °C, t = 20 hr, methanol/oil = 20:1, catalyst content = 10% | Methyl ester yields = 85% |
Helwani et al., (2009) reviewed various technological methods to produce biodiesel being used in industries and academia. Their focus was catalytic transesterification, the most common method in the production of biofuel. They reviewed both homogeneous and heterogeneous types of catalysts used in batch and continuous processes. Although batch production of biodiesel is favored over continuous process in many laboratory and larger scale efforts, the latter is expected to gain wider acceptance in the near future, considering its added advantages associated with higher production capacity and lower operating costs to ensure long term supply of biodiesel. Table 5.7 lists different heterogenous catalysts for transesterification of vegetable oils reported in the literature, as collected by Helwani et al., (2009). Most of these catalysts are alkali or alkaline oxides supported over large surface area supports. Similar to their homogeneous counterparts, solid basic catalysts are more active than solid acid catalysts. CaO, used as a solid basic catalyst, possesses many advantages such as long catalyst lifetimes, higher activity and requirement of only mild reaction conditions. The reaction rate, however, was slow in producing biodiesel (Liu et al., 2009).
Table 5.7 Different heterogeneous catalysts used for transesterification of vegetable oils (from helwani et al., 2009).
| Vegetable oil | Catalysts | Ratio MeOH/Oil |
Reaction time, h |
Temperature, °C |
Conversion, % |
| Blended vegetable oil | Mesoporous silica loaded with MgO | 8 |
5 |
220 |
96 |
| Soybean oil | WO3/ZrO2, zirconia–alumina and sulfated tin oxide | 40 |
20 |
200–300 |
90 |
| Soybean oil | Calcined LDH (Li–Al) | 15 |
1–6 |
65 |
71.9 |
| Palm oil | Mg–Al–CO3 (hydrotalcite) | 30 |
6 |
100 |
86.6 |
| Soybean oil | La/zeolite beta | 14.5 |
4 |
160 |
48.9 |
| Soybean oil | MgO MgALO | 3 |
10 |
65 |
57 |
| Sunflower oil | NaOH/alumina | 6–48 |
1 |
50 |
99 |
| Soybean oil | MgO, ZnO, Al2O3 | 55 |
7 |
70, 100, 130 |
82 |
| Soybean oil | Cu and Co | 5 |
3 |
70 |
|
| Sunflower oil | CaO/SBA-14 | 12 |
5 |
160 |
95 |
| Jatropha Curcas oil | CaO | 9 |
2.5 |
70 |
93 |
| VO | Cs-heteropoly acid, SO42−/ZrO2, SO42−/Al2O3, SO42−/SiO2, WO3/ZrO2 | 19.4 |
1 |
75 |
70 |
| Rape oil | Mg–Al HT | 6 |
4 |
65 |
90.5 |
| Soybean oil | CaO, SrO | 12 |
0.5–3 |
65 |
95 |
| Soybean oil | ETS-10 | 6 |
24 |
120 |
94.6 |
| Cotton Seed oil | Mg–Al–CO3 HT | 6 |
12 |
180–210 |
87 |
5.2.2.3 Future Trends and the Impact on the Environment
Biodiesel has been popularized due to its reputation in emitting less carbon monoxide and other pollutants to the atmosphere. The main process of biodiesel manufacturing is transesterification in the presence of triglycerides and alcohol. If these chemicals are not produced with sustainable technologies, the product would not become sustainable. More importantly, it is the choice of catalyst that would dictate the fate of the product. Both catalyst and raw material selection play a significant role in the cost of biodiesel production. Because it is the economics that governs the selection, it is important to note the future developments in light of economic appeal. The heterogeneous catalyst offers a wide option for the catalytic selection because of its high selectivity and reusability characteristics. If they are selected from natural materials and used before value addition through refining is made, the price can come down significantly (Chhetri and Islam, 2009). In reducing costs, waste vegetable oil has become popular (Chhetri et al., 2008). Many studies have investigated for the use of waste cooking oil, greases, animal fats, tallow and spent bleaching for the biodiesel production. Next, The barrier in commercialization of biodiesel production is the cost of feedstock. The use non-edible oils has become popular due to several advantages such that, they are inexpensive and does not possess any threat to the environment, and most importantly do not compete with the food supply.
Table 5.8 lists the major feedstocks for biodiesel. In this table, the sustainability requirements are pointed out, along with shortcomings. As can be seen from this table, very few sources are sustainable and inexpensive.
Table 5.8 Biodiesel feedstocks and economic feasibility.
| Type of oil | Feedstock | Oil content % (w/w) |
Requirement for sustainability/shortcoming |
| Edible | Soybean | 15–20 |
Orgnic source/expensive |
| Rapeseed | 38–46 |
Organic source/expensive | |
| Sunflower | 25–35 |
Organic source/expensive | |
| Peanut oil | 45–55 |
Organic s source/expensive | |
| Coconut | 63–65 |
Organic s source/expensive | |
| Palm | 30–60 |
Organic s source/expensive | |
| Non-Edible | Jatrropha seed | 35–40 |
Naturally grown/not common |
| Pongamia pinnata | 27–39 |
Naturally grown | |
| Neem oil | 20–30 |
Expensive | |
| Castor | 53 |
Expensive | |
| Other sources | Rubber seed | 40–50 |
Expensive |
| Sea mango | 54 |
inexpensive | |
| Cotton seed | 18–25 |
Organic source/Expensive, | |
| Microalagae | 30–70 |
Organic source |
The role of microwaves was also presented. Recently, ultrasonic treatment has been popularized as a concept. Ultrasound during transesterification creates cavitation between oil and alcohol phases and makes the mixing is efficient. This process uses less energy consumption when compared to other conventional mechanical stirring process. It is reported that the cavitation of bubbles due to ultrasonication enhances the mass transfer rates with the record of high yield (Fan et al., 2010). The ultrasonic wave provides high temperature and pressure such that it increases the catalytic surface area with low frequency modes (Yu et al., 2010). Solid catalyst was reported to be efficient for ultrasonic assisted transesterification, as the wave energy breaks the catalyst into fine small particles (Georgogianni et al., 2008). The literature supports the use of ultrasound assisted transesterification using sunflower as major feedstock. The 95% biodiesel yield was reported at 60 °C in 20 min. The frequency maintained throughout the process was 24 kHz with molar ratio of 7:1 (Georgogianni et al., 2008). The ultrasound assisted transesterification was reported to be highly suitable as stability of the catalyst were expected to have a longer lifetime.
The presence of catalyst increases the rate of the reaction consequently yield of the product also increases. The catalysts used for the transesterification reaction are grouped into three categories as homogeneous, heterogeneous and enzymes as catalyst. Transesterification can also be carried by non-catalytic mechanism under super critical conditions. The overview of various catalysts for the production of biodiesel have been discussed the following chapters in detail and summarized their merits and demerits as listed in Table 5.9. In this table, the last columns refers to sustainability. The sustainability criterion of Khan and Islam (2007) was applied1. Only natural (but not cooking oil) sources are deemed potentially sustainable. It is assumed that these sources are organic and no chemical fertilizer or pesticide has been used. Also, the catalyst used must be organically produced. For the waste cooking oil, the only way it can be sustainable if it is organic – an unlikely occurrence. Soybean even if organic isn’t considered to be sustainable because it is a food and should not used as a fuel for energy production.
Table 5.9 List of homogeneous catalyst used for transesterification reaction.
| Catalyst | Source | Yield % (wt/wt) |
Comment |
| NaOH | Waste cooking oil | 86 |
Unsustainable |
| CH3ONa | Soybean | 94 |
Unsustainable |
| KOH | Pongamia pinnata | 92 |
Likely sustainable |
| H2SO4 | Jatropha | 99 |
Likely sustainable |
Acid catalyzed transesterification reaction requires high of amounts of alcohol for high yield of esters. The advantage of acid catalyst is that they are performed at low temperature and pressure. Bronsted acids such sulfuric and sulfonic acids are used for transesterification reaction yielding high biodiesel. On protonation of ion the methyl/ethyl ester are obtained with the displacement of alcohol. The use of sulfuric acid for the completion of a transesterification reaction was achieved in single step acid catalysis reaction with high FFA content (Palligarnai and Briggs, 2008). The effect of canola oil conversion to biodiesel under various operating conditions was studied. The AlCl3 and ZnCl2 were used as catalyst and compared. The catalytic activity of AlCl3 is higher than that of ZnCl2 (Soriano et al., 2009). Water content seems to great challenge for the acid catalyzed reaction as the presence of water content deactivates the catalyst. This issue arises due to polar carboxylic group present in the feedstock. The other noted disadvantage with acid catalysis is that they lead to corrosion and decreasing the yield of the product. Homogeneous catalysts frequently used for the production of biodiesel are NaOH, KOH, H2SO4 and CH3Na. Table 5.10 shows optimal conditions for operations of various catalysts. Note that Khan and Islam (2007) criterion is used to determine sustainability. Also, see the logic used in describing Table 5.9.
Table 5.10 List of heterogeneous catalyst commonly used for biodiesel production.
| Catalyst | Source | Yield % (wt/wt) |
Comment |
| KI/Mg-Al-mixed metal oxides | Soybean | >90 |
Sustainable |
| CaO/Al2O3 | Palm oil | 98.6 |
Sustainable |
| CaO | Sunflower oil | 80 |
Likely sustainable |
| Mg-Al hydrotalcite | Jatropha | 95.2 |
Likely sustainable |
| K2CO3 supported MgO | Soybean | 99.5 |
Likely sustainable |
| Mg/Zr | Sunflower | 98 |
Sustainable |
| Fe-Zn Double metal cyanide (DMC) complex | Sunflower | 98.3 |
Unsustainable |
| Na/BaO | Canola oil | 97.5 |
Unsustainable |
| SO42-/TiO2 | Jatropha | 97 |
Unsustainable |
| WO3/ZrO2 | Sunflower oil | 97 |
Unsustainable |
| ZS/Si | Waste cooking oil | 98 |
Unsustainable |
| Vanadium | Soybean | 80 |
Unsustainable |
| Al2O3/ZrO2/WO3 | Soybean | 90 |
Unsustainable |
The most notable progress in catalyst technology has been in using nanosized catalyst because of high stability over the repeated use. Nanocatalyst solves various bottleneck problems associated in the production of biodiesel (Baskar and Aiswarya, 2016). Nanocatalyst plays a crucial role in the field of energy and environment. Increased stability, activity and reusability are important characteristics of nanocatalyst. Nanocatalyst has high selectivity due to nano-dimensional pores on the surface (Baskar and Aiswarya, 2015). The nanoparticle tends to reduce the diffusion limitations and provides an efficient surface to volume ratio for enzyme loading. Nanocomposites has gained attention as they possess a large surface area with enhanced interaction between the reactant and catalyst. Sodium titanate nanotubes was used as catalyst for the production of biodiesel. The surface area of the catalyst was found to have 200 m2/g with a pore volume of 0.61 cm3/g. The yield was around 98% with 1% catalyst loading and 20:1 of methanol to oil ratio (Hernandez-Hipolito et al., 2014). Shahraki et al., (2015). Reported 95% yield when they used nono-solid based catalyst, KF/γ-Al2O3 for production of biodiesel. The reaction was performed in the sonication mode of 45 W. This is a significant improvement, which can be explained by the fact that sonification increases the surface area of the reactants thus increasing the reaction rate. Besides, surface modification of the nanoparticles through immobilization provides bifunctional characteristics for the production of biodiesel. The reaction was found to be efficient because of the stability of the nanobiocatalyst (Macario et al., 2013). Lipase-coated magnetic nanoparticle was reported for the production of FAME from soybean and olive oil. The enzyme from species Candida rugosa on magnetic nanoparticle was reported with maximum yield of 94% (Xie et al., 2009). Immobilization of enzyme over the support is not sufficient for the high yield of methyl esters. Selection of a reactor is also important in applying to industrial level. Scale-up of the transesterification reaction was achieved by using the packed bed reactors for the efficient synthesis of biodiesel using nanobiocomposite (Wang et al., 2011). Magnetic nanocarriers were found to be efficient. But the availability of enzyme and binding capacity in terms of stabilization creates termination at industrial scale (Ngo et al., 2013). Supercritical technology/Non-catalytic transesterification reaction Supercritical processes do not require a catalyst for the reaction to initiate. Supercritical method has a greater advantage than transesterification reaction using the catalysts. The final purification of the product is not required because the supercritical process does not involve the use of catalyst. This method can be used for the feedstock which has high-water content, as the presence of water does not influence the reactions at supercritical condition. The reaction time for the supercritical process is 2–4 min (Saka and Kusdiana, 2001). Production of biodiesel from palm oil under supercritical method along with monitoring of different variables was reported. The major drawback in using supercritical process for commercial biodiesel production is the high operating temperature and pressure with a high ratio of oil to methanol (Tan et al., 2010).
The most preferred technique for the quantification of the components in biodiesel is GC–MS due to its increased accuracy. HPLC has an advantage that it can be used for different feedstocks. Spectroscopic techniques used for characterization are NMR and IR. NMR is most commonly applied for the determination of blend level. Particularly 1 H-NMR was reported to monitor the yield of transesterification where the conversion is calculated from the peaks corresponding to the different ppm range. The other analytical method is IR spectroscopic techniques, which gives details about the estimation of both FAME and triglycerides simultaneously. The quality of the biodiesel is assessed from the physio-chemical data analysis, such as kinematic viscosity, density, cloud point and pour point in accordance with ASTM.
5.2.3 Greening of the Biodiesel Process
The connection between greening and climate change (or CO2 concentration in the atmosphere) is in the fact that a sustainable process produces CO2 that are readily absorbed by the environment. The difficulty arises from the lack of a comprehensive sustainability criterion that indeed can identify the process, which renders CO2 non-absorbable in the environment. Before true greening can be discussed, one should consider this scientific sustainability criterion. In this chapter, we gave reference to the sustainable criterion that characterizes most of the biofuel technologies as unsustainable. This criterion was developed by Khan and Islam (2007), which was used to test sustainability of the green biodiesel (Khan et al., 2007). According to this criterion, to consider any technology sustainable in the long term, it should be environmentally appealing, economically attractive, and socially responsible. The technology should continue for infinite time, maintaining that the indicators function for all time horizons. For a green bio-diesel, the total environmental benefits, social benefits, and economics benefits are higher than the input for all time horizons. For example, in the case of environmental benefits, burning green bio-diesel produces “natural” CO2 that can be readily synthesized by plants.
The products generated during bio-diesel burning are also not harmful because there are no toxic additives involved in the bio-diesel production process. The plants and vegetables for bio-diesel feedstock production also have positive environmental impacts. Thus, switching from petrodiesel to bio-diesel fulfils the condition:
(5.1)
where Cn is the total environmental capital of the life cycle process of bio-diesel production. Similarly, the total social benefit, where Cn is the total environmental capital of the life cycle process of bio-diesel production. Similarly, the total social benefit,
(5.2)
and economic benefit,
(5.3)
become positive by switching from mineral diesel to bio-diesel. Bio-diesel can be used in practically all areas
Chhetri and Islam (2006) presented the recipe for the truly green biodiesel model. They pointed out that existing bio-diesel production process is neither completely “green” nor renewable. In addition to using fossil fuel as a feedstock to producing methanol, it uses catalytic agents that taint the process. Each of the artificial chemicals used will lead to the emission of oxides and other products that will not be absorbed by the ecosystem.
The current biodiesel production uses fossil fuel at various stages such as agriculture, crushing, transportation, and the process itself (Carraretto et al., 2004). Figure 5.16 shows the share of energy use at different stages from farming to biodiesel production. Approximately 35% of the primary energy is consumed during the life cycle from agriculture farming to biodiesel production. This energy basically comes from fossil fuels. To make the biodiesel completely green, this portion of energy also has to be derived from renewable sources. For energy conversion and crushing, direct solar energy can be effectively used while renewable biofuels can be used for transportation and agriculture.
Figure 5.16 Share of energy at different stages of biodiesel production (From Chhetri and Islam, 2008).
Table 5.11 shows that biodiesel has all kinds of emissions as does petrodiesel, but the emission level is significantly reduced when biodiesel is replaced by petrodiesel. Islam et al., (2010) reported that the CO2 produced from fossil fuel burning may be heavier than CO2 produced from fresh biological sources. Biological sources produce new CO2, which is easily taken up by plants. Plants discriminate heavier CO2 during photosynthesis (NOAA, 2005). The use of toxic chemicals and catalysts during the biodiesel production process increases the possibility of generating heavier CO2, which are less likely acceptable for plants (Islam et al., 2010). Similarly, methanol synthesized from natural gas is also different from methanol produced from starch or grains fermentation because of their isotopic difference. Fresh biomass-based renewable alcohol is less toxic and more easily degradable compared to petroleum-based methanol.
Table 5.11 Difference in average toxic effects at two biodiesel blend levels (from chhetri and Islam, 2008).
| Average % change compared to base fuel | ||
| Toxins | 20% biodiesel |
100% biodiesel |
| Acetaldehyde | –7.10 |
–14.40 |
| Acrolein | –1.50 |
–8.50 |
| Benzene | 16.50 |
–0.80 |
| 1,3-Butadiene | 39.00 |
–12.30 |
| Ethylbenzene | –44.90 |
–61.00 |
| Formaldehyde | –7.80 |
–15.10 |
| n-Hexane | –48.70 |
–12.10 |
| Naphthalene | –13.80 |
–26.70 |
| Styrene | –3.70 |
39.30 |
| Toluene | 19..90 |
13.30 |
| Xylene | –12.30 |
–39.50 |
The pathway for conventional bio-diesel production and petrodiesel production follows a similar path (Figure 5.1). Both fuels have similar pollutants in their emissions, such as benzene, acetaldehyde, toluene, formaldehyde, acrolein, PAHs, and xylene. Figure 5.17 depicts a simplified block flow diagram for a typical biodiesel production process using base catalysis.

Figure 5.17 A simplified block flow diagram for a typical base-catalyzed process for the production of biodiesel (from Helwani et al., 2009).
A simplified block flow diagram of the acid process is shown in Figure 5.18.

Figure 5.18 A simplified block flow diagram of the acid-catalyzed process for the production of biodiesel (from Helwani et al., 2009).
The key to greening is introducing all natural sources of mass as well as energy at each stage. 100% greening appears to be a remote goal because it would mean the use of organically grown vegetable oil (or fully free-range animal fat) along with heating with solar or wood burning in a clay stove with all containers made of natural products, such as naturally processed metals, clay, and ceramics.
In the interim, Chhetri and Islam (2008) developed a process that renders the biodiesel production process truly “green,” using waste vegetable oil as bio-diesel feedstock. The catalysts and chemicals used in the were non-toxic, inexpensive, and natural. The catalysts used were sodium hydroxide, obtained from the electrolysis of natural sea salt, and potassium hydroxide, obtained from wood ash. The new process substituted the fossil fuel-based methanol with ethanol produced by grain-based, renewable products. The use of natural catalysts and non-toxic chemicals overcame the limitation of the existing process. Fossil fuels were replaced by direct solar energy for heating, making the bio-diesel production process independent of fossil fuel consumption. Chhetri and Islam (2008) proposed a new approach, based on the use of alcohol produced from renewable sources. Methanol is produced by utilizing microbes to convert methane to methanol (Figure 5.19). Methane produced from anaerobic digestion is acted upon by methanotrops that convert methane into methanol. Hanson and Hanson (1996) and Murrell (1994) reported that methanotrophs are aerobic bacteria which utilize methane as their sole carbon and energy source. Methanotrophs are physically versatile and can be effectively utilized to produce methanol from a wide variety of wastes. Use of ethyl alcohol fermented from grain-based biomass such as corn or sweet sorghum and molasses from sugar for alcoholysis of vegetable oils or fats is also a sustainable option (Figure 5.20). Both of these alternatives eliminate the consumption of huge amount of natural gas to make methanol. No expensive or toxic chemicals are involved in these processes.
Figure 5.19 Production of methanol from methane by microbial conversion.

Figure 5.20 Flow process for production of ethyl alcohol (from Helwani et al., 2009).
Figure 5.21 is the schematic diagram of proposed new concept of biodiesel production. In this process, potassium hydroxide derived from wood ash or sodium hydroxide derived from sea salt is used as the catalyst along with bio-based methanol or ethyl alcohol. Waste cooking oil is preheated up to 50 °C before mixing the methanol and potassium hydroxide mixture in it. After mixing, the mixture is heated to 60 °C at which the reaction takes place to form biodiesel and glycerin. The glycerin is separated by gravity separation. The crude biodiesel is heated to 65 °C to evaporate and recover methanol to reuse in the process. In case ethyl alcohol is used as medium for alcoholysis, it should be heated to 80 °C to evaporate the ethyl alcohol. The biodiesel is then washed with either mist or bubbles and then dried to evaporate all water vapor before sending to storage or usage.
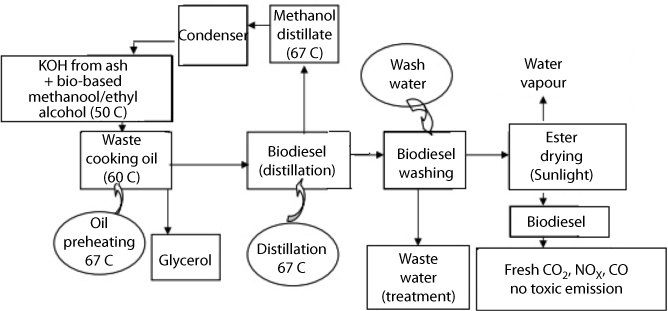
Figure 5.21 Schematic diagram of a green biodiesel production process (from Helwani et al., 2009).
The biodiesel produced from this process is a nontoxic product because all the chemicals and catalysts are nontoxic. The CO2 produced is “new” CO2, with lighter isotopes, and it is not contaminated by any chemicals because the biological sources produce new CO2 (Islam, 2004). Plants will synthesize this “fresh” CO2 and complete the carbon cycle. Similarly, the NOx and CO produced during combustion of nontoxic biodiesel is not harmful compared to the petrodiesel and conventional biodiesel. Formaldehyde and other emissions from biodiesel combustion are different from those emitted from petrodiesel or conventional biodiesel.
5.3 Conclusions
Conventionally, biofuels are considered to be renewable and hence inherently sustainable. This conclusion is reached only because New Science fails to include all salient features of biofuel production. This chapter uses biodiesel as an example and shows biofuels are not renewable. Such unsustainability comes from the source material of biofuels. Previous chapters have shown that the use of chemical fertilizers and pesticides causes organic materials to become inherently toxic to the environment. This chapter shows that further toxicity arises from the current methods of material processing, which is highly toxic. This toxicity comes from the highly toxic chemicals and catalysts used in the process. As new types of catalysts are introduced, the level of toxicity increases. This is because the focus has been economic benefit, while it is taken for granted that long-term sustainability is assured. Because New Science does not include the role of trace components, such as catalysts that are used in a small quantiity, conventional analyses fail to evaluate the role of these catalysts in altering long-term qualities of the products.
In this chapter, a new process for biodiesel production is presented and its impact discussed. This process makes the biodiesel production independent of fossil fuels as well as toxic chemicals, relying instead on natural sources of both mass and energy. This new process proposes bio-based methanol/ethyl alcohol for alcoholysis of waste cooking oil/animal fat. The catalysts proposed in this process are completely natural. Potassium hydroxide derived from wood ash and sodium hydroxide derived from sea salt are proposed to replace the synthetic sodium or potassium hydroxide as catalysts. Ash is a very cheap source and a by-product of biomass burning. Naturally occurring potash, sodium carbonates, titanium oxide, and other heterogeneous catalysts are considered for the transesterification process. Since this process follows a completely natural path, the biodiesel is inherently nontoxic and environmentally friendly. The direct application of solar energy to convert vegetable oil into biodiesel is the cost effective and environmentally friendly option. This concept makes the biodiesel production truly green.
