Thermoplastic polyurethane (TPU)-based polymer nanocomposites
Abstract:
Thermoplastic polyurethanes (TPU) are a commercially important class of thermoplastic elastomers, which have an inherent nanostructured morphology. This chapter introduces thermoplastic polyurethanes before summarizing the benefits of introducing various types of nanofillers using various processing methods and highlighting the engendered properties and performance. A detailed discussion of the influence of nanofillers on TPU morphology is provided, as well as a section on biomedical applications and nanofiller toxicity.
11.1 Introduction: the potential of thermoplastic polyurethane (TPU) nanocomposites
11.1.1 Polyurethanes and thermoplastic polyurethanes
Polyurethanes, first discovered by Otto Bayer in 1937, encompass the series of polymers whose molecular backbone contains a significant number of urethane linkages, regardless of the content of the rest of the macromolecule.1 The urethane linkage is formed via the reaction between an isocyanate group and a hydroxyl group. Initial studies into these polymers focused on forming linear polyurethanes by reacting diisocyanates with diols, but it was very quickly realized that a multitude of polymers with wide-ranging properties could be produced.2 Early work focused on polyester-based polyols; however, the enhanced hydrolytic stability and immense versatility afforded by polyether-based polyols saw them become a preferred precursor in polyurethane synthesis.2 These days, the vast selection of polyols, isocyanates, and chain extenders allows polyurethanes to be varied from soft thermoplastic elastomers to adhesives, coatings, flexible foams, and rigid thermosets. This chapter will focus solely on thermoplastic polyurethane (TPU) elastomers as host polymers for polymer nanocomposite systems. These TPUs are often called ‘segmented polyurethane block copolymers,’ and form a subset of a large and commercially important family of thermoplastic elastomers (TPEs) comprising styrene block copolymers (SBCs), thermoplastic olefins (TPOs), thermoplastic vulcanizates (TPVs), and copolyester elastomers (COPEs). TPEs combine the flexibility and resilience of rubbers with the processability of plastics. Global demand for TPEs is forecast to increase 6.3% pa through 2011 to 3.7 million tonnes. TPU materials currently comprise approximately 15% of the volume of TPE polymers sold annually.3 They are at the premium end of this class of materials and are known for their high strength, durability, and tremendous versatility in terms of tailored formulations.
Some common applications for TPUs include automotive interiors, footwear, flexible hose and tubing, cellphone buttons, closures, seals and o-rings, adhesives, cable jacketing, sport and leisure items, textiles and textile coatings, implantable medical device components, mining and mineral processing equipment, laminates for impact glazing, photovoltaic cell encapsulation, and wastewater treatment equipment, just to name a few.
The clever incorporation of functional nanofillers of various types into TPU host polymers can extend typical TPU property profiles, improving mechanical and tribological performance,4’5 dimensional stability at higher operating temperatures,6 gas barrier and flame retardancy performance,7,8 electrical properties,6,9 and biological properties.10–12 This chapter reviews some of the exciting progress being made in these areas.
11.1.2 TPU chemistry, morphology, and properties
TPUs are linear, block copolymers of alternating hard and soft segments. The soft segments (SSs) are composed of long-chain diols (most typically polyester, polyether, or polycaprolactone-based) with a molecular weight of 1000–4000 g/mol, whilst the hard segments (HSs) commonly consist of alternating diisocyanates and short-chain extender sequences.1 Thermodynamic incompatibility between the segments results in phase separation, and subsequent organization into hard and soft domains with a nanoscale texture, which gives TPUs their distinct mechanical properties and thermoplastic utility.13–15
The flexible, amorphous soft domains primarily influence the elastic nature of the TPU, but still have some influence on the hardness, tear strength, and elastic modulus of the polymer. They are typically above their glass transition temperature (Tg) at room temperature and thus control TPU performance at low temperatures. In contrast, the rigid and typically semi-crystalline hard domains primarily influence elastic modulus, hardness, tear strength, and melt processability.1 However, the hard domains also act as physical cross-links, imparting elastomeric properties to the soft domain phase.16 Thus, the mechanical properties of TPUs are strongly influenced by the complex microphase morphology of the hard and soft domains. These are, in turn, dependent on such factors as the hard/soft segment composition ratios, the segmental solubility parameters and associated compatibility, molecular weight and polydispersity of the hard and soft segments, and the thermal and processing history of the TPU. 17–23 The unique microphase morphology confers on TPUs a higher tensile strength and toughness when compared with most other elastomers,22 and the absence of covalent cross-linking allows TPUs to be both melt and solution processed. However, under continuous or cyclic loading the absence of chemical cross-linking gives way to a degree of plastic flow whereby the hard domains can restructure, which results in large hysteretic losses and poor creep resistance.24’25 The clever introduction of nanofillers into this complex nanoscale morphology, and the characterization of the level of interplay between the various system components, represents a fascinating challenge for current researchers. Similarly, the typically moderate hard domain Tg in these systems seriously limits the upper use temperatures and high temperature performance and chemical or dimensional stability in aggressive environments. Again this is believed to be an area where innovation in TPU nanocomposite systems can effectively address these limitations.
11.1.3 TPU nanocomposites versus traditional microcomposites
The polymer industry is continually researching to find new materials that offer increased performance at lower costs, often necessitating the introduction of fillers. There have been various attempts to improve the mechanical performance, creep resistance, and permanent set of TPUs either by varying the composition of the hard and soft segments19,26 or by introducing macro- or micro-sized particulate fillers,27,28 with the majority of these studies yielding suboptimal results. Generally microfillers significantly stiffen TPUs and also substantially reduce elongation to break. They can also be associated with major wear of polymer processing equipment and dies due to the high loading levels required (up to 40% by weight) and the highly abrasive nature of these fillers.
Nanofillers offer significant advantages over macro-sized or micro-sized fillers, including vastly superior reinforcement efficiency, a greater surface area to mass ratio, low percolation threshold, and often very high aspect ratios, and as such there has been significant research and investment into the production of polymeric nanocomposites. Recently, a range of nanoparticles has been used in TPU-based composites, with varying levels of success. The bulk of this work has involved the use of carbon nanotube or layered silicate-based nanofillers.
11.2 TPU nanocomposites: structure, processing, properties, and performance
11.2.1 Mechanical influence of nanofillers on TPU stiffness and ultimate tensile strength
Figure 11.1 and 11.2 compare the mechanical properties of many of the best literature examples of TPU nanocomposites reinforced with layered silicate nanofillers or carbon nanotubes or nanofibres. We have chosen only the most impressive results from the academic and commercial literature. This is because we found that many studies, while presenting large percentage changes as a result of nanofiller reinforcement, did not benchmark particularly well to typical commercial TPU property ranges. While clearly there are many other material properties of great interest for TPU nanocomposites which will be discussed elsewhere in this chapter, these figures do present some very noteworthy trends.

11.1 Tensile strength versus elastic modulus for various TPU nanocomposites reinforced with carbon nanotubes (![]() ) or layered silicates (
) or layered silicates (![]() ). Source references are given beside each point.
). Source references are given beside each point.

11.2 Tensile strength versus stress at 50% strain for various TPU nanocomposites reinforced with carbon nanotubes (![]() ) or layered silicates (
) or layered silicates (![]() ). Source references are given beside each point.
). Source references are given beside each point.
First, Fig. 11.1 plots ultimate tensile strength versus elastic modulus. Even taking into account obvious differences in the particular TPU matrix materials, nanofillers and nanofiller loadings used in each study, the data clearly shows a more pronounced stiffening effect for carbon nanotubes and a more pronounced strengthening effect for layered silicates. The one exception is the very stiff nanocomposite prepared by solvent exchange at an unusually high loading (20 wt%) of unmodified synthetic hectorite.291 The highest tensile strengths achieved (anything above 45 MPa is considered very good in the industry) were all layered silicate reinforced systems, with the strongest being prepared by reactive extrusion.30
Figure 11.2 plots tensile strength against the more industrially acceptable parameter for TPUs of stress at 50% strain. The grouping of the layered silicate data in the top right quadrant tells us that nanofiller-TPU molecular interactions are more substantial and perhaps ‘cooperative’ during deformation in layered- silicate reinforced systems, and that carbon nanotube (CNT)-based nanofillers need further optimization for utility in TPU systems.
A TPU property profile which engenders strength and toughness while maintaining flexibility and resilience has been traditionally very difficult to achieve via the composite approach due to substantial stiffening, and moving to softer TPU matrix polymers is not possible because they tend to suffer from a lack of strength due to the limits of segmental demixing evident at low hard segment composition ratios. In addition, typically if a stiffer, harder TPU is required, a higher hard- segment composition ratio can readily be formulated. Therefore stiffening of this class of polymers without the accompaniment of other improved functional properties or performance benefits is not generally of commercial interest.
11.2.2 TPU nanocomposites employing nanofillers other than layered silicates
There are reports in the literature of TPU nanocomposites incorporating nanosilica in the form of either fumed silica,31,32 to improve the mechanical or adhesive properties of TPUs, or in the form of polyhedral oligomeric silsesquioxane POSS components to improve either biostability10 or resistance to thrombosis.10,11 Magnetite nanofillers have recently been dispersed and solution cast to produce TPU nanocomposite films with superparamagnetic properties/ and zinc oxide- TPU nanocomposites have recently been prepared and fabricated into electrospun web constructs, which demonstrated enhanced UV absorption and antimicrobial performance for potential use in protective clothing.12
There is also a growing body of work on TPU nanocomposites incorporating single-walled carbon nanotubes (SWNTs),33 double-walled carbon nanotubes,34 and multi-walled carbon nanotubes (MWNTs).8 The manipulation and incorporation of large numbers of individual CNTs into polymeric systems is a difficult task because the high molecular weights and strong intertube forces (both van der Waals and electrostatic) promote the formation of micron-sized bundles and ropes,i5 leading to phase separation and poor mechanical properties. As a result both surfactant and covalent functionalizations have been employed in an attempt to fragment and disperse these micron-sized inclusions.33,36–40 The majority of these TPU-CNT nanocomposites have been formulated via solution processing techniques,8’33’41–49 although in situ polymerization46’50–53 and melt processing8 have been reported as well. Despite the superior mechanical performance of SWNTs, MWNTs are the most common variety of CNT studied and a wide variety of surface functionalizations has been investigated. Koerner and co-workers reported the first study into TPU-CNT nanocomposites, in which the researchers attempted to improve the stress-recovery characteristics of Morthane (a commercially available, shape memory TPU) by adding 1–5 vol.% MWNT via solution processing.45 The researchers demonstrated that the nanocomposite could store and subsequently release up to 50% more recovery stress than the unmodified TPU. The nanocomposite also demonstrated the ability to recover stress, above that of the host TPU, when exposed to increased temperatures, infrared radiation, and electrical current. The authors theorized that the absorption of energy by MWNTs caused localized heating, which in turn melted the strain-induced TPU soft segment crystals, increasing stress recovery.
Sen and co-workers were the first to investigate the fabrication of TPU nanocomposites incorporating functionalized SWNTs33 The solution-processed nanocomposites demonstrated significantly increased mechanical properties over the host TPU. Whilst the final material was mechanically inferior to several commercially available TPUs without nanoparticle reinforcement, the study was the first to demonstrate both the possibility of increased dispersion in a TPU-CNT nanocomposite and that TPU-CNT interactions can be enhanced by CNT functionalization.
As a result, the majority of research into TPU-CNT nanocomposites has focused on using functionalized CNTs; however, there have been several studies published describing the use of pristine CNTs.8,43,48 Importantly, these investigations have demonstrated poor CNT dispersion and phase separation between the SWNTs and the host TPU and suggest that the future of TPU-CNT nanocomposites lies with modification of the CNT surface. A wide variety of functional groups have been attached to CNTs in an attempt to overcome these problems. CNT surface modifiers are typically either short hydrophilic groups like COOH,47,49 NH2,46 and OH41 capable of significant hydrogen bonding with the host polymer, or long- chain hydrophobic groups designed to break apart CNT aggregates and increase dispersion.33,54 Recently, significant results have been achieved by Deng and co-workers,42,55 who demonstrated dramatic mechanical enhancements with very small loadings of MWNTs modified with novel functional groups. Several research groups have also attempted to use CNTs as cross-linking agents, covalently bonding individual CNTs to the TPU backbone. However, results have been mixed due to the fine stoichiometric control required to produce nanocomposites with the same molecular weights and hard/soft segment ratios as the control TPU.50,52
Despite the abundance of work into TPU-CNT nanocomposites, the only published investigations of TPU-functionalized CNT nanocomposites formulated by melt processing techniques have been conducted by Chen and co-workers56 and more recently Barick and Tripathy.57 Chen et al. demonstrated that acid-treated MWNTs had dramatically increased mechanical properties with an eightfold increase in Young’s modulus and a 2.4-fold increase in tensile strength due to the strong interfacial interactions and good MWNT dispersion within the melt-drawn fibers. Barick and Tripathy reported substantial stiffening and toughening of a soft aliphatic grade of TPU by melt compounding vapor-grown carbon nanofibres (VGCNF), which had been purified, treated with acid and heat, into the host polymer. They also observed significant shifts in TPU thermal transitions and thermal stability, suggesting strong interfacial effects. Significantly, these results corroborate computational studies that suggest melt processing may induce a residual thermal radial stress (through differences in the coefficients of thermal expansion between the CNT and the host polymer), forcing the polymer into closer contact with the tube surface and thereby enhancing molecular interactions.58
11.2.3 TPU layered silicate nanocomposites
The vast majority of work on TPU nanocomposites has involved the use of layered-silicate-based nanofillers. Therefore, the remainder of this chapter will focus upon this class of nanocomposites and their chemistry, processing, structure, performance and safety or nanotoxicology.
11.2.4 Layered silicates as nanofillers
The incorporation of layered silicates into polymer matrices has been performed for over 50 years. During recent years, polymer nanocomposites containing natural clays and synthetic layered silicates have been most widely investigated, since the starting clay materials have been readily available in various sizes and shapes, and because their intercalation chemistry and interactions with surfactants and polymers have been studied for several decades.59 In particular, the development of instrumentation to characterize polymer nanocomposites at small length scales, such as scanning force, laser scanning fluorescence, and electron microscopes, have spurred researchers into probing the influence of particle size, shape, and surface chemistry on the properties of these new materials.
11.2.5 Fabrication techniques for TPU-clay nanocomposites
Typically smectite-type clays are usually used as fillers. Both natural clays (montmorillonite and hectorite) and synthetic clays (synthetic hectorites and fluoromicas) have been investigated, and TPU nanocomposite processing has seen many different approaches. The most common dispersion methods60 are solvent casting,61 melt processing,57 in situ polymerization,62 and reactive extrusion.30,38
In solvent casting, a polymer is solubilized in an organic solvent, then the nanoclay is dispersed in the resulting solution, often assisted with high shear homogenization or ultrasonic energy. The solvent is then allowed to evaporate, leaving the nanocomposite behind, typically as a thin film. The solvent imparts the enhanced mobility the polymer needs in order to intercalate between the silicate layers, while thermodynamic compatibility combined with physical mixing gives rise to a dispersed system. There are limitations to solvent casting. The selected solvent must be able to completely dissolve the polymer and disperse the nanoclay. This approach can also lead to poor clay dispersion, as well as other problems such as high costs due to the large amounts of solvent required to achieve appreciable filler dispersion, technical phase separation problems, and health and safety problems.60,63
In situ polymerization involves the dispersion of clay layers into the matrix by polymerization, mixing the silicate layers with the monomer, in conjunction with the polymerization initiator or the catalyst. This method, which was used by Usuki et al.64 in their pioneering research, takes advantage of the relative ease with which a small monomer molecule can intercalate silicate layers compared with a much larger polymer molecule.
Melt processing refers to the process of single or twin screw extrusion, whereby polymer chains are melted to increase their mobility and then the nanoclays are dispersed into the melt to allow intercalation of the nanoclays. Twin screw extruders or Brabender mixers require much higher shear energy, so care is needed when processing thermally sensitive TPU materials, as aggressive melt processing inevitably leads to a loss in molecular weight and a reduction in properties and performance. The selection of thermally stable organoclay modifiers is paramount, and careful washing away of excess exchanged alkylammonium cations is also important to avoid unwanted TPU degradation in the extruder.65 Melt processing of TPU nanocomposites can be easily scaled to manufacturing quantities, but care must be taken to avoid TPU degradation.66
Reactive extrusion of TPU involves the in situ polymerization of TPU precursors (polyol, diisocyanate, and a chain extender) in a twin screw extruder. Nanofillers can be either side fed as dry powders or pre-dispersed into the polyol liquid precursor.30,38 This method is used for chemical modification of existing polymers and the function of the extruder is as a continuous chemical reactor for polymerization.67 This technique requires chemical reaction control besides conventional screw extruder parameter control (of materials conveying, melting, and mixing). Compared with conventional solution casting, reactive extrusion has the following advantages: no solvent is required, high-viscosity polymers can be used, and processing conditions, such as mixing time and temperature, are flexible.67 Importantly, it avoids TPU reprocessing and thermal degradation, potentially avoids the handling of dry, inhalable nanopowder feeds, and effectively shifts the TPU nanocomposite processing route further up the TPU supply chain.30 The majority of commercial TPU materials available today are manufactured using reactive extrusion.
11.2.6 TPU clay nanocomposite structure–property relations
The dispersion of clay nanolayers and the morphology of the nanocomposite depend on various factors, such as the nature of the components used (layered silicate, surface modifier, and polymer matrix) and method of preparation (mixing method, time, and temperature).63,68,69 Generally, four main nanocomposite structures can be obtained, as illustrated in Fig. 11.3.60

11.3 Principal polymer layered silicate nanocomposite structures. (adapted from Alexandre and Dubois60)
In an intercalated structure, the polymer chains are sandwiched in between the silicate layers, which display limited dispersion. On the other hand, exfoliated nanocomposites demonstrate separated, individual silicate layers, which are more or less uniformly dispersed in the polymer matrix. The ordered structure is lost and the uniform dispersion of the anisotropic nano-sized particles can lead to a large interfacial area between the constituents at extremely low loadings of the nanoparticles. This large interfacial area and the nanoscopic dimensions between constituents differentiate polymer nanocomposites from conventional composites and filled plastics. Hence, new mechanical, optical, and electrical properties can be developed, which may not occur in the macroscopic counterparts.60 In fact, exfoliation of the platelets is the desirable objective of dispersion, because it achieves optimum enhancement of the properties. As well as these two well- defined structures, other intermediate organizations can exist presenting both intercalation and exfoliation.
Compared with the large body of research into polymer-clay nanocomposites, the number of studies conducted on TPU nanocomposites is relatively small. The use of TPU as the nanocomposite matrix presents some interesting challenges in understanding the nanoscale and microscale morphology, due to the pre-existing nanoscale morphology of the segmented TPU domain (the so-called soft and hard segments). TPU nanocomposites have been prepared predominantly through 3n situ polymerization,62’70–74 solvent casting,6’75–77 melt processing,78,79 and to a lesser extent via reactive extrusion.80,81
Previous research shows that TPUs can be tailored to meet the specific property requirements by incorporating organically modified smectic clays. The first examples of TPU nanocomposites with greatly improved performance were reported by Wang and Pinnavaia.62 The nanocomposites were prepared via in situ polymerization and alkylammonium-exchanged montmorillonites were used as nanofillers. They showed that the tensile strength, modulus, and strain at break all increased by more than 100% at a loading of only 10 wt% organoclay.
Meng et al.82 employed different molecular weight polyether-based TPUs and organically modified montmorillonite (OMMT) in their investigation of the exfoliation of OMMT in a TPU matrix by melt processing. They concluded that optimized exfoliated nanocomposites can be obtained if there is adequate shear stress and appropriate molecular diffusion to accommodate interaction between the OMMT and the TPU.
Low permeability TPU nanocomposites were produced by Runt and co-workers61 using polyether-based TPU and octadecylammonium-modified montmorillonite (MMT) by solvent casting. The water vapor permeabilities of the nanocomposites were reduced by as much as a factor of five at the highest OMMT content (20 wt%) and the enhancement of modulus was achieved without loss of ductility.
Cai et al.81 produced TPU-MMT nanocomposites by reactive extrusion, which involved a one step direct polymerization-intercalation technique with a twin screw extruder. Morphological characterization of the nanocomposite indicated that there were delaminated nanocomposite structures with well-dispersed nanoclays in the TPU matrix. Improvements in thermal stability, flame retardancy, and tensile strength were achieved with the addition of up to 4 wt% MMT.
Other successful TPU nanocomposites have been reported in the following publications. Depending on the system, increases in modulus62’75’77’83’84 tensile strength,70,74,75,78,83 elongation,6,74 flame resistance and thermal stability,70,72,83 barrier resistance,70,75,83 tear strength,85 abrasion resistance,72 fracture toughness,72 fatigue resistance,86 and corrosion resistance87 have been reported.
11.2.7 Hard and soft segment interactions with nanofillers
Knowledge in the TPU nanocomposite area has progressed recently with several research papers discussing the effects of nanofillers on the underlying complex TPU morphology, highlighting the importance of understanding the specific hard and soft segment interactions with the nanofillers in correlation with the resulting nanocomposite properties. Some of the literature in this field is summarized in Table 11.1.
Table 11.1
Summary of studies of TPU/organofiller nanocomposites with specific hard-segment or soft-segment interactions



The nanofiller in a nanocomposite should be distributed on a nanoscale level and therefore undergo substantial interactions with polymer chains and sequences. There is therefore tremendous potential ‘reinforcement efficiency’ due to the very high nanofiller aspect ratio and surface area, the local confinement of molecular motion,88,89 and the tailoring of specific hard or soft segment interactions with the nanofiller surface.34,90,91 However, due to the layered structure of silicate nanofillers, the organization of the resultant nanocomposite depends on the thermodynamic compatibility of the polymer and the layered silicate, the intercalation kinetics, and the processing conditions.65,92 The types of structures encountered are shown in Fig. 11.3. Virgin layered silicate particles are naturally hydrophilic, and as such are largely immiscible with TPUs, which contain a significant hydrophobic component in the form of the soft segments. This natural repulsion between the untreated silicate and the TPU discourages the formation of an exfoliated system, which is required for an effective nanocomposite. Instead a phase-separated composite or microcomposite is formed, whose properties do not exceed those of traditional composites.
In order to prepare effective nanocomposites, the hydrated cations in the interlayer spacing are replaced with organic cations to render the surface more organophilic and improve wetting by the polymer matrix60,93 Furthermore, this substitution allows swelling of the gallery between the silicate layers, which in turn allows the polymer to enter the galleries during the formation of the nanocomposite. An unswollen silicate has an interlayer spacing of the order of 1 nm, which is smaller than the host polymer’s radius of gyration.94,95 The control and enhancement of both the polymer-silicate interaction and the swelling of the gallery layers are thus critical factors in the production of any polymeric nanocomposite, particularly so for TPUs, which generally possess both hydrophilic (hard segments) and hydrophobic (soft segment) components, both of which are of a length scale much smaller than even the smallest synthetic clay-based nanofillers (~ 25 nm).
In 1998, Wang and Pinnavaia62 reported the first examples of TPU-layered silicate nanocomposites. The nanocomposites were prepared via in situ polymerization using montmorillonite (ion exchanged with alkylammonium salts) as the nanofiller. X-ray diffraction (XRD) patterns indicated that intragallery polymerization contributed to the dispersion of the organosilicates, and improvements in tensile strength, elongation, and modulus were observed.
The commercially available Cloisite® series of organosilicates have been used in a large number of TPU nanocomposite studies. Earlier studies employed solvent casting54,61,96,97 to assist in overcoming some of the abovementioned thermodynamic barriers, and also to avoid complications due to thermal degradation of the alkylammonium modifiers.98,99 Interestingly, the more hydrophobic Cloisite 15A generally did not disperse well, although increases in tensile and barrier properties96,97 were reported with this nanofiller. Finnigan et al. reported that the more hydrophilic Cloisite 30B produced nanocomposites with relatively better dispersion, and very large associated increases in stiffness, tensile hysteresis, and permanent set.77 This increase in hysteresis in nanocomposites has not been well documented in the literature, but is an important observation because many dynamic applications of TPUs require that resilience is maintained. This study was also the first to directly compare identical TPU nanocomposite formulations prepared via both solution and melt processing. However, the very small lab scale conical twin screw extruder employed caused extreme thermal degradation and loss of ultimate properties.
Due to the more commercially scalable nature of melt compounding, several workers have investigated TPU-Cloisite® nanocomposites prepared via melt compounding on a larger scale.7,57,100,101 The relatively hydrophilic Cloisite® 30B and Cloisite® 10A generally disperse and delaminate well in TPUs.7,54,57 This is due to their respective polar hydroxyl and benzyl functionalities, which promote thermodynamically favorable enthalpic driving forces, such as H bonding, for intercalation.54,34,103 Cloisite® 25A, in the middle of the hydrophilicity/ hydrophobicity range, has also resulted in good dispersion and property enhancement.75,83 Dan et al. achieved the best property improvements with Cloisite® 30B, and also found that higher tensile properties could be achieved in an ester-based TPU with respect to an ether-based TPU, despite better dispersion in the latter.102 They also reported the influence of some nanofillers on hard/soft segment demixing and morphology, as well as a decrease in observed properties after a second melt processing step, implying thermal degradation.
Chavarria and Paul100 further probed the effect of organoclay structure by modifying a series of montmorillonite-based nanofillers with various alkylammonium surfactants, and studying the resulting structure–property relations for a high-hardness ether-based versus a medium-hardness ester-based host TPU. They concluded that for both host TPU systems: (a) one long alkyl tail on the ammonium ion rather than two, (b) hydroxyl ethyl groups on the amine rather than methyl groups, and (c) a longer alkyl tail as opposed to a shorter one, all lead to improved clay dispersion and TPU nanocomposite stiffness. However, although the harder ether-based (and presumably higher melt viscosity) TPU host polymer had better nanofiller dispersion, the percentage increase in properties remained higher in the softer TPU. Meng et al.82 also showed that the degree of dispersion of the nanofiller (Cloisite 30B) was improved by processing the TPU host so that it had a higher molecular weight and higher viscosity due to improved shear. They also showed that an optimum Brabender melt processing time was required to achieve the highest combination of nanofiller dispersion and tensile stress at 500% strain, due to excessive thermal degradation at longer processing times. Ma et al. organically modified a less well-known class of layered silicate, rectorite, and performed melt compounding studies on a polyester-based TPU and reported substantial increases in both tensile strength and tear strength.101 Most interestingly, the strongest formulations did not necessarily provide the best tear strength, suggesting that toughening mechanisms in these systems are complex and that non-delaminated clay tactoids may play more of a role in toughening than discrete organoclay platelets.
Whilst much of the research has focused on ionic substitution of bulky organic surfactants, several attempts at covalent modification of the silicate surface have been attempted.74’104 An initial report from Tien and Wei74 utilized organosilicates with one to three hydroxyl groups per surfactant molecule, which were capable of participating in the polymerization process and promoting delamination from within the layers. Since then, other researchers have used this approach with Cloisite® 30B.104 The tethering of TPU chains to the organosilicate surface has also been used to achieve a strong interfacial bond. However, it is difficult to reach the full potential for property improvements via this route, because the isocyanate groups can also react with bound water in the silicate interlayer, and with hydroxyl groups on the silicate edges, which, if not correctly accounted for, can lead to reduced molecular weight and matrix cross-link density.105 The size of the layered silicate has been shown to influence the resulting nanocomposite morphology. Due to the relative scale of the hard domains compared with the most common silicates employed, few groups have investigated this area. However, research into smaller silicates has shown tensile strength can be significantly enhanced without significantly altering Young’s modulus or resilience.78’106 In particular, the number of polymer-filler interactions and entanglements increases as the platelet size decreases, because the smaller platelets have a larger surface area and a smaller interparticle distance. This can have a significant effect on the mechanical properties, particularly at high strains where smaller platelets can more readily rotate without overcrowding, and therefore can more readily interact with the matrix by communicating in shear. Finnigan et al78 clearly demonstrated this by performing in situ strained synchrotron small-angle X-ray scattering measurements on a series of solution-cast TPU nanocomposites incorporating organically modified fluoromica nanofillers of controlled aspect ratio, which had been prepared via high energy milling. In a follow-up study they showed that superior resistance to stress relaxation could also be achieved by the incorporation of low aspect ratio organoclays. 106 They explained that, as the particle size and aspect ratio of the filler increase, the TPU chains in the interfacial region are more restrained and experience greater localized stresses, which result in an increase in strain-induced slippage at the polymer-filler interface. This technology has been patented,108 and a startup company, TenasiTech Pty Ltd, formed to commercialize the technology. TenasiTech has now substantially scaled up TPU nanocomposite production via reactive extrusion.30 Typical performance gains for a common polyether-based aromatic TPU reinforced with a 2(w/w)% fluoromica-based nanofiller are shown in Table 11.2.
Table 11.2
Overall performance enhancement for a TPU nanocomposite prepared by reactive extrusion, with respect to the unreinforced host polymer30,107
| Property | Host | Nanocomposite |
| Tensile strength (MPa) | 53 | 67 |
| Elongation at break (%) | 665 | 748 |
| Shore hardness (D) | 43 | 43 |
| Stress at 100% strain | 10 | 11 |
| Stress at 300% strain | 16 | 16 |
| Tear strength (MPa) | 87 | 106 |
| Creep modulus (MPa) | 2.27 | 3.49 |
| Creep modulus (MPa) 60C | 1.73 | 1.93 |
| Compression set (%) at room temp | 27 | 15 |
Notably, the property improvements reported are evidence that well-engineered nanofiller systems can not only avoid stiffening and hardening effects; they can also have a positive influence on critical viscoelastic properties such as creep and compressive set, in addition to ultimate tensile strength and tear strength.
Using a Monte Carlo simulation, Pandey et al.109 predicted that smaller clay platelets should be more easily delaminated and dispersed in a compatible solventhost polymer than high aspect ratio clays. Furthermore, computational models by Balazs and co-workers on block copolymers indicated that a change in microphase texture and properties would be expected since nanofiller size scales with the phase domain size of the block copolymer.110 McKinley and co-workers29,91 incorporated unmodified, low aspect ratio hectorite (Laponite) into a number of TPUs at up to 20 wt% using a novel solvent-exchange technique. They reported remarkable mechanical properties, heat distortion temperature, and morphological changes in fully delaminated nanocomposite systems. Some degree of ‘tuned’ segmental association of the Laponite® was demonstrated, depending on the solubility parameter of the chosen soft segment.91
11.3 TPU nanocomposites as potential biomaterials
Thermoplastic polyurethane is the material of choice for many biomedical applications due to the relative ease of fabrication into devices, flexibility, biocompatibility, biostability, and electrical insulation properties. Polyether-based TPUs have been the materials of choice for certain types of medical implants for many years. However, there have been some cases in which the TPU degraded and led to surface or deep cracking, stiffening, erosion, or the deterioration of mechanical properties such as tensile and flexural strength.111–114 These eventually caused implant malfunction. Polydimethylsiloxane (PDMS)-based elastomers were then gradually commercialized and introduced to overcome these problems. A PDMS/ poly(hexamethylene oxide)-based TPU based on an optimized formulation (Elast-Eon™) from AorTech International plc exhibited properties comparable to those of medical grade polyether-based TPUs such as Pellethane 80A.115 Elast-Eon TPUs are now widely accepted as being the most biostable of all TPUs and as such are imminently suitable for long-term implantation.116
Few researchers have recognized the potential of TPU nanocomposites as biomaterials even though recent studies have revealed that TPU nanocomposites have the potential to be developed for biomedical device components due to the enhancement of thermal, mechanical, and barrier properties4,61’117’118 and their capability in tuning cell-material interactions.191,120 Furthermore, nanofillers can introduce new functionality, such as antimicrobials.121,122 One research group directly studying TPU-organosilicate nanocomposites as biomaterials is led by James Runt at Pennsylvania State University. This group has published two papers describing the preparation and properties of solution-cast nanocomposites prepared from the biomedical TPU Biospan™ (chemically similar to poly(ether urethane) (PEU)) and Cloisite® 15 A.61’97 The nanocomposites demonstrated an enhancement in tensile strength, modulus, and elongation at break when the nanoclay content increased from 0 wt% to 20 wt%. Styan123 also prepared biomedical TPU organosilicate nanocomposites from PEU with organically modified MMT loadings in the range of 1 wt% to 15 wt%, using solution casting, and found that partially exfoliated nanocomposites were produced using 15 wt% Cloisite® 30B. These nanocomposites displayed several advantageous properties, namely significant antibacterial activity, reducing Staphylococcus epidermidis adherence after 24 h to ~ 20% and enhanced biostability. Liff et al.19 employed a novel solvent-exchange approach to efficiently exfoliate synthetic smectic clays (Laponite® RD) in a biomedical poly(ether urethane) (Elasthane™ 80A). Elasthane™ 80 reported an ultimate tensile strength increase of 50% when 10 wt% of unmodified Laponite® RD was incorporated into the Elasthane matrix using a labor intensive solvent exchange technique.
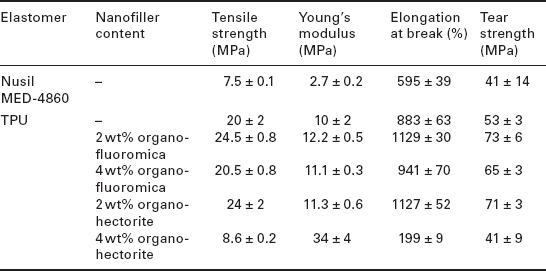
The next generation of cochlear-implant electrode arrays will require easily processable insulating materials with substantially improved mechanical performance. Currently, soft silicone materials are employed as insulation for implantable cochlear electrode arrays. In an attempt to produce new materials with improved properties, we recently generated PDMS-based TPU nanocomposites containing low aspect ratio synthetic hectorite (Lucentite SWN from Kobo Products) and high aspect ratio synthetic fluoromica (Soma sif™ ME100 from Kobo Products) with a hydrophobic surface modification.124 The mechanical properties of the solvent-cast TPU (blank and nanocomposites) are summarized in Table 11.3, compared with Nusil MED 4860, a biomaterial that is currently used for insulation in cochlear implants. In general, this PDMS-based TPU displays greater mechanical properties compared with Nusil MED-4860, with an increase of 167% in tensile strength, 48% in elongation at break, and 29% in tear strength. Adding 2 wt% of modified nanofillers further increased the mechanical properties in this system. Both high and low aspect ratio nanofillers successfully increased the tensile strength, elongation at break, and tear strength by ~ 20%, 28%, and 34% respectively (Table 11.3 and Fig. 11.4. These preliminary results show that PDMS-based TPU nanocomposites are promising materials for biomedical applications, and may allow thinner and more intricate electrode arrays to be designed, while still maintaining structural integrity. To this end, we are currently investigating the morphology of this PDMS-based TPU and associated nanocomposites in attempt to develop an in-depth understanding of the structure- property relations of these materials, most importantly the interplay between the TPU nanophase domains and the engineered low and high aspect ratio nanofillers.
Table 11.3
Tensile properties of E5-325 TPU host and nanocomposites incorporating 2 and 4% low-aspect-ratio (hectorite) and high-aspect-ratio (fl uoromica) synthetic organoclays, and Nusil MED-4860 silicone for comparison
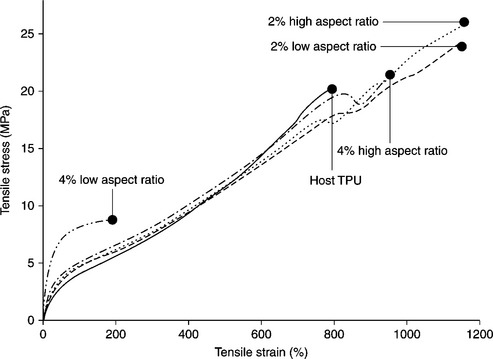
11.4 Representative stress–strain curves for the TPU host polymer and nanocomposites incorporating 2% and 4% low- and high-aspect- ratio organoclays
Organomodifiers are commonly ion exchanged with cations in the interlayer galleries of the clay to improve compatibility between the clay and the polyurethane matrix. Pool-Warren et al. showed that antimicrobial functionality can be introduced by incorporating chlorhexidine-diacetate-modified montmorillonite in nanocomposites. When dodecyl-amine-modified montmorillonite was used, the adhesion of both platelets and fibroblasts decreased, indicating the potential use of polyurethane nanocomposites as bioactive materials.125
Some studies show that the release of quaternary alkyl ammonium from organoclays could induce toxicity.126’127 Rueda et al. reported that the presence of 2 wt% Cloisite® 30B in polyurethane nanocomposites caused a dramatic reduction in the number of proliferating cells in contact with an extract of the nanocomposites.117 Styan et al. mentioned that methyl tallow bis-2-hydroxyethyl ammonium, which was released from Cloisite® 30B, was capable of inhibiting cell growth.118 In contrast, Mishra et al. showed different results. Cells were able to grow on polyurethane nanocomposites containing Cloisite® 30B within 48 h. Furthermore, the authors showed that the nanocomposites were not genotoxic by observing the localization of the enzyme HIPK2. HIPK2 is an apoptosis activator. The presence of HIPK2 in a nucleus indicates genotoxicity stress. In this paper, HIPK2 was found in cytoplasm and thus incapable of transfecting the apoptosis gene.128 Unfortunately, direct comparison of the previously mentioned studies is not possible because the authors used different cell lines to investigate toxicity.
In order to ensure the biocompatibility of our TPU nanocomposites, we are investigating the cytotoxicity of nanofillers on mouse neuroblasts (neuro N2A) using a CellTiter 96AQueous non-radioactive cell proliferation assay (MTS). Preliminary results show that some of organo-fluoromica are is significantly less toxic than Cloisite 30B. The half maximal inhibitory concentration (IC50) of the least toxic organo fluoromica is 90 μg/ml, while that of Cloisite 30B is 20 μg/ml, both concentrations normalized to clay (unpublished work). Styan et al. showed that, when montmorillonite modified with amino undecanoic acid was used, cell growth was not severely inhibited because at pH 7.4 this organomodifier was negatively charged. Thus, the possibility of interacting with cell membranes and causing cell death was reduced. These imply that suitable material selection is important when incorporating organoclays in nanocomposites. In addition, the method of processing the materials is another crucial factor. Edwards et al. showed that some commercial organoclays contained excess surfactants, which could be released and thus induce toxicity.99
The susceptibility of polyurethanes to degrade in vivo is useful for the development of bioresorbable implants intended for short term implantation. The hydrophilic nature of clays is utilized in implanted polyurethane nanocomposites to attract water, which induces hydrolysis and consequently leads to polyurethane degradation. Da Silva et al. showed that employing unmodified montmorillonite could enhance the rate of biodegradation and subsequently the release of drugs incorporated in the implant.129,130
The above findings show that TPU nanocomposites can provide a combination of biostability and advantageous mechanical properties. They are, therefore, promising candidates for use as biomaterials in the fabrication of a wide range of medical devices.
11.4 Future trends
While the nanocomposite approach has undoubtedly led to several encouraging examples of new functional and mechanical property profiles, most examples have failed to live up to their theoretical potential. An excellent recent review paper by Schaefer and Justice 131 points out that in the majority of polymer nanocomposites today ‘large scale disorder is ubiquitous.’ Consistently with some of the reports discussed here, they suggest that the fixation on high aspect ratio nanofillers is unproductive due to: (a) problems with agglomeration and dispersion of the high aspect ratio particles, and (b) the potential for tailored molecular self- assembly involving a higher degree of ‘cooperation’ between the nanofiller and matrix. The nature of more recent investigations suggests that a more systematic and exacting approach is now being adopted by TPU nanocomposite researchers, guided by a growth in multiscale theoretical modeling. New experimental techniques need to be developed and applied to better probe and understand the subtle structures and conformations at the TPU-nanofiller interface. Synchrotron X-ray and neutron scattering will continue to be important. Highly engineered model nanoparticle and TPU systems may be required to elucidate these questions. There is also a need for the development of new classes of layered inorganic nanofillers that are more readily delaminated and more thermally stable. Metal phosphonates have been proposed as one possible new class 132 and there have also been some efforts to employ covalent organic modification 133 using silane grafting.
Historically, the mechanical testing performed on polymer nanocomposites has been limited to simple tensile experiments, and thereby has largely ignored many of the properties critical to the true service performance of the materials. Therefore, the long-term mechanical, thermal, and other environmental ageing, tear and cut- growth resistance, and fatigue performance of TPU nanocomposite materials are other areas greatly in need of attention.
The effects of processing on nanocomposite structure and properties, and the use of rheology to elucidate the effects of nanocomposite formation, are new and exciting fields of research in nanocomposites. Traditionally much research (highlighted above) has focused on the properties of nanocomposites produced from one type of process or processing condition. Clearly the effects of processing on nanocomposite formation and the understanding of the rheology of nanocomposites are some of the next key scientific steps needed to fully understand and control TPU nanocomposite properties and performance in various applications. An optimistic perspective would see future applications of TPU nanocomposites in biomedical implants (e.g., softer, tougher, implantable electrode insulation for pacemakers), 124 better creep and abrasion resistant mining screens, drive and conveyer belts, more durable TPU seals, o-rings, and hoses for fuels. Further uses are stronger, lighter, melt spun Spandex, thin aliphatic gas barrier coatings for encapsulating the next generation of thin-film photovoltaics, tougher, more scuff-resistant covers on golf balls and protective wear, and many others. Nanocomposite technology could also see TPUs remain at the ‘top of their class’ with respect to the many other TPEs available.
11.5 References
1. Hepburn, C. Polyurethane Elastomers. London, New York: Elsevier, 1991; 441.
2. Oertel, G., Abele, L. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties, 2nd edition, Munich, New York, Cincinnati: Hanser/Gardner; 1994:688.
3. Sullivan, F.A. p. A750-37. U.S. Thermoplastic Elastomers (TPE) Markets. 2004.
4. Song, H.J., Qi, H.A., Li, N. Tribological behavior of carbon nanotubes/polyurethane nanocomposite coatings. Micro & Nano Letters. 2011; 6(1):48–51.
5. Yusoh, K., Jin, J., Song, M. Subsurface mechanical properties of polyurethane/ organoclay nanocomposite thin films studied. Progress in Organic Coatings. 2010; 67(2):220–224.
6. Silva, G.G., Rodrigues, M.F., Fantini, C. Thermoplastic polyurethane nanocomposites produced via impregnation of long carbon nanotube forests. Macromolecular Materials and Engineering. 2011; 296(1):53–58.
7. Berta, M., Saiani, A., Lindsay, C., Gunaratne, R. Effect of clay dispersion on the rheological properties and flammability of polyurethane-clay nanocomposite elastomers. Journal of AppliedPolymerScience. 2008; 112(5):2847–2853.
8. Heraidan, M., Shishesaz, M.R., Kassiriha, S.M. Study on the effect of ultrasonication time on transport properties of polyurethane/organoclay nanocomposite coatings. Journal of Coatings Technology and Research. 2011; 8(2):265–274.
9. Ashjari, M., Mahdavian, A.R. Efficient dispersion of magnetite nanoparticles in the polyurethane matrix through solution mixing and investigation of the nanocomposite properties. Journal of Inorganic and Organometallic Polymer and Materials. 2010; 20(2):213–219.
10. Kannan, R.Y., Salacinski, H.J., De Groot, J., Clatworthy, I., Bozec, L., et al. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules. 2006; 7(1):215–223.
11. Kannan, R.Y., Salacinski, H.J., Odlyha, M., Butler, P.E., Seifalian, A.M. The degradative resistance of polyhedral oligomeric silsesquioxane nanocore integrated polyurethanes: An in vitro study. Biomaterials. 2006; 27(9):1971–1979.
12. Lee, S. Multifuctionality of layered fabric systems based on electrospun polyurethane/ zinc oxide nanocomposite fibers. Journal of Applied Polymer Science. 2009; 114(6):2658–3652.
13. Chu, B., Gao, T., Li, Y.J., Wang, J., Desper, C.R., et al. Microphase separation kinetics in segmented polyurethanes - effects of soft segment length and structure. Macromolecules. 1992; 25(21):5724–5729.
14. Elwell, M.J., Ryan, A.J., Grunbauer, H.J.M., VanLieshout, H.C. In-situ studies of structure development during the reactive processing of model flexible polyurethane foam systems using FT-IR spectroscopy, synchrotron SAXS, and rheology. Macromolecules. 1996; 29(8):2960–2968.
15. Ryan, A.J., Willkomm, W.R., Bergstrom, T.B., Macosko, C.W., Koberstein, J.T., et al. Dynamics of (micro)phase separation during fast, bulk copolymerization – Some synchrotron SAXS Experiments. Macromolecules. 1991; 24(10):2883–2889.
16. Lilaonitkul, A., Cooper, S.L. Properties of thermoplastic polyurethane elastomers. Advances in Urethane Science and Technology. 1979; 7:163–183.
17. Abouzahr, S., Wilkes, G.L., Ophir, Z. Structure property behavior of segmented polyether MDI butanediol based urethanes – Effect of composition ratio. Polymer. 1982; 23(7):1077–1086.
18. Harrell, L.L., Abstracts of Papers of the American Chemical Society. Segmented polyurethanes - Properties as a function of segment size and distribution. 1969:PO57 ff.
19. Martin, D.J., Meijs, G.F., Renwick, G.M., Gunatillake, P.A., McCarthy, S.J. Effect of soft-segment CH.IO ratio on morphology and properties of a series of polyurethane elastomers. Journal of Applied Polymer Science. 1996; 60(4):557–571.
20. Miller, J.A., Lin, S.B., Hwang, K.K.S., Wu, K.S., Gibson, P.E., et al. Properties of polyether polyurethane block copolymers – Effects of hard segment length distribution. Macromolecules. 1985; 18(1):32–44.
21. Samuels, S.L., Wilkes, G.L. Rheo-optical and mechanical behavior of a systematic series of hard-soft segmented urethanes. Journal of Polymer Science Part C: Polymer Symposium. 1973; 43:149–178.
22. Speckhard, T.A., Cooper, S.L. Ultimate tensile properties of segmented polyurethane elastomers – Factors leading to reduced properties for polyurethanes based on nonpolar soft segments. Rubber Chemistry and Technology. 1986; 59(3):405–431.
23. Wang, C.B., Cooper, S.L. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules. 1983; 16(5):775–786.
24. Lee, H.S., Hsu, S.L. Structural changes and chain orientational behavior during tensile deformation of segmented polyurethanes. Journal of Polymer Science Part B: Polymer Physics. 1994; 32(12):2085–2098.
25. Reynolds, N., Spiess, H.W., Hayen, H., Nefzger, H., Eisenbach, C.D. Structure and deformation behavior of model poly(ether-urethane) elastomers. 1. Infrared studies. Macromolecular Chemistry and Physics. 1994; 195(8):2855–2873.
26. Martin, D.J., Meijs, G.F., Gunatillake, P.A., McCarthy, S.J., Renwick, G.M. The effect of average soft segment length on morphology and properties of a series of polyurethane elastomers. 2. SAXS-DSC annealing study. Journal of Applied Polymer Science. 1997; 64(4):803–817.
27. Torro-Palau, A., Fernandez-Garcia, J.C., Orgiles-Barcelo, A.C., Pastor-Blas, M.M., Martin-Martinez, J.M. Comparison of the properties of polyurethane adhesives containing fumed silica or sepiolite as filler. Journal of Adhesion. 1997; 61(1–4):195–211.
28. Varma, A.J., Deshpande, M.D., Nadkarni, V.M. Angewandte Makromolekulare Chemie. Morphology and mechanical properties of silicate filled polyurethane elastomers based on castor oil and polymerix MDI. 1985; 132(JUN):203–209.
29. Liff, S., Kumar, N., McKinley, G. High-performance elastomeric nanocomposites via solvent-exchange processing. Nature Materials. 2007; 6(1):76.
30. Marshall, R., Martin, D. Nanocomposite polyurethanes for extreme applications in oil and gas. Houston, USA, 2011 . 2011.
31. Petrovic, Z.S., Javni, I., Waddon, A., Banhegyi, G. Structure and properties of polyurethane-silica nanocomposites. Journal of Applied Polymer Science. 2000; 76(2):133–151.
32. Vega-Baudrit, J., Sibaja-Ballestero, M., Vazquez, P., Torregrosa-Macia, R., Martin- Martinez, J.M. Properties of thermoplastic polyurethane adhesives containing nanosilicas with different specific surface area and silanol content. International Journal of Adhesion and Adhesives. 2007; 27(6):469–479.
33. Sen, R., Zhao, B., Perea, D., Itkis, M.E., Hu, H., et al. Preparation of single-walled carbon nanotube reinforced polystyrene and polyurethane nanofibers and membranes by electrospinning. Nano Letters. 2004; 4(3):459–464.
34. Balazs, A.C., Singh, C., Zhulina, E. Modeling the interactions between polymers and clay surfaces through self-consistent field theory. Macromolecules. 1998; 31(23):8370–8381.
35. Tagmatarchis, N., Prato, M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. Journal ofMaterials Chemistry. 2004; 14(4):437–439.
36. Barisci, J.N., Tahhan, M., Wallace, G.G., Badaire, S., Vaugien, T., et al. Properties of carbon nanotube fibers spun from DNA-stabilized dispersions. Advanced Functional Materials. 2004; 14(2):133–138.
37. Hilding, J., Grulke, E.A., Zhang, Z.G., Lockwood, F. Dispersion of carbon nanotubes in liquids. Journal of Dispersion Science and Technology. 2003; 24(1):1–41.
38. Lin, T., Bajpai, V., Ji, T., Dai, L.M. Chemistry of carbon nanotubes. Australian Journal of Chemistry. 2003; 56(7):635–651.
39. Ortiz-Acevedo, A., Xie, H., Zorbas, V., Sampson, W.M., Dalton, A.B., et al. Diameter- selective solubilization of single-walled carbon nanotubes by reversible cyclic peptides. Journal of the American Chemical Society. 2005; 127(26):9512–9517.
40. Dieckmann, G.R., Dalton, A.B., Johnson, P.A., Razal, J., Chen, J., et al. Controlled assembly of carbon nanotubes by designed amphiphilic peptide helices. Journal of the American Chemical Society. 2003; 125(7):1770–1777.
41. Buffa, F., Abraham, G.A., Grady, B.P., Resasco, D. Effect of nanotube functionalization on the properties of single-walled carbon nanotube/polyurethane composites. Journal of Polymer Science Part B: Polymer Physics. 2007; 45(4):490–501.
42. Deng, J., Zhang, X., Wang, K., Zou, H., Zhang, Q., et al. Synthesis and properties of poly(ether urethane) membranes filled with isophorone diisocyanate-grafted carbon nanotubes. Journal ofMembrane Science. 2007; 288(1–2):261–267.
43. Foster, J., Singamaneni, S., Kattumenu, R., Bliznyuk, V. Dispersion and phase separation of carbon nanotubes in ultrathin polymer films. Journal of Colloid and Interface Science. 2005; 287(1):167–172.
44. Koerner, H., Liu, W.D., Alexander, M., Mirau, P., Dowty, H., et al. Deformation- morphology correlations in electrically conductive carbon nanotube thermoplastic polyurethane nanocomposites. Polymer. 2005; 46(12):4405–4420.
45. Koerner, H., Price, G., Pearce, N.A., Alexander, M., Vaia, R.A. Remotely actuated polymer nanocomposites – Stress recovery of carbon nanotube filled thermoplastic elastomers. NatureMaterials. 2004; 3(2):115–120.
46. Kuan, H.C., Ma, C.C.M., Chang, W.P., Yuen, S.M., Wu, H.H., et al. Synthesis, thermal, mechanical and rheological properties of multiwall carbon nano tube/ waterborne polyurethane nanocomposite. Composites Science and Technology. 2005; 65(11–12):1703–1710.
47. Kwon, J.Y., Kim, H.D. Preparation and properties of acid-treated multiwalled carbon nanotube/waterborne polyurethane nanocomposites. Journal of Applied Polymer Science. 2005; 96(2):595–604.
48. Meng, J., Kong, H., Xu, H.Y., Song, L., Wang, C.Y., et al. Improving the blood compatibility of polyurethane using carbon nanotubes as fillers and its implications to cardiovascular surgery. Journal of Biomedical Materials Research Part A. 2005; 74A(2):208–214.
49. Sahoo, N.G., Jung, Y.C., Yoo, H.J., Cho, J.W. Effect of functionalized carbon nanotubes on molecular interaction and properties of polyurethane composites. Macromolecular Chemistry and Physics. 2006; 207(19):1773–1780.
50. Jung, Y.C., Sahoo, N.G., Cho, J.W. Polymeric nanocomposites of polyurethane block copolymers and functionalized multi-walled carbon nanotubes as crosslinkers. Macromolecular Rapid Communications. 2006; 27(2):126–131.
51. Xia, H., Song, M., Jin, J., Chen, L. Poly(propylene glycol)-grafted multi-walled carbon nanotube polyurethane. Macromolecular Chemistry and Physics. 2006; 207(21):1945–1952.
52. Xu, M., Zhang, T., Gu, B., Wu, J.L., Chen, Q. Synthesis and properties of novel polyurethane-urea/multiwalled carbon nanotube composites. Macromolecules. 2006; 39(10):3540–3545.
53. Xiong, J., Zheng, Z., Qin, X., Li, M., Li, H., et al. The thermal and mechanical properties of a polyurethane/multi-walled carbon nanotube composite. Carbon. 2006; 44(13):2701–2707.
54. Smart, S., Fania, D., Milev, A., Kannangara, G.S.K., Lu, M., et al. The effect of carbon nanotube hydrophobicity on the mechanical properties of carbon nanotube- reinforced thermoplastic polyurethane nanocomposites. Journal of Applied Polymer Science. 2010; 117(1):24–32.
55. Deng, J., Cao, J., Li, J., Tan, H., Zhang, Q., et al. Mechanical and surface properties of polyurethane/fluorinated multi-walled carbon nanotubes composites. Journal of Applied Polymer Science. 2008; 108(3):2023–2028.
56. Chen, W., Tao, X., Liu, Y. Carbon nanotube-reinforced polyurethane composite fibers. CompositesScience and Technology. 2006; 66:3029–3034.
57. Barick, A.K., Tripathy, D.K. Effect of nanofiber on material properties ofvapor- grown carbon nanofiber reinforced thermoplastic polyurethane (TPU/CNF) nanocomposites prepared by melt compounding. Composites Part A: Applied Science and Manufacturing. 2010; 41(10):1471–1482.
58. Wong, M., Paramsothy, M., Xu, X.J., Ren, Y., Li, S., et al. Physical interactions at carbon nanotube-polymer interface. Polymer. 2003; 44(25):7757–7764.
59. Akelah, A., Moet, A. Polymer-clay nanocomposites: Free-radical grafting of polystyrene on to organophilic montmorillonite interlayers. Journal of Materials Science. 1996; 31(13):3589–3596.
60. Alexandre, M., Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Materials Science and Engineering: R: Reports. 2000; 28(1–2):1–63.
61. Xu, R.J., Manias, E., Snyder, A.J., Runt, J. Low permeability biomedical polyurethane nanocomposites. Journal of Biomedical Materials Research Part A. 2003; 64A(1):114–119.
62. Wang, Z., Pinnavaia, T. Nanolayer reinforcement of elastomeric polyurethane. Chemistry of Materials. 1998; 10(12):3769–3771.
63. Bhattacharya, S., Kamal, M., Gupta, R. Hanser Gardner Pubns. Polymeric Nanocomposites: Theory and Practice. 2007.
64. Usuki, A., Kojima, Y., Kawasumi, M., Okada, A., Fukushima, Y., et al. Synthesis of nylon 6-clay hybrid. Journal of Materials Research(USA). 1993; 8(5):1179–1184.
65. Dennis, H., Hunter, D., Chang, D., Kim, S., White, J., et al. Effect o fmelt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer. 2001; 42(23):9513–9522.
66. Giannelis, E. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. AppliedOrganometallicChemistry. 1998; 12(10–11):675–680.
67. Xanthos, M., Biesenberger, J. Hanser Munich. Reactive Extrusion: Principles and Practice. 1992.
68. Mittal, V. Optimization of Polymer Nanocomposite Properties. Wiley-VCH; 2010.
69. Takeichi, T., Zeidam, R., Agag, T. Polybenzoxazine/clay hybrid nanocomposites: Influence of preparation method on the curing behavior and properties of polybenzoxazines. Polymer. 2002; 43(1):45–53.
70. Choi, W., Kim, S., Jin Kim, Y., Kim, S. Synthesis of chain-extended organifier and properties of polyurethane/clay nanocomposites. Polymer. 2004; 45(17):60456057.
71. Ma, J., Zhang, S., Qi, Z. Synthesis and characterization of elastomeric polyurethane/clay nanocomposites. J. Appl. Polym. Sci.. 2001; 82(6):1444–1448.
72. Pattanayak, A., Jana, S. Thermoplastic polyurethane nanocomposites of reactive silicate clays: Effects of soft segments on properties. Polymer. 2005; 46(14):51835193.
73. Rhoney, I., Brown, S., Hudson, N., Pethrick, R. Influence of processing method on the exfoliation process for organically modified clay systems. I. Polyurethanes. J. Appl. Polym. Sci.. 2003; 91(2):1335–1343.
74. Tien, Y., Wei, K. High-tensile-property layered silicates/polyurethane nanocomposites by using reactive silicates as pseudo chain extenders. Macromolecules. 2001; 34(26):9045–9052.
75. Chang, J., An, Y. Nanocomposites of polyurethane with various organoclays: Thermomechanical properties, morphology, and gas permeability. Journal of Polymer Science Part B: Polymer Physics. 2002; 40(7):670–677.
76. Chen, T.K., Tien, Y.I., Wei, K.H. Synthesis and characterization of novel segmented polyurethane/clay nanocomposites. Polymer. 2000; 41(4):1345–1353.
77. Finnigan, B., Martin, D., Halley, P., Truss, R., Campbell, K. Morphology and properties of thermoplastic polyurethane composites incorporating hydrophobic layered silicates. J. Appl. Polym. Sci.. 2005; 97(1):300–309.
78. Finnigan, B., Jack, K., Campbell, K., Halley, P., Truss, R., et al. Segmented polyurethane nanocomposites: Impact of controlled particle size nanofillers on the morphological response to uniaxial deformation. Macromolecules. 2005; 38(17):7386–7396.
79. Mishra, J., Kim, I., Ha, C. Newmillable polyurethane/organoclay nanocomposite: Preparation, characterization and properties. Macromolecular Rapid Communications. 2003; 24(11):671–675.
80. Kim, T., Kim, B., Kim, Y., Cho, Y., Lee, S., et al. The properties ofreactive hot melt polyurethane adhesives modified with novel thermoplastic polyurethanes. Journal of Applied Polymer Science. 2009; 114(2):1169–1175.
81. Cai, Y., Hu, Y., Song, L., Liu, L., Wang, Z., et al. Synthesis and characterization of thermoplastic polyurethane/montmorillonite nanocomposites produced by reactive extrusion. Journal of Materials Science. 2007; 42(14):5785–5790.
82. Meng, X., Du, X., Wang, Z., Bi, W., Tang, T. The investigation of exfoliation process of organic modified montmorillonite in thermoplastic polyurethane with different molecular weights. Composites Science and Technology. 2008; 68:1815–1821.
83. Kim, B., Seo, J., Jeong, H. Morphology and properties of waterborne polyurethane/ clay nanocomposites. European Polymer Journal. 2003; 39(1):85–91.
84. Moon, S., Kim, J., Nah, C., Lee, Y. Polyurethane/montmorillonite nanocomposites prepared from crystalline polyols, using 1, 4-butanediol and organoclay hybrid as chain extenders. European Polymer Journal. 2004; 40(8):1615–1621.
85. Varghese, S., Gatos, K., Apostolov, A., Karger-Kocsis, J. Morphology and mechanical properties of layered silicate reinforced natural and polyurethane rubber blends produced by latex compounding. J. Appl. Polym. Sci.. 2004; 92(1):543551.
86. Song, M., Hourston, D., Yao, K., Tay, J., Ansarifar, M. High performance nanocomposites of polyurethane elastomer and organically modified layered silicate. J. Appl. Polym. Sci.. 2003; 90(12):3239–3243.
87. Chen-Yang, Y., Yang, H., Li, G., Li, Y. Thermal and anticorrosive properties of polyurethane/clay nanocomposites. Journal of Polymer Research. 2005; 11(4):275–283.
88. LeBaron, P.C., Wang, Z., Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Applied Clay Science. 1999; 15(1–2):11–29.
89. Padmanabhan, K.A. Mechanical properties of nanostructured materials. Materials Science and Engineering A: Structural Materials Properties Microstructure and Processing. 2001; 304:200–205.
90. Edwards, G., Optimisation oforganically modified layered silicate based nanofillers for thermoplastic polyurethanes. 2007.
91. Korley, L.T.J., Liff, S.M., Kumar, N., McKinley, G.H., Hammond, P.T. Preferential association of segment blocks in polyurethane nanocomposites. Macromolecules. 2006; 39(20):7030–7036.
92. Giannelis, E., Krishnamoorti, R., Manias, E., Polymer-silicate nanocomposites: Model systems for confined polymers and polymer brushes. Polymers in Confined Environments. 1999:107–147.
93. Giannelis, E.P. Polymer layered silicate nanocomposites. Advanced Materials. 1996; 8(1):29 ff.
94. Bergaya, F., Lagaly, G. Surface modification of clay minerals. Applied Clay Science. 2001; 19(1–6):1–3.
95. Zilg, C., Thomann, R., Mulhaupt, R., Finter, J. Polyurethane nanocomposites containing laminated anisotropic nanoparticles derived from organophilic layered silicates. AdvancedMaterials. 1999; 11(1):49–52.
96. Finnigan, B., Martin, D., Halley, P., Truss, R., Campbell, K. Morphology and properties of thermoplastic polyurethane composites incorporating hydrophobic layered silicates. Journal of Applied Polymer Science. 2005; 97(1):300–309.
97. Xu, R., Manias, E., Snyder, A., Runt, J. New biomedical poly (urethane urea) layered silicate nanocomposites. Macromolecules. 2001; 34(2):337–339.
98. He, H.P., Duchet, J., Galy, J., Gerard, J.F. Influence of cationic surfactant removal on the thermal stability of organoclays. Journal of Colloid and Interface Science. 2006; 295(1):202–208.
99. Edwards, G., Halley, P., Kerven, G., Martin, D. Thermal stability analysis of organo-silicates, using solid phase microextraction techniques. Thermochimica Acta. 2005; 429(1):13–18.
100. Chavarria, F., Paul, D.R. Morphology and properties of thermoplastic polyurethane nanocomposites: Effect of organoclay structure. Polymer. 2006; 47(22):7760–7773.
101. Ma, X.Y., Lu, H.J., Liang, G.Z., Yan, H.X. Rectorite/thermoplastic polyurethane nanocomposites: Preparation, characterization, and properties. Journal of Applied Polymer Science. 2004; 93(2):608–614.
102. Dan, C.H., Lee, M.H., Kim, Y.D., Min, B.H., Kim, J.H. Effect of clay modifiers on the morphology and physical properties of thermoplastic polyurethane/ clay nanocomposites. Polymer. 2006; 47(19):6718–6730.
103. Vaia, R.A., Giannelis, E.P. Lattice model of polymer melt intercalation in organically-modified layered silicates. Macromolecules. 1997; 30(25):7990–7999.
104. Pattanayak, A., Jana, S.C. Synthesis of thermoplastic polyurethane nanocomposites of reactive nanoclay by bulk polymerization methods. Polymer. 2005; 46(10):3275–3288.
105. Choi, M.Y., Anandhan, S., Youk, J.H., Baik, D.H., Seo, S.W., et al. Synthesis and characterization of in situ polymerized segmented thermoplastic elastomeric polyurethane/layered silicate clay nanocomposites. Journal of Applied Polymer Science. 2006; 102(3):3048–3055.
106. Finnigan, B., Casey, P., Cookson, D., Halley, P., Jack, K., et al. Impact of controlled particle size nanofillers on the mechanical properties of segmented polyurethane nanocomposites. International Journal of Nanotechnology. 2007; 4(5):496–515.
107. McCarthy, S., Meijs, G., Mitchell, N., Gunatillake, P., Heath, G., et al. In-vivo degradation of polyurethanes: Transmission-FTIR microscopic characterization ofpolyurethanes sectioned by cryomicrotomy. Biomaterials. 1997; 18(21):1387–1409.
108. Martin, D., Edwards, G., Granted International Patent Application AU 2005279677. Polymer Composite. 2011.
109. Pandey, R.B., Anderson, K.L., Farmer, B.L. Exfoliation of stacked sheets: Effects of temperature, platelet size, and quality of solvent by a Monte Carlo simulation. Journal of Polymer Science Part B: Polymer Physics. 2006; 44(24):3580–3589.
110. Ginzburg, V.V., Qiu, F., Balazs, A.C. Three-dimensional simulations of diblock copolymer/particle composites. Polymer. 2002; 43(2):461–466.
111. Lelah, M., Cooper, S. Polyurethanes in Medicine. CRC Press, Inc, 1986; 225.
112. Szycher, M. Biostability of polyurethane elastomers: A critical review. Journal of Biomaterials Applications. 1988; 3(2):297.
113. Szycher, M., McArthur, W., ASTM International. Surface Fissuring of Polyurethanes Following in vivo Exposure. 1985.
114. Williams, D., Definitions in biomaterials: Proceedings of a consensus conference of the European Society for Biomaterials. ChesterProgress in Biomedical Engineering. Amsterdam: Elsevier, 1987.
115. Gunatillake, P., Meijs, G., Mccarthy, S., Adhikari, R., Poly (dimethylsiloxane)/ poly (hexamethylene oxide) mixed macrodiol based polyurethane elastomers. I. Synthesis and properties. J. Appl. Polym. Sci.. 2000; 76(14):2026–2040. http://www.aortech.com/technology/elast-eon
116. Rueda, L., Garcia, I., Palomares, T., Alonso-Varona, A., Mondragon, I., et al. The role of reactive silicates on the structure/property relationships and cell response evaluation in polyurethane nanocomposites. Journal of Biomedical Materials Research Part A. 2011; 97A(4):480–489.
117. Styan, K.E., Martin, D.J., Poole-Warren, L.A. In vitro fibroblast response to polyurethane organosilicate nanocomposites. Journal of Biomedical Materials Research Part A. 2008; 86A(3):571–582.
118. Kannan, R.Y., Salacinski, H.J., Sales, K.M., Butler, P.E., Seifalian, A.M. The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites – An in vitro study. Cell Biochemistry and Biophysics. 2006; 45(2):129–136.
119. Wang, W., Guo, Y.-I., Otaigbe, J.U. The synthesis, characterization and biocompatibility of poly(ester urethane)/polyhedral oligomeric silesquioxane nanocomposites. Polymer. 2009; 50(24):5749–5757.
120. Deka, H., Karak, N., Kalita, R.D., Buragohain, A.K. Bio-based thermostable, biodegradable and biocompatible hyperbranched polyurethane/Ag nanocomposites with antimicrobial activity. Polymer Degradation and Stability. 2010; 95(9):1509–1517.
121. Hsu, S.H., Tseng, H.J., Lin, Y.C. The biocompatibility and antibacterial properties of waterborne polyurethane-silver nanocomposites. Biomaterials. 2010; 31(26):6796–6808.
122. Styan, K. Polyurethane organosilicate nanocompositesfornoveluse as biomaterials. Thesis . 2006.
123. Osman, A.F., Andriani, Y., Schiller, T.L., Padsalgikar, A., Svehla, M., et al, Australasian Polymer Symposium (APS 2011). Assessing thermoplastic polyurethane nanocomposites for biomedical devices. 2010.
124. Poole-Warren, L.A., Farrugia, B., Fong, N., Hume, E., Simmons, A. Controlling cell-material interactions with polymer nanocomposites by use of surface modifying additives. Applied Surface Science. 2008; 255(2):519–522.
125. Li, P. R., Wei, J. C., Chiu, Y. F., Su, H. L., Peng, F. C., etal., Evaluation on cytotoxicity and genotoxicity of the exfoliated silicate nanoclay. ACS Applied Materials & Interfaces. 2(6): pp. 1608–1613
126. Lordan, S., Kennedy, J.E., Higginbotham, C.L. Cytotoxic effects induced by unmodified and organically modified nanoclays in the human hepatic HepG2 cell line. Journal of Applied Toxicology. 2011; 31(1):27–35.
127. Mishra, A., Das Purkayastha, B.P., Roy, J.K., Aswal, V.K., Maiti, P. Tunable properties of self-assembled polyurethane using two-dimensional nanoparticles: Potential nano-biohybrid. Macromolecules. 2010; 43(23):9928–9936.
128. Da Silva, G.R., Ayres, E., Orefice, R.L., Moura, S.A.L., Cara, D.C., et al. Controlled release of dexamethasone acetate from biodegradable and biocompatible polyurethane and polyurethane nanocomposite. Journal of Drug Targeting. 2009; 17(5):374–383.
129. Da Silva, G. R., Da Silva-Cunha, A., Behar-Cohen, F., Ayres, E., and Orefice, R. L., Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. PolymerDegradation andStability. 95(4): pp. 491–499
130. Schaefer, D.W., Justice, R.S. Hownano are nanocomposites? Macromolecules. 2007; 40(24):8501–8517.
131. Rule, M. Patentno. US 7199172 B2. MetalPhosphonatesandRelatedNanocomposites. 2007.
132. He, H. Grafting of swelling clay materials with 3-aminopropyltriethoxysilane. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2005; 288(1):171–176.
