[1] Rogers J, Webster S, Lue L.-F, Brachova L, Harold Civin W, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer's disease pathogenesis. Neurobiol Aging. 1996;17:681–686.
[2] Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259.
[3] Hebert L.E, Beckett L.A, Scherr P.A, Evans D.A. Annual Incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–173.
[4] Hausdorff M, Lertratanakul A, Cudkowicz E, Peterson L, Kaliton D, Goldberger L. Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. J Appl Physiol. 2000;88:2045–2053.
[5] Yunfeng W, Krishnan S. Statistical analysis of gait rhythm in patients with Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2010;18:150–158.
[6] Banaie M, Pooyan M, Mikaili M. Introduction and application of an automatic gait recognition method to diagnose movement disorders that arose of similar causes. Expert Syst Appl. 2011;38:7359–7363.
[7] Berger H. Über das Elektrenkephalogramm des Menschen. Archiv für Psychiatrie Nervenkrankheiten. 1929;87:527–570.
[8] Ferri C.P, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes P.R, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2005;366:2112–2117.
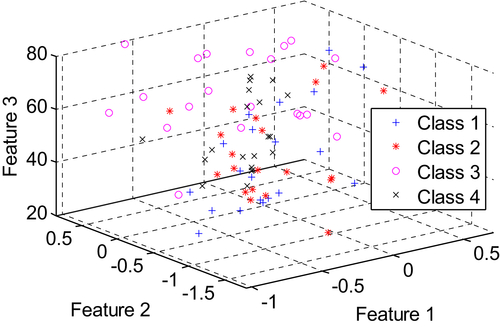
[9] Ganji F, Abadeh S, Hedayati M, Bakhtiari N. Fuzzy classifcation of imbalanced data sets for medical diagnosis. In: 17th Iranian Conference of Biomedical Engineering (ICBME). 2010:1–5.
[10] Marlin B.M. Missing data problems in machine learning. University of Toronto; 2008.
[11] Zhao X.-M, Li X, Chen L, Aihara K. Protein classification with imbalanced data. Proteins. 2008;70:1125–1132.
[12] Zhi-Hua Z, Xu-Ying L. Training cost-sensitive neural networks with methods addressing the class imbalance problem. IEEE Trans Knowledge Data Eng. 2006;18:63–77.
[13] Ani A, Deriche M. A New Technique for Combining Multiple Classifiers using The Dempster-Shafer Theory of Evidence. J Artificial Intelligence Res. 2002;17:333–361.
[14] Meriggi P, Castiglioni P, Rizzo F, Gower V, Andrich R, Rabuffetti M, Ferrarin M, Di Rienzo M. Potential role of wearable, ambulatory and home monitoring systems for patients with neurodegenerative diseases and their caregivers. In: 5th International Conference on Pervasive Computing Technologies for Healthcare. 2011:316–319.
[15] Harter A, Hopper A, Steggles P, Ward A, Webster P. The Anatomy of a Context-Aware Application. Wireless Networks. 2002;8:187–197.
[16] Armstrong R.A, Cairns N.J, Patel R, Lantos P.L, Rossor M.N. Relationships between β-amyloid (Aβ) deposits and blood vessels in patients with sporadic and familial Alzheimer's disease. Neurosci Lett. 1996;207:171–174.
[17] Beck J. Parkinson's Disease Foundation. 2012. Available: http://www.pdf.org/en/about_pd.
[18] Hou J.-G.G, Lai E.C. Non-motor Symptoms of Parkinson's Disease. Int J Gerontol. 2007;1:53–64.
[19] Neela D, Rangarajan K. Hybrid Workflow Net Based Architecture for Modeling Huntington's Disease. In: Bio-Inspired Computing: Theories and Applications (BIC-TA), 2011 Sixth International Conference on. 2011:319–323.
[20] Wang Y, Chen Q, Hu W. Behavior Selection Mechanism of Two Typical Brain Movement Disorders: Comparative Study Using Robot. In: International Conference on Digital Manufacturing and Automation. 2010:319–323.
[21] Matías-Guiu J, Galán L, García-Ramos R, Barcia J.A, Guerrero A. Cerebrospinal fluid cytotoxicity in lateral amyotrophic sclerosis. Neurología (English Edition). 2010;25:364–373.
[22] Dugdale D.C. Amyotrophic lateral sclerosis and other motor neuron diseases. 2010. Available: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001708/.
[23] Madarame T, Tanaka H, Inoue T, Kamata M, Shino M. The development of a brain computer interface device for amyotrophic lateral sclerosis patients. In: IEEE International Conference on Systems, Man and Cybernetics. 2008:2401–2406.
[24] Woon W.L, Cichocki A, Viallate F, Musha T. Techniques for early detection of Alzheimer's disease using spontaneous EEG recordings. In: Presented at the IOP Publishing. 2007.
[25] Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: Evidence from resting state fMRI. NeuroImage. 2006;31:496–504.
[26] Knauer U, Meffert B. Evaluation based combining of classifiers for monitoring honeybees. In: Presented at the Workshop on Applications of Computer Vision (WACV), Germany. 2009.
[27] Ghanem A.S, Venkatesh S, West G. Multi-class Pattern Classification in Imbalanced Data. In: Presented at the Proceedings of the 20th International Conference on Pattern Recognition. 2010.
[28] Fernández A, García S, Jesus M.J.d, Herrera F. A study of the behaviour of linguistic fuzzy rule based classification systems in the framework of imbalanced data-sets. Fuzzy Sets Syst. 2008;159:2378–2398.
[29] Babiloni C, Frisoni G.B, Pievani M, Vecchio F, Lizio R, Buttiglione M, Geroldi C, Fracassi C, Eusebi F, Ferri R, Rossini P.M. Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage. 2009;44:123–135.
[30] Frisoni G.B, Padovani A, Wahlund L.O. The predementia diagnosis of Alzheimer disease. Alzheimer Dis Assoc Disord. 2004;18:51–53.
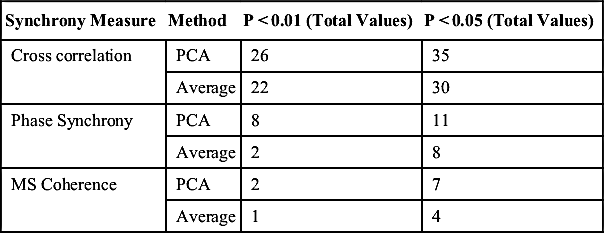
[31] Gallego-Jutgla E, Elgendi M, Vialatte F, Sole-Casals J, Cichocki A, Latchoumane C, Jaesung J, Dauwels J. Diagnosis of Alzheimer's disease from EEG by means of synchrony measures in optimized frequency bands. In: International Conference of the IEEE Engineering in Medicine and Biology Society. 2012:4266–4270.
[32] Breakspear M. Nonlinear phase desynchronization in human electroencephalographic data. Hum Brain Mapp. 2002;15:175–198.
[33] Anghinah R, Kanda P.A, Jorge M.S, Lima E.E, Pascuzzi L, Melo A.C. Alpha band coherence analysis of EEG in healthy adult's and Alzheimer's type dementia patients. Arq Neuropsiquiatr. 2000;58:272–275.
[34] Locatelli T, Cursi M, Liberati D, Franceschi M, Comi G. EEG coherence in Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1998;106:229–237.
[35] Dauwels J, Vialatte F, Cichocki A. A Comparative Study of Synchrony Measures for the Early Detection of Alzheimer’s Disease Based on EEG. In: Neural information processing. vol. 4984. Berlin: Springer; 2008:112–125.
[36] Oppenheim A.V, Schafer R.W, Buck J.R. Discrete-time signal processing. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 1999.
[37] Güntekin B, Saatçi E, Yener G. Decrease of evoked delta, theta and alpha coherences in Alzheimer patients during a visual oddball paradigm. Brain Res. 2008;1235:109–116.
![]() (1)
(1)![]() (2)
(2)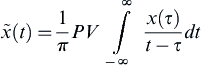 (3)
(3)![]() (4)
(4)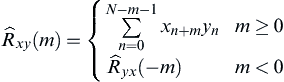 (5)
(5)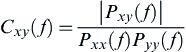 (6)
(6)![]() (7)
(7)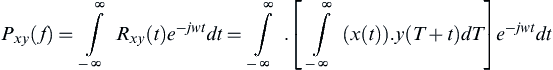 (8)
(8)![]() (9)
(9)![]() (10)
(10)