Nanotitanium Oxide as a Photocatalytic Material and its Application
Titanium dioxide, or titania (TiO2), is one of the more versatile nanomaterials, with applications in photocatalytic water remediation, solar water splitting, and energy harvesting. Titania is a wide band-gap semiconductor, and thus exhibits many interesting light-driven properties. In Chapter 6, aspects of titania nanoparticles are discussed, including the physical principles of photocatalysis, preparation of nanoscale titania, and the various applications of titania.
Keywords
TiO2; photocatalysis; water remediation; sol–gel
In recent years, the word “photocatalyst” has appeared frequently in the literature. What is it exactly? Strictly speaking, a photocatalyst is a substance that can promote chemical reactions through the conversion of light irradiation but the photocatalyst itself does not change. The promise of photocatalysis is in its ability to use solar energy to convert high-density chemical or electrical energy. Similarly, it is possible to use photoactived chemical species directly in the degradation, mineralization, and hydration of various pollutants in the air. As such, photocatalysis has great potential in environment remediation and renewal energy applications.
The most commonly used photocatalysts are TiO2, CdS, WO3, ZnO, ZnS, Fe2O3, and SnO2. TiO2 catalysts have been the most studied and used photocatalytic materials because of their high catalytic activity, chemical stability, low cost, and nontoxicity.
This chapter is an overview of the development of photocatalytic technology and focuses mainly on TiO2 materials.
6.1 Principle of TiO2 Photocatalysis [1]
6.1.1 Development of Photocatalytic Technology
In 1972, Fujishima and Honda [2] discovered the photocatalytic decomposition of water on n-type semiconductor TiO2 electrodes, thus initiating the study of photocatalysis. This photocatalytic effect is sometimes referred to as the Honda–Fujishima effect. In 1976, Garey removed the chlorine in polychlorinated biphenyls (PCBs) using TiO2 photocatalyst. In the following year, Frank and Bard [3] achieved the photocatalytic oxidation of CN− into OCN−, symbolizing the start of the research of photocatalytic technology being applied in environmental remediation. Over the past decade, semiconductor photocatalysis technology has been developing rapidly in applied research on environmental remediation and solar fuel production.
With highly prospective applications, the current TiO2 photocatalytic technology may cover the following main research areas:
1. Preparation of nanocatalysts for efficient wastewater treatment and air purification;
2. Applications of TiO2 photocatalyst in coatings aiming to achieve application and industrialization in “self-cleaning” external and internal surfaces;
3. Research and application of TiO2 photocatalyst in ceramic sanitary ware aiming to realize the industrialization of antibacterial sanitary ware;
4. TiO2 photocatalyst applications in household appliances to achieve the application and industrialization of a self-cleaning series of appliances;
5. Use of TiO2 photocatalyst featured with the surface superhydrophilicity and photocatalytic activity for research and development of nanophotocatalytic thin film materials with self-cleaning and fog-resistance performance.
6.1.2 Principles of Semiconductor (TiO2) Photocatalysis [4]
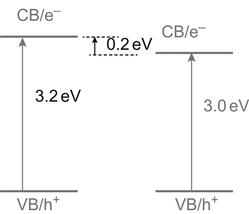
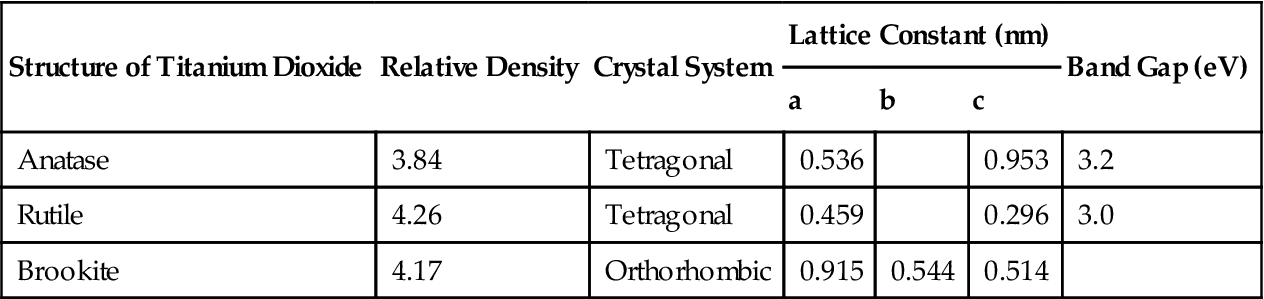
The basic physical properties of titanium dioxide crystals are as shown in Table 6.1. Titanium dioxide crystals may exist in three phases, anatase, rutile, and brookite, in which TiO2 with the anatase and rutile phases may have energy band structures as shown in Figure 6.1.
Table 6.1
Basic Properties of Titanium Dioxide Crystals
| Structure of Titanium Dioxide | Relative Density | Crystal System | Lattice Constant (nm) | Band Gap (eV) | ||
| a | b | c | ||||
| Anatase | 3.84 | Tetragonal | 0.536 | 0.953 | 3.2 | |
| Rutile | 4.26 | Tetragonal | 0.459 | 0.296 | 3.0 | |
| Brookite | 4.17 | Orthorhombic | 0.915 | 0.544 | 0.514 | |
From Figure 6.1 we can see that the anatase and rutile phases share the same position of the valence band, with a photo-generated hole of the same oxidation capacity. However, the anatase conduction band has more negative potential and more capability in photo-electron reduction. After being irradiated with photons with a wavelength of less than 387.5 nm on the surface, the electrons in the valence band will be excited onto the conduction band, resulting in highly active photo-generated holes (h+) and photo-generated electrons (e−) respectively on the valence band and the conduction band. Under different conditions, h+ and e− will be isolated from each other and migrate to the surface of particles at different locations. Through a series of reactions, they will generate oxygen-containing small-molecule active species (O2−, ·OH, H2O2, etc.). The TiO2 catalytic reaction equation is as follows:
In the degradation of water and organic pollutants in the air, the active photocatalytic oxidation of TiO2 has been proven to show remarkable results. Organic matter and bacteria can be decomposed and oxidized to CO2 and H2O2. Because the photocatalytic reaction will generate OH radicals (i.e., ·OH), and because their energy is usually larger than the bonding energy from the CH, OH, CC, and C![]() Cl compositions of general organic matter, they can easily cut off the bond between molecules.
Cl compositions of general organic matter, they can easily cut off the bond between molecules.
Practical application often involves the use of a mixed crystal effect based on these two-phase materials. Anatase and rutile mixed crystals may present a higher photocatalytic activity. This is because in the mixed crystal, a rutile thin layer will be formed on the surface of titanium dioxide anatase. This is a kind of coated composite structure effective in improving the separation of electron–hole pairs. The P25 variant of TiO2 is a popular example of an effective photocatalyst with mixed anatase/rutile phases.
Photocatalyst particles can effectively enhance the quantum yield, which favors the catalytic reaction. Photocatalysts can show the following nanoeffects:
1. Quantum Effects
When the semiconductor particle size is reduced to a particular critical value, the energy gap between the conduction band and valence band will be widened, and the light electrons and holes may have higher energies, and the oxidation–reduction capacity will increase accordingly.
2. Carrier Diffusion Effect
The smaller the particle size, the less time required for photo-induced electrons to spread from the crystal to the surface. This reduces the chances for recombination of electrons and holes, increasing photocatalytic efficiency.
3. Effect of Surface Area Increase
The factors of reduced particle size and increased surface area will enhance photocatalytic reactivity by exposing more reactive surfaces and allowing for more substrate adsorption.
TiO2 has the following characteristics: high photocatalytic activity, strong absorption of ultraviolet light (due to the large band gap), strong reduction and oxidation of photo-generated electrons and holes, chemical stability (against acid and alkali and photochemical corrosion), biological nontoxicity, no absorption in the visible area, availability for the production of white or transparent films, and a rich supply of raw materials.
Nano-sized titanium dioxide TiO2 surfaces contain a number of dangling bonds, which could constitute defect levels in the energy gap, enabling a very high surface activity of nano-TiO2. This can have a great impact on the optical properties of nano-TiO2.
Studies have found that nano-TiO2 can be equally effective in photocatalytic reactions with considerable pollutants in water, such as halogenated aliphatic hydrocarbons, dyes, nitroaromatics, substituted anilines, polycyclic aromatic hydrocarbons, heterocyclic aromatic compounds, hydrocarbons, phenols, surface-active agents, and pesticides. Therefore, TiO2 can be used for the detoxification, decolorization, and mineralization of water.
General bacteria (fungi, viruses) and odor are mainly composed of organic compounds, so the functions of deodorizers, antibacterials, and antivirals can be achieved through photocatalysis. Professor A. Fujishima and associates at the Faculty of Engineering at Tokyo University conducted some experiments and proved that nano-sized TiO2 has a strong bactericidal effect against Pseudomonas aeroginosa, coliform, and Staphylococcus aureus and can be used in decontaminating hospital operating tables, walls, jar tiles, and so on.
Nano-TiO2 can generate strong reducing capacity to restore heavy metal ions in water through light-generated electrons. Cr6+ in wastewater has strong carcinogenicity, with its toxicity being 100 times higher than that of Cr3+. TiO2 photocatalysis can restore 85% of Cr6+. This is of great significance in practical applications dealing with wastewater containing Cr.
Currently, nano-TiO2 is potentially one of the most effective optical catalysts. However, due to its wide band gap, TiO2 can only work in the ultraviolet region. As such, there is active research to find new photocatalytic materials that are active under visible/solar light, with much of the work focused on the modification of titanium dioxide [5–7]. In the early stage of studies, the idea of getting the TiO2 redshift to the visible light is concentrated in doping with metal ions or other metal-oxide semiconductor compounds. After 20 years of research, it was finally proved that while the modified cation is able to reduce the TiO2 band gap, it also brings about significant reduction of the optical quantum efficiency. This is because the doped metal ions themselves induce electron–hole recombination. Researchers at Toyota Motor Corporation proposed that doping with anions can improve the photocatalytic properties of titanium dioxide in 2001. They found that by replacing oxygen with nitrogen, the light absorption capability of the obtained TiO2−xNy photocatalytic materials was substantially more increased than that of pure titanium dioxide [5]. In September 2002, researchers from the United States found that carbon may be used to replace a portion of oxygen to obtain C-doped TiO2, which also experienced a substantial increase of its optical absorption in the visible region [6]. Although these studies have shown some increase in the absorption of TiO2 in the visible region with good photocatalytic efficiency, such methods are not suitable for practical purposes because the injected modifier N or C is unstable and easily breaks down under the illumination. So far, researchers have focused on the design of narrow-band TiO2 while improving its catalytic capacity [7].
6.2 Preparation of TiO2 Materials
TiO2 powder can be prepared through physical or chemical methods. The physical method includes the building method (e.g., gas condensate) and the crushing method (high-energy ball milling method). Chemical preparation of TiO2 materials and the comparison effects are shown in Table 6.2.
Table 6.2
Comparison of Titanium Dioxide Nanomaterials Prepared Using Chemical Methods
| Method | Precursor | Features |
| Precipitation | Ti(OBu)4 TiCl4 | Small size, evenly dispersed |
| Hydrolysis | TiCl4 | By control of reaction conditions, mixed crystalline and amorphous porous titania are available |
| Spray pyrolysis | TiCl4 | Electrolytes may affect the morphology and the size of aggregates |
| Sol–gel method | Ti(OBu)4 | Doping can be easily achieved, but the particle size is widely distributed |
| Oxidation–reduction | Ti+H2O2 | The mixed crystal with anatase and rutile can be obtained by calcining at 200 |
| Hydrothermal method | TiCl4 | Titanium dioxide in different crystalline phases can be prepared with a good degree of crystallinity |
TiO2 thin films can also be prepared in different ways, as shown in Table 6.3.
Table 6.3
Comparison of Preparation Methods of TiO2 Thin Films
| Method | Precursor | Features |
| Liquid deposition | Ammonium titanium fluoride | Transparent, uniform particle distribution |
| Sol–gel method | Ti (OBu)4 | Coating thickness is closely related to the number of coatings, plus dense thin film, small holes, particle of uniformity |
| Chemical vapor deposition | Titanium isopropoxide | All in anatase, without the rutile phase structure |
| Pyrolysis | Titanium isopropoxide | Film thickness can be effectively increased under control, with smooth film surface and uniform particles |
| Magnetron sputtering | Pure Ti | DC magnetron sputtering, with thickness of approximately 200 nm; high quality, high density, good combination, and strength |
TiO2 materials can also be prepared by modification of titanium dioxide and porous titanium in mesoporous materials:
1. Assembly of titanium oxide in zeolite (molecular sieves)
TiO2 is a polar molecule with surface polarization. This is not conducive to contact with nonpolar organic molecules. Vector may cause an increase of the catalytic activity of TiO2 due to its acidic surface or its participation in the intermediate process of the catalytic reaction. It is prepared by impregnation.
2. Pore surface modification of mesoporous titanium oxide materials
Mesoporous molecular sieves have a high specific surface area (>200 m2/g), in which the surface area in contact with titanium dioxide and the substrate molecule can be increased to improve its catalytic activity, in situ synthesis, or secondary synthesis.
3. Preparation of porous titania
Because the aperture is in the mesoporous range, substrate and resultants may rapidly proliferate in heterogeneous catalysis; titanium oxide nanocrystals in the powder can constitute micron-level aggregates with mesoporous holes inside, which not only have a higher activity but also are easy to filter and are quite significant for recovery and regeneration of photocatalysts. With high hydrothermal stability and thermal stability, its specific application can achieve further modification of its surface. Sol–gel synthesis preparation comprises two steps. The first is to prepare Ti–Si mixed oxides with controlled structure, and the second is to dissolve the silica contained within.
Light absorption of TiO2 is limited to the ultraviolet range, limiting its use of sunlight. In addition, it is easy to recombine the photo-carrier to bring about some impact on photocatalytic efficiency. To address these problems, some methods such as surface modification can be used to prepare composite TiO2 materials such as photocatalytic materials. In recent years, studies have shown that TiO2 optical absorption can benefit by the introduction of impurities or defects from deposition of precious metals, doping transition metal ions, organic dye photosensitization, as well as compound semiconductors and other methods. Meanwhile, quantum efficiency and photocatalytic reaction rate can also be increased accordingly [8].
1. Compound Semiconductors
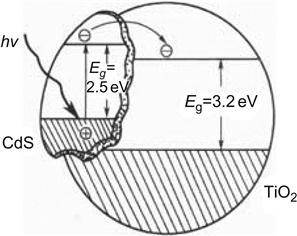
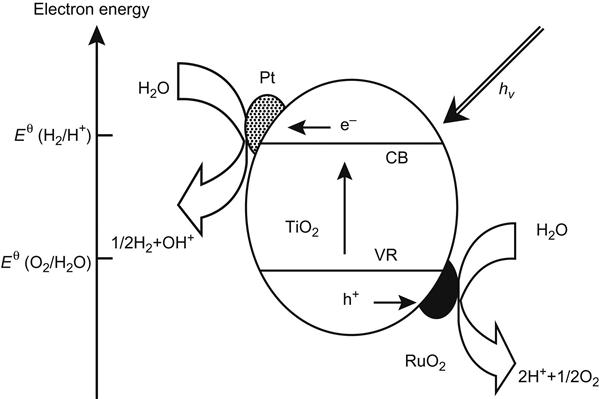
Compound semiconductors can effectively improve the charge separation efficiency and extend the spectral response range. The semiconductor compound methods for the modification of TiO2 include a simple combination of doping and multilayer structure, and the combination of different phases, as well as ion implantation and other methods. In recent years, CdS–TiO2, SnO2–TiO2, WO3–TiO2, ZrO2–TiO2, and V2O5–TiO2 have been reported to have shown photocatalytic properties that are superior to that of a single semiconductor [9,10] (Figure 6.2).
2. Metal Deposition
Pt, Pd, Au, Ag, and Ru are all commonly used inert metals, of which the most commonly used is Pt. The deposition of a layer of precious metals on the TiO2 surface is like building a short-circuit micro-cell with TiO2 and inert metal micro-electrodes, so that photo-generated electrons and photo-generated holes can be effectively separated to improve the photocatalytic properties of the catalyst. Studies show that the surface of TiO2 thin films deposited with silver, platinum, gold, palladium, ruthenium, and other metals can effectively improve its photocatalytic properties.
3. Ion Modification
The introduction of an appropriate number of metal ions can inhibit the recombination of photo-electronics and photo-holes to improve photocatalytic efficiency. Because metal ions can serve as an effective acceptor of electrons, they can capture the electrons in the conduction band. Competition of metal ions on electrons may reduce the raw carrier recombination, thereby enhancing the activity of the catalyst. Transition metal elements exist with multiple valencies. A small amount of transition metal ions (e.g., Fe3+, Cu2+), when doped into TiO2, can result in the change of TiO2 crystal lattice to introduce the lattice defects. Furthermore, this will form shallow potential wells to capture the photo-generated electron–hole pairs and lower the recombination risk of cavities and electrons to extend the recombination time, thereby enhancing the photocatalytic activity of TiO2. For the improvement of the quantum efficiency of TiO2 and the expansion of the light response range, a variety of metal ions can be chosen to conduct single-doping or multi-doping processes. Moreover, changing the concentration of the doped ions is combined with the use of different doping methods in an attempt to obtain the best possible doping effect.
6.3 Application of TiO2 as Photocatalytic Material
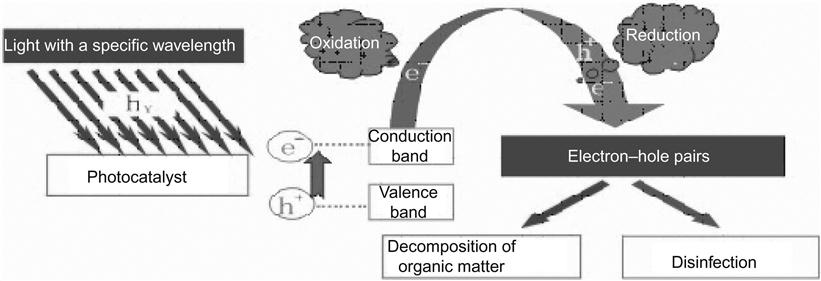
TiO2 photocatalytic material is applicable in many areas, such as sewage treatment devices, air purifiers, anti-fog and self-cleaning coatings, antibacterial materials, and photocatalytic decomposition of water [11]. Figure 6.3 illustrates the basic principles for photocatalytic purification.
1. Sewage Treatment: Solar Reactor for Wastewater Treatment
Traditional water treatment methods are constrained by limitations like low efficiency and high cost, in addition to secondary pollution and other problems. The development and application of nanotechnology may solve this problem. In addition to the aforementioned use of TiO2 photocatalytic reduction in dealing with wastewater containing Cr6+, solar reactors for wastewater treatment are also considered an important element.
Solar reactors use sunlight as the radiation source to conduct the photocatalytic reaction, which can degrade the organic pollutants in wastewater to reduce water pollution levels. Table 6.4 lists the various types of solar photoreactors with respect to their characteristics. The main organic photocatalytic degradation reactions are shown in Table 6.5.
2. Air Purifiers
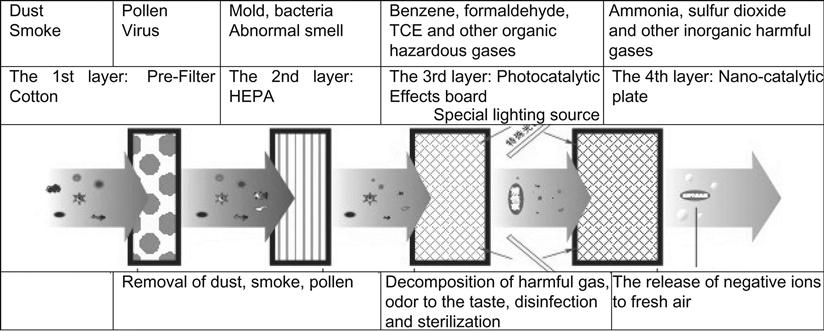
At room temperature, photocatalytic oxidation technology can be used to remove air pollutants with the help of air, water vapor, and oxygen. TiO2 as an air purification material is effective in degrading indoor and outdoor organic pollutants, and its oxidation functions can remove chlorine oxide, sulfide, and a variety of middle-atmospheric ozone. Also, TiO2 is effective in degrading indoor harmful gases, such as formaldehyde released from decorative materials, as well as methyl mercaptan, hydrogen sulfide, and ammonia arising from the environment. Figure 6.4 shows the basic purification process of a photocatalytic air purifier.
3. Applications in Anti-Fogging and Self-Cleaning Coatings
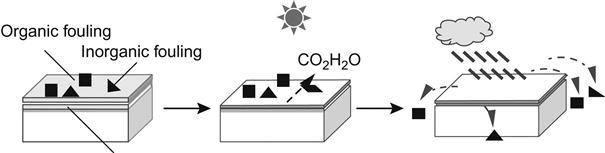
Under normal circumstances, the TiO2-coated surface has a larger contact angle with water and oily liquids such as ethylene glycol, n-hexadecane, and triolein. However, after UV irradiation, the water contact angle may decrease to less than 5°, or even down to 0°. This means they can fully seep into the surface of TiO2. Therefore, the surface of TiO2 shows affinity to both water and oil, and is a so-called superamphiphilic interface material. Therefore, bathroom mirrors, auto glass, and mirror surfaces, once coated with a layer of titanium oxide thin films, will have an anti-fogging effect. Because titanium oxide film may generate strong oxidation capacity and superhydrophilicity in sunlight, window glass, outer walls of buildings, highway guardrails, street lamps, and other surfaces coated with it will also have a self-cleaning effect (Figure 6.5).
4. Antibacterial Materials
Active superoxide ion radicals and hydroxyl radicals are able to penetrate the bacterial cell wall to enter bacteria by degrading cellulose. In this way, they may prevent the transmission of film-forming material through blocking their respiratory system and electronic transmission system, thus killing bacteria effectively. The present study of antibacterial materials includes TiO2 photocatalytic effects on bacteria, viruses, fungi, algae, and cancer cells. For example, there are antibacterial ceramic tiles and sanitary ceramics coated with nano-TiO2 films that are used in hospitals, food processing sites, and other sites; the walls of some hospitals and public places are coated with antibacterial materials containing TiO2 photocatalyst to inhibit the spread of germs. Some other applications include dentures containing nano-oxide titanium material, new types of dental bleaching agents containing nanometer titanium dioxide powder, and antibacterial fiber containing nano-titania powder.
5. Photocatalytic Decomposition of Water
Titanium dioxide can be used for photocatalytic decomposition of water to produce hydrogen and oxygen to provide clean, efficient, and safe energy (Figure 6.6).
Table 6.4
Solar Reactors Used for Wastewater Treatment
| Nature of the Sun’s Rays | Photocatalyst Status | |
| Suspended | Fixed | |
| Enhanced | Parabolic trough photoreactor | Parabolic trough photoreactor |
| Drop-down film photoreactor | ||
| Nonenhanced, with a reflective device | Compound parabolic concentration photoreactor | Tubular photoreactor |
| Optical fiber photoreactor | ||
| Nonenhanced, without a reflective device | Solar pool | |
| Tubular photoreactor | ||
| Plate photoreactor | Plate photoreactor | |
| Drip-style flat photoreactor | Drip-style flat photoreactor | |
| Double-shell flat-plate photoreactor | Double-shell flat-plate photoreactor | |
| Drop-down film photoreactor | ||
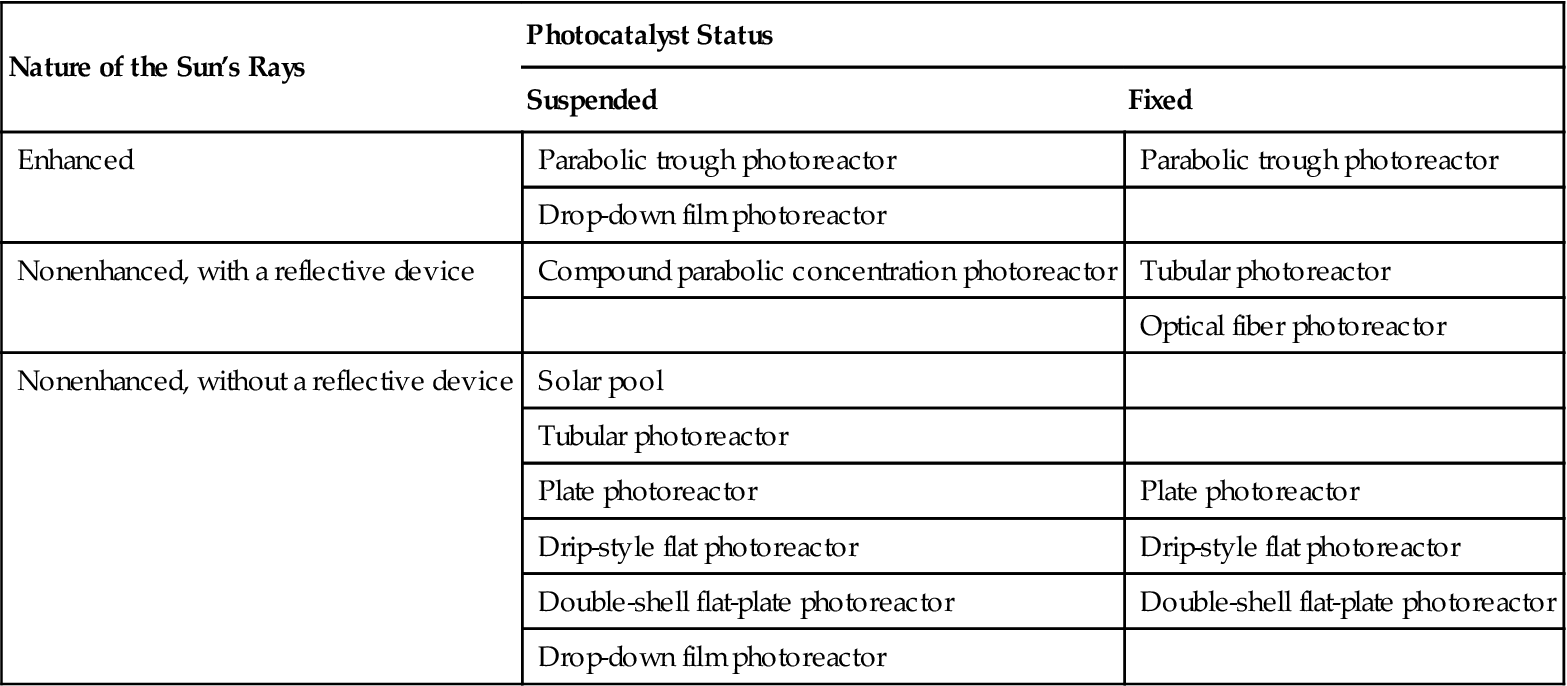
Table 6.5
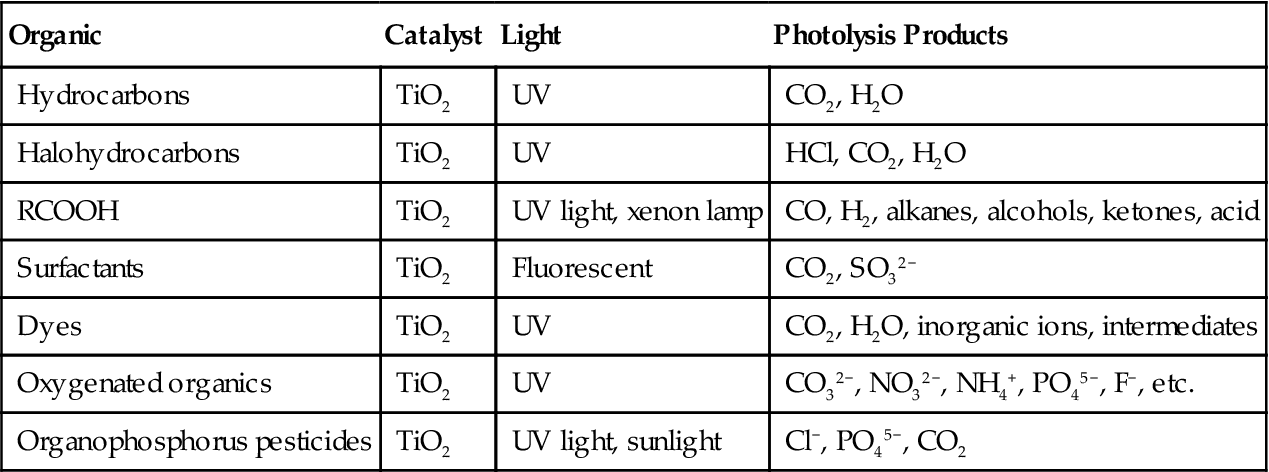
Photocatalytic Degradation Reactions of the Major Organics
| Organic | Catalyst | Light | Photolysis Products |
| Hydrocarbons | TiO2 | UV | CO2, H2O |
| Halohydrocarbons | TiO2 | UV | HCl, CO2, H2O |
| RCOOH | TiO2 | UV light, xenon lamp | CO, H2, alkanes, alcohols, ketones, acid |
| Surfactants | TiO2 | Fluorescent | CO2, SO32− |
| Dyes | TiO2 | UV | CO2, H2O, inorganic ions, intermediates |
| Oxygenated organics | TiO2 | UV | CO32−, NO32−, NH4+, PO45−, F−, etc. |
| Organophosphorus pesticides | TiO2 | UV light, sunlight | Cl−, PO45−, CO2 |