Nanocomposites
Nanocomposites, which can be defined as composite materials with at least one nanoscale material dispersed in a matrix, are perhaps the most developed nanomaterials with applications in structural materials. Chapter 11 deals with the important aspects of nanocomposites, which includes nanoparticle surface modification, composite formation, and the synthesis of core–shell nanomaterials.
Keywords
Nanocomposites; core–shell particles; surface modification
11.1 Concept and History
The history of humanity is closely related to the development of materials. Historians view the use of tools and tool-making materials as a sign of the progress of civilization and divide the history of human development accordingly into the Stone Age, Pottery Age, Bronze Age, and Iron Age.
Ever since the beginning of human civilization, mankind has continued to develop new materials in advancing production and science. As for materials, any single material may come along with certain advantages and obvious shortcomings. Sometimes, it is difficult to improve the properties of materials and avoid their shortcomings. In the past three decades, the rapid development of science and technology has placed an increasing demand on the performance of materials. Materials made of single elements are therefore no longer capable of meeting this demand. Studies proved that two or more materials combined in some manner can be made into new materials (composite materials), with the advantages of the original components retained in many cases. These new materials are made to overcome or compensate for the shortcomings of the original elements and, in some cases, with new properties.
Composite materials first emerged in 5,000 BC, when people in the Middle East learned to mix asphalt with reeds for shipbuilding. In 3,000 BC, the ancient Indians were already able to produce composite boards with shellac resin. In China, a composite adhesive was used in the construction of the Forbidden City. Composite material made of thatch and soil in building houses is another early example. However, as a discipline and a new kind of industry, composite materials did not emerge until the 1940s. In 1940–1960, glass fibers, the so-called reinforced plastics or glass fiber-reinforced plastic, were introduced together with the emergence of boron fibers and carbon fiber-reinforced plastics. These are known as the first generation of composite materials. The period of 1960–1980 witnessed the advent of fiber-reinforced plastics, Kevlar fiber-reinforced plastics, SiC, and metal fiber-reinforced plastic Al2O3, which belong to the second generation of composite materials. This period underwent the development of advanced composite materials. In the period of 1980–2000, the advanced composite materials were well developed. This was called the third generation of composite materials, which have undergone rapid development in aviation and aerospace fields, and wide applications are found in various fields. At the same time, fiber-reinforced metal and nanomaterials were known to people as equivalent dispersion composite materials.
The definitions of composite materials vary. Some define a composite material as “a material composed of two or more kinds of single materials and it displays a number of new properties.” This interpretation is easy to understand, but scientifically it is imperfect and imprecise. The most sound definition was given by the ISO (International Organization for Standardization): “composite materials (or composites for short) are engineered materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct on a macroscopic level within the finished structure.”
Nanocomposites [1] are one of the new composite materials formed by nanometer-sized materials that are dispersed in a 3-D substrate. They can provide obvious quantum size effects (e.g., the blue shift of the optical absorption edge) and an increased third-order nonlinear optical coefficient, which are expected to have applications in optical bistability, fast response, phase conjugation, optical waveguides, and other optoelectronic devices.
In contrast to the nanophase materials from single-component nanocrystalline materials, nanocomposite materials can be either intragranular nanocomposite (i.e., nanocrystalline phase comes into the larger phase grains) or nanocomposite in the grain boundary (i.e., nanograin distribution in the grain boundary over the larger grains of the parent phase); the latter applies to ceramics, metal, and polymer materials.
11.2 Surface Modification of Nanomaterials [2,3] and Their Applications
In the preparation process of nanocomposite materials, nanoparticles are very easy to join together because of their large specific surface area and high surface energy; however, nanoparticles have poor affinity with the substrate with a relatively low surface energy. The two are not compatible in mutual mixing, leading to interface voids, that is, the phenomenon of phase separation. Only after a satisfying solution is found for the agglomeration of nanoparticles in the material can the special effects of nanoparticles be well applied in the materials. Ultimately, the material performances in the mechanical, optical, thermal, and other aspects will be greatly improved. To ensure the nanosized existence of nanoparticles in the material, nanoparticle surface modification has become a critical goal of nanopowder research surface modification of nanotechnology and is an important part of surface engineering.
11.2.1 Nanosurface Engineering [4]
Surface engineering is an integrated design as a system of the surface and substrate of a material; it is system engineering involving the use of a variety of surface technologies that enable the surface of the material to have the desired performance not present in the material itself.
Nanosurface engineering technology is built on the basis of surface scientific theory. It is an advanced technology derived from the combination of modern physical and chemical methods and conventional surface technologies, involving all areas of surfaces and interfaces, as well as a variety of metals and nonmetal, ceramic, composite materials. Nanosurface engineering includes surface coating technology, surface thin film technology, and surface modification technologies. At the same time, modern surface technology also involves the preparation of coatings and thin film materials, processes and equipment, process and quality control, surface analysis, surface properties and evaluation, as well as testing technology, testing methods and standards, and the process of environmental injury and its mechanism.
With the development of nanotechnology, coupled with the growth of design and manufacture of microelectromechanical systems, manufacturing technology has moved from the sub-micron level to the nanoscale and even atomic or molecular level. Electromechanical systems like nanorobots, nanotweezers, and nanomotors may involve considerable surface science and technology issues. Meanwhile, with the size reduction and the appearance of surface effects, traditional surface design and processing methods are no longer adaptable. There are a growing number of cases that require materials to be serviced under special conditions, such as ultrahigh temperature/low temperature, high pressure, high vacuum, strong oxidation, and reduction or the corrosive environment, and in the presence of radiation, sound absorption, signal shielding, and bearing point load. As nanomaterials are presented with characteristics different from those of macromaterial in the aspects of power, electricity, sound, light, heat, and magnetism, the surface of nanotechnology is particularly important to traditional materials, and thus nanosurface engineering has emerged.
Nanosurface engineering is a systematic type of engineering that equips materials with the surface of nanotechnology via specific processing. The nanostructure process enables the material surface to be strengthened or modified or gives new properties to the surface to produce smart surfaces, nanointellectual surfaces, and devices with nanoscale surfaces.
Compared with traditional surface engineering, nanosurface engineering is characterized by factors that depend on the substrate properties being weakened, freedom for surface treatment, modification, and functional properties to expand, and the more prominent role of the surface processing technology and higher value-added products. Attributed to its nanosurface engineering characteristics and the properties that may be acquired, it has a broader range of applications, virtually covering every aspect of industrial and civilian use in high-tech areas, such as aerospace, aviation, marine, computer technology, electronics, information, transportation, oil, chemistry, construction, irrigation, and machinery industries, as well as all other areas of people’s lives. Statistics show that industries directly related to surface technology have accounted for 7% of national economic output. With the development of nanomaterials and nanotechnology, the use of the related research achievements in the development of surfaces in nanotechnology and engineering is an important direction of the development of surface technology and also an important part of nanotechnology today.
11.2.2 Mechanism of Surface Modification of Nanoparticles
Surface modification of nanoparticles refers to the reaction between the surface of nanoparticles and surface modifier to improve the solubility of the nanoparticle and enhance the interfacial compatibility of nanoparticles in the medium, so that nanoparticles can be easily dispersed in organic compounds or water. The molecular structure of a surface modifier must have the characteristics of the substrate group, which easily reacts with the surface of nanoparticles. This characteristics group can be obtained from the design of the molecular structure of the surface modifier. According to the reaction mode between nanoparticles and the surface modifier, the modified mechanism can be divided into coated modification and coupling modification.
11.2.2.1 Coating Modification
Coating modification specifically refers to the surface-coated treatment of nanoparticles with inorganic or organic compounds (water-soluble or oil-soluble polymers, fatty acid soap, etc.), aiming to weaken or block the agglomeration of nanoparticles. Coated materials can generate a steric hindrance repulsion, which makes it quite difficult to obtain agglomeration of particles to achieve the purpose of modification. The mechanism used in coating can be coated adsorption, attachment, simple chemical reaction, or deposition of the coating. The preparation of nano-TiO2 may introduce the hydroxypropyl cellulose modifier, whereby modifier molecules adsorbed on the TiO2 particles may show a stereo-hindrance effect that can effectively prevent the particles from further accumulating growth, thus improving the dispersion and uniformity of the hydrated TiO2 particles. At the same time, a particle surface with adsorption of these molecules can thoroughly “shadow” the nonbridging carbonyl and the absorbed water between particles to reduce the surface tension and become less prone to aggregation. Polyvinyl alcohol (PVA) may be added in the preparation of nanometal oxides. PVA contains a large amount of free, strong polar hydroxyl groups, which may form a chelate bond with metal ions in aqueous solution, which is closely coated around the metal ions. A finite structure can be formed with PVA chains to limit the size of the synthesis of nanoparticles, achieving the purpose of modification. In the preparation of nanosilver particles, polyvinylpyrrolidone (PVP) may be doped to have coordination between PVP molecules and surface atoms of nanosilver particles through the N and O atoms, leaving behind a long chain of CH stretching around to prevent the mutual agglomeration of nanosilver particles between them. With this method, silver powder with an average particle size of 25 nm can be prepared with good dispersion and distribution in a uniform particle size.
11.2.2.2 Coupling Modification
Coupling modification refers to the chemical coupling reactions that may occur on the surface of nanoparticles. In addition to the interactions of van der Waals forces, hydrogen bonds, or coordination bonds, there is also a combination of covalent bonds or ionic bonds between the two components. Via treatment with a coupling agent, the surface of nanoparticles can produce very good compatibility with organic matter. Coupling agent molecules must have two kinds of groups: one has a chemical reaction with the surface of inorganic nanoparticles or the precursors to prepare the nanoparticles, and the other (organic functional groups) can be reactive or compatible with the organic substrate, such as dioxin acetate titanate (2-n-octyl coke phosphate), vinyl triethoxysilane, and so on. Coupling modification can be easier to operate and have more choices of coupling agents, so the method is more often applied in nanocomposite materials. The preparation of polymethyl methacrylate–silica nanocomposite material requires methyl-propionyl oxypropyltrimethoxysilane to act as coupling agents, with its carbon–carbon double bond copolymerized with the polymethyl methacrylate (PMMA). At the same time, the trimethoxysilane group is hydrolyzed with tetraethoxysilane to produce a silica bond, so that a composite system can be uniformly dispersed and remain stable.
11.2.3 Surface Modifiers of Nanoparticles
11.2.3.1 Inorganic Compounds for the Surface Modification of Nanoparticles
Usually, Al2O3, SiO2, and ZnO are used as modifiers for the surface modification of nano-TiO2. After proper treatment, anatase TiO2 will exhibit strong UV absorption capabilities and can be safely applied to cosmetics, paper, paint, and other fields. The fluoride-modified α-Al2O3 can generate alumina powder that is uniformly dispersed, with an average particle size less than 50 nm. In the Surface Engineering Research Center for military equipment maintenance in China, the nickel-coating method is used for the treatment of nano-Al2O3, SiC, and diamond powder surfaces to improve the conductivity of nanoparticles. It has been proven that this method can increase the amount of nanopowder deposition in the nickel-based composite coating and enhance the uniformity of nanopowder coating.
11.2.3.2 Surface Modification with Nanoparticles
Surface modification with nanoparticles is actually the use of a compound between nanoparticles to improve some aspect of the performance of the nanoparticles being processed. For example, the sol–gel method can be used for preparation of composite nanoparticles. Specifically, the sol–gel method is first used to make n-butyltitanate particles into nano-TiO2, which is then made into a transparent solution. Before this solution changes into a gel, it is added with another precursor, by which the sol–gel method can be used to form nanoparticles. An example is using ammonium solution as WO3 tungsten precursors. After the formation of gel in the mixture, a compound of nanoparticles WO3/TiO2 can be formed through heat treatment. The new layer of nanoparticle film formed on the surface of nanoparticles will act to stabilize the inner nanoparticles and give a new performance to the particles. By using the gel method, Fe2O3 nanoparticles coated with a layer of SiO2 film can significantly improve the dispersity of modified nanoparticles in PMMA solution.
11.2.3.3 Surface Modification with Organic Compounds
Organic compounds are a major modifier of nanoparticles that can bring about unique changes in properties. CdS nanoparticles are capable of producing weak red light. Modification with a small amount of alkylamine can significantly enhance the fluorescence of CdS nanoparticles and produce a blue shift. However, the high concentration of alkyl amines may quench the CdS fluorescence; mercaptan also functions in the same way as alkyl amines. TiO2 nanoparticles coated with stearic acid will have a very low degree of crystallinity, and the obtained TiO2 nanoparticles have an average particle size of 5–6 nm. Meanwhile, the absorption spectra of TiO2 nanoparticles will be generated with a clear blue shift and light-induced luminescence may occur at room temperature. When resinate RS-2 and fatty acid salt RS-1 are used in the surface modification of nanocalcium carbonate, the modifier coated on the surface of calcium carbonate will increase their oleophilic properties, and its dispersion in nonpolar medium can be improved accordingly. Because nano-ZnO is dispersed in nonaqueous media, the surfactants sodium dodecyl benzene sulfonate and sodium laurate can be added as a surface modifier, which will be adsorbed on the nano-ZnO to ensure a stable and homogeneous dispersion. Stearic acid-modified CdO nanoparticles may have an absorption blue shift with strong luminescence. In the sol–gel preparation process of SiO2 nanoparticles, when the polymer in the nonhydrophobic chain (polyethylene glycol) is used as surfactant for particle modification, sol clusters will form a rod-like network structure; with hydrophobic–hydrophilic polymers (fatty alcohol polyoxyethylene ether) as surfactant for modification, sol clusters will form a rod-like network structure; with polyoxyethylene–polyoxypropylene ether–polyoxyethylene triblock copolymers as surfactant for modification, a dendritic network structure will be formed. Increase of the hydrophobic part (polyoxypropylene ether) helps form the network structure and keeps a consistent nanoparticle size.
11.2.3.4 Surface Modification with Polymers
Surface modification of nanoparticles with polymers is the use of a polymer network to acquire the stability of nanoparticles. After a polymer network is treated with carboxylic acid salts (zinc, separated), sulfonates (zinc, cadmium, copper, and their multicomposite metal ions, etc.), and hydrogen sulfide gas-flow processing to generate the sulfide nanoparticles, the obtained particles may have an average size of only a few nanometers. Under the 3-D protection of polymer networks, the stability of nanoparticles can be increased to achieve micro-control of the special nature of nanoparticles. Because of its excellent optical properties and easy processability, polymers can be a good supportive agent in the formation of nanoparticles. In the synthetic process of ZnO nanoparticles, PVP demonstrated protection of nanoparticles. This can be helpful in the synthesis while improving the properties of nanoparticles. MoO2 nanoparticles synthesized in γ-radiation aqueous solution, by adding PVA as a surfactant, can be significantly better than the surfactant sodium dodecyl sulfate with respect to purity and thermal stability.
11.2.4 Implementation of Nanoparticle Modification
Means for the implementation of modifying nanoparticles can be divided into mechanical dispersion, ultrasonic dispersion, and high-energy processing methods. Mechanical force dispersion mainly uses mechanical effects by external shearing force or impact force, and the special surface structure of nanoparticles is prone to chemical reactions, so that nanoparticles may undergo chemical changes with the surrounding medium (e.g., the surrounding solid, liquid, or gas) to form a layer of branched-chain organic compounds or a protection layer on its surface to make it easier for the dispersion of nanoparticles. Fe3O4 powder and micron polyvinyl chloride (PVC), when dispersed in high-energy ball milling, are able to form α-Fe3O4/PVC nanocomposites, where α-Fe3O4 may have a particle size of 10 nm. Ultrasound is widely used in chemistry, playing an important role in the synthesis of compounds, polymer degradation, and the dispersion of particulate matter. When nano-CrSi2 particles (with an average diameter of 10 nm) are added to tetrahydrofuran solution containing acrylonitrile–styrene copolymer via ultrasonic dispersion, nanocrystals may become available for coating the polymer materials. The high--energy approach indicates the use of ultraviolet, infrared, corona discharge, and the plasma radiation method to perform the surface modification of nanoparticles. In addition, methyl methacrylate can be grafted onto nano-MgO with the help of UV radiation. Such surface modification can greatly improve the dispersion of nanopowder in high-density polyethylene (PE).
Plasma is a system comprising a large number of charged particles (ions, electrons) from ionized gas and the neutral particles in excited states (atoms, molecules). The system contains the same total number of positively and negatively charged particles. Over the past 20 years, plasma technology has made fruitful achievements in the fields of chemical synthesis, new materials development, fine chemicals, and surface treatment. In recent years, there have been more reports on studies and research on using plasma technology for surface modification of nanoparticles for biomedical purposes [5]. Plasma technology is relatively low cost, easy to operate, and useful to control material modification by optimizing process conditions. In theory, it can achieve the surface modification of the materials of any nature in any shape without changing the physical properties of the nanomaterial.
There are two main types of reactions between plasma and the material surface, namely plasma polymerization and plasma surface treatment. By way of plasma polymerization, organic monomer can be converted into a plasma state to produce various active species (free radicals) for surface polymerization. Plasma surface treatment involves the use of energy particles and reactive species in the plasma of nonconvergent gas (e.g., argon, nitrogen, oxygen) to react with the surface of the material to be processed, resulting in the surface producing a particular functional group (e.g., –OH, –N2H). These two types of reactions can change the surface composition and structure, aiming to achieve the purpose of material surface modification.
With the development of medical research, a variety of transplant technologies for therapeutic, diagnostic, and orthopedic surgeries were developed, and the implants had to satisfy many requirements, such as biocompatibility, permeability, anti-aging and nontoxic properties, and so on. Many polymer materials usually have satisfactory mechanical properties such as bulk properties, but they cannot be used as implants because their surface properties often fail to meet these requirements. Over the years, many domestic and foreign researchers have been trying to introduce a variety of physical, chemical, or biological means to perform surface modification on biological materials. Plasma technology has been proven to be a very effective approach.
11.2.5 Application of Modified Nanoparticles
11.2.5.1 Application in Plastics
Due to the small size effect of nanoparticles, as well as large surface area and strong interfacial bonding, nanomaterials can be effective in increasing strength and toughness, as well as in improving the aging resistance of plastics. When SiO2 (with a particle size of 14 nm) treated with dimethyl silane has a volume fraction that is 4% of that of PE, the casting method can be used to prepare SiO2/PE composite materials, which exhibited a tensile strength approximately double that of the substrate. Polypropylene filled with CH-IA-treated nano-CaCO3 powder will show marked improvement in composite material toughness and impact resistance. By surface modification of high-energy radiation, the obtained SiO2-filled polypropylene composites will also have increased modulus and strength, whereas their toughness can also be improved significantly.
11.2.5.2 Application in Composite Fire-Retardant Materials
After traditional inorganic flame retardants are changed into nano-based materials, with nano-Sb2O3 as the carrier, a type of highly efficient flame retardant can be made by way of the surface modification, with its oxygen index being several times that of common flame retardants. In addition, the nano-Sb2O3 and polyolefin can be perfectly matched with plastic. It has features of good thermal stability, nontoxicity, and durable resistance against fire.
11.2.5.3 Application in Composite Catalysts
Nanoparticles are small, so the surface/volume ratio is large, which makes the bonding state and the electronic state of the surface different from that inside the particles; the surface smoothness may deteriorate and form uneven atomic levels. Meanwhile, this will increase the contact surface for chemical reactions and produce incomplete atomic coordination, leading to an increase in active surface sites. All these properties are the basic conditions that cause it to be a type of catalyst. Copper chromite is a good catalyst that can promote ammonium perchlorate decomposition. However, it is likely to contain agglomeration of previously prepared copper chromite and perchloric acid ultrafine particles. Ammonium perchlorate crystals used for coated nanocopper chromite to form composite particles provide a better solution to this problem.
11.2.5.4 Application in the Field of Lubrication
The application of nanomaterials in lubrication is a new area of research. Because nanomaterials have large surface/volume ratio, high proliferation, low sintering, and melting point reduction, the new lubricating materials, which are prepared with nanomaterials and applied in the friction system, will have a lubricating function that is different from that of traditional load additives. In addition to the formation of a layer of film that is easy to cut on the friction surface to reduce the friction coefficient, the new lubricating materials can help fill and restore the surface, thus enhancing the resistance of the system.
11.2.5.5 Applications in Composite Coating
The unique role of nanomaterials will have a far-reaching impact on paint applications. The combination of nanomaterials with traditional paint in manufacturing nanocomposite coatings is an important direction for paint development. The excellence of new composite coatings is increasingly reflected in a variety of nanocomposite coatings that have been successfully developed. After surface modification, nanomaterials can be produced with hydrophobic and oleophobic features. Nissan and Toyota have applied nanomaterials with self-cleaning and anti-fog properties as the mirror surface coating for their automobiles.
11.2.5.6 Application in Rubber
After rigid nanoparticles are added to the rubber-toughening system, the special effects of nanoparticles will provide the rubber with reinforcement, barrier, and processing properties. Rubber and the modified SiO2 nanoparticles in composite materials may be dispersed very evenly. In addition, the dispersed phase can be controlled with respect to its chemical composition and structure, size and distribution, and surface characteristics. The prepared nanocomposite material has extremely high tensile and tear strength, as well as excellent heat and dynamic lag/static compression performance. In the most optimum conditions, its overall performance can significantly surpass that of rubber nanocomposites reinforced with carbon black and silica. By using this technology, the mixing process can also be partly omitted.
11.3 Core–Shell Structure Composite Nanomaterials [6–9]
As researchers’ understanding of nanomaterials expands, the continuous research also undergoes a gradual transition from the simple preparation of nanoparticles to the design and controlled synthesis of nanocomposite materials that have specific functional properties. Composition of materials in the nanometer scale is a popular research topic. Nanocomposites with core–shell structures are a typical form of composite material of this kind. Over the past 10 years, making core–shell structures in materials has stimulated the interest of scientists. Core–shell material is formed with the core and the external shell having exceptional composition, size, and structure, and it can provide better performance than that of conventional materials. Core–shell structured nanomaterial has an excellent range of adjustable features. To prepare different types of core and shell, new materials have become the focus of studies in many countries.
11.3.1 Characteristics of Core–Shell Composite Structures
Core–shell nanoparticle composite structures are an ordered assembly of nanoscale structures. This new structure is formed by using a particular nanomaterial to coat another kind of nanomaterial via chemical bonds or other interactions. It is a higher level of composite nanostructure. This structure provides many new properties beyond the reach of single nanoparticles, which are widely appreciated for wider application prospects than single nanoparticles. The design and controllable synthesis of composite nanomaterial with core–shell structures has been an increasingly important forefront of material science in recent years. As functional materials with a new structure, such materials are favored by researchers because they have many unique properties, such as single dispersion, core–shell operability, stability, control, self-assembly, and the capabilities involved in light, electricity, magnetic, catalytic, chemical, and biological responses. Thus, by providing a reasonable design of experimental conditions, regulation can be largely achieved for many properties of composite nanomaterials.
Core–shell nanomaterials can be divided into different categories by applying different criteria. Depending on whether a chemical reaction may occur between core–shell particles, it can be divided into physical coating and chemical coating types. Depending on the different core–shell components, it can be divided into three categories: organic–inorganic, inorganic–organic, and inorganic–inorganic types. These core–shell structures are designed for specific purposes. The relatively stable nature of the shell is used to protect the core particle from the occurrence of physical and chemical changes; another potential objective of the shell is to improve the surface activity of the core particles, as well as stability, dispersion, and so on. Through surface coating, core particles can have magnetic, optical, and catalytic properties that are unique to shell particles.
11.3.2 Composite Method
With the in-depth research supported by improved experimental means, core–shell structure composites can be prepared using a variety of methods such as surface modification, polymerization, deposition film formation of the most commonly used in situ composite, self-assembly technology, electroless plating, and so on. These methods not only can lead to shell thickness and uniformity control but also can prepare multishell structured composite materials. A few commonly used methods are given below.
11.3.2.1 Polymerization Chemical Reaction
Polymerization chemical reaction usually refers to the polymerization reaction of organic monomers in solution containing the particles to be coated with the formed polymer deposited on the particle surface to form a coating layer. It includes monomer adsorption polymerization and emulsion polymerization.
In monomer polymerization, a core with high catalytic activity is usually used as coating particles, such as α-Fe2O3, CeO2, CuO, and SiO2. Strong interactions may exist between monomers and the coated particles, which can be directly adsorbed to the surface of inorganic particles, and then trigger monomer polymerization to complete the coating. The key to the use of monomer-coated particles is that polymerization must take place at the particle surface. Experiments show that the thickness of the coating can be adjusted by changing the time of contact reaction between the core and organic matter. This method is simple to operate and applicable for a wide range of conditions.
Additionally, a low-molecular-weight surface-active agent is capable of forming double-layer micelles at the particle surface. Based on this, monomers can be inclusive in the micelles to cause polymerization to achieve surface modification of particles. With this method, a thin polymer coating layer (2–10 nm) can be formed on the organic or inorganic particle surface, especially for particles with an irregular surface shape. It can maintain a certain thickness in thin-layer coatings along the contours of the surface of the particles.
11.3.2.2 Biological Macromolecular Method
Biological macromolecules are another candidate for nanoparticle coatings. Their main purpose is to enable ordinary particles with special genes and response capabilities that may be found in certain proteins or organisms and can be widely applied to clinical analysis, immunological tests, and the study of biological characteristics. There are several techniques that can be used to help biological macromolecules adhere to the surface of solid particles, such as nonvalence-bond adsorption, valence-bond adsorption, sol–gel capture, and electrostatic self-assembly. One of the most commonly used methods is valence-bond adsorption. It can be used for the coating of various types of proteins and antibodies against solid particles (e.g., polystyrene, polyaniline). Nonetheless, such coating usually is not solid enough and may be accompanied by loss of activity. This is especially true for smaller biological particles. The sol–gel method can achieve a variety of complex forms of coating that may be difficult for a general coating method; for some complex biological systems, the coating can be completed via infiltration of sol–gel solution with the requirements of nondestruction of its structure and function.
11.3.2.3 Surface Deposition and Surface Chemical Reaction Method
In particles coated using surface deposition techniques, it is required that the coating and coated particles are both dispersed in aqueous solution. By conditioning pH or heating, coated materials may undergo precipitation or hydrolysis, followed by deposition on the core material to form a core–shell structure, or specific functional groups can be used to complete the direct reaction on the surface for coating. This method can be used to prepare inorganic coating layers, such as SiO2, basic yttrium carbonate, TiO2, ZrO2, and so on. Studies of the TiO2 surface coated with SiO2 showed that a large number of particles may be agglomerated with the deposition of SiO2. Meanwhile, the sol–gel combined hydrogen reduction method can be used to prepare Fe/SiO2 core–shell nanoparticles. Based on this, the acetylene pyrolysis deposition method is introduced to prepare core–shell structured carbon-coated Fe/SiO2 particles; iron nanoparticles can be evenly coated in silica and carbon shell to further improve the thermal stability, as shown in Figure 11.1.
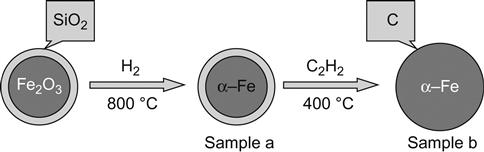
11.3.2.4 Controlled Deposition of Inorganic Colloidal Particles on the Core Particle Surface
Controlled deposition of inorganic colloidal particles coated on the core particle surface is generally a result of the surface static interaction between inorganic nanoparticles and large particles. For example, under solvent-controlled deposition, a polystyrene (PS) colloidal surface can be coated with a layer of CdTe nanocrystals. When modified with mercapto-glycine, CdTe nanocrystals will have a negatively charged surface, –COO, which via electrostatic action will be adsorbed to the surface of PS particles positively charged with –NH3 to form a single-layer coating. The coating can then be completed by the following condensation of nanocrystals. A certain thickness of sedimentary layer is available by controlling the rate of cohesion. The thickness and shape of the inorganic coating layer are determined by the initial concentration of reactants, aging time, and temperature.
11.3.2.5 Ultrasonic Chemical Method
Sonochemical methods have been considered to be quite effective in the preparation of new materials. The mechanism is largely due to the ultrasonic cavitation effect, for example the formation of liquid in microbubbles and raising and collapse by implosion. In present experiments, numerous nanocoated materials have been chemically synthesized with ultrasound, including iron oxide nanoparticles coated on carbon balls, gold nanoparticles deposited on SiO2 microballs, Eu2O3 and Tb2O3 coated on SiO2, Al2O3, and ZrO2, oxides of the transition metals Fe, Co, and Ni deposited on SiO2 or Al2O3 microballs, Eu2O3 coated on TiO2 nanoballs, SiO2 coated on ZnS particles, and so on. A series of experiments showed that ultrasound may increase the chemical interaction between the surfaces of the coating material and the particles being coated. This is conducive to the formation of chemical bonds.
11.3.2.6 Self-assembly
Self-assembly is an effective method in the preparation of core–shell structured composite nanoparticles. In self-assembly, nanoparticles are made first, and are then taken as a template for surface coating. Finally, core–shell structured composite nanoparticles are constructed. For example, a spherical biodegradable polymer is taken as a template. The first step is the use of the polymer electrolyte to be modified to make the smooth surface charged with static electricity, and then to attach gold nanoparticles and silica particles to the surface, followed by repeated centrifugation, washing, and removal of the particles not being adsorbed. These steps can be repeated to achieve uniform and compact multicoated gold and silica nanoparticles. The dissolved template can generate a material with special optical properties. In addition, the vapor deposition method and chemical plating are both often used in the preparation of core/shell-type materials. For instance, prepared by chemical vapor deposition, SiOx-coated FeCoNi nanowires will have excellent soft magnetic properties and good thermal stability and can be used in nanodevices for high-density magnetic recording. In addition, the chemical plating method has also been cited in reports regarding its application to carbon nanotubes with Ag coating. Due to the low reactivity of carbon nanotubes, adequate surface oxidation, sensitization, and activation treatment are required before conducting chemical silvering to attain a smooth uniform coating. Meanwhile, reactions should be performed at the lowest possible rate.
11.3.3 Mechanism of Formation of Core–Shell Structures
The mechanism of formation of the core–shell structure through the coating of organic or inorganic particles is described in the following subsection.
11.3.3.1 Mechanism of Chemical Bonding
In research on SiO2-coated TiO2, we find that the two are combined through the formation of the Ti2O2Si bond. This is because inorganic oxide nanoparticles in water, such as SiO2 and TiO2, may be hydrated with water molecules to produce hydroxides such as Si–OH on the surface of silica particles. These groups are susceptible to chemical reaction with the hydroxyl groups on other inorganic particle surfaces, or some of the functional groups of the polymer chain (such as –COOH, –OH), thus enabling the formation of chemical bonds. In the reaction system, the introduction of the coupling agent also allows the formation of chemical bonds between the coating material and the coated material. For example, in studies on the preparation of Au coated with SiO2, Au nanoparticles cannot exist stably in solution, and there is no affinity between Au and SiO2, making it impossible to apply the coating directly; therefore, citric acid is used and adsorbed on the surface of Au nanoparticles to prevent their agglomeration, followed by doping with 3-aminopropyl methyl siloxane coupling agent and sodium silicate. Thus, the process of coating Au nanoparticles with SiO2 can be completed with the help of chemical bonds.
11.3.3.2 Mechanism of Coulomb Electrostatic Force
According to this mechanism, coating agent accompanies a charge that is opposite to that on the substrate surface. Affected by Coulomb attraction, this charge enables particles of the coating agent to be adsorbed on the surface of the particles being coated.
11.3.3.3 Mechanism of Adsorption Layer Media
Inorganic particles can undergo surface treatment to form a layer of organic adsorbed layer. With this kind of particle as core, the adsorption layer may act as the medium to improve the compatibility between inorganic particles and organic matter. Thus, the organic monomer can be aggregated to obtain complex encapsulated particles. For example, using citric acid Y2O3/Eu for surface modification can make the surface adsorbed with an organic layer. By the polymerization of styrene in the subsequent process, we can obtain PS-coated Y2O3/Eu composite particles.
Coating of the particle surface, inorganic or organic, generally involves the use of the mechanism mentioned previously, and some coating processes may involve the combination of two or more mechanisms. For example, electrostatic adsorption and chemical bonding can be combined to enable Au nanoparticles to be coated on the surface of PS particles. First, the positive poly(ethylene imine)-static charge is adsorbed on the negatively charged PS latex particle surface, followed by the bonding of Au nanoparticles with amino groups of polyethylene imine, which is coated with the PS particle surface. Next, the PS particles are used as crystal seeds in the reaction with NH4OH and HAuCl4 to improve the coverage of Au particles on the PS particle surface.
11.3.4 Changes in Material Properties
Coated nanomaterials have physical and chemical properties that differ from those of noncoated material and show some special optical, electrical, magnetic, or biological properties.
11.3.4.1 Changes in Optical Properties
Semiconductor nanocrystals can be used in biological fluorescence labeling and optoelectronic devices, but they require a very high fluorescence quantum efficiency and strong stability of light degradation. Coating semiconductor nanocrystalline particles with semiconductor material with a broader band gap is an effective way to improve these properties. In this way, shell modification can greatly enhance the fluorescence quantum yield of the core and the stability of the material, with the band gap energy being adjustable in certain bands. For example, at room temperature, CdS/Cd (OH)2 may have a fluorescence quantum yield of 50%, which is much higher than that of CdS itself.
11.3.4.2 Increase in the Stability of Particles
A nanoparticle is characterized by its small size and large specific surface area. This brings about properties such as active nature, large surface energy, and ease of aggregation. The most widespread application of surface coating is to improve the chemical stability of the material being coated. Some metal nanoparticles are easily oxidized in the air, or even result in spontaneous combustion. The solution is to coat such nanoparticles with one or more layers of inert compounds that are able to isolate them from the external environment. Magnetic nanoparticles, such as Fe2O3, are widely used in magnetic fluid but are easy to aggregate and susceptible to acid corrosion. Coating its surface with a layer of inert material (e.g., SiO2) can improve its stability and prevent acid corrosion. For example, after the surface of iron nanoparticles is coated with SiO2 and Al2O3, its stability in air is greatly improved. As the volume of the coating increases, the stability becomes enhanced. As shown in Figure 11.2, iron particles with a diameter of 30–40 nm, through the coating, are able to provide a steady presence in the air and can effectively prevent oxidation from occurring.
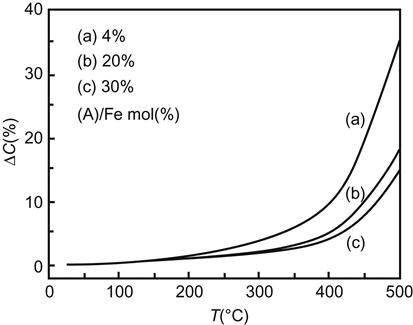
11.3.4.3 Catalyst Stability and Changes in Catalytic Activity
TiO2 is widely used as a white coating, catalyst, and catalyst carrier. As a catalyst, TiO2 with high surface area is thermally unstable and can easily aggregate, leading to the reduction of the specific surface area. A common practice to improve thermal stability of TiO2 is to coat particles with a high surface area with TiO2. It was reported that TiO2 catalyst on SiO2-coated surfaces can be stabilized to 1,058 K, with an increase of two orders of magnitude in 12-propanol dehydrogenation reactivity.
11.3.4.4 Changes in Magnetic
The magnetic core can be coated with nonmagnetic, antiferromagnetic, or ferromagnetic/ferrimagnetic shells. Nonmagnetic coating is mainly used to increase the magnetic stability of the core or to achieve surface function for biomedicinal purposes. Ferromagnetic core coated with antiferromagnetic may lead to exchange bias (hysteresis loop shift along the field direction) and improved thermal stability. For the core and shell made of strong magnetic (ferromagnetic or ferrimagnetic) materials, close contact between the core and shell may lead to an effective exchange coupling, making the magnetism adjustable. It is reported that FePt core coated with an MFe2O4 (M=Fe, Co) layer with a thickness of 0.5–3 nm, under the action of an external electric field, will have a smoother magnetic hysteresis curve, with the coercivity depending on the volume ratio of the hard and soft magnetic phases. Magnetic properties can be adjusted by changing the chemical composition of the coating and thickness. The soft magnetic properties of the magnetic core made with iron and their alloys coated with SiO2 and Al2O3 can be significantly improved. Meanwhile, the resistivity of the material can also be greatly improved, thereby reducing eddy current losses. In addition, the high-frequency soft magnetic properties will be significantly enhanced accordingly, and the real part of magnetic conductivity can be maintained constant even up to 1 GHz, as shown in Figure 11.3.
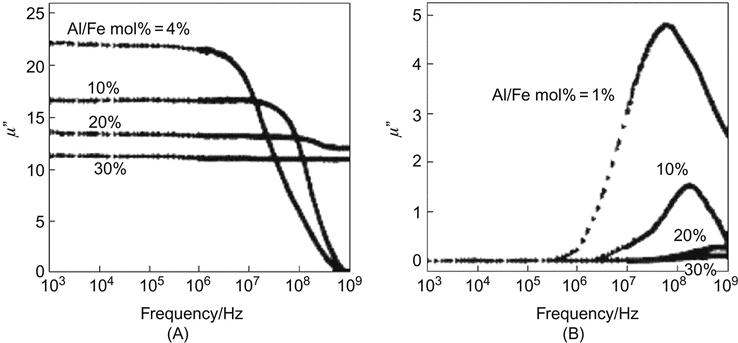
11.3.5 Applications of Core–Shell Composite Nanomaterials
As an ordered composite structure, core–shell composite nanomaterials have many properties that single nanomaterials do not have, and they have many new applications as well. The differences in the categories of nanomaterials that constitute a core–shell may lead to various target properties and applications. For example, the two kinds of semiconductor nanoparticles with different energy bands can be combined as indicated, and narrow band gap semiconductor particles can be used in the sensitization of semiconductor nanoparticles with a wide band gap. The result of such a match can improve material properties in applications like photovoltaic conversion, nonlinear optical properties, electric color conversion, solar cells, and high-density information storage devices. In recent years, some breakthroughs have been made in the preparation of water-soluble semiconductor nanomaterials. Compared with single-core semiconductor nanometer quantum dots, the core–shell structured compound semiconductor nanoparticles have shown superior optical properties; for example, core–shell nanoparticles may have their fluorescence quantum yield significantly increased and their stability greatly enhanced. In recent years, computer performance and computational tools have been and are being improved constantly. Researchers have been able to perform calculations based on the first principle of the core–shell structured compound semiconductor nanoparticles. These theoretical studies have greatly enhanced the understanding of the properties of core–shell structured compound semiconductor nanoparticles [10,11].
Core–shell nanocomposite structures composed of biocompatible polymers can protect the biological activity of bioenzymes, DNA, and other bioactive substances as carriers for controlled release of these substances to achieve targeted therapy. Nanoparticles embedded by using block copolymers or end-group functionalized polymers can have many new properties and reactivity on the surface, which means that the surface of the nanoparticles is modified. As for magnetic Fe3O4 nanocomposite structured particles coated with metal Au, because Fe3O4 is superparamagnetic, nano-Au can be used as a biologically active agent in clinical tests. Nanoparticles of this composite structure are expected to have superparamagnetic features plus the advantages of easy separation and easy modification of gold surface, making it easier to use. Regarding catalysis, the core–shell structured catalysts can be controlled to achieve a catalytic reaction. This structure can also protect the core material from chemical erosion from the external environment and prevent agglomeration of nanoparticles. By removing the core material, we can get a hollow nanocage, which can be used for nanoparticle synthesis reactors and separators.
