Nanobiological Materials
Nanoscale materials are smaller than many cellular components of the body. As such, there is massive potential to use nanomaterials for therapeutic applications, including drug delivery, tissue engineering, and medical diagnosis. Chapter 9 is dedicated to the use of nanoscale materials in the field of medical treatment and diagnosis. Topics included are biocompatible nanomaterials, targeted drug delivery, medical imaging techniques, and sensors for diagnosis.
Keywords
Biocompatible nanomaterials; nanocarriers for drug delivery; quantum dots; magnetic hyperthermia
Based on life science, modern biotechnology refers to the design and building of novel materials or systems with specific expected performances by using the features or functionality of organisms (or tissues, cells, and other components of organisms). It also integrates technologies with engineering principles to process goods or to provide services. The scope of this technology is very wide, including gene technology (e.g., “cloning”), which is used for reforming or restructuring the biological genes and enabling the recombinant gene to be expressed in cells to generate new materials that humans need: biological molecules engineering technology, which starts from the simple, common raw materials, is used to design the best route and select the appropriate enzymes in the synthesis of essential functional products; biological production technologies (e.g., fermentation) that use biological cells in a large number of processing, manufacturing products; biological coupling technology, by which the biological molecules and the electronic, optical, or mechanical systems can be linked together while the information captured by biological molecules can be amplified, transmitted, and converted into optical, electric, or mechanical information; nanobiotechnology, which can be used to study the fine structure of biological macromolecules and their functions in the nanoscale and to transform their structure to assemble molecular devices; and bionic technology that simulates biological objects or biological systems, organizations, and organ structure.
As an integration of nanotechnology and biotechnology, nanobiotechnology will become an important component of modern bioengineering. It has promising potential applications in the biomedical sector, electronics, materials, and environmental science.
Nanobiotechnology research is mainly focused on two aspects: first is the use of existing biological molecules such as DNA, proteins, and ion channels for the development of self-assembly nanotechnology and exploration of nanobiology; and the second is the use of processing nanomaterials and technologies, such as gold nanoparticles, branched molecules, quantum dots (QDs), and magnetic nanoparticles (magnetic fluid), and through appropriate chemical modification or in connection with biological molecules, making them applicable in detection, drug delivery or release treatment, biomimetic roles, gene transfer, cell separation, specific biological molecules, or separate calibration.
Nanoparticles for biological applications can be divided into two categories: organic and inorganic. Organic nanoparticles, such as the gelatin nanoparticles made from protein, can bond with DNA or peptides by modifying their surface amino acid content. Another example is the polymer nanoparticles from monomer polymerization, such as acrylic acid and styrene, in addition to the biomedical research purposes.
Inorganic nanoparticles, such as silica nanoparticles as a carrier of DNA and protein by chemical modification, are applicable to analysis detection and gene transfer purposes. Gold nanoparticles, magnetic nanomaterials, branch molecules, and QDs also fall in this category.
9.1 Nanobiological Materials [1,2]
9.1.1 Overview
Related to both life science and material science, biomaterials are natural or synthetic materials that are used for diagnosis, treatment, replacement, or repair of damaged tissues or to enhance function. Current applications cover a variety of biological materials, such as inert or active implant materials, drug delivery materials, dental materials, sutures, wound membranes, catheters, adhesives, and other materials that may have contact with body fluids. In addition, there are sensor materials, probing materials, and electrode materials that can be used in medical diagnostics and medical equipment.
In the context of this chapter, biological materials largely comprise biopolymers, with the rest including metallic materials for medical purposes, bioceramic materials, and biocomposite materials. Depending on function, biological materials can be divided into bioinert materials, biologically active materials, biodegradable materials, and bioabsorption materials.
Biocompatibility is the key to biological materials in research and applications. It refers to the chemical, physical, and mechanical reactions in the interaction of biological materials and organisms, including immune response, blood reaction, tissue reaction, and biochemical reaction.
There are two major types of applications of nanobiological materials. Novel nanomaterials that are developed with functional biological molecules are exploited for their self-assembly properties to create multifunctional materials. Other nanomaterials are suitable for biomedical applications and may or may not have biological activity without causing adverse reactions. The main materials are polymer nanoparticles, inorganic nanoparticles, and the nanostructured biomaterials in tissue engineering with specific identification and orientation-induced functions.
Polymer nanobiomaterials may include nanoparticles, nanomicrocapsules, nanomicelles, nanofibers, and nanopore structured biological materials. Polymer nanoparticles have particle sizes in the range of 1–1,000 nm, with a large surface area and the emergence of new properties and new features different from common materials. Polymer nanobiological materials can be synthesized by using methods such as microemulsion. Polymer nanobiomaterials exhibit their applications mainly in immunoassays, drug and gene carriers, DNA nanotechnology, gene therapy, nanoliposomes (the drug delivery system for imitated biological cells), biomolecular adsorption separation, invasive diagnosis, and treatment. Immunoassay here refers to the quantitative analysis of protein, antigen, antibody, and the cell as a whole. It can be divided into fluorescence immunoassay, radioactive immunoassay, and enzyme-linked analysis by means of the markers used in the process.
Immunoassay operations can be performed through the following three steps: in a specific carrier, covalent bonding corresponds to the immune affinity molecules of the analysis object; solution containing the analysis object is cultured with the carrier; and by detecting the amount of free carriers through a microscope, accurate quantitative analysis can be performed on the analysis object. Immunoassay carriers are usually selected as the polymer nanoparticles, particularly some of the particles with a hydrophilic surface. They have a very small amount of adsorption of nonspecific protein and are widely used as a marker carrier.
9.1.2 Drug and Gene Carrier Nanomaterials
In nanobiomaterial research, the current hotspots are drug nanocarrier and nanoparticle gene delivery technology. This technique is based on nanoparticles as drug and gene transfer vectors, by which the molecules for gene therapy, such as drugs, DNA, and RNA, are wrapped in the nanoparticles or adsorbed on the surface. At the same time, they couple with specific target molecules on the surface, such as specific ligands and monoclonal antibodies. The target molecules are combined with specific cell surface receptors and can find a way into specific cells for safe and effective targeting of gene therapy.
Drug nanocarriers come with the advantages of a highly accurate degree of targeting and high level of drug release control and can improve the dissolution rate of insoluble drugs and absorption rate, drug efficacy, and lower toxicity. Nanoparticles as gene vector have the following significant advantages: they can be used for wrapping, concentrating, and protecting nucleotides from degradation via nucleases; they have a larger specific surface area plus biocompatibility; they easily couple to specific targeting molecules on the surface to achieve specificity of gene therapy; cycle time in the circulatory system is significantly prolonged as compared with ordinary particles and, in a certain period of time, it will not be rapidly cleared by phagocytic cells like ordinary particles; they allow a slow release of nucleotide and effectively extend the time of action and maintain an effective concentration of the product to enhance the transfection efficiency and bioavailability of the transfected products; they have fewer metabolites, fewer side effects, and no immune rejection reactions.
Biodegradability is one of the features that cannot be ignored for drug carriers or gene vectors. Through degradation, carriers and drug/gene fragments can be directed into the target cell, keeping the surface of the carrier biodegradable, while the drugs contained in the core are released to provide a curative effect. In this manner, drug release in other tissues can be avoided.
Drug nanoparticle carrier (nanoparticle drug delivery) technology, as one of the important development directions of nanobiotechnology, will bring great changes to the treatment of diseases like malignant tumors, diabetes, and Alzheimer’s disease. Nanomaterials as drug carriers are largely divided into the following two categories: synthetic polymer materials, such as poly-alkyl cyanoacrylate ester (PACA, including methyl, ethyl, butyl, iso-ester, hexyl ester, as well as dissidents ester, hexadecyl ester) and polyester (mainly including polylactic acid (PLA), polylactide, polycaprolactone (PCL), PCL ester, poly-hydroxybutyric acid, glycolic acid, etc.) and their derivatives and copolymers. Drug-loaded nanoparticles prepared from these materials mainly include the following types.
9.1.2.1 Nanolilmsome
Nanolilmsome (lipid vesicles) has become one of the research hotspots in recent years. It is easy to prepare, convenient to use, and can be used for multipurpose administrations. It is a carrier with a phospholipid bilayer structure similar to that found in natural systems. Nanolilmsome as a drug carrier has unique advantages, including protecting the drug from degradation, helping drugs get to target sites, and reducing toxic side effects. Nanolilmsomes have had some issues, such as low encapsulation efficiency, rupture-prone liposome membrane, leakage, poor reproducibility, and in vivo instability and quick release. Preparation of nanoliposomes is mainly dependent on the ultrasonic dispersion method and reverse-phase evaporation method.
9.1.2.2 Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are prepared by the use of carriers of a variety of lipid materials, such as fatty acids, fatty alcohols, and phospholipids; the medicines are wrapped in lipid materials to make solid particles. SLNs have a certain sustained-release effect, mainly suitable for the wrapping of insoluble drugs. They are used for intravenous injection or local administration to achieve target positioning and controlled release, thus avoiding drug degradation and leakage. SLNs are mainly applicable to lipophilic drugs; for hydrophilic drugs, they have the defect of low encapsulation efficiency. SLNs are commonly prepared by the high-pressure emulsion smoothing method and the microemulsion method.
9.1.2.3 Nanocapsules and Nanospheres
Nanocapsules (NCs) and nanospheres (NSs) are mainly prepared by using biodegradable polymer materials, such as PLA, polylactide/glycolide, chitosan, and gelatin. They can be used to wrap hydrophilic or hydrophobic drugs. The route of administration depends on the performance of different materials, such as their intravenous targeting effect and intramuscular or subcutaneous sustained-release and controlled-release effects. NCs and nanoparticles in oral delivery can also be nondegradable materials, such as ethyl cellulose and acrylic resin. Preparation of such carriers is mainly through precipitation, emulsification-solvent evaporation, and other methods.
9.1.2.4 Polymer micelles
Polymer micelles are novel nanocarriers that have been developed in recent years, with both hydrophilic groups and hydrophobic groups. Dissolved in water, they will spontaneously form polymer micelles and complete the solubilization and wrapping of drugs containing a hydrophilic shell and hydrophobic core suitable for carrying different types of drugs. In addition, they enable drugs to escape phagocytosis by mononuclear macrophages, that is, they have a “stealth” feature. The hydrophilic chain segment is commonly made from polyethylene glycol (PEG), polyoxyethylene (PEO), and polypropylene, whereas the hydrophobic chain segments are made from PLA (P), polylactide glycolide, chitosan, and so on. The current study highlights the block copolymer PLA–PEG of PLA and PEG, whereas chitosan and its derivatives are being closely studied because of their excellent biodegradable properties.
Another type of nanoscale drug carrier is prepared from natural materials such as lipids, sugars, DNA, proteins, and so on. DNA nanotechnology is designed on the physical and chemical principles of DNA mainly used in molecular assembly. Self-assembly of nanoparticles is conducted in the following ways: a single strand of the DNA fragments is connected to the surface A of nanogold particles with diameter of 13 nm, and then another single-stranded DNA fragment with a complementary sequence is connected to the surface B of nanogold particles. A and B are mixed, then, in the DNA heterozygous condition, A and B are automatically linked together. Finally, based on the complementary features of double-stranded DNA, the automatic assembly of nanoparticles is completed.
Plasmid DNA can be used for gene therapy. After entering the target cells, it can repair the genetic errors or produce therapeutic factors (e.g., peptides, proteins, antigens). Nanotechnology enables DNA targeting in cells through an targeting role, and plasmid DNA will be condensed to the size of 50–200 nm and applied with negative electricity, contributing to the effective invasion of the nucleus. The process depends on the nanoprocess techniques available.
9.1.3 Bioceramic Nanomaterials
Bioceramic nanomaterial was developed in the mid 1980s. This type of new materials is composed of microstructures at the nanoscale level, with its grain size, grain boundary width, second phase distribution, pore size, and defect size only limited to the order of 100 nm.
Bioceramics are nontoxic, free from side effects, and have good compatibility with biological tissues. They are applicable in the production of artificial bones, bone nails, artificial teeth, dental implants, and nails within bone marrow, ranging from short-term replacement and filling to permanent transplantation. They have been developed from bioinert materials in bioactive materials. Nanobioceramics can enhance biological properties and mechanical properties, showing a unique superplasticity, whereas the increased chemical activity leads to higher biological activity.
Bone-like nanoapatite crystals and polyamide synthetic polymer can be synthesized into a complex as artificial nanobone, which can bond with the natural bones and grow tightly together with human muscle and blood vessels. In addition, it can induce cartilage formation, showing a variety of features equivalent to those of human bones. Bioceramic nanomaterials can also be used in making artificial eyes; by using nanoceramic material in the making of the shell of the construct, the artificial eye can be controlled by the muscles to move around, or electrical pulses are applied to stimulate the nerves of the brain to achieve the visual effect.
Bioceramic nanomaterials can be used for nanocell separation technologies, cell staining, as well as antibacterial function and sterilization. In nanocell separation, the first step is to produce amorphous SiO2 nanoparticles, with the size controlled within 15–20 nm. Its coated monomolecular layer works as the adhesion layer, and the finished size of coating is approximately 30 nm; then, in the second step, PVP colloidal solution containing multiple cells is produced by uniformly mixing the two and using centrifuge technology to separate out the desired cells. The intracellular staining technique requires selection of the type of antibody. Nanogold particles are mixed with prepurified antibodies or the monoclonal antibody to prepare a variety of (nanogold antibody) complexes; respectively, composite particles are combined with various intracellular organs and skeletal systems to form different complexes, by which certain featured colors will be rendered in the irradiation of white or monochromatic light, and thus different colored labels are affixed to various combinations.
Once biological materials are used in the human body, the surrounding tissue may be at risk of infection. Therefore, antibacterial operation and sterilization are especially important. In the reaction to synthesize hydroxyapatite (HA) nanoparticles, silver, copper, or another aqueous solution of soluble salts is added in the reaction, so that antibacterial metal ions are able to get into the apatite crystals. A typical representative of nanomaterials with bactericidal or antivirus features is known as photocatalyst titanium dioxide (TiO2) and works only in ultraviolet radiation, as described in greater detail in Chapter 10.
9.1.4 Magnetic Nanoparticles
Magnetic nanoparticles have a good surface effect, which is characterized by surge of specific surface, larger functional group density, and selective adsorption capacity, so the capacity for carrying drugs or genes is increased. In the physical and biological sense, paramagnetic or superparamagnetic iron oxide nanoparticles may experience a temperature increase up to 40–45°C under the influence of an external magnetic field, which can kill tumor cells.
Practical application of magnetic nanoparticles for biomedicine covers the following aspects: coating magnetic nanoparticles with polymer materials and combining them with targeting moieties. Then, they can be used as drug carriers in the human body. Under the action of an external magnetic field, magnetic navigation is applied to move the carriers to lesions to complete the treatment; 10–50 nm of the Fe2O3 surface coated with methacrylic acid may have its size increased to 200 nm and is able to carry proteins, antibodies, or drugs for cancer diagnosis and treatment. Its local treatment effect is good, with few side effects.
9.1.5 Biocomposite Nanomaterials
Inorganic–organic nanocomposite materials are most commonly found in biological organisms. Biomineralization refers to the process of minerals (biominerals) being formed inside a living body. In the biomineralization process, through the interactions on the interface of organic macromolecules and inorganic ions, inorganic mineral precipitation is controlled at the molecular level, so that biominerals have a special multilevel structure and assembly modes. During the process of biological mineralization, cell secretion can complete the auto-assembly of organic matter, which acts as a template for the formation of inorganic compounds, so that inorganic mineral has a certain shape, size, orientation, and structure.
To a large extent, the manufacture of nanobiological materials is inspired by the biomineralization process. In nature, the cell membrane of some bacteria may be mineralized at different levels. Outside the cell membrane, the protein molecules in regular arrangements can be used as a template to induce the synthesis of microstructured nanomaterials. Biomineralization provides an effective means for the design and processing of nanobiomaterials. US scientists have found a gender-peptide molecule; one end is the hydrophilic arginine-glycine-aspartic acid (RGD), and the other end is amino acids containing phosphorylated residues. RGD helps the adhesion of materials and cells, whereas the phosphorylated amino acid residue can interact with calcium ions.
Tissue engineering is the research and development of organism alternatives aimed at restoring, maintaining, and improving organizational functions by the application of the basic principles and methods of engineering and life science. The idea is first to isolate cells in vitro for culturing, and then a certain amount of the cells are grown onto a three-dimensional biological material to be cultured further; eventually, some tissues and organs with a certain structure can be formed. Stent materials play an important role in tissue engineering, because adherent-dependent cells are only adhesive to the materials before they can grow and divide. By imitating the natural extracellular matrix-collagen structure, nanofibers containing biodegradable materials are used in vitro and in animal experiments in tissue engineering; they demonstrate an attractive application potential. In composition, the currently reported nano-HA/collagen composite is an imitation of inorganic and organic components in natural bone matrix, with a nanoscale microstructure similar to natural bone matrix. The 3-D stent formed from porous nano-HA/collagen composite provides a microenvironment similar to the body for osteoblasts. Cells are able to grow well and secrete the bone matrix on such stents. Experiments in vitro and animal experiments show that such an HA/collagen composite is a good nanobiological material for bone repair.
As the biodegradable polymer material for tissue regeneration template, nanostructured tissue engineering stent material has the following properties: (1) good biological compatibility; (2) cells having good adsorption and proliferation at the material surface; (3) capability of inducing cell growth by prefabricated patterns; and (4) after tissues are grown, the materials can be degraded and excreted through metabolism (Table 9.1).
9.2 Nanobiomedical Materials
There are a wide range of nanobiomedical materials, from nanobiomedical materials (including drug carrier, drug release control, composite materials) to the nanodrugs getting into cells, as well as nanotechnology that is conducive to drug absorption, targeting, and controlled release. The following is a detailed description of both the inorganic and organic aspects.
9.2.1 Nanobioinorganic Materials
Nanoinorganic biological materials mainly include ceramic oxides, alumina, titanium dioxide, porous glass granules, nanocarbon materials, nanosilicon oxide (SiO2), and nanomagnetic particles.
1. Ceramic Oxides
Traditional ceramics have long been used to make artificial bones in clinical practice, as well as bone screws, artificial teeth and dental implants, artificial sponge free from impact of bearing weight, and bone intramedullary fixation materials. As for nanoceramics, its strength, hardness, toughness, and plasticity are significantly increased, so it has great future potential for artificial organs and other clinical applications.
2. Alumina, Titanium Dioxide, and Porous Glass Granules
The adhesion of alumina (Al2O3) and titanium dioxide (TiO2) on bone cells has been greatly improved, so they can be better combined with living cells. Nanoporous glass powder is a novel inorganic nanobiological material. Its applications in the biomedical field include functional matrix materials, microreactors, or a biochemical separation matrix, bioenzyme catalyst carrier, or the carrier of the drug controlled release system.
3. Nanocarbon Materials
Carbon is an essential element in human tissues. Studies of artificial blood vessels revealed that carbon has an excellent antithrombotic function. Nanocarbon materials are featured with fewer defects, large surface area, and compact structure. When it is used in artificial organs, artificial bones, artificial teeth, and artificial tendons, the strength, hardness, and toughness can be increased. It can also be used in blood purification systems to remove particular viruses or components.
4. Nanosilica
Nanosilica (SiO2) can be used to separate a very small amount of fetal cells from the blood samples of pregnant women at approximately 8 weeks of gestation and then accurately determine whether there are genetic defects in the fetus. It can also be used to detect cancer cells in the blood of a tumor in its early stages or check the muscle protein in blood to help treat heart disease.
5. Nanomagnetic Particles
Iron oxide (Fe3O4) with a diameter of approximately 10–50 nm, after being coated on polymer material, followed by externally binding with protein, can be injected into organisms. Nanomagnetic particles with polymer and protein can be used as a drug carrier. Through intravenous injection, such drug carriers can enter organisms. Navigated by an external magnetic field, it can carry the drug to the lesions.
9.2.2 Nanoorganic Biological Material
9.2.2.1 Nanopolymeric Biological Materials
Nanopolymeric biological materials can be generated from microemulsion polymerization, with particle diameters of approximately 10 nm. As such nanoparticles are much smaller than red blood cells and can move freely in the blood, and they can be injected into all parts of organisms for inspection and treatment of diseases. Also, they are suitable for the production of drug carriers in different forms and the biofilters used to separate biological substances. Among this type of materials, lactic acid–acid polymers can be used as drug carriers. Animal experiment results show that carriers containing dexamethasone may be effective in the treatment of arterial stenosis by arterial administration. Polymers containing the antiproliferative drug are appropriate in the prevention of coronary artery stenosis by coronary delivery.
Polymer materials can also be used for drug delivery. Nanoparticles with inclusive or surface-combined vaccines can provide sustained release of the wrapped antigens. Polymethacrylate nanoparticle anticancer drugs in nanoparticle sustained release are able to extend the retention time of the drug in the tumor, preventing the growth of the tumor. As tumors normally have higher vascular permeability, nanodrugs administered by intravenous operation can be transmitted largely within tumors, thus improving efficacy and reducing dosage and toxicity.
Nanopolymer materials can be modified to increase the targeting specificity toward the tumor. For instance, PEG-modified nanoparticles can reduce the uptake by the reticuloendothelial system while increasing the uptake of tumor tissue to avoid the toxicity caused by nonspecific aggregation. By the use of antibiotic therapy of intracellular infection, a study has shown that nanoparticle-coated Ampicillin may have an effect 20 times higher than the dissociative Ampicillin.
Polymer materials can be used as gene delivery systems as well. The use of poly-cyanoacrylate alkyl to adsorb oligonucleotides has been proven to play a role against RNase in both the buffer solution and a cell culture medium to prevent nucleic acid degradation. Cholesterol can be adsorbed to poly-cyanoacrylate alkyl lipid nanoparticles and then transfected into human bladder cancer cells. This complex can complement the proto-oncogene mutation area, and thus inhibit the proliferation of bladder cancer cells in culture medium.
9.2.2.2 Nanobiocomposite Materials
Many nanobiocomposite materials are derived from natural tissues, such as the human body; most such tissues are composite materials, whereas teeth and bones are nanocomposite materials composed of nanoapatite crystals and polymers. Through the imitation of natural hard tissues, some medical composite materials have been developed.
In 1994, British scholar W. Bonfield made high-molecular-weight polyethylene and nano-HA. In the study, he indicated that this kind of nanocomposite had somewhat worse mechanical properties. Dutch scholars studied the composite material from nano-HA needle crystals and polyactive polymer. Because of the use of a dry HA needle crystal, this kind of composite material is easy to gather into clumps that may affect the dispersion in the composite materials.
In natural bones, nanoscale HA materials deposit in the collagen matrix. In 1992, Yu-bao Li and associates, by using the hydrothermal synthesis process, obtained nanoscale synthetic bone-like apatite crystals, which are very similar to natural bone apatite crystals in shape, size, composition, and structure.
A solution environment can be directly used to prepare the polyamide (PA66)/nanocrystalline nano-HA bioactive composite. Nano-HA can be uniformly dispersed in the composite, with a content up to 60%. This figure is close to the ratio of natural bone apatite, able to form a bond at the interface between the two phases. Composite material is close to human cortical bone in respect to compressive strength, flexural strength, and modulus of elasticity, and it has cartilage-inducing properties. Animal experiments demonstrated that nano-HA/PA66 has good biocompatibility and biological activity and is an ideal material for bone repair.
9.2.3 Nanotechnology in Drugs
Nanomedicine is essentially a nanocomposite material, which is the nanostructural system assembled and synthesized according to the design of the human body. They are made of nanowire or tubes as the basic unit that is assembled and arranged in one-dimensional, two-dimensional, or three-dimensional spaces, and are associated with stability, gastrointestinal irritation, toxic side effects, high drug utilization, targeted drug delivery, and sustained-release effect.
We know that smaller drug particles have a greater dissolution rate than larger drug particles. So, by controlling drug particle size, we can control the dissolution rate of particles. For water-soluble drugs as oral tablets made of granules, particle size is the key to controlling a drug’s pharmacological effect. For nonwater-soluble drugs, stable water suspensions can be made for subcutaneous injection into the body and can travel with the blood circulation. In this case, the size of drug particles should be controlled very strictly to not block the blood circulatory system. For gas sol–spray pharmaceutical agents, particle size is the key factor determining its effectiveness. The current pharmaceutical particles can only reach the micron level.
In applying nanotechnology in pharmacy, QDs and magnetic nanomaterials have enjoyed a high priority in recent years. QDs are nanoscale metals and can be used as novel fluorescent labeling materials because of their light-emitting characteristics. Magnetic nanomaterials are mainly iron oxide nanomaterials with excellent biosecurity and biocompatibility. Because the nanoscale can lead to an improvement of magnetic properties, iron oxide nanomaterial has become an independent development in the field of biomedical testing and treatment.
9.2.4 Biochips
The biochip is an integrated assembly of one or a variety of biological activities on a small surface; by using tiny samples of physical or biological matter, it can simultaneously detect and study different biological cells, biomolecules, and DNA characteristics, as well as the interaction between them to acquire the life patterns of microactivity. The biochip can be approximately divided into a cell chip, protein chip (biological molecules chip), and gene chip (A-chip), all of which have the advantages of integration, parallel and rapid detection. It has become cutting-edge biomedical engineering technology in the twenty-first century. DNA chip, a representative of the biochip, has been developing rapidly recently and is typically symbolized by nanotechnology, integration, and multifunction. The scope of its use covers molecular biology, disease prevention, diagnosis and treatment, drug development, the development of biological weapons, judicial appraisal, and monitoring and supervision of environmental pollution and food hygiene.
The biochip in a narrow sense is a microarray, including DNA microarrays, oligonucleotide arrays, protein microarrays, and microarrays of small-molecule compounds. Addressable elements are fixed by way of a lattice to the surface of a certain size of substrate (silicon, glass, plastic, etc.). Each dot in the lattice can be regarded as a sensor probe. Biochip operation typically includes the following steps: first, the object of detection can be labeled using a chemical fluorescence method, enzyme label method, isotope marking method, or electrochemical method; second, the labeled object of detection getting into the biochip will respond at the appropriate address, and this specific address will be displayed; third, a scanner is used to search for biochip marks on the display for analysis with calculating software.
Biochips, in a general sense, can be used for rapid parallel processing and analysis of biological components or molecules. They are a kind of solid-thin device with a size of less than a cubic centimeter. Typical biochips include the microarray chip, filtration separation chip, dielectrophoresis separation chip, biochemical reaction chip, and capillary electrophoresis chip.
Instruments commonly used for observing and testing the biochip are the transmission electron microscope and scanning probe microscope. Transmission electron microscopy is required to be conducted in a high vacuum to observe dry samples. There are many types of scanning probe microscope, such as scanning tunneling microscope, atomic force microscope, magnetic force microscope, and scanning electrochemical microscope.
9.2.5 Future Development of Nanobiomedical Materials
The development of biomedical materials at the nanoscale is mainly focused on nanorobots, nanotargeted drugs, invasive diagnostic capabilities and intelligence, drug delivery systems, and medical complex material.
9.2.5.1 Nanorobots
Nanorobots are the most attractive field in nanobiology. Controlled by nanomicroelectronics, coupled with nanobiomedical materials and nanomedical inorganic materials and crystal structure, nanorobots will be made at a size even smaller than red blood cells of humans. This will certainly bring about a profound revolution in human health care. The first generation of nanorobot is an organic combination of biological and mechanical systems, such as a combination of enzymes and nanogears. Such nanorobots can be injected into veins to become molecular robots able to complete operations in blood vessels. These robots may get energy from glucose and oxygen dissolved in the blood, and they are able to explore any object they have encountered according to preprogrammed procedures. Molecular robots can perform a full medical check, clearing a thrombus and the fat deposits in heart arteries, eating bacteria, killing cancer cells, or monitoring the body’s diseases. The second generation of nanorobots is nanoscale molecular devices, which are assembled directly from the original elements, presented with specific functions. The third-generation nanorobot is a nanocomputer. This is a device equipped with the function of performing human–machine dialog. Such nanorobots, once successfully developed, would be able to complete several billion calculations within 1 s, thus bringing about a revolutionary change to humanity.
9.2.5.2 Targeted Nanomedicine
Drugs made in nanosize are directly injected into the lesion and will greatly enhance the medical effect and reduce side effects. As such, drug targeting will be a hot topic.
9.2.5.3 Capabilities and Intelligence of Invasive Diagnosis
Nanobiomedical materials will enable interventional diagnosis and treatment to develop in the direction of microtype, trace, and mini-invasive or noninvasive, and will help develop specific and intelligent materials.
9.2.5.4 Drug Delivery Systems
Nanodelivery systems can have a slow-release effect and create a novel route of administration. Under the premise of ensuring drug action, the system is designed to reduce the dose to reduce or avoid toxicity. Furthermore, the system can improve the stability of drugs, making them easy to store; a nano-controlled release system, through proper modification, can also come through the blood–brain barrier to deliver drugs to the central nervous system. Pills, such as “mini-pills” or “drug-loaded nanoparticles,” which contain sensors, storage capsules, and micro-pressure pumps, can be developed; “mini-pills” are pumped to the designated location inside the body to release drugs, which is a more effective method than the traditional ways of injecting or ingesting medicine. There are more advanced “nanomagnetic particles.” The United States has successfully developed a targeting drug with nanomagnetic materials as a drug carrier, known as a “biomissile.” Namely, drugs may be carried on the surface of the protein, wrapped with magnetic Fe2O3 nanoparticles, and injected into human blood vessels. Navigated through the magnetic field, it is transported to the lesions to be released. This can prevent the liver, spleen, and kidney from suffering side effects of drugs. Therefore, the nanodelivery system is a very promising form of drug dosage.
9.2.5.5 Medical Composite Materials
Nanobiocomposite material is widely present in the whole organism (e.g., bamboos, shells, bones, teeth), but real nanobiocompound synthetic materials are rare. With the simulation of human tissue composition, the structural and mechanical properties and bionic medical composite material of nanobioactivity appear to be very important in research.
9.3 Magnetic Particles in Medical Applications [3]
Miniaturization is the most significant advantage of nanomaterials in biomedical applications, but it also makes the separation of such materials more difficult. Although magnetic nanomaterials are also nanoscale particles but with magnetic properties, they are easily separated from other materials. This is the advantage of magnetic nanomaterials.
Magnetic nanomaterials are usually composed of iron oxide, e.g., Fe2O3 and Fe3O4. Among the current products, it is common to use embedded or coated magnetic particles in combination with antibodies in the cell separation and purification steps.
There are two ways to prepare magnetic nanomaterials: (1) if Fe2+ and Fe3+ are heated as a mixture, magnetic nanomaterials can be derived from the reaction precipitation, and (2) the mixture of Fe3+ and SO32− under alkaline conditions can lead to the formation of Fe3O4 nanoparticles.
Magnetic nanoparticles for biomedical purposes must be dispersed in aqueous solution. However, magnetic nanoparticles are generally composed of a single crystal, such as Fe3O4, MnFe2O4, or CoFe2O4. These single crystals are not soluble in water, so magnetic nanoparticles need to be coated on the surface with a layer of hydrophilic surfactant, so that magnetic nanoparticles can be stably dispersed in water. The solution is the so-called magnetic fluid. In magnetic fluid, magnetic nanoparticles are subject to the effect from the thermal fluctuation of water molecules. In the absence of an external magnetic field, the fluid has no spontaneous magnetic dipole. But when a magnetic field is applied to the fluid, the magnetic moment of magnetic particles in the liquid tends to follow the direction of the external magnetic field, resulting in a magnetic dipole. When the applied field is removed due to the thermal fluctuation of water molecules, magnetic nanoparticles will again show a zero magnetic dipole. This phenomenon is called superparamagnetism. It enables magnetic particles to have a strong magnetic field in the presence of a magnetic field, but after the removal of the magnetic field, magnetic properties will also disappear accordingly. Because of this, magnetic nanoparticles are traceable, recyclable, and can also be analyzed quantitatively. Fe3O4 was also the first to be used in biomedical magnetic resonance imaging (MRI).
Magnetic nanoparticles have another important feature, i.e., their size is similar to that of biological molecules. Therefore, the proper transformation of the biochemical characteristics of the particle surface will enable the combination of particles with specific biological molecules, giving biological molecules a magnetic marker. At present, this characteristic is often used in biomedical technology for immunoassay, cell separation, and transgenic applications.
Regarding cells, particular cells may have particular biological molecules on the membrane. For example, the surface of stem cells has CD34, a unique biological molecule. Some antibodies corresponding to these particular biomolecules, therefore, can be coated on the magnetic nanoparticles and mixed with blood containing the separation cells. These specific cells will be coated with magnetic nanoparticles. As shown in Figure 9.1, the black circles represent the magnetic marked cells and the white circles indicate other kinds of cell. So, when a magnet passes near the blood, due to the magnetic attraction, magnetic nanoparticles will make that particular cell (Figure 9.1, black circle) adsorbed by the magnet. Then, deionized water is used for washing the blood to remove the other kinds of cells adsorbed by the magnet, followed by removal of magnets; that is, the required specific cells will be separable out of blood.
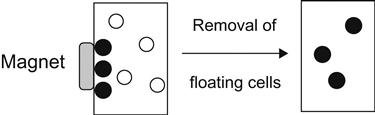
The black circles are the separated cells that have been magnetically marked, and the white circles are the other kinds of cells.
Compared with traditional separation technology, magnetic separation technology is simple and requires less time. Moreover, it has higher separation efficiency and specificity. This technique can also be applied to the purification of proteins and other biological molecules, and it has a considerable impact on the biological and chemical industries, so it is highly valued by academia and industry.
Advantages in magnetic drug delivery are numerous. First, the dosage of drug is less. The drugs can be delivered to the lesions highly concentrated without the need of surgery. This will not affect the rest of the body with side effects. Its combination with MRI can be used for development of new drugs for the analysis of the disease on cell activity, side effects, and usage.
But magnetic nanoparticles also have some disadvantages. For example, the drape modification method used in preparing them will make the particle size increase from 5–10 to 5,000 nm. So, further development is needed for this method.
Magnetic nanomaterials have great development potential as immobilized carriers of enzymes and proteins, DNA and RNA used for the reagents and tracing agents of MRI, as well as purification and separation agents for biochemicals, cell separation, calibration and control, drug delivery or gene therapy, and biochips.
The following highlights the application of the surface nanomagnetic particles coated with bioactive agents in drug delivery and medical treatment.
We know that cancer patients after tumor excision still need to receive radiation therapy regularly to kill cancer cells in the body. However, radiotherapy will inevitably harm normal cells, especially the bone marrow cells in the body, which are responsible for hematopoietic function and the immune system. Therefore, radiation therapy requires the patient’s bone marrow to be extracted first. After conducting radiation therapy on the cancer lesions, bone marrow will then be implanted. For terminal cancer patients, cancer-diseased cells often have spread to the bone marrow. After radiation treatment, cancer lesions will still be retained in the bone marrow that is to be replanted back into the matrix, so transplanting the matrix will just make the treatment procedures useless. So, before the bone marrow fluid is transplanted back to the matrix, the cancer cells must first be separated from the bone marrow fluid.
The following experiment is an example of separation with mouse bone marrow liquid to illustrate how magnetic nanoparticles work in cancer treatment processes. The experiment involved using Fe3O4 nanoparticles with particle size of approximately 50 nm and polystyrene with outer cladding diameter of 3 μm.
The experimental process of mouse bone marrow liquid separation can be summarized as follows:
1. Start by taking out antimouse antibody Fc, or immunoglobulin, from the sheep body.
2. Combine into complex molecules with magnetic nano-Fe3O4 particles coated with organic polymer.
3. Remove the bone marrow containing normal cells and cancer cells from the mouse body, and add in the hybrid antineuroblastoma antibodies, which will only combine with the cancer cells in bone marrow fluid.
4. Extract this antibody and adsorb it onto the magnetic nanoparticles covered with polymer. The obtained composite particles are injected into the bone marrow fluid containing the cancer cells, and then the antibodies on the magnetic particles will only combine with the cancer cells with antigen.
5. Using magnetic separation devices with nanomagnetic particles, cancer cells can be easily separated from the bone marrow fluid, with a differential rate of more than 99.9%.
In addition to cell separation, magnetic nanoparticles also have their application values in disease detection and drug treatment. Magnetic nanoparticles can be used as a drug carrier to enter into the animals through intravenous injection. Then, an appropriate magnetic field is applied in vitro in the animal to control the movement of nanomagnetic particles in the body. This is so-called magnetic navigation, which can guide the drugs to be released in a suitable location.
9.4 Nanoparticles in Bioanalysis
The rapid development of life science has highlighted many new issues of analytical chemistry currently focused on the analysis of peptides, proteins, nucleic acids, and other biological macromolecules, as well as biopharmaceutical analysis, ultra-trace analysis of bioactive substances, and even microbiological analysis. Therefore, biochemical analysis has become one of the most important frontiers in the development of modern analytical chemistry. Many researchers in analytical chemistry are trying their best to find new methods and techniques. The application of nanoparticles is one key initiative.
Fluorescence analysis is commonly used in the clinical determination of the content of certain elements in biological samples, of which the determination of RNA and DNA is of most importance. Fluorescent probes are commonly used in this method.
Normally, the fluorescent agent in such probes is an organic dye but, in most cases, their excitation spectra are narrow, so it is difficult to stimulate a variety of components at the same time, and the distribution of the spectra is asymmetric. Using nanoparticles as biological fluorescent probes can address these issues well. Compared with conventional fluorescent probes, nanocrystals have wider excitation spectra and a continuous distribution, whereas the emission spectrum is symmetrically distributed with narrow width and adjustable color; that is, nanocrystals of different sizes can be excited by a single wavelength of light to show different colors of light. Meanwhile, it has higher photochemical stability and is resistant to photolysis.
There are three types of nanoparticles that can be used as fluorescent tags:
1. Metal nanoparticles with optical activity.
The following is an overview of these three kinds of nanoparticles and their development prospects in bioanalysis.
1. Use of metal nanoparticles in bioanalysis
When gold (Au) particles reach the scale below 10 nm, the surface activity will be much larger, making it qualified as a catalyst. With the change in the size of gold nanoparticles, the phenomenon of wavelength shift will arise from its optical color due to the differences in quantum effects, from large to small, including yellow, orange (100 nm), green (50 nm), and red (13 nm). The 13-nm gold nanosolution, after the inclusion of NaCl, will result in condensation effects that change it from the original dark red solution to a blue one.
Preparation of Au nanoparticles can be achieved using the following three methods: (1) laser shot stripping method, which uses a laser to break gold blocks into nanoparticles; (2) gas phase synthesis, whereby gold atoms are changed into gas through gasification and condensed into nanoparticles during aggregation; and (3) chemical reduction, which uses hydrogen tetrachloroaurate and sodium citrate to produce the gold nanoparticles by mixing them in an appropriate proportion.
Gold nanoparticles are mainly used to probe the genetic labeled material and as a fluorescent quencher.
Recently, Elghanian and colleagues proposed a highly selective colorimetric method for polynucleotide detection. They used metal nanoparticles as the receptor, which was modified with polynucleotide alkanethiol. After hybridization, polynucleotide probes not only bind with the target polynucleotide by a specific sequence but also form a converged network with each receptor cell connected to a number of short double-helix fragments.
With the progress of hybrid, system colors will vary with the change in the optical properties of nanoparticles, because part of the optical properties of nanoparticles depends on their distance in the aggregation network. When this distance is much greater than the average diameter of particles, it appears to be red; and when they are approximately equal, it is blue.
This change is caused by the resonance of metal nanoparticles on the surface plasma. Hybridization can shorten the distance between particles, resulting in a corresponding color change and the formation of nanoparticle aggregates. Thus, by the color change, it can be determined whether hybridization has occurred.
Elghanian and colleagues also found that the DNA-marked gold nanoparticles, when placed at a high temperature (80°C) or in a higher concentration of salt solution (0.1 mol/L of NaCl solution), can remain stable for several days. This is mainly due to the surface of gold nanoparticles linked to DNA that prevents their integration with each other. This is very important for the hybrid, because DNA hybridization needs to be performed in salt solutions with high concentrations.
It was found in experiments that prehybridization solution was red; after hybridization, it is pink (or purple), and after being dried it is blue. If no hybridization occurs or if the temperature exceeds the thermal decomposition temperature, then it will show a pink color. Moreover, according to the change of the hybrid system color at approximately the thermal decomposition temperature, it is discernible whether a hybrid is an exact match.
This method is effective in detecting ultra-traces (10 fmol, i.e., 10–14 mol) of oligonucleotides, which can be widely used in designing a high-resolution nucleic acid detection system; moreover, because the equipment is low cost and easy to operate, it is particularly suitable for small laboratories.
2. Fluorescent latex NSs in bioanalysis
In contrast to single-dye molecules, each of the fluorescent latex nanoparticles (or nanoparticles) contains approximately 100–200 molecules, and each molecule also contains chromophores protected by the external environment. The protective effect of the emulsion arises from separation of different chromophores (as different chromophores generally contain conjugated ring structures, which, if not isolated, often resulting in π–π stacking, so that peaks will be widened and a red shift will result).
These fluorescent nanoparticles are difficult to break down and can emit stable fluorescent light (no flickering).
Taylor and colleagues used proteins labeled with nanoparticles to determine the specific order of the individual DNA molecules straightened.
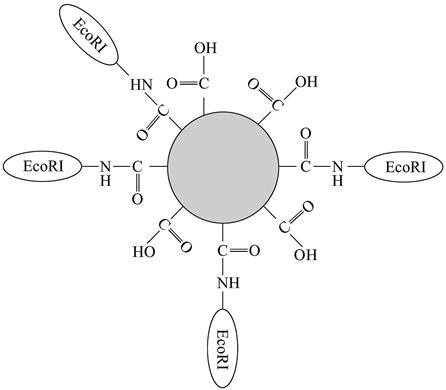
EcoRI enzyme can identify a specific sequence GAATTC of double-helix DNA molecule through 12 hydrogen bonds; it can combine with the 20-nm fluorescent nanoparticles through amide bonds (Figure 9.2).
This combination undergoes a reaction with the λ-DNA (there are five positions that can be combined), and then the DNA molecules after the reaction are straightened by applying the fluid mechanics theory and fixed onto the glass slides coated with polylysine. The experiment showed that EcoRI enzymes labeled by fluorescent nanoparticles can recognize and split a single λ-DNA molecule. Using a multicolor fluorescence microscope, we can see the green (530 nm) single DNA molecule and yellow orange (580–620 nm) NS. In this way, fluorescent images can reveal the location of connected nanoparticles. Experimental results showed that more specific orders on the same DNA molecule can be determined simultaneously.
Nanoparticles combined with organisms are very conducive to real-time observation and dynamic studies of the proteins and enzymes on a single DNA molecule.
3. Light-emitting QDs in bioanalysis
In recent years, the potential value of nanocrystal methods has caused great concern in immune biology and clinical testing studies. Nanocrystals are a special kind of nanoparticle characterized by a neat arrangement of atoms and a bulk crystal structure. A QD is a kind of nanocrystal that is a class of semiconductor nanoparticle made from semiconductor material. It is stable and soluble in water, with a radius of less than or close to the exciton Bohr radius. The next section is devoted to presentation of the light-emitting QDs in biological analysis. It must be emphasized that the application of the nanoparticles in biochemistry is a new area that deserves high priority. The development of nanoparticles in biological markers has brought new opportunities for a large number of multicolor experiments and diagnostics; they have tunable optical characteristics that make them available to be directly used as a probe or as a traditional probe sensitizer. Of course, there are still some problems using nanoparticles as biological fluorescent markers. For example, stable and efficient light-emitting nanoparticles are difficult to prepare, and their biological compatibility and accessibility of large molecules have yet to be further improved.
9.5 QDs in Biological and Medical Analysis [4]
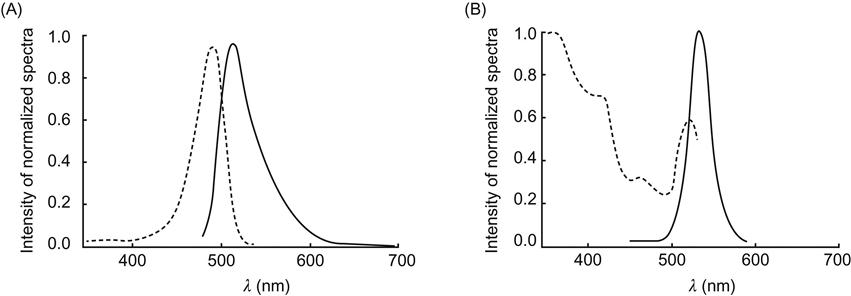
QDs have unique optical properties. A number of semiconductor QDs (nanocrystals) can emit laser-induced fluorescence, and fluorescent colors (peak position on the fluorescence spectroscopy) are controlled by the physical scale of QDs. They can be synthesized by using either a top-down crystal surface etching method or a bottom-up chemical method. In the 1970s, its application was mainly focused on the electronic and optical sectors. In the 1980s, biologists began to have a strong interest in QDs. But because of its lower fluorescence quantum yield, the work was focused on the research of the fundamental characteristics of QDs. Since 1997, preparation technology of QDs continued to improve and their application in biological research became possible. QDs available as biological probes can be traced back to two research groups: Alivisatos et al. [5] and Chan et al. [6] in 1998. The functions of QDs were further discovered and promoted, making them a popular field of biological research. QD emission has a wide wavelength range and a sharp emission peak, and the emission wavelength can be adjusted through nanoparticle size. This is particularly suited for markers of biological systems (Figure 9.3).
9.5.1 QDs in Biological and Medical Analysis
Fluorescence is a widely used analytical method, particularly in the clinical determination of the content of certain elements in biological samples. The traditional fluorescent labels’ absorption and emission wavelength are often restricted to a narrow range, with the intensity in continuous decline. If we use fluorescence spectroscopy directly to study them, then the bases and nucleic acids may have very low fluorescence quantum efficiency. Only tryptophan, tyrosine, and phenylalanine have natural fluorescence, so the best way to detect them is to use a variety of fluorescent probes.
At present, the fluorescent agent in such probes is an organic dye; however, in most cases, their excitation spectra are narrow, so it is difficult to stimulate a variety of components at the same time and the distribution of the spectra is asymmetric. The most serious flaw is the poor photochemical stability. QDs can absorb a wider range of wavelengths of light and emit a single specific wavelength. QDs have a structure of approximately hundreds to thousands of semiconductor atoms, with the size generally within 10 nm. This gives QDs atomic-like discrete energy levels. After QDs absorb the photon irradiation, an electron can skip the energy gap to an excited state. When falling from the excited state back to the ground state, the appropriate wavelength of photons can be launched. It is possible to design the QDs in different light colors. QDs can be created using group II–IV, CdSe, CdTe, CdS, and ZnSe materials, but also can be formed from group III–V, InP and InAs materials. In 1993, Bell Labs developed a highly efficient synthesis of light-emitting semiconductor QDs, thus beginning the utilization of QDs.
Compared with conventional fluorescent probes, the excitation spectra of QDs are wide and distributed continuously; that is, nanocrystals of different sizes can be excited by a single wavelength of light and emit the light in different colors, while the emission spectrum is narrow and distributed symmetrically. Meanwhile, it has higher photochemical stability and is resistant to photolysis. QDs as fluorescent probes have shown broad application prospects in the field of biomarkers and diagnostics.
QDs generally used in the biochemistry and medical fields function via QD bioconjugation. It is based on QDs as the core, followed by the formation of an organic outer layer by way of coating or chemical modification, which then combines with biological molecules to form a conjugate.
Bioconjugated QDs can be made in the following ways: the use of bifunctional base cross-linking agent for modification with a ZnS disulfide chain; hydrophilic adsorption; surface silylation; surface ionization; and micronanospheres.
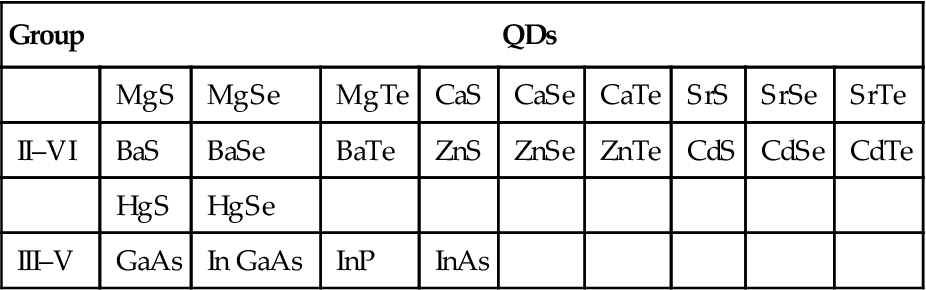
QDs highlighted in the research are mainly composed of group II–VI or III–V elements (Table 9.2).
Table 9.2
QDs Composed of the Elements in Groups II–VI or III–V
| Group | QDs | ||||||||
| MgS | MgSe | MgTe | CaS | CaSe | CaTe | SrS | SrSe | SrTe | |
| II–VI | BaS | BaSe | BaTe | ZnS | ZnSe | ZnTe | CdS | CdSe | CdTe |
| HgS | HgSe | ||||||||
| III–V | GaAs | In GaAs | InP | InAs | |||||
A QD is usually a core–shell-type nanobody, with CdSe as the nucleus and CdS or ZnS as the shell. Compared with traditional organic dyes, it has unique properties; QDs feature a large Stokes shift and a narrow, symmetrical fluorescence spectrum (Figure 9.4).
QDs have a broad range of applications, including a variety of fields and instruments, but their greatest application is for cell and disease detection. Compared with ordinary fluorescent substances, QDs are presented with a strong luminescent capacity and a longer light-emitting time. Nontoxic and easy to detect, they have a broad absorption wavelength range but only emit a single wavelength. They are valued as a future hot commodity to supersede fluorescent materials. QDs are available with a variety of colors in light emission. The color depends on the size of QDs. For the same excitation wavelength, there will be a range of excitation lights qualified for simultaneously detecting multiple targets. Other features of QDs include resistance against light-induced bleaching, safety, low toxicity to cells, suitable for living cells and in vivo studies, and long fluorescence (the fluorescence time is thousands of times longer than that of ordinary fluorescent molecules, which facilitates long-term tracing and storage of the results).
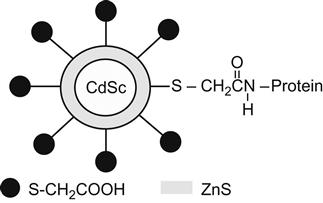
QDs can be used for ultra-sensitive detection of nonisotope-labeled biological molecules. For example, the surface of QDs can be connected to mercaptoacetic acid (HS–CH2COOH), so that QDs not only can be water soluble but also are able to be combined with biological molecules (e.g., proteins, multipeptides, nucleic acids). Then, QDs can be detected by photoluminescence, thus enabling biomolecular recognition of some specific substances (Figure 9.5).
QDs of different fluorescent characteristics can be combined into hollow polymer balls, creating fluorescent nanoparticles with different spectral characteristics and brightness features available to be labeled to biological macromolecules. Taylor and associates once used nanoparticle-labeled protein to determine straightened individual DNA molecules, where EcoRI enzyme could be combined with the fluorescent nanoparticles at a size of 20 nm through amide bonds and identify the specific sequence of double-helix DNA molecule through 12 hydrogen bonds.
Temperature may directly affect the size of QD particles. In general, the higher the T, the smaller the QD particles obtained, and the shorter the fluorescence wavelength. So, QD particles of different sizes can be displayed in different colors (Figure 9.6). This character can be used to track the activity and proliferation of amino acid receptors in nerve cell membranes.

The diversity of QD colors is able to meet the requirements of analyzing biological polymers (protein, DNA) that contain vast amounts of information. Polymers and QDs can be combined to form polymer beads, which are free to carry QDs of different sizes (colors) and begin to emit on irradiation. Transmitted through prism refraction, a variety of spectral lines of specified density (bar codes) can be formed. This type of bar code has a promising application potential in gene chip and protein chip technology.
QDs can also be used to detect the characteristics of DNA and proteins. Researchers have been able to bring a mixture of a variety of QDs encapsulated into rubber balls of millionths of a meter in diameter each, and they can radiate different colors of light. Researchers can use these rubber balls as different markers of gene sequences or antibodies to identify different DNA or antibody proteins. This provides a new approach to further probe the nature of DNA or antibody proteins. In addition, the hydrophobic modified polyacrylic acid used to coat QDs, combined with immunoglobulin G and streptavidin–biotin, can acquire accurate results for marking on the cell surface protein, cytoskeleton proteins, and the proteins in the nucleus. Its antibleaching performance is often of great value for the quantitative detection of fluorescent molecules and biosimulation of living cells.
Detection of tumor cells with QDs is also one of the hotspots in the current studies. Scientists may have the transferrin and QDs covalently cross-linked, so that cervical cancer cells can be “swallowed” into the cell. Then, QDs connected with transferrin still have biological activity to achieve long-term observation of single-color fluorescence labeling. They use two kinds of QDs of different sizes in marking the mouse fibroblasts: one emits green fluorescence and the other emits red fluorescence. Meanwhile, the QDs emitting red fluorescence are specifically marked on the cell actin filament, while QDs emitting green light are combined with urea and acetic acid. Such QDs have high affinity with the nucleus, and simultaneously the red and green fluorescence in cells can be observed to achieve the two-color fluorescent labeling.
Also, other applications of QDs in biological detection have been frequently reported. For instance, QDs were linked with biotin, urea, acetic acid salts, and certain antibodies, and they successfully achieved specific cell structures. A variety of molecules can be used as guidance materials for QDs, including nucleic acids, lipids on the cell membrane, proteins closely connected with the carrier protein or carrier sugar, as well as some drugs, by which QDs can be guided to a specific cellular structure. Researchers are committed to the application of QDs in neurotransmitter studies (for the understanding of neural signal transduction). They marked the QDs on an important neurotransmitter, 5-hydroxytryptamine, and then observed how the transporter protein in the process of promoting neurotransmitters returned to the cell after the intermittent signal was transmitted through adjacent nerve cells. This can be applied to medical imaging technology.
In general, because of its unique marking characteristics, QD technology will certainly develop into a cutting-edge technology for future biomolecular detection, providing a more advanced approach for DNA testing (DNA chips), protein detection (protein chips), and the exploration of the mechanisms of protein–protein reactions (antigen–antibody, ligand–receptor, enzyme–substrate). Furthermore, this technology will greatly enhance the development of bioimaging technology and biopharmaceutical technology, leading to a huge step forward for disease diagnosis and treatment.
9.5.2 QDs for In Vivo Studies
Early detection of cancer is a major issue in the modern-day medical profession. Traditional detection methods required the tumors to reach a certain size before they could be discovered, and the best treatment is often adversely affected by time. The ideal situation is when just a small amount of cancer cells arise in a local area, and they can be detected and treated.
Different CdSe/ZnS core–shell-type QD surfaces modified with different peptides can be injected into mice for postbiopsy analysis. The results showed that different peptide-modified QDs can be specifically applied to the lungs and vascular system of a normal or a tumor-afflicted mouse. These results indicated that QDs can possibly be utilized in studies of disease diagnosis and drug delivery (Figure 9.7).
Recently at the University of Cincinnati, Professor Donglu Shi and colleagues developed a multifunctional nano combination device [8,9]. Such nanoscale structure uses nanotubes as a substrate. After surface treatment, the outer surface of nanotubes can be coupled to connect QDs, which can be used to partially trace cells in vivo. Due to the strong luminance, QDs can be used for the imaging of the depth tissues (in vivo imaging) and characterization. After a special plasma coating, the nanotubes are loaded with an antibody that can indentify cancer cells on the outer surface to complete the so-called targeting role. The interior of the hollow nanotubes can be used to store anticancer drugs, which can be transported to the vicinity of cancer cells for controlled release to kill cancer cells, thus achieving a local treatment effect. This new method is far superior to conventional chemotherapy.
9.6 Research Progress of Nanomagnetic Materials in Hyperthermia
9.6.1 Background of Hyperthermia
After surgery, radiotherapy, and chemotherapy, the hyperthermic treatment of tumors has become a novel “green therapy” in treating cancer. Hyperthermia used as a treatment modality has a long history that can be dated back to 5,000 BC. It is said that breast tumors treated with heat were documented in the manuscripts of a Danish doctor named Edwin Smith. Legend has it that Hippocrates, the famous ancient Greek doctor, had used heat therapy in treating tumors and he had a proverb: “The disease which cannot be cured with iron can be cured with fire; the disease that fire cannot cure is incurable.”
In 1884, Bruns reported that a case of advanced melanoma infected with erysipelas was accompanied by high fever of 40°C; a few days later, the tumor disappeared. This contributed to survival of 8 more years. Later, people began to use artificial bacterial infection or the injection of a chemical-induced heat source to cause the patients to have a high fever. The most renowned research was performed by Coley, who published a number of articles in 1893 that described his artificial methods of infection-induced fever of 38–42°C. As a result, 12 of the reported 38 cases of advanced cancer were cured, which caused a sensation.
Nineteenth-century German scholars have presented many reports and literature on hyperthermia. Heating technology was then rudimentary and even used inferior methods such as hot needles, burning surface lumps with a small hot iron, or soaking limbs with hot water and local infusion with hot water for heating, and so on. With the application of the electric knife since the nineteenth century, Westermark took the initiative to use radiofrequency coils as radiators for hyperthermia in cervical cancer. After World War II, the microwave technology developed rapidly. In the 1960s, more systematic studies were performed regarding the heating technology in electromagnetic hyperthermia. Despite a long history of hyperthermia, its development was limited as a result of underdeveloped science and technology and backward heating methods and equipment. Until the 1970s, with the rapid development of electronic technology and multidisciplinary involvement and coordination, modern hyperthermic oncology gradually formed its own disciplinary system on the basis of a large amount of basic and clinical research. Ultimately, it developed itself into an emerging discipline integrating oncology, biothermal methods, thermal physics, and electrical and mechanical disciplines with computer and other technologies.
In recent years, the United States, Russia, and other European countries have performed a wide range of research on tumor hyperthermia, showing that hyperthermia is indeed effective against cancer. Hyperthermia in China began in the late 1970s and is currently available in forms of microwave hyperthermia, focused ultrasound hyperthermia, radiofrequency hyperthermia, and the latest generation of endogenous field hyperthermia. Microwave heating can only be applied to superficial cancer treatment because of its shallow penetration depth. Focused high temperature of ultrasound hyperthermia may be 90°C or more. With a higher penetrating power, it can be used to treat deep tumors. However, the limited nature of ultrasound prevents it from either penetrating the gas-bearing tissues or passing through bones, and therefore it is not a candidate for the treatment of lung and esophagus cancers or liver cancer that is blocked by ribs. Moreover, ultrasound must also rely on water as the mediator, because it cannot reach the tumor without going through water on the surface of the focus. Therefore, the location of the disease plays a decisive role and the cost of this kind of therapy is high. Radiofrequency hyperthermia can also be used to heat deep tumors, but it has to be applied through local water cooling. Meanwhile, subcutaneous fat is prone to overheating and pain, resulting in rapid development and difficulties in operation. Endogenous field hyperthermia is the latest generation of the hyperthermia system that is a combination of the advantages of various types of hyperthermia at home and abroad, without the need of water cooling. Patients can be safe and comfortable in a supine position, and this therapy is applicable to the treatment of tumors in the chest, abdomen, pelvis, and other deep positions. Its significant effect has greatly enhanced the patient’s quality of life.
Hyperthermia is the use of a variety of physical energy (e.g., microwave, radiofrequency, ultrasound) to produce thermal effects, so that the tissue temperature increases to the treatment temperature of 43°C and above to accelerate cell death. The role of hyperthermia in tumors includes the destruction of tumor blood vessels, tumor blood clots, and the inhibition of tumor angiogenesis. Statistics show the following: between 39°C and 40°C, the growth of most tumor cells is significantly inhibited; between 40°C and 41.5°C, tumor cell survival rate decreased rapidly; between 41.5°C and 43°C, tumor cells will die within a short period; and between 70°C and 120°C, the tumor cells burst instantaneously. Therefore, hyperthermia is a highly effective means of cancer treatment; it is safe, noninvasive, and is not associated with toxic side effects and infection. Currently, hyperthermia can be divided into two categories: whole body hyperthermia and local hyperthermia. The operation of whole body hyperthermia is less used because it is complicated and would cause strong systemic reactions, producing a certain degree of risk. Currently, local hyperthermia is the most applicable clinical tumor therapy; the most frequently used heat sources include IR, hot water bath, hot bath, ultrasonic waves, radiofrequency, microwave, and so on. Some experimental studies have shown that local hyperthermia not only can directly kill tumor cells but also can enhance the immune function. This possibly results from the high temperature applied locally that may lead to tumor degeneration and necrosis, and the absorption of necrotic tumor products will stimulate the body’s immune function.
There is a need for comprehensive treatment strategies rather than a single treatment of cancer, whereas surgery, radiotherapy, and hyperthermia are all local treatments. Systemic treatment with drugs, for example chemotherapy with traditional medicine, can provide better efficacy. Cancer is a systemic disease. If a local tumor has grown to a volume of 1 cm3, then other parts of the body may have small metastases that cannot be seen with the naked eye. Even with surgical excision, radiotherapy, or hyperthermia, there is still a need for coupling with systemic drugs before residual cancer cells in vivo can be wiped out, so that they cannot be left behind as the scourge of metastasis or have any recurrence in some parts of the body. Because hyperthermia is not a radical means and needs to be combined with systemic chemotherapy or traditional Chinese medicine treatment, efficacy could be enhanced by several times or even dozens of times. Here, the drug amounts are only one-third or one-half of the commonly used dose; therefore, the toxic side effects of the drugs are significantly reduced, whereas the treatment efficacy can be improved greatly. Patients with cancer are happy to accept such treatment because it can greatly improve their quality of life and allow new hope.
In this section, we highlight the use of nanomagnetic materials for hyperthermic treatment (Figure 9.8).
9.6.2 Magnetic Hyperthermia [10–13]
In magnetic hyperthermia, magnetic particles are transported to the treatment area; in an external alternating magnetic field, a hyperthermic effect is generated by the heat due to magnetic loss of the magnetic particles.
Magnetic hyperthermia has the following advantages. First is targeting within the tissues, and second is the bystander effect of heat treatment. A very good biocompatibility of materials can work to improve the efficacy of cancer chemotherapy or radiotherapy.
The main types of magnetic hyperthermia include embolism magnetic hyperthermia, liposome magnetic hyperthermia, intracellular hyperthermia, and whole body hyperthermia.
1. Embolism Magnetic Hyperthermia
In embolization therapy, iodized oil or hot brine is injected via a catheter into tumor blood vessels of liver cancer patients. Magnetic hyperthermia plus embolization hyperthermia yields magnetic embolization hyperthermia. Embolism magnetic hyperthermia is an organic combination of embolization and hyperthermia; it can play the role of combined therapy to improve efficacy and also enhance the targeting of hyperthermia.
2. Liposome Magnetic Hyperthermia
A liposome is a small body of closed vesicles, composed of a lipid bilayer with the aqueous phase inside. According to the structural properties of lipid membrane, liposomes can be divided into heat-sensitive liposomes, pH-sensitive liposomes, common liposomes, and reliposomes. As a targeted drug delivery system functioning with magnetic orientation, magnetic nanoliposomes have excellent drug-targeting capabilities and the unique role of local hyperthermia under the influence of an external magnetic field, showing attractive application prospects. Magnetoliposomes can combine hyperthermia with chemotherapy. Kubo achieved satisfactory efficacy by heating adriamycin containing 10 nm Fe3O4 and uracil for the treatment of the tumor osteosarcoma in hamsters.
3. Intracellular Hyperthermia
A variety of modifications of the surface of magnetic nanoparticles may improve the efficiency and selectivity of magnetic nanoparticles in gaining access to the tumor cells. And the role of the alternating magnetic field in vitro may promote the magnetic nanoparticles within cells to generate heat and kill the tumor cells. This method is called intracellular magnetic hyperthermia.
Studies show that cancer cells may absorb an amount of magnetic particles equivalent to 8 times to 400 times that of normal cells. Tumor cells containing ferromagnetic nanoparticles are susceptible to antimagnetic hyperthermia, so intracellular hyperthermia has excellent targeting features.
4. Whole Body Hyperthermia
Babincova and colleagues injected dextran nanomagnetic particles into the patient’s blood flow to perform whole body hyperthermia and effectively killed the cancer cells. This method can be used to treat malignant tumors with systemic blood metastasis or lymphoma-related diseases.
The development of magnetic hyperthermia has opened new avenues for cancer therapy. Drug-loaded magnetic liposomes with targeted controlled release successfully solved the problems of cancer chemotherapy with severe systemic side effects and rapid elimination of drugs in vivo; magnetic microspheres with small particle size and their targeting characteristics provide a solution to the problem of embolization failure and ectopic embolization in tumor embolization. The bystander effect of heat treatment is expected to solve the problem of uniform heating of cancer cells; tumor cells with high efficiency in absorption of ferromagnetic nanoparticles make magnetic hyperthermia unique to other forms of hyperthermia in uniformity and efficiency in killing tumor cells.
9.6.3 Magnetic Materials for Hyperthermia
Magnetic materials for hyperthermia are required to have a high specific absorption rate (SAR). Used in the human body, their biological safety must be guaranteed. In addition, the product performance should maintain certain stability. At present, common magnetic materials for magnetic hyperthermia include the following three kinds: Fe3O4; γ-Fe2O3; and carbonized iron, iron powder, and glass–ceramic iron oxide. These magnetic materials can be synthesized using physical or chemical methods. Physical methods are the colloid mill, jetting, impact, and tear-ultrasonic methods, whereas chemical methods include coprecipitation, precipitation oxidation, sol–gel, and metal organic decomposition.
Magnetic materials for magnetic hyperthermia have SAR related to the particle size. The SAR is high when the particle diameter is approximately 10 nm; if the particle size is between 100 nm and 1 μm, then the SAR is low. To improve the SAR, surface modification is typically needed for the magnetic materials to be prepared in magnetic fluids. There are two common surfactants used for the surface modification: water-based magnetic fluids (dextran, polyvinyl alcohol, PEG) and oil-based magnetic fluids (oleic acid, polyester).
9.6.4 Thermogenesis Mechanism of Magnetic Materials for Magnetic Hyperthermia
In the magnetization process and the magnetization reversal process, part of the energy in magnetic materials would be irreversibly transformed into heat and the loss of energy is called magnetic loss. Therefore, for magnetic materials for magnetic hyperthermia, the heat is sourced from the magnetic loss. Magnetic loss Wm includes the eddy current loss We, hysteresis loss Wh, as well as other loss arising from the residual magnetic relaxation or magnetic after-effect Wr, that is, Wm=We+Wh+Wr. Under normal circumstances, magnetic losses in ferrites are mainly residual loss and hysteresis loss; in metallic magnetic materials, mainly eddy current loss and hysteresis loss occur.
For a magnetic conductor in an alternating magnetic field, electromagnetic induction may generate eddy currents. This causes magnetic field strength H and magnetic induction intensity B to have an uneven distribution of amplitude and phase inside the materials. Meanwhile, this will make the phase of B lag behind that of H, thus increasing part of the energy loss, known as eddy current loss. Experimental studies show that for some metallic magnetic materials, the measured magnetic loss is much greater than the sum of theoretical calculations of the eddy current loss and quasi-static losses. The difference between experiment and theory causing the additional loss is called abnormal loss. Abnormal loss comes as part of the micro-eddy current induced in the vicinity of the domain wall by electromagnetic induction in the domain wall movement; it also partially results from the deformation of domain walls or domain wall pinning. It is noteworthy that abnormal loss accounts for a large part of the total loss in a number of metallic magnetic materials (e.g., silicon steel sheet).
Hysteresis loss comes from the irreversible magnetization process existing in the magnetic material (the irreversible displacement of the domain wall and irreversible dynamic behavior of the magnetic domain). In the quasi-static magnetic case, the hysteresis loss is proportional to the area of the hysteresis loop. In medium and strong alternating magnetic fields, the hysteresis loss of some metal-based magnetic materials is suitable for empirical formula Steinmetz ![]() , where f is the frequency and η and n are material-related constants. For example, for 3%Si–Fe alloy,
, where f is the frequency and η and n are material-related constants. For example, for 3%Si–Fe alloy, ![]() and
and ![]() .
.
Residual loss means all other losses apart from eddy current loss and hysteresis loss. It is caused by the magnetic relaxation processes in different mechanisms. In the low-frequency and weak magnetic fields, the residual loss is mainly magnetic after-effect loss and has nothing to do with the frequency. High-frequency residual losses include losses caused by size resonance, domain wall resonance, and the natural resonance. The residual loss dominates in ferrites.
Residual loss caused by magnetic after-effect is proportional to the frequency, domain wall displacement, and rotation damping of the magnetization vector. This loss is divided into two categories: Richter type and Jordan type. The former is related to temperature and frequency; the latter is slightly dependent on temperature and frequency. Richter-type loss is mainly caused by the induced anisotropy generated by the proliferation of impurities. Jordan-type losses are mainly caused by thermal fluctuations. Ferrite Richter loss is due to the proliferation of valence electrons between the ions.
In the high-frequency and ultrahigh-frequency areas at 104 Hz and above, the imaginary component of permeability on the ferrite spectrum related to magnetic loss μ″ may present several absorption peaks in different frequency regions. They correspond to the resonance loss, which is also a kind of relaxation loss. As the frequency increases, these absorption peaks will be caused by size resonance, domain wall resonance, natural resonance, and natural exchange resonance.
Studies have shown that magnetic particles approximately 10 nm in diameter will mainly have relaxation loss, showing a higher SAR; and hysteresis loss mainly occurs in magnetic particles of 100 nm–1 μm diameter, showing a lower SAR rate.
Two examples of the application of magnetic hyperthermia are described. Shinkai and colleagues used cationic liposomes (including Fe3O4 with a particle size of 10 nm) in the hyperthermia of rat glioma. Tumors in more than 80% of the rats disappeared completely. Brusentsov and associates used dextran-coated γ-Fe2O3 magnetic fluid (including γ-Fe2O3 with a diameter of 10 nm) in the hyperthermia of MX2 tumors in rats. Tumors in 33% of the rats showed complete recession, with the term of survival being extended by 150%.
Although the use of nanomaterials promoted the development of magnetic hyperthermia, there are still many problems in the application of magnetic hyperthermia. First is the need to improve the targeting of hyperthermia, the SAR of the materials, and the degree of biosecurity. It must be combined with other tumor treatment methods to achieve a satisfactory effect. Also, we must consider that the magnetic fluid should have the minimal residue in the body after treatment is completed. In general, nanomagnetic materials can be naturally excreted by the body. Furthermore, we can take advantage of the so-called magnetic field guidance technology to exclude the potential residual material. In addition, after the magnetic fluid enters the body, part of the fluid will inevitably find a way into normal tissue cells. We also need to conduct further studies on the impact of nanomagnetic materials on normal tissues, that is, their side effects.