DNA Nanotechnology
Nanoscale materials are not exclusively synthetic materials. Every living thing on Earth consists of nanoscale materials, such as cellular components, DNA, proteins, and so on. DNA in particular is an interesting nanoscale biopolymer, as not only is it the “molecule of life”, but has many other interesting properties making themselves useful in many nonbiological applications. Chapter 12 summarizes the research done by the nanotechnology community, including DNA-based electronics and metallic nanowire formation.
Keywords
DNA; DNA-FET; metallic nanowires; molecular motors
12.1 Basics of DNA
12.1.1 Unique Structure of DNA
DNA is a biomacromolecule that plays a very important role in storing the genetic information of organisms. It is formed by four neucleotides, each with a unique side change base: adenine (A), guanine (G), cytosine (C), and thymine (T). The commonly envisioned picture of DNA consists of a helical structure of two individual DNA strands brought together by a series of nucleotides. Between the two single strands of DNA, nucleotides will form hydrogen bonded pairs of bases at corresponding positions. These nucleotides are mutually complementary: A combined with T, C combined with G. Through this base-pairing, the single strands twist together to form the famous double helix.
The DNA sequence (the series of bases along the polymeric chain) contains all the genetic information of organisms that is ultimately responsible for the organism’s growth, reproducibility, adaptability, and function. Specific fragments of DNA are known as genes or coding regions that guide cellular protein synthesis. Other areas of note on the DNA molecule are the noncoding region (with no specific role or an undiscovered role) and some regulation and control areas. The human DNA sequence is as long as 3 billion nucleotides, consisting of approximately 30,000 genes, with a noncoding region accounting for more than 75% of all nucleotides. The shortest known sequences belong to viruses, which number in the range of a few thousand nucleotides. By comparison, bacterial DNA commonly contains approximately a few million nucleotides. These short sequences exhibit a lower percentage of noncoding DNA, which is sometimes zero for most efficient use.
Since the discovery of the DNA double-helix structure by Watson and Crick, the biochemical function of DNA has been extensively studied. Molecularly, the structure is modestly simple, consisting of two single chains that are wound into a double helix directed by base-pairing. By various treatment methods, the double helix can easily be separated into the two single chains. Moreover, two individual DNA strands containing matched base pairs (sometimes referred to as complementary chains) can be brought together to form a helical structure. This attribute of DNA is useful in the study of biological and chemical properties of DNA molecules and make the material useful in the fields of nanotechnology and nanoelectronics.
12.1.2 DNA Conductivity [1,2]
The electrical nature of DNA was proposed as early as the 1960s. While the fundamental electrical properties of DNA can help assist in the understanding of the biological properties and processes this material has in nature, others have expressed interest in the material for the electronics field. Presently, 90-nm scale processes are widely used in commercial integrated circuit manufacturing, with smaller size scales of 65 and 45 nm now also arriving on the market. Although there is a push for smaller scale materials to increase the performance of integrated microchip technology, current manufacturing techniques have been developed using classical physics. Quantum effects would gradually become evident as the size scales continue to shrink, resulting in unpredictable and often undesirable materials features. Therefore, as the sizes of electronic devices are pushed to 10 nm or less, a paradigm shift is required with completely different materials and technology. DNA may play an important role in this field, which is discussed in detail later.
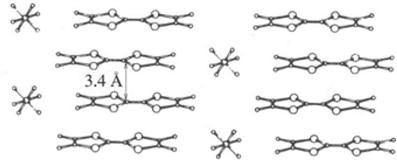
In the double-helix structure, the base pairs are parallel to each other and arranged axially due to strong intramolecular π-electron interactions in the bases (sometimes referred to as the π-stack). The average distance between the base pairs is 0.34 nm (Figure 12.1), similar to the spacing between the planes of carbon atoms in graphite. Due to the interactions of π-electrons between lamellar regions of graphite, electron conductivity is achievable in the lamellar direction. DNA base pairs have electrons in a similar orientation, with base pair spacing of identical length compared with that of graphite. Electron transfer between the base pairs of DNA molecules is dependent on the direction of electron mobility, wherein longitudinal electron movement along the DNA axis gives the material conductive properties. In fact, some conductive organic molecules and polymers have a similar atomic structure; however, these materials typically exhibit higher degrees of intramolecular π–π interactions and are more crystalline in nature than DNA. Disordered systems lacking periodicity tend to result in localization of electrons that impede electron mobility and, hence, are not conductive; this is called Anderson localization. That stated, DNA sequences are not completely disordered systems, so the electrical properties can be between those of a conductor and those of an insulator.
Interest in DNA conductivity is not only driven by fundamental knowledge of biological processes but also driven by the potential of DNA in electronic applications. The current integrated circuit (IC) manufacturing industry has reached feature sizes of 90 nm with respect to the process of the main volume (circuit wire width) and is continuing to push toward smaller sizes of 65 and 45 nm. Nevertheless, we know that quantum effects will become more evident as the size continues to be reduced. At this scale, traditional processes and materials will fail to produce usable technology utilizing extremely small scales. Therefore, electronic devices with fewer features require completely different materials and technology.
To determine whether DNA can be used as nanoscale wires in future ICs, direct electrical measurements of single DNA molecules are required to establish possible viability. To accomplish this, the DNA molecule can be directly tethered between the two electrodes as shown in Figure 12.2. To perform this experiment successfully, two aspects of the experiment require careful attention. The first critical aspect is the preparation of electrodes, wherein smaller electrode spacing can provide more reliable measurements, with a minimum value of only a few nanometers (Figure 12.2). The next step is to reliably link the DNA molecules to the electrode while maintaining good contact between them.
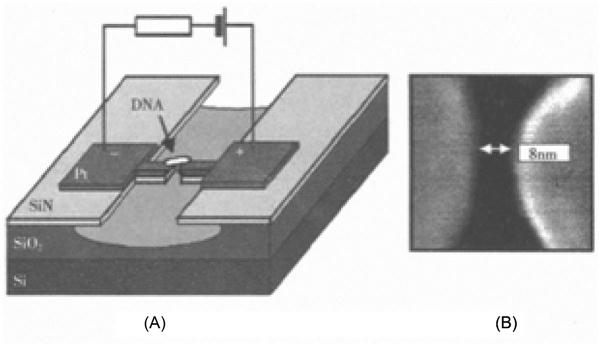
There have been numerous experimental studies of the electrical properties of DNA, with reports of insulating, semiconducting, conducting, or even superconducting properties. Such variation is not surprising because the experiments were performed using different DNA sequences, preparation, and measurement methods and conditions. As stated, an electron microscope experimental setup can be used to study a DNA chain between two metal electrodes for direct measurement of the current–voltage relationship. This experimental setup can be problematic, unfortunately. Contact quality between DNA and the electrode will seriously affect the measurement. If a poor DNA–electrode interface is obtained, then the results are unreliable. Alternatively, if a short circuit is present between the electrodes, then the results of measurements may show DNA conductivity, even if the DNA is in fact insulating. In this regard, current experimental techniques still need further advancements.
Theoretical studies are an attractive route to study DNA conductivity and are most commonly pursued using two methods: first principles calculations and model calculations. First principles calculations rely solely on the basic laws of physics, namely of quantum mechanics. Specifically, the first principles calculation takes into account all the atoms (more accurately the outer electron shell) and all types of interaction for the entire system to solve the Schrödinger equation. From introductory quantum physics, one should remember that an exact solution is not directly available but can be approximated using the Hartree–Folk (HF) method or density functional theory to seek the approximate solution. The advantage of this approach is that it is straightforward, starting from the basic laws of quantum mechanics without the addition of adjustable parameters. The main drawback, however, is that the complexity of the calculation increases exponentially with an increasing number of atoms. This often results in the modeling of smaller systems. In the context of DNA, this method is usually only used to calculate properties of very short sequences because larger sequences are computationally expensive. The addition of solution and counter ions, making the simulation more realistic, further compounds this issue. As such, it would be impractical to use first principle calculations to study the conduction of electric charges in longer DNA (more than a dozen nucleotides).
Model calculation is also known as the “effective model” approach. Regarding DNA conductivity, one approach is to use experimental data or first principles calculations to simplify a model DNA system for the establishment of a relatively simple model. For example, first principles calculations can obtain all electronic energy levels and wave functions for the small segment of a DNA molecule. At room temperature, electrons at the lower molecular orbitals are very difficult to move, so we only need to consider the energy of electrons in the highest occupied molecular orbital (HOMO) and the energy level and potential occupation at the lowest unoccupied molecular orbital (LUMO). Further approximations can then be made. Each nucleotide is considered an individual unit (or an imaginary “atom,” if you will), with each unit possessing two energy levels: the LUMO and HOMO mixed via the adjacent LUMO and HOMO orbitals, and the respective ionization potentials of four different nucleotides. Thus, the calculation can be governed by the charge on the potential energy as a stop at each nucleotide as well as the kinetic energy as it moves to the adjacent nucleotides. With this approximation, DNA molecular structure has been greatly simplified and thus can be utilized for calculations of longer DNA sequences. Of course, the results from these calculations must be taken with a grain of salt, given that the level of simplification may not be entirely accurate in a physical system.
These methods have advantages and disadvantages, with the results being heavily influenced by lack of current computational power or oversimplification. Ultimately, the merit of computational results depends on how well the calculations reproduce experimental data without user bias.
12.1.3 Simplest Equivalent Model of DNA Conduction [3]
The simplest theoretical treatment defines a DNA molecule as a one-dimensional periodic structure. With this approximation, concepts in solid-state electronic band theory can be used to theorize the conductivity of DNA. Depending on the implementation of theory, DNA is either a semiconductor or an insulator.
The method introduced in the following part is one of the effective tight-binding models; the hole (in the DNA, the hole is the charge for electrical conductivity) has a Hamiltonian that can be approximated as follows:
(12.1)
Physicists are very familiar with the mathematical form of this Hamiltonian, which is, to them, the Anderson localization model used in the study of disordered systems. Here, ![]() is the operator for generation (elimination) of an i-point electron (hole).
is the operator for generation (elimination) of an i-point electron (hole). ![]() is a random diagonal block that follows a certain distribution in the [−W/2, W/2] interval, and W is the so-called disorder degree. N is the number of grid points and
is a random diagonal block that follows a certain distribution in the [−W/2, W/2] interval, and W is the so-called disorder degree. N is the number of grid points and ![]() is the transition matrix element. If
is the transition matrix element. If ![]() is disordered and if
is disordered and if ![]() is a constant, then we refer to it as diagonal disorder; if
is a constant, then we refer to it as diagonal disorder; if ![]() is ordered and
is ordered and ![]() is disordered, then it is referred to as nondiagonal disorder; if
is disordered, then it is referred to as nondiagonal disorder; if ![]() is disordered and
is disordered and ![]() is also disordered, then it is called total disorder.
is also disordered, then it is called total disorder.
Equation (12.1) can be rewritten in the form of a tight-binding Schrödinger equation:
(12.2)
This equation is then rewritten in matrix form:
(12.3)
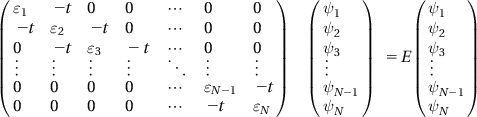 (12.3)
(12.3)
Through diagonalization of this Hamiltonian, we can get the intrinsic energy and wave function of the system.
For DNA, potential energy ![]() is not entirely generated by random numbers like disordered systems but depends on the arrangement of the nucleotide sequences of DNA. In Eq. (12.1), the first item (
is not entirely generated by random numbers like disordered systems but depends on the arrangement of the nucleotide sequences of DNA. In Eq. (12.1), the first item (![]() ) is the kinetic energy of the hole for a transition from the lattice point i to lattice point i+1. In a strict definition, this term is concerned with the nucleotide species at the two points; however, to simplify the model, all current transition items are first treated as equal, that is
) is the kinetic energy of the hole for a transition from the lattice point i to lattice point i+1. In a strict definition, this term is concerned with the nucleotide species at the two points; however, to simplify the model, all current transition items are first treated as equal, that is ![]() . The second term (
. The second term (![]() ) is the potential energy for the hole to remain stationary at the lattice point i, related to nucleotide types at that point:
) is the potential energy for the hole to remain stationary at the lattice point i, related to nucleotide types at that point: ![]() .
.
In this model, according to the results of first principles calculation, ![]() is 8.24, 9.14, 8.87, or 7.75 eV for A, T, C, and G nucleotides respectively, and represents the potential energy at the lattice point i. It is important to note that these values are calculated on “gaseous” nucleotides, that is, isolated nucleotides in vacuum conditions. In biological systems, the potential energy values will become lower. As for the kinetic energy
is 8.24, 9.14, 8.87, or 7.75 eV for A, T, C, and G nucleotides respectively, and represents the potential energy at the lattice point i. It is important to note that these values are calculated on “gaseous” nucleotides, that is, isolated nucleotides in vacuum conditions. In biological systems, the potential energy values will become lower. As for the kinetic energy ![]() , its value lies in the range of 0.03–0.4 eV. Because of the influence of the chemical and biological environments, however, it will result in larger
, its value lies in the range of 0.03–0.4 eV. Because of the influence of the chemical and biological environments, however, it will result in larger ![]() values. With these considerations, the energy parameter
values. With these considerations, the energy parameter ![]() actually has the aforementioned value, but
actually has the aforementioned value, but ![]() is in the range of 0.4–1.0 eV.
is in the range of 0.4–1.0 eV.
As mentioned, the potential energy sequence of DNA is different from the periodic structure, yet it is not a completely disordered system. Accordingly, we need to build a model that is quasi-periodic. Albuquerque and associates used the RS model to generate a four-element (G, C, A, T) quasi-periodic sequence that is based on the general rules as G→GC; C→GA; A→TC; T→TA. Starting from G (the first generation), the initial generations are G, GC, GCGA, GCGAGCTC, GCGAGCTC GCGATAGA.., making Fi the number of elements of the R-S sequence of generation i, and then we have Fi+1=2Fi(![]() ). Therefore, the number of elements in the first few generations follows the sequence of 1, 2, 4, 8, 16,…, and for the 12th generation the number of lattice points (elements) will be 2,048.
). Therefore, the number of elements in the first few generations follows the sequence of 1, 2, 4, 8, 16,…, and for the 12th generation the number of lattice points (elements) will be 2,048.
12.1.4 Advantages of DNA Molecular Devices
DNA is considered one of the best candidates for the next generation of nanoelectronics given its variety of electrical properties. The electrical properties of DNA are directly related to the nucleotide sequence, and therefore sequence design can lead to the desired conduction properties. Electrical conductivity is critical for circuit design.
Although scientists have been able to produce nanoelectronic devices in a research laboratory setting, adaptation of these methods to large-scale production is impossible in most instances. For example, the use of an STEM tip to move atoms or atomic groups in a particular arrangement is most definitely not suitable for mass production. Due to the development of biotechnology, the mass production of DNA with desired specific nucleotide sequences is obtainable. Moreover, the ability to fold DNA molecules into a variety of different shapes is easily accomplishable with many known techniques. As such, there is great potential for the use of DNA in the electronics industry, provided that there is complete understanding of the electrical properties of DNA and that they translate well to manufacturing electronic devices.
Irrespective of the electrical properties of DNA, the charged phosphate backbone is a useful template for other materials. One such example is the creation of metallic nanowire using DNA as a template. Metal ions in solution can be adsorbed on the DNA molecule, allowing for easy assembly of metallic nanowires. Such processes make DNA “conductive,” regardless of the particular DNA sequence. Note that this method likely results in the loss of biological function of DNA. The creation of Ag nanowires using DNA templates is detailed here. Note that this method can be used, in theory, for most metallic materials.
First, DNA molecules are deposited in the substrate and are immersed in the solution containing Ag+. The negatively charged phosphate backbone of DNA electrostatically interacts with the Ag+ in solution. A reducing agent is used to create zero-valent Ag nanowires, templated in a parallel arrangement in the DNA backbone. Electrical measurements have shown that metallic nanowires made using this method have good conductivity. Using this method, metalized DNA molecules can be used to construct components of next-generation ICs.
12.2 DNA Nanotechnology [4–7]
In the DNA replication process, the simplicity and near-constant matching of base pairs coupled with the diversity of genetic information along with the conformational specificity are all desired principles in the design of nanoscale materials. The mutual noncovalent bonding between single-strand DNA molecules via the complementary bases leads to self-assembly of DNA and the further production of a functional assembly of aggregates. Nanotechnology based on the physical and chemical properties of DNA can be referred to as DNA nanotechnology.
DNA used for gene chips has long been the focus of research. Apart from gene chips, DNA molecules are applied in two main areas of nanotechnology. In one area, DNA is used as a template to assemble nanoparticles. Nanoparticles may be incorporated in a DNA template in many different ways. One of the early and main applications of nanoparticle assembly in this regard is the aggregation of Au nanoparticles functionalized with DNA, which was pioneered by the team of Chad Mirkin. Taking advantage of the base-pairing seen in DNA, complementary DNA strands can assemble Au nanoparticles together, changing the system’s optical properties and allowing for applications in medical diagnostics and sensing. Likewise, manipulation of the sequence and structure of DNA can yield complex patterns and assemblies of nanoparticles, such as in the preparation of molecular wires.
12.2.1 DNA for the Assembly of Nanoparticles
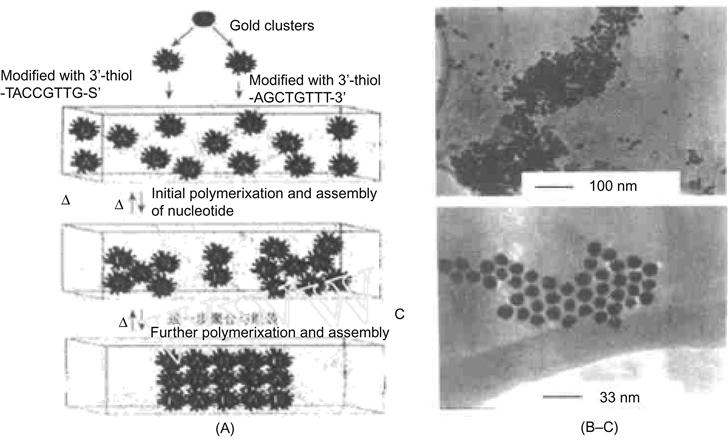
In certain applications, the assembly and arrangement of nanoparticles are crucial. Assembly methods for nanoparticles can be divided into two categories, namely the colloidal crystal method and the template method. Among them, the template method is the most intensively studied and therefore most developed. The concepts of biomimicry and bio-inspired molecular recognition are becoming a very promising method for assembling nanoparticles. Furthermore, biomolecular templating methods may be more accurate in the control of generating particles with the desired structure, size, and shape to influence properties. Using DNA as a template for sophisticated spatial arrangements, research on DNA-functionalized Au nanoparticles was the first area of aggressive research. As stated, pioneering work in this area was reported by the group of Chad Mirkin at Northwestern University [8]. In their work, a thiol was introduced to the ends of two noncomplementary oligonucleotides and absorbed onto Au nanoparticles using the known affinity of thiols with metallic surfaces. A specifically designed DNA connector was introduced into the system containing a double-strand area and two single-chain areas that are complementary with the two oligonucleotides on the surface of the Au nanoparticles. The addition of this DNA connector causes accumulation and slow sedimentation into a DNA gel. The aggregates contain a large number of DNA molecules with reasonable structural order that can be identified by transmission electron microscopy (TEM). TEM images show a closely assembled monolayer and two-dimensional colloidal aggregates formed by the Au nanoparticles.
Nanoparticle assembly using DNA has considerable advantages over traditional approaches, because DNA can be used as templates for very unique assembly arrangements. As shown, an appropriate choice of a DNA sequence can lead to the precise assembly of nanoparticles through complementary base-pairing. Additionally, because the driving force for assembly is dependent, in part, on the length of the oligonucleotide, this method is suitable for the assembly of aggregates of different sizes.
12.2.2 Driving Force for Self-Assembly of DNA Templates
The earliest reports on applications of DNA as the template for successful self-assembly of nanoparticles were performed using quantum dot materials. Using low concentrations of plasmid DNA mixed with Cd2+ solution, the negatively charged phosphate backbone would electrostatically interact with the metal cation. On exposure to H2S gas, CdS quantum dots were obtained. This early report clearly shows that DNA molecules can play a role in self-assembly and nanoparticle synthesis through electrostatic interactions with the DNA backbone. Using similar methodologies, other self-assembled nanoparticle structures can be made.
When electrostatic interactions are used to create nanomaterials, the self-assembled nanostructures are often in the form of one-dimensional metallic nanowires. For example, electrostatic deposition of Ag+ onto DNA, followed by reduction of Ag+ to Ag, successfully created silver nanowires with widths of 100 nm and lengths up to 12 μm. Such metallic nanostructures cannot be made via conventional synthesis techniques. The resulting DNA-templated Ag nanowire exhibited promising electrical properties, suggesting that such materials have promise in future nanoelectronic devices. Using similar methods, Pd, Pt, and Au nanowires have also been prepared.
For inorganic nanomaterials, if the driving force of self-assembly is from biotic–abiotic interactions or base-pair recognition between oligonucleotides, then two-dimensional or three-dimensional networks of assembly systems of nanoparticles can be obtained. For example, a DNA-based reversible assembly process was used to make a macroscopic network of Au nanomaterials [4]. Starting with a gold nanoparticle sol, two thiol-containing nucleotides were added to the Au, wherein the corresponding bases were noncomplementary to each other and thus were noninteracting. By adding a connector DNA exhibiting the complementary bases of both DNA-functionalized Au nanoparticles, the Au nanoparticle would become attached during the sol, changing from a red to a purple color. As the DNA-functionalized Au nanoparticles aggregated beyond the point of colloidal stability, a magenta precipitate would settle out of solution. TEM images (Figure 12.3) show a network structure of Au nanoparticles connected with DNA. Through the use of higher temperatures, this process was reversible if the temperature was greater than the melting temperature (Tm) of DNA. The input of additional energy would disrupt the hydrogen bonding between complementary bases, resulting in dissolution of the composite biological material while generating individual Au particles that were redistributed in solution. Such control of this self-assembly process could have interesting applications in electronic and optical properties.
12.2.3 DNA as a Template to Prepare Molecular Wire
A molecular wire can be defined as a bridge between molecular devices or between molecular devices and a macroscopic transducer. Effective molecular wires are a key component in making future molecular circuits and must meet the following criteria: reasonable conductivity, a length with low polydispersity, connection points that can be tethered at different components in a system, oxidation–reduction chemistry available at the endpoints, and insulation from the surroundings to prevent any transfer of electrons.
There are many different types of molecular wires, with current research focused on polyphenylacetylene (PPE) and its derivatives, carbon chain-type molecular wires, porphyrin molecular wires, and DNA molecular wires. An obvious common theme of these materials is the delocalization of a π conjugation system for the high mobility of electrons. Many issues currently exist in the field of molecular wires, including chemical synthesis, characterization, performance testing, and appropriate theoretical calculations. Fortunately, new breakthroughs have been made in these areas in recent years.
The structure and properties of DNA are tunable through modifications of the base sequences. Such tunable properties include overall size, molecular rigidity, and double-strand or single-strand confirmations of the overall conformation of DNA. Such modifications can result in a regular two-dimensional or three-dimensional nanoscale network. Such versatility makes DNA very useful in biotechnology and possibly for future nanoelectronic devices. One such example is the use of Au nanoparticles assembled into two-dimensional DNA templates for electronic applications, setting the foundations of a DNA-based molecular memory device. The fabrication of DNA onto a silicon chip can lead to potential applications in semiconductor devices as well. Jiang Xiao-Hua and colleagues performed electric field-induced absorption by use of different types of DNA on the surface of highly regular pyrolytic graphite (HOPG) and obtained the nano-network structures of different morphologies with the goal of preparing a DNA-based molecular wire. Monson and Woolley [7] deposited copper onto surface-absorbed λ-DNA, which created similar structured nanowires. To accomplish this, λ-DNA is adsorbed on the surface of silicon substrate and treated with a solution of Cu(NO3)2. Via electrostatic interactions previously described, Cu nanowires were formed after reduction with ascorbic acid. Atomic force microscopy observations show that additional copper and/or ascorbic acid results in a denser metal layer on the DNA surface. These copper nanowires could serve as a connector used in the nanometer integrated circuits and have laid a foundation for the production of functional electronic devices, such as electronic switches and dipoles. Various other metals can be produced in this fashion, including Ag, Pt, and Pd, while DNA is capable of intercalating Zn2+, Ni2+, and Co2+ ions, leading to another route to conductive DNA nanowires.
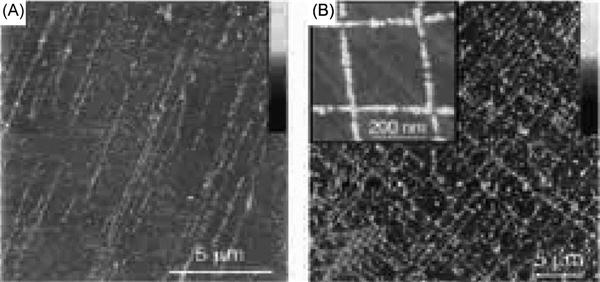
Many applications in nanoscience often require highly ordered nanowire or nanotube arrays. Creating such structures in a controlled and repeatable fashion has been challenging thus far, particularly regarding nanoelectronic applications. Using DNA, such hurdles may be overcome. By aligning DNA molecules on a mica surface using mechanical stresses in the form of compressed air, DNA can template nanowire arrays in an ordered orientation, as shown in Figure 12.4.
12.3 DNA Molecular Motors
12.3.1 Drexler Conjecture
K. Eric Drexler is a renowned space technology expert at Massachusetts Institute of Technology. In 1986, he published the book The Engines of Creation. In the book he says that the continuous progress of one’s ability in controlling substances will result in the birth of molecular-sized mechanical components that can be assembled into micro-machines that are much smaller than cells, so that humankind can have a direct effect on biological mechanisms. He also points out that the organism is but a combination of molecular machinery derived from natural evolution. Human-made “cell repair machines” manipulated by nanoscale computers may do a better job than the forces of nature. It can fix atoms one-by-one, correct DNA errors, and perform maintenance of all the ingredients of the individual cells. Based on this, he declared in his book, “With the help of such man-made machines as ‘cell repair machines,’ aging can be terminated, and humankind could eventually overcome death.”
Are nanoscale machines realistic and feasible? Can humans develop the technology to build nanomachinery? Would such nanomachines be miniaturized copies of current machines or run with totally different mechanisms?
To answer these questions, we should first clearly define machinery. The machines that are mentioned here are mechanical devices. Large mechanical devices, ranging from aircraft and submarines to toasters and microwave ovens, are very familiar. Mechanical devices have many definitions, but in this analysis a mechanical device is “a device designed for the implementation of mechanical motion.” Accordingly, we know that a mechanical force must be used; its mode of operation is determined by its manufacturer. Note that here we do not deliberately draw a line between machinery and machine. In fact, the machine is a special kind of mechanical device; it is a “special mechanical device that can run a specific requirement of mechanical motion with a specific energy input.” The concept of a molecular motor is such a machine.
According to this definition of machinery, nanoscale machinery is found in living cells where functional molecular devices, such as proteins or RNA molecules, perform a specific task of mechanical motion. So, the answer to whether nanoscale machinery exists has already been answered by nature.
In cells, there are some molecule devices that are similar to human-made machinery, such as the rotary motor on bacterial cell membranes, which looks like an electric motor. There is also molecular machinery that is slightly similar to some of our human-made machines. One example is the combination of RNA and protein, namely the ribosome is the “machinery” to make proteins in an assembly line fashion. Some molecular machinery, such as topoisomerase (a protein with double-strand DNA that can be entangled together with helicase), has no similarity with mechanical devices in the macro-world. The machinery in the cells is involved in efficient synthesis of macromolecules, including the manufacturing process of molecular self-assembly. This process is much more complicated than the mechanical assembly we are familiar with on the macroscale; therefore, some are inspired by such processes to create models of economic and organizational processes based on nature’s complexity.
12.3.2 Molecular Motors [10–13]
The development of modern medical equipment is an integral part of the development of medicine. Medical devices based on nanoscale components will open a new territory of nanomedicine. At present, much research effort is focused on molecular motors. Molecular motors can be defined as molecular machinery with a composite structure of larger molecules with mobility at a molecular level that also uses the smallest entity that can be used as mechanical components. They are driven by way of an external stimulus (such as the use of chemical, electrochemical, photochemical, and other methods to change the environment) to bring significant change to the molecular structure, configuration, or conformation, and it must be ensured that this change is controlled and modulated, akin to human-made machinery.
Current molecular motors comprise biological macromolecules and a nanoassembly that uses chemical energy to perform mechanical work. Natural molecular motors, such as kinesin, RNA polymerase, and myosin, are involved in a series of important life activities in organisms, such as cytoplasmic transport, DNA replication, cell division, and muscle contraction. Molecular motors fall into two general categories: linear progression and the rotating type. Linear molecular motors are biological molecules that can convert chemical energy into mechanical energy and move in a linear fashion. This class includes myosin, kinesin, DNA helicase, and RNA polymerase (and many others), among which muscle myosin is more often studied. With actin representing a linear track, the process of movement is coupled with ATP hydrolysis. The kinesin takes tubulin as the track to move along the microtubules, and thus completes the different transfer functions inside and outside cells. To elucidate the mechanism for kinesin movement, a “hand-over-hand” model was proposed: kinesin’s two heads are alternately combined with the microtube, moving on foot along the microtubule. In the course of their “walk,” certain conformational changes will occur to convert chemical energy into mechanical energy, achieving linear movement of molecules themselves. At present, between ATP hydrolysis and myosin or kinesin, the chemical and mechanical coupling relationships of their mechanical movement are still unclear. Recent studies have found that they share the same central core structure, and ATP energy is converted into protein conformational movements under similar changes. DNA helicase is a linear molecular motor, with DNA molecules as a “track,” and it is coupled with the energy released by ATP hydrolysis. In the release of ADP and phosphate at the same time, the DNA double-strand is separated into two complementary single strands. RNA polymerase in the DNA transcription process will rapidly move along the DNA template, with the consumed energy coming from the nucleotide polymerization and RNA folding reactions.
Rotary molecular motors can convert chemical energy directly into mechanical energy via the hydrolysis reaction. Their structure can be more appropriately called a “motor,” because their motion is similar to that seen in human-made motors. The typical rotary molecular motor is the F1-ATP enzyme. ATP enzymes consist of two parts: one is integrated in the mitochondrial membrane, known as F0, and the other part is outside the membrane, known as F1. The subunits a, b, and c in the F0-ATP enzyme constitute the channel flowing through the membrane proton. When the proton flows through F0, the torque will be generated to facilitate the rotation of the g subunit in the F1-ATP enzyme. The clockwise and counterclockwise rotations of subunit g are associated with the ATP synthesis and hydrolysis respectively. The F1-ATP enzyme has a diameter of less than 12 nm, and it can produce forces greater than 100 pN, with a no-load speed of up to 17 rev/s. The F1-ATP enzyme, in combination with the nanomicroelectromechanical system, has become a new kind of nanomechanical device.
12.3.3 Basic Principle of Molecular Motors
The concept of molecular motors was first proposed by the Polish scientist M. Smoluchowski. In the beginning, the feasibility of molecular motors was widely questioned. Later, its feasibility was proven in the studies by Feynman and Magnasco. More molecular motors have been found to exist in biological systems, and the design of artificial molecular motors is also of immense interest to the scientific community.
The concept of the molecular motor has a very broad definition, such as the Brownian motor, molecular motors, atomic machines, ion pumps, and others. Also included are the quantum interval charge pump and the spin pump.
As the name would suggest, the Brownian motor is related to Brownian motion. At the nanoscale and microscale, collisions constantly occur between particles; therefore, particle motion would not follow a fixed path (Figure 12.5) and would undergo random motion. Similarly, a particle’s own energy is not fixed, but rather is a randomly distributed range. Random collisions are the physical mechanism causing such an energy distribution. Any random collisions will constitute the driving force found in micro-world movement. Meanwhile, the collision at a certain moment during the process of the particle motion is also likely to have slightly higher energy than the average energy. In addition to the collision, the particles found between the interaction are called viscous force, which may result in the dissipation of particle energy. Figure 12.5 shows the so-called Einstein fluctuation–dissipation theorem.
Random collisions in the microscopic world reflect the nature of the particle movement, sometimes referred to as spontaneous fluctuations. As long as the temperature is sufficient, there is a fluctuation force. Therefore, the “on-table” particles of ![]() -type (Figure 12.6) are likely to fall “under-table” through spontaneous acts of pushing, and it might be squeezed from “under-table” up to “on-table.” This can explain why there is a difference between the microscopic world and macroscopic world.
-type (Figure 12.6) are likely to fall “under-table” through spontaneous acts of pushing, and it might be squeezed from “under-table” up to “on-table.” This can explain why there is a difference between the microscopic world and macroscopic world.
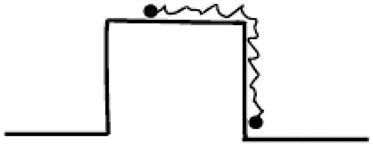
 -type potential energy, • for Brownian particles. From Ref. [11].
-type potential energy, • for Brownian particles. From Ref. [11].This random fluctuation is also called “white noise.” This kind of force is not directional, so there is no effective power in it. The molecular motor provides the movement at molecular orientation in the absence of an external macroscopic force, which can translate the energy provided by the outside world, for example chemical energy into power to achieve directional movement.
Here, we use a simple model to explain why molecular motors can generate directional movement in the microscopic world, a key basic principle of molecular motors. Feynman has proposed a ratchet potential model (Figure 12.7) to illustrate the feasibility of molecular motors. Here, we analyze the details of its principles.
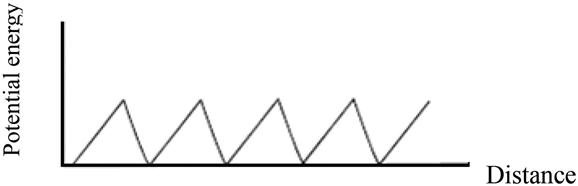
We already know that on the microscale, when particles are pressed, their energy will increase. On movement, the length of the path is not a concern, for example ![]() or
or ![]() (Figure 12.8); however, only the energy level of point B is of interest. Therefore, in the absence of external forces and under the condition of random fluctuation, there is a chance that the particle distribution at A is equivalent to that found at C, which is referred to as the “detailed balance” principle.
(Figure 12.8); however, only the energy level of point B is of interest. Therefore, in the absence of external forces and under the condition of random fluctuation, there is a chance that the particle distribution at A is equivalent to that found at C, which is referred to as the “detailed balance” principle.
To make more particles distributed at point C than at point A, more work is needed. Rather than providing a fixed external force (macroforce), we can use an external fluctuating force with an average of zero external forces over time; however, unlike thermal noise, it is unrelated. Because the particle has a force on ![]() that is the same on
that is the same on ![]() , the average effect of the external fluctuation forces on the right and left are offset by each other, so there will be no net effect. Therefore, it does not undermine the delicate balance. As mentioned, once the force on
, the average effect of the external fluctuation forces on the right and left are offset by each other, so there will be no net effect. Therefore, it does not undermine the delicate balance. As mentioned, once the force on ![]() is not equal to that on
is not equal to that on ![]() , thermal noise will not be able to produce such an imbalance to disrupt the system; however, after the external fluctuation force is combined with the forces on
, thermal noise will not be able to produce such an imbalance to disrupt the system; however, after the external fluctuation force is combined with the forces on ![]() and
and ![]() , the forces on the right and left are no longer in balance and will produce a net force. This results in net flow for promotion of the particle.
, the forces on the right and left are no longer in balance and will produce a net force. This results in net flow for promotion of the particle.
12.3.4 DNA Molecular Motors
12.3.4.1 DNA Applications in Molecular Devices
In this section, molecular devices refer to devices at the molecular scale or molecular level, for example molecular wires, molecular switches, molecular rectifiers, molecular memory, molecular motors, molecular sensors, molecular logic, molecular computers, and so on. In recent years, molecular devices have been increasingly studied regarding the function of a single molecule. Another trend lies in the use of organic materials to supersede inorganic materials for enhancing the flexibility of molecules. Clearly, among the various materials, DNA is undoubtedly the most appropriate choice. Therefore, DNA has been given more and more attention in the study of molecular devices. For example, DNA molecules have a natural length, which has been seen as an ideal molecular wire and as the materials to build a molecular wire. Second, DNA molecules based on single-molecule transistors, sensors, molecular motors, and biochips have also been favored by more and more scientific researchers. Here, we focus on DNA molecular motors.
12.3.4.2 DNA Molecular Motors
As mentioned, molecular motors have an interesting potential application. Micromolecular motors can be used as building blocks for nanorobots or nanodevices and components in power sources. The DNA-based molecular motor is one hotspot of the research performed on molecular motors. There have been reports on various methods of using DNA molecular assembly of DNA molecular motors [5–17]. Here, we provide a brief introduction of the work performed by Li and Tan, with the details published in Nano Letters (2002) [14].
The key to create a molecular “motor” is the realization of the “automatic” elements. Li and Tan used synthetic single-strand DNA molecules to assemble a DNA molecular motor. Such a DNA molecular motor works based on the changes of DNA molecules in two different configurations: intramolecular tetraplex (TE) configuration and intermolecular duplex (DU) configuration (Figure 12.9). Interconversion of these two configurations is achieved through DNA hybridization interactions with the strand exchange reaction, so that DNA molecules can be flexible, like an inchworm. Typically, they use the DNA molecule, which contains 17 units and is naturally present in a fourfold chain form. Its two ends are closer, equivalent to the “contracted” state of molecular motors. When this “contracted” DNA molecule finds the target pairing DNA chain ![]() , its fourfold chain will naturally stretch to form a double strand of DNA with
, its fourfold chain will naturally stretch to form a double strand of DNA with ![]() (process I in Figure 12.9), which is equivalent to a “stretched” state of the molecular motor. Then, with the emergence of another DNA chain,
(process I in Figure 12.9), which is equivalent to a “stretched” state of the molecular motor. Then, with the emergence of another DNA chain, ![]() , as a result of the chain substitution reaction, the original chain in the double-strand DNA will be replaced by
, as a result of the chain substitution reaction, the original chain in the double-strand DNA will be replaced by ![]() (process II in Figure 12.9), where
(process II in Figure 12.9), where ![]() and
and ![]() will form a longer, more stable αβ chain. The original chain is released (Figure 12.9 in the process of III), which will reform a fourfold chain structure (process IV in Figure 12.9). Processes I–IV form the complete working course of this molecular motors.
will form a longer, more stable αβ chain. The original chain is released (Figure 12.9 in the process of III), which will reform a fourfold chain structure (process IV in Figure 12.9). Processes I–IV form the complete working course of this molecular motors.
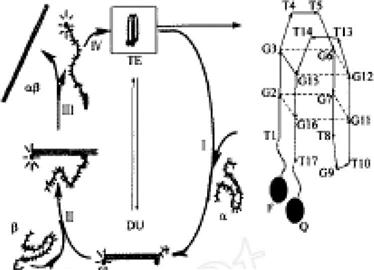
How does one quantify the movement of such DNA molecular motors? We can allow the DNA molecular motor to be connected with an organic fluorophore (abbreviated as F) and a fluorescence quencher (abbreviated as Q) on both of its sides. This movement of the DNA molecular motor can be directly monitored by the fluorescence signal (Figure 12.9, right).
The DNA molecular motor described is a typical one, featuring a simple structure, stable performance, and easy operation, and it can work in solution or on the surface of nanoparticles.