Adhesives for Special Adherends
This chapter describes the adhesives used for specific adherend types. Tables are occasionally published listing large numbers of adhesive types recommended for specific adherends; however, such tables can be misleading in supplying information needed to provide strong durable bonds, because the user may not know that some combinations of adhesives and adherends are superior in durability and resistance to others in particular environments. This chapter places emphasis on a listing of the adhesives believed to provide strong, lasting bonds.
Keywords
Metals; thermoplastics; thermosetting plastics; plastic foams; rubbers
6.1 Introduction
This chapter describes the adhesives for specific adherend types. Tables are occasionally published listing large numbers of adhesive types recommended for specific adherends. Such tables can be misleading in supplying information needed to provide strong durable bonds, because the user may not know that some combinations of adhesives and adherends are superior in durability and resistance to other environments. This chapter places emphasis on a listing of the adhesives believed to provide strong lasting bonds. Chapter 5 discusses all adhesives in detail.
6.2 Metals
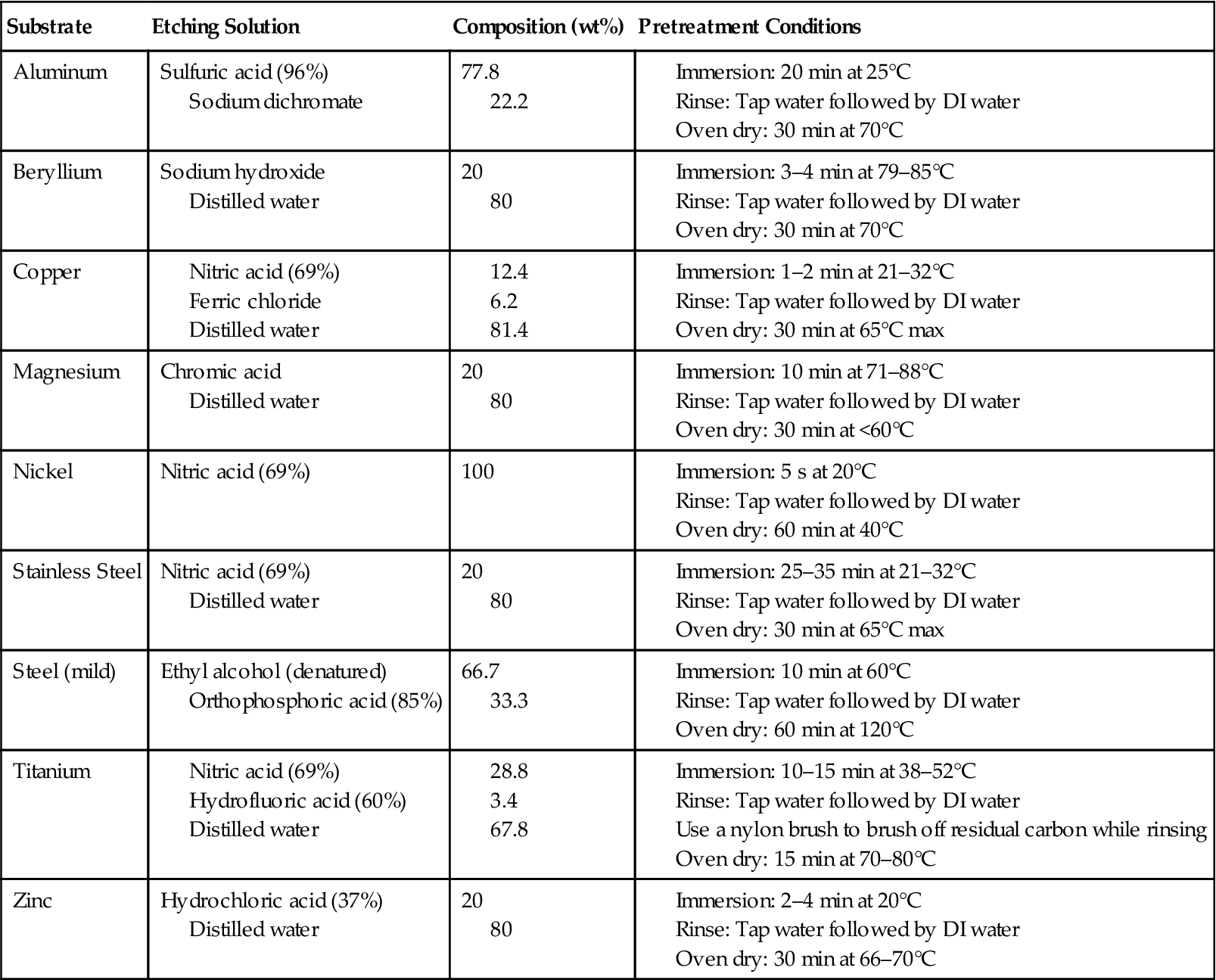
Metals require surface preparation to form strong bonds to another substrate using adhesives. Surface treatment methods for metals have been covered in Chapter 3 and elsewhere extensively [1]. Surface treatment of metals often involves use of strong chemicals that can cause injury. It is strongly recommended to the readers to consult the surface preparation procedures and the relevant safety, health, and environmental information before attempting to treat metals. Table 6.1 provides highly abbreviated procedures for the surface preparation of a few common metals.
Table 6.1
Abbreviated Procedures for the Surface Preparation of a Few Common Metals [2]
6.2.1 Aluminum and Alloys
Aluminum and many of its alloys react with oxygen to form a stable, extremely hard surface coating that protects the base metal from further corrosion. The anodizing process exploits this phenomenon to build up the oxide layer to a thicker coating, which is tightly bound to the base aluminum alloy. The resulting aluminum oxide layer can offer electrical insulation, protection from corrosion, improved abrasion resistance, provide a lasting decorative finish, and offer a stable surface for bonding, coating, or other secondary operations [3].
A wide range of adhesives can be used to bond aluminum to aluminum or to other materials. Adhesives recommended include modified epoxies, modified phenolics, epoxy-phenolics, neoprene-phenolics, second-generation acrylics, cyanoacrylates, silicone rubbers, and vinyl plastisols. Sell [4] has ranked a number of adhesives in the order of decreasing durability with aluminum adherends as follows:
• 121°C-curing rubber-modified epoxies
• Two-part, room-temperature-curing epoxy paste with amine cure
Brewis [5] has discussed the nature of adhesives used for aluminum. The two major aluminum manufacturers, Aluminum Company of America (ALCOA) and Reynolds, has published small useful volumes on all aspects of aluminum bonding, although these volumes are not recent [3,6,7]. Another excellent detailed discussion of aluminum adhesives, particularly from the viewpoint of durability, is given by ALCOA [6].
6.2.2 Beryllium
Beryllium is a very light, hard, steel-gray metal [3]. It features high strength, stability at elevated temperatures and reflectivity. It offers outstanding thermal conductivity and is transparent to X-rays. Beryllium is nonmagnetic, corrosion resistant at ambient temperatures, and has a rigidity about 50% greater than that of steel.
Applications of beryllium include:
• Structural components for aircraft, satellites, and space vehicles
Adhesives recommended include epoxy-phenolics, nitrile-phenolics, epoxies (RT cure, contact pressure), epoxy-nylon, polyimide (PI), polybenzimidazole (PBI), epoxy-nitrile, and polyurethane. As beryllium retains significant strength at temperatures up to 538°C, the high-temperature application area is significant for this somewhat exotic metal. PBIs are relatively stable in air at temperatures up to 288°C for short periods of time. PIs can be used at somewhat lower temperatures for longer periods. The more conventional adhesives listed above are much more temperature sensitive than PBI and PI, but are considerably stronger at room temperature, and have equivalent or even slightly higher strength at 121°C [7,8]. Bond strengths of >30 MPa in shear and tension can be obtained by adhesive-bonding beryllium, with fracture being due to cohesive failure within the adhesive [9].
6.2.3 Brass and Bronze
Adhesives used with copper and copper alloys (see Section 6.2.5) can also be used with brass and bronze, although the surface preparation methods may be different.
6.2.4 Cadmium (Plated on Steel)
Cadmium is a unique metal that resists corrosion, especially to salt and alkaline environments [2]. Bluish-white in color, it offers thermal/electrical conductivity and high/low temperature serviceability. It has a low melting point of 320.9°C. Soft, malleable, and ductile, cadmium is often used as a protective layer for other metals including iron, brass, and aluminum. These coatings are commonly in use in the mining, electronic, aerospace/defense, and offshore oil industry. End uses of cadmium primarily include batteries, pigments, and stabilizers in addition to coating applications. Adverse health, safety, and environmental issues are of much concern and limit usage. Adhesives recommended include epoxies, nitrile-phenolic, and anaerobics.
6.2.5 Copper and Copper Alloys
Adhesives recommended include epoxies, polyurethane, silicone, nylon-epoxy, nitrile-phenolic, neoprene-phenolic, acrylic, cyanoacrylate, anaerobics, and partially hydrogenated polybutadiene (for bonding copper to polyethylene) [3].
Only heat-cured epoxies containing dicyandiamide (DICY) or melamine should be used. DICY has been shown to be beneficial, either when used as the sole-curing agent with epoxy resins, when mixed with other curing agents, or when used to pretreat the copper surface before bonding. Even when simply added to coatings (e.g., phenolic-cured epoxies, which cure by a different mechanism), both DICY and a melamine compound increased time to adhesive failure significantly on either bare or alkaline permanganate-treated copper [10].
6.2.6 Gold
Adhesives recommended include epoxies, epoxy-phenolic, polyvinyl alkyl ether, and anaerobics (need a primer to activate the system).
6.2.7 Lead
Adhesives recommended include epoxies, vinyl alcohol–vinyl acetate copolymer, polyvinyl alkyl ether, polyacrylate (carboxylic), polyurethane (two-part), epoxy-phenolics, silicones, and cyanoacrylates. The high-strength thermoset and alloy adhesives are rarely justified for bonding lead. Even where other properties recommend these adhesives, the designer should check to see whether some lower-cost or easier-to-use adhesive is also suitable. An exception is terne (an alloy made of lead (80%) and tin (20%), used for coating steel). This is a much stronger metal than lead, and lap-shear strengths exceeding 2.1 MPa have been reported for adhesive joints with terne [11,12].
6.2.8 Magnesium and Magnesium Alloys
Magnesium is a silvery white metal that is lightweight, strong, and dimensionally stable. It is ductile, malleable when heated, and has superior resistance to impact. Damping characteristics are outstanding. Magnesium welds easily, is machinable, and recyclable. Its melting point is 648.8°C. When exposed to air, magnesium tarnishes and forms a thin oxide coating [2].
Magnesium is one-third lighter than aluminum. It is widely used in alloys with zinc, silicon, copper, aluminum, and zirconium. Improvements in strength, vibration absorption, durability, resistance to corrosion, and weight savings are often achieved. Common applications are for automotive/truck components, electronic devices, and aerospace.
Adhesives recommended include epoxies, epoxy-phenolics, polyurethanes, silicones, cyanoacrylates, polyvinyl acetate, vinyl chloride–vinyl acetate copolymer, vinyl-phenolic, nitrile-phenolic, neoprene-phenolic, and nylon-epoxy. A wide variety of adhesives can be used for bonding magnesium as long as proper corrosion protection is maintained in keeping with joint design and end-use requirements. Because of magnesium’s sensitivity to moisture and the galvanic couple, water-based adhesives would be expected to cause problems. Surface preparation should always be carried out to ensure that the adhesive itself does not react with the alloy to create a corrosive environment. Another important observation is that high-modulus adhesives tend to provide lower bond strengths than lower-modulus adhesives [13].
6.2.9 Nickel and Nickel Alloys
Nickel and nickel-based alloys play an important role in the economy [2]. Nickel is usually used in alloy form. Such alloys offer special properties and are widely used in the assembly of a variety of products including:
Today about 3,000 nickel alloys and superalloys are available for use. They provide cost-effective solutions and meet the vital needs of industry.
Nickel is hard, malleable, and ductile. It conducts electricity and heat well, resists corrosion/oxidation, and is magnetic at ambient temperatures. Silvery white in color, it has a melting point of 1,453°C. Additionally, nickel can be electroplated on to other metal parts. Two component epoxy adhesives for bonding nickel to nickel and nickel to dissimilar substrates. These products are usually designed because of high-performance capability and easy processing.
Adhesive bonding of nickel-based alloys encounters problems when these alloys are used at temperatures above the service temperature of organic adhesives, or under corrosive conditions. Inorganic adhesives of sufficient ductility and sufficiently low maturing temperatures have not been developed to compete effectively with brazing and welding for joining high-temperature structures [14]. Epoxy adhesives are the most common adhesives used to bond nickel and its alloys. In all likelihood, PBI and PI adhesives can also be used for high-temperature applications. Other adhesives used include epoxy-nylon, polyamides, nitrile-phenolic, vinyl-phenolic, polyisocyanates, melamines, and neoprenes [15].
6.2.10 Plated Metals
See cadmium (Section 6.2.4) and zinc (Section 6.2.19).
6.2.11 Platinum
Platinum is a precious, silvery-white metal known for its wear, corrosion, and high-temperature resistance properties. It is dense, malleable, and ductile. Nontoxic and biocompatible, it is employed in many medical applications. Platinum has a melting point of 1,722°C.
6.2.12 Silver
Adhesives recommended include epoxies, polyvinyl alkyl ether, polyhydroxy ether, and neoprene rubber.
6.2.13 Steel, Mild, Carbon (Iron)
Adhesives recommended include acrylics, epoxies, nitrile-phenolic (high moderate-temperature strength, but drops off rapidly at higher temperatures), PBI (high strength over a wide temperature range), PI, and epoxy-phenolic for high-strength applications. For lesser-strength applications, use thermoplastics and rubber-based materials such as chlorinated natural rubber, reclaimed rubber, styrene–butadiene rubber (SBR), butadiene-acrylonitrile rubber, neoprene, butyl rubber, polyisobutylene, polyurethane rubber, polysulfide, and silicone rubber [16]. Bitumen and soluble silicates are also used for some applications.
6.2.14 Stainless Steel
Although surface preparation methods are usually different, the adhesives used for mild steel can generally be used for stainless steel.
6.2.15 Tin
Adhesives recommended include casein glue, epoxies, polyvinyl alkyl ether, polyacrylate (carboxylic), SBR, and polyisobutylene.
6.2.16 Titanium and Titanium Alloys
Titanium is a low-density, high-strength, tough, corrosion-resistant, silver-colored metal. It is twice as strong as aluminum and 45% lighter than steel. It possesses good heat transfer characteristics and does not become magnetized. Titanium has a melting point of 1,725°C. Nontoxic and biocompatible, titanium is often used in the health-care industry. Lightweight titanium alloys feature enhanced strength, toughness, and thermal stability capabilities [2].
Adhesives recommended include epoxies, nitrile-epoxy, nitrile-phenolic, PI, and epoxy-phenolic. PI adhesives provide strengths of 11.0–12.4 MPa at 316°C. These adhesives are not used for skin-to-core bonds because the temperature environment is not high enough to make them attractive, and because of the inherent problems caused by high volatiles release during cure. Epoxy-phenolics (novalacs) and nitrile-epoxies are normally tested at 177°C. Nitrile-phenolics, because of their high peel strengths, are recommended for use in metal-to-metal bonds at the cost of lap-shear strength at temperatures above 177°C, provided the application permits a reduction in shear strength. Nitrile-epoxies are recommended for skin-to-core applications because less volatile compounds are released during cure than with epoxy-phenolics. The volatiles released during cure by the latter adhesives and by PIs create internal pressure, which can result in core-node bond and skin-to-core bond failure [17,18].
The use of titanium adhesive-bonded structures for high-temperature 200–300°C applications has been limited because of the rapid degradation of the adhesive at these temperatures. PI adhesives have been developed with terminal acetylenic groups. These adhesives have been found to retain 45–50% of their original strength after 1000 h of thermal aging at 260°C. In another approach, the introduction of perfluoro-alkylene groups into aromatic PIs has resulted in a high degree of strength retention after 5000 h at 300°C. To improve the oxidation resistance at elevated temperatures, many formulations are pigmented with fine alumina powder. The only really high-temperature adhesive not based on PI resin is polyphenylquinoxaline. An adhesive based on this heteroaromatic polymer showed a decrease of only 25% of its original strength after 500 h at 370°C [19]. Keith [20] has covered all aspects of titanium adhesive bonding, including adhesive selection.
6.2.17 Tungsten and Tungsten Alloys
Little information has been found on adhesives recommended for tungsten, although nitrile rubber and epoxies have been used in the past [15].
6.2.18 Uranium
Epoxies have been used to bond this exotic material [15].
6.2.19 Zinc and Zinc Alloys
Zinc is used in multiple applications. It is widely employed for galvanizing steel and iron against rust. It is also used for die casting and forming alloys including brass and bronze. This lustrous blue-white metal is hard/brittle at ambient temperatures. It is malleable at 100–150°C, conducts electricity and heat, is anticorrosive, and has a relatively low melting point (419.5°C). Zinc is the fourth most common metal today. Adhesives recommended include nitrile-epoxies, epoxies, silicones, cyanoacrylates, and rubber-based adhesives [21].
6.3 Thermoplastics
With these materials, solvent cementing or thermal-welding methods are often preferable alternatives to adhesive bonding. However, where dissimilar materials are being bonded, or where the thermoplastic is relatively inert to solvents, adhesive bonding is recommended.
6.3.1 Acetal Copolymer
Acetal homopolymer is a highly crystalline thermoplastic manufactured by polymerization of formaldehyde and capping the two ends of the polymer chain with acetate groups (Table 6.2). It is called polyoxymethylene (POM) and has a backbone consisted of repeating ![]() CH2O
CH2O![]() units. Acetal copolymers are produced by copolymerization of trioxane and small amounts of a comonomer. The comonomer randomly distributes carbon–carbon bonds in the polymer chain, which stabilizes the resin against environmental degradation. The low cost of acetals compared to other polymers with similar performance and their mechanical, chemical, and electrical properties, allows them to replace metal and other structural materials in many applications.
units. Acetal copolymers are produced by copolymerization of trioxane and small amounts of a comonomer. The comonomer randomly distributes carbon–carbon bonds in the polymer chain, which stabilizes the resin against environmental degradation. The low cost of acetals compared to other polymers with similar performance and their mechanical, chemical, and electrical properties, allows them to replace metal and other structural materials in many applications.
Table 6.2
Acetal Manufacturers and Trademarks
| Trademark | Manufactures |
| Celcon® | Celanese Ticona |
| Delrin® | DuPont |
| Iupital® | Mitsubishi Engineering Plastics |
| Kemlex® | Ferro Corporation |
| Tenac® | Asahi Chemical |
| Ultraform® | BASF |
Although thermal welding is ordinarily used for bonding this material to obtain optimum bond strength, adhesives are used under certain conditions. Three types of adhesives are used: solvent, structural, and nonstructural. Hexafluoroacetone sesquihydrate is used for solvent cementing. Structural adhesives are generally thermosets. Many of these adhesives can be used continuously at temperatures up to 177°C, which is higher than the recommended continuous-use temperature of 104°C of the copolymer.
Structural adhesive types recommended are epoxy (up to 71°C), polyester with isocyanate-curing agent (up to 121°C), and cyanoacrylate (up to 82°C). Structural adhesives for bonding acetal copolymer to itself have yielded shear strengths of 4.1–5.5 MPa. Nonstructural adhesives are usually one-component, room-temperature-curing systems based on either thermoplastic resins or elastomeric materials dispersed in solvents. They are normally used in applications that will not have to sustain heavy and/or continuous loading and will not reach temperatures above 82°C. Neoprene (polychloroprene) rubber adhesives have been used to provide shear strengths of 2.24 MPa to sanded surfaces and 2.1 MPa to unsanded surfaces. As in structural adhesives, a reduction in strength can be expected under peeling load [22].
6.3.2 Acetal Homopolymer
Adhesives used to bond acetal homopolymer (Delrin®) to itself and to other materials, such as aluminum, steel, natural rubber, neoprene rubber, and Buna rubber, include polyester with isocyanate-curing agent, rubber-based adhesives, phenolics, epoxies, modified epoxies, and vinyls. Solvent cementing cannot be used unless the surfaces are specially roughened, because of the high solvent resistance of this material [23]. Other adhesive types sometimes used are resorcinol, vinyl-phenolic, ethylene vinyl acetate, cyanoacrylates, and polyurethane.
6.3.3 Acrylonitrile–Butadiene–Styrene
Bodied solvent cements are usually used to bond acrylonitrile–butadiene–styrene (ABS). Adhesives recommended include epoxies, urethanes, second-generation acrylics, vinyls, nitrile-phenolics, and cyanoacrylates [24,25].
6.3.4 Cellulosics
These plastics (cellulose acetate, cellulose acetate butyrate (CAB), cellulose nitrate, cellulose propionate, and ethyl cellulose) are ordinarily solvent cemented, but for bonding to nonsolvent-cementable materials, conventional adhesives must be used. Adhesives commonly used are polyurethanes, epoxies, and cyanoacrylates. Cellulosic plastics may contain plasticizers that are not compatible with the adhesive selected. The extent of plasticizer migration should be determined before an adhesive is selected [24]. Recommendations for conventional adhesives for specific cellulosic types are as follows:
• Cellulose acetate: natural rubber (latex), polyisobutylene rubber, neoprene rubber, polyvinyl acetate, ethylene vinyl acetate, polyacrylate (carboxylic), cyanoacrylate, polyamide (versamid), phenoxy, polyester+isocyanate, nitrile-phenolic, polyurethane, and resorcinol-formaldehyde.
• CAB: natural rubber (latex), polyisobutylene rubber, nitrile rubber, neoprene rubber, polyvinyl acetate, cyanoacrylate, polyamide (versamid), polyester+isocyanate, nitrile-phenolic, resorcinol-formaldehyde, and modified acrylics.
• Cellulose nitrate: same as for CAB above.
• Ethyl cellulose: cellulose nitrate in solution (or general-purpose household cement), epoxy, nitrile-phenolic, synthetic rubber, or thermoplastic resin combined with thermosetting resin, and resorcinol-formaldehyde.
6.3.5 Ethylene-Chlorotrifluoroethylene
See Section 6.3.7.
6.3.6 Fluorinated-Ethylene Propylene (Teflon®)
See Section 6.3.7.
6.3.7 Fluoroplastics
Epoxies and polyurethanes give good bond strengths with properly treated fluoroplastic surfaces [1,24].
6.3.8 Ionomer (Surlyn®)
Adhesives recommended are epoxies and polyurethanes.
6.3.9 Nylons (Polyamides)
There are a number of types, based on their chemical structure, but the most important and most widely used is nylon 6,6. The best adhesives for bonding nylon to nylon are solvents. Various commercial adhesives, especially those based on phenol-formaldehyde (phenolics) and epoxy resins, are sometimes used for bonding nylon to nylon, although they are usually considered inferior to the solvent type because they result in a brittle joint. Adhesives recommended include nylon-phenolic, nitrile-phenolic, nitriles, neoprene, modified epoxy, cyanoacrylate, modified phenolic, resorcinol-formaldehyde, and polyurethane. Bonds in the range of 1.7–6.9 MPa, depending on the thickness of the adherends, have been obtained [24,25].
6.3.10 Perfluoroalkoxy Resins
See Section 6.3.7.
6.3.11 Phenylene-Oxide-Based Resins (Noryl®)
Although solvent cementing is the usual method of bonding these resins, conventional adhesive bonding can be used. Epoxy and acrylic adhesives are generally recommended because of the versatile product lines and cure-rate schedules. Other adhesives recommended include cyanoacrylates, polysulfide-epoxy, room-temperature vulcanizing (RTV) silicones, synthetic rubber, and hot melts. The manufacturer, General Electric, has recommended specific commercial designations of these types. The cure temperatures of the adhesives selected must not exceed the heat-deflection temperature of the Noryl resin, which ranges from 85°C to 158°C, depending on the formulation. Adhesives not tested for compatibility with Noryl resins should be avoided or tested. Such testing should consider operational conditions of temperature and stress [26].
6.3.12 Polyaryl Ether (Arylon T)
This material is normally joined by solvent cementing.
6.3.13 Polyaryl Sulfone (Astrel 360; 3M Co.)
Hysol EA 9614 (modified epoxy on a nylon carrier) has been used to create good steel–plastic–steel bonds [1]. Curing is at 71°C for 4 h, or 1 h at 93–121°C at 0.21 MPa pressure. Bonds with strengths up to 14 MPa have been obtained with solvent-cleaned surfaces.
6.3.14 Polycarbonate
Polycarbonate is usually solvent cemented, but it can be bonded to other plastics, glass, aluminum, brass, steel, wood, and other materials using a wide variety of adhesives. Silane primers may be used when joining polycarbonates with adhesives to promote adhesion and ensure a dry surface for bonding [25]. Adhesives recommended include epoxies, urethanes, silicones, cyanoacrylates, and hot melts. Generally, the best results are obtained with solvent-free materials such as epoxies and urethanes. Polycarbonates are very likely to stress crack in the presence of solvents. When cementing polycarbonate parts to metal parts a nontemperature-curing adhesive should be used to avoid creating strains in the adhesive caused by the differences in the coefficients of thermal expansion. This differential causes adherend cracking and considerably decreases expected bond strengths. Under no circumstances should curing temperatures exceed 132°C, the heat-distortion temperature of standard polycarbonate resins [27].
6.3.15 Polychlorotrifluoroethylene (Aclar®)
Epoxy-polyamide and epoxy-polysulfide adhesives have been used successfully for bonding properly treated polychlorotrifluoroethylene (PCTFE). An epoxy-polyamide adhesive (Epon 828/Versamid 125 60:40 ratio) cured for 16 h at room temperature followed by 4 h at 74°C has given tensile-shear strengths of 19.6–20.8 MPa for various grades of PCTFE resins treated with sodium naphthalene etch solutions and also abraded [28,29].
6.3.16 Polyester (Thermoplastic Polyester)
Solvent cementing is usually used with these materials. Conventional adhesives recommended include single- and two-component polyurethanes, cyanoacrylates (Loctite 430 Superbonder), epoxies, and silicone rubbers.
6.3.17 Polyetheretherketone
Epoxy adhesives such as Huntman’s Araldite AW 134 with HY 994 hardener (cured for 15 min at 120°C) and Araldite AV 1566 GB (cured for 1 h at 230°C) give the best results with this engineering resin. Other adhesives that can be used are cyanoacrylate (Loctite 414 with AC primer), anaerobics (Loctite 638 with N primer), and silicone sealant (Loctite Superflex). The highest lap-shear strength was obtained with Araldite AW 134. This adhesive has balanced properties, good resistance to mechanical shock, thermal resistance to 100°C, and reasonable stability in the presence of aliphatic and aromatic solvents. Some solvents, particularly chlorinated hydrocarbons, will cause deterioration of the bond [30].
6.3.18 Polyetherimide (ULTEM®)
Adhesives for this engineering plastic are polyurethane (cured at room temperature to 150°C), RTV silicones, hot-melts (polyamide types) cured at 205°C, and epoxies (nonamine type, two-part) [31].
6.3.19 Polyethersulfone
Polyethersulfone may be solvent cemented. Conventional adhesives recommended by the manufacturers include epoxies (Huntman’s Araldite AV 138 with HV 998 hardener and Araldite AW 134B with HY 994 hardener), Hysol 9340 two-part epoxy paste, and Silcoset 153 RTV silicone sealant supplied by ACC Silicones Europe with primer OP and Silcoset RTV2 with Superflex primer supplied by F. Ball and Company [32]. The highest lap-shear strength was obtained with the Araldite AW 134B [30].
Other adhesives recommended are 3M Company’s Scotch Weld 2216 two-part epoxy, Amicon’s Uniset A-359 one-part aluminum-filled epoxy, American Cyanamid’s BR-89 one-part epoxy, Bostik’s 7026 synthetic rubber and 598-45 two-part adhesive, Momentive’s Silgrip SR-573, and Bostik’s Vitel polyester with isocyanate-curing agent [30].
6.3.20 Polyethylene
Acceptable bonds have been obtained between polyethylene surfaces with polar adhesives such as epoxies (anhydride- and amine-cured and two-component-modified epoxies) and solvent cements containing synthetic rubber or phenolic resin. Other adhesives recommended include styrene-unsaturated polyester and solvent-type nitrile-phenolic.
6.3.21 Polymethylmethacrylate
Ordinarily solvent cementing or thermal welding is used with polymethylmethacrylate (PMMA). These methods provide stronger joints than with adhesive bonding. Adhesives used are cyanoacrylates, second-generation acrylics, and epoxies, each of which provides good adhesion but poor resistance to thermal aging [24].
6.3.22 Polymethylpentene (TPX® by Mitsui Chemicals Co.)
No information has been found on adhesives for bonding TPX, but it is likely that the adhesives used for polyethylene will prove satisfactory for this polyolefin.
6.3.23 Polyphenylene Sulfide (Ryton®)
Adhesives recommended by the manufacturer (Phillips Chemical Company) include anaerobics (Loctite 306), liquid two-part epoxies (Lord Corporations’s Chemlok 305), and a two-part paste epoxy (Henkel’s Hysol Eccobond 104). Also recommended are USM’s BOSTIK 7087 two-part epoxy and 3M Company’s liquid two-part polyurethane EC-3532 [33].
6.3.24 Polypropylene
In general, adhesives recommended are similar to those used for polyethylene. Candidate adhesives include epoxies, polyamides, polysulfide epoxies, nitrile-phenolics, polyurethanes, and hot melts [25].
6.3.25 Polystyrene
This plastic is ordinarily bonded by solvent cementing. Polystyrene can be bonded with vinyl acetate/vinyl chloride solution adhesives, acrylics, polyurethanes, unsaturated polyesters, epoxies, urea-formaldehyde, rubber-based adhesives, polyamide (Versamid® by BASF), PMMA, and cyanoacrylates [24,25,34]. The manufacturer provides information about particular cements for both nonporous and porous surfaces. Cements are recommended for the fast-, medium-, and slow-setting ranges [35].
6.3.26 Polysulfone
Polysulfone is transparent, heat-resistant, ultra-stable, high-performance engineering thermoplastic. It has low flammability, smoke emission, and rigidity at high temperatures. Typical applications include food handling equipment, coil bobbins, and chemical processing equipment.
Adhesives recommended by the manufacturer include epoxies, acrylics, phenolics, polyurethanes, polyesters, and vinyl. Specific adhesives recommendations can be obtained from the adhesives suppliers [36].
The Scotch-Grip 880 elastomeric adhesive is recommended for bonding polysulfone to canvas, and Uralane 8615 for bonding polysulfone to polyethylene.
6.3.27 Polytetrafluoroethylene (Teflon®)
See Section 6.3.6. Other adhesives used include nitrile-phenolics, polyisobutylene, and silicones, of which the last two are pressure-sensitive adhesives [1].
6.3.28 Polyvinyl Chloride (PVC)
Solvent cementing is usually used for polyvinyl chloride (PVC). Because plasticizer migration from vinyls to the adhesive bond line can cause problems, adhesives selected must be tested for their compatibility with the plasticizer. Nitrile rubber adhesives are particularly good in this respect, although polyurethanes and neoprenes are also useful. 3M Company’s Scotch-Grip 2262 adhesive (synthetic resin in solvent) is claimed to be exceptionally resistant to plasticizer migration in vinyls.
A number of different plasticizers can be used with PVCs, so an adhesive that works with one plasticizer may not work with another [24]. Even rigid PVC contains up to 5% plasticizer, making it difficult to bond with epoxy and other nonrubber-type adhesives. Most vinyls are fairly easy to bond with elastomeric adhesives after proper surface preparation. Cyanoacrylates can be used with rigid PVC. The highest bond strengths with semirigid or rigid PVC are obtained with two-component, room-temperature-curing epoxies. Other adhesives used with rigid PVC include polyurethanes, modified acrylics, silicone elastomers, anaerobics, polyester-polyisocyanates, PMMA, nitrile-phenolics, polyisobutyl rubber, neoprene rubber, epoxy-polyamide, and polyvinyl acetate.
6.3.29 Polyvinyl Fluoride (Tedlar®)
Adhesives recommended include acrylics, polyesters, epoxies, elastomers, and pressure-sensitive adhesives.
6.3.30 Polyvinylidene Fluoride (Kynar®)
See Section 6.3.7.
6.3.31 Styrene-Acrylonitrile (Lustran®)
Solvent cements are frequently used for styrene-acrylonitrile. Commercial cements include cyanoacrylate, epoxy, and the following 3M Company elastomeric adhesives:
Several other commercial adhesives not specified as to type can be found in Ref. [37].
6.4 Thermosetting Plastics (Thermosets)
Most thermosetting plastics are not particularly difficult to bond. As these materials are not soluble, solvent cementing cannot be used. In some cases, however, solvent solutions can be used to join thermosets to thermoplastics. In general, adhesive bonding is the only practical way to join a thermoset to a thermoplastic, or to another thermoset. Epoxies or modified epoxies are the best adhesives for this purpose.
6.4.1 Diallyl Phthalate
Suggested adhesives include urea-formaldehyde, epoxy-polyamine, neoprene, nitrile-phenolic, styrene-butadiene, phenolic polyvinyl butyral, polysulfides, furans, polyesters, and polyurethanes.
6.4.2 Epoxies
Suggested adhesives include modified acrylics, epoxies, polyesters, resorcinol-formaldehyde, furane, phenol-formaldehyde, polyvinyl formal-phenolic, polyvinyl butyral, nitrile rubber-phenolic, polyisobutylene rubber, polyurethane rubber, reclaimed rubber, melamine-formaldehyde, epoxy-phenolic, and cyanoacrylates. For maximum adhesion primers should be used. Nitrile-phenolics give excellent bonds if cured under pressure at temperatures of 149°C. Lower-strength bonds are obtained with most rubber-based adhesives.
6.4.3 Melamine-Formaldehyde (Melamines)
Adhesives recommended are epoxies, phenolic-polyvinyl butyral, epoxy-phenolic, nitrile-phenolic, polyurethane, neoprene, butadiene-nitrile rubber, cyanoacrylates, resorcinol-polyvinyl butyral, furane, and urea-formaldehyde.
6.4.4 Phenol-Formaldehyde (Phenolics)
Adhesives recommended are neoprene and urethane elastomers, epoxies, and modified epoxies, phenolic polyvinyl butyral, nitrile-phenolic, polyester, cyanoacrylates, resorcinol-formaldehyde, phenolics, polyacrylates, modified acrylics, PVC, and urea-formaldehyde. Phenolic adhesives give good results, but require higher cure temperatures and are less water-resistant than resorcinol-based adhesives.
6.4.5 Polyester (Thermosetting Polyester)
These materials may be bonded with neoprene or nitrile-phenolic elastomer, epoxy, epoxy-polyamide, epoxy-phenolic, phenolic, polyester, modified acrylic, cyanoacrylates, phenolic-polyvinyl butyral, polyurethane, butyl rubber, polyisobutylene, and PMMA.
6.4.6 Polyimide
Adhesives for bonding PIs include epoxy, polycarbonate copolymer that includes reacted resorcinol, siloxane, and bisphenol-A. A NASA study has evaluated six adhesives for this purpose [38–40].
6.4.7 Polyurethane
Elastomeric adhesives are prime candidates for polyurethanes, and polyurethane elastomer adhesives are particularly recommended [25]. Other suitable adhesives include cyanoacrylates, epoxies, modified epoxies, polyamide-epoxy, neoprene, and resorcinol-formaldehyde. The latter offers excellent adhesion but is somewhat brittle and can fail at relatively low loads [24,41].
6.4.8 Silicone Resins
These are generally bonded with silicone adhesives, either silicone rubber or silicones. Primers should be used before bonding.
6.4.9 Urea-Formaldehyde
Adhesives recommended are epoxies, nitrile-phenolic, phenol-formaldehyde, urea-formaldehyde, resorcinol-formaldehyde, furane, polyester, butadiene-nitrile rubber, neoprene, cyanoacrylates, and phenolic-polyvinyl butyral.
6.5 Reinforced Plastics/Composites
Adhesives that bond well to the base resin can be used to bond plastics reinforced with such materials as glass fibers or synthetic high-strength fibers. Reinforced thermoplastics can also be solvent cemented to themselves or joined to other thermoplastics using a compatible solvent cement. For reinforced thermosets, in general, the adhesives recommended above for thermosetting plastics apply.
6.6 Plastic Foams
Solvent cements are usually preferable to conventional adhesives for thermoplastic structural foams. Some solvent cements and solvent-containing, pressure-sensitive adhesives will collapse thermoplastic foams. Water-based adhesives based on SBR, polyvinyl acetate, or neoprene are frequently used. Solvent cementing is not effective on polyethylene foams because of their inertness. Recommendations for adhesives for thermoplastic foams are:
• Phenylene oxide-based resins (Noryl): epoxy, polyisocyanate, polyvinyl butyral, nitrile rubber, neoprene rubber, polyurethane rubber, polyvinylidene chloride, and acrylic.
• Polyethylene-nitrile rubber, polyisobutylene rubber, flexible epoxy, nitrile-phenolic, and water-based (emulsion) adhesives.
• Polystyrene: for these foams (expanded polystyrene), aromatic solvent adhesives (e.g., toluol) can cause collapse of the foam cell walls. For this reason, it is advisable to use either 100% solids adhesives or water-based adhesives based on SBR or polyvinyl acetate [24]. Specific adhesives recommended include urea-formaldehyde, epoxy, polyester-isocyanate, polyvinyl acetate, vinyl chloride–vinyl acetate copolymer, and reclaim rubber. Polystyrene foam can be bonded satisfactorily with any of the following general adhesive types:
Water-based (emulsion): best for bonding polystyrene foam to porous surfaces.
Contact-bond: for optimum initial strength. Both the water- and solvent-based types may need auxiliary heating systems for further drying. Solvent types are recommended for adhering to metal, baked enamel, and painted surfaces.
Pressure-sensitive adhesives: these will bond to almost any substrate. Both water- and solvent-based types are used. However, they are not usable in applications requiring long-term resistance to stress or resistance to high heat levels.
100% solids adhesives: these are two-part epoxies and polyurethanes. They form an extremely strong heat- and environment-resistant bond.
• PVC: epoxy, polyester-isocyanate, unsaturated polyester, vinyl chloride–vinyl acetate copolymer, polyvinyl acetate, polyvinyl alkyl ether, ethylene-vinyl acetate, nitrile rubber-phenolic, neoprene rubber, polyisobutylene rubber, polyurethane rubber, and polysulfide rubber. See discussion in Section 6.3.28 concerning migration of plasticizers in PVC.
Recommendations for thermosetting foams are:
• Epoxy (including syntactic foams), heat-cured epoxies (one-part).
• Phenolic: epoxy, polyester-isocyanate, polyvinyl acetate, vinyl chloride–vinyl acetate copolymer, polyvinyl formal-phenolic, nitrile rubber, nitrile rubber-phenolic, reclaim rubber, neoprene rubber, polyurethane rubber, butyl rubber, melamine-formaldehyde, neoprene-phenolic, and polyvinyl formal-phenolic.
• Polyurethane: epoxy, polyester, polyacrylate, polyhydroxyether, nitrile rubber, butyl rubber, water-based (emulsion), polyurethane rubber, neoprene, SBR, melamine-formaldehyde, and resorcinol-formaldehyde are specific types. Generally, a flexible adhesive should be used for flexible polyurethane foams. Synthetic elastomer adhesives with fast-track characteristics are available in spray cans. Solvent-based neoprenes are recommended for resistance to stress, water, and weathering. Solvent-based nitriles are recommended for resistance to heat, solvents, and oil. Water-based adhesives generally dry too slowly for most industrial applications, unless accelerated equipment is used. For immediate stress resistance, contact bonding is preferred. In this method, the adhesive is applied to the foam and to the other substrate by spraying or brushing. Wet bonding can be used where the adhesive is applied to the other surface. This reduces “soak-in” on the highly absorbent and porous foam [37].
• Urea-formaldehyde: urea-formaldehyde, resorcinol-formaldehyde.
6.7 Rubbers (Elastomers)
Bonding of vulcanized elastomers to themselves and to other materials is generally accomplished using a pressure-sensitive adhesive derived from an elastomer similar to the one being bonded. Adhesives used include the following rubber-based materials: natural, chlorinated, reclaim, butyl, nitrile, butadiene-styrene, polyurethane, polysulfide, and neoprene rubber, as well as acrylics, cyanoacrylates, polyester-isocyanates, resorcinol-formaldehyde, phenolic-resorcinol-formaldehyde, silicone resin, epoxies, polyisocyanates, furanes, nitrile-phenolics, neoprene-phenolic, polyvinyl formal-phenolic, and flexible epoxy-polyamides [15,24]. Neoprene and nitrile rubber adhesives are particularly recommended for bonding rubber. Neoprene adhesives are good all-around adhesives for rubber. Nitrile adhesives are particularly recommended for gaskets formulated with nitrile or polysulfide rubber [37].
6.8 Ceramics and Glass
This section has been graciously contributed by The Welding Institute, www.twi.co.uk.
Engineering ceramics such as silicon nitride, silicon carbide, and a large number of oxides are used in industries ranging from aerospace to automotive and biomedical to electronics. These materials are used because they possess a range of properties that are attractive for particular applications. These include:
The excellent stability of ceramics under extreme chemical and thermal environments is often the primary reason for their selection. However, the ceramic component must be joined to the rest of the device. There are many joining techniques that can be utilized; these range from mechanical attachment to direct bonding methods such as brazing or adhesive bonding. With all these methods, the correct design criteria for ceramic materials must be followed. These criteria must address issues such as:
• The inherent brittle nature of ceramics
• Their low fracture toughness
• Their low tolerance to high shear and tensile stresses
• Their low coefficient of thermal expansion compared to other materials.
The technique selected depends on whether the ceramic is to be joined to a similar or dissimilar material, and on the expected operational conditions at the joint. If the joint temperature is not expected to exceed 150°C or to only have very short-term excursions to ~200°C, and the environment is not too chemically aggressive, organic adhesives offer an attractive joining solution.
There is a wide range of adhesives that are commercially available, such as epoxy compounds or cyanoacrylates, which can be used to bond ceramics. Each of these has its optimum application method and curing regime to give maximum performance. Optimization may involve the use of a primer or other additive. For example, oxide ceramics are generally porous structures with slightly acidic surfaces. This acidity tends to inhibit the polymerization of cyanoacrylate adhesives, while the porosity requires these surface-initiating species to extend across relatively large gaps. Both problems can be overcome by the use of small quantities of basic species such as amines, which activate polymerization of the cyanoacrylate. Other adhesive systems also give enhanced bonding properties when used in conjunction with surface modifying primers, or keying agents, such as silane compounds.
The use of adhesive bonding for ceramics has both pros and cons.
• Joints can be weak when subjected to peel load
• Limited service temperature, typically <150°C or <200°C in special applications
• Poor electrical and thermal conduction, although loading with metal particles improves performance
• Joint integrity is sensitive to cleanliness of the mating surfaces and service environment
With correct joint design and material selection and consideration of operational conditions, adhesive bonding of ceramics can be used highly successfully. Probably, the most famous application of advanced ceramics used adhesive bonding. The NASA Space Shuttle employed 24,000 ceramic tiles as a thermal protection system to keep the temperature inside the vehicle relatively constant. The external temperature of the tiles can vary from −80°C during orbit to 1250°C during reentry. These tiles were adhesively bonded through a strain isolation pad to the aluminum skin of the shuttle, which had a design limit of 175°C. The success of this system not only allowed the shuttles to operate but also permitted them to be reused.
