Bio-chem-FETs: field effect transistors for biological sensing
N. Chaniotakis and M. Fouskaki, University of Crete, Greece
Abstract:
Field effect transistors (FETs) are electrochemical transducers upon which micro-sized solid-state chemical sensors and biosensors, the so-called Bio-chem-FETs, can be developed. The chapter first discusses the key issues of FET operation. It then describes the ways of introducing chemical and biochemical sensitivity and selectivity to analytes, using either chemically activ sensing elements or biological recognition elements. The new advances in Bio-chem-FET design, based on novel carbon and inorganic nanomaterials, are then presented. Finally, the current analytical limitations are presented, followed by a discussion on the future trends and possible improvement strategies of the Bio-chem-FETs in relation to low detection limits, high sensitivity, in-vivo applications and long operational lifetimes.
Key words
chemical sensor; biosensor; field effect transistor (FET); semiconductor; gate; chemical recognition; enzyme catalysis
7.1 Introduction
7.1.1 Key issues and terminology
Biologically and chemically sensitive field effect transistors (Bio-chem-FETs and Chem-FETs) are sensing systems which are based on microelectronics and sensing technologies. The biological or chemical recognition on the one hand, and the electrochemical or semiconductor field effect transduction on the other, are two completely different sciences, and for this reason their successful combination requires multidisciplinary scientific actions. It is thus a very challenging technological and scientific task. This chapter deals with the development of Bio-chem-FETs. Starting with the historical prospective, we will then analyze the technologies involved, as well as the chemical, biological and electronic components required.
Systems that combine different technologies, such as Bio-chem-FETs, are also characterized as hybrid devices. Their design is based on the successful fusion of a purely biochemical process, biochemical recognition, with a purely physical process, the field effect phenomenon of the transducer (the transistor). This fusion utilizes the strengths of both technologies: highly selective biological recognition and the micro to nano-size of the highly sensitive FETs. The combination of ahighly complex biological or chemical species with the physical or ‘inorganic’ transduction surface generates unique detection systems capable of numerous in-vivo, remote or other specialized applications. For these specialized applications, the analytical characteristics of Bio-chem-FETS are of primary importance. Transduction efficiency, lifetime, signal drift and biocompatibility are some of the characteristics involved. Optimizing the physical and analytical characteristics of these devices is the aim of many research groups, since the current state of the art in selectivity, sensitivity, detection limit and especially lifetime is sometimes not ideal. As the science improves in the areas of biocompatibility, nanotechnology, material science, semiconductors and electronics, so will the Bio-chem-FET devices, vastly expanding their range of applications.
Following is a list of the important terminology we will encounter in this chapter.
• Sensing element: The site at which the chemical or biochemical recognition takes place.
• Ionophore: The chemical species that performs the chemical recognition.
• Biological element: The biological species that performs the biochemical recognition or, in most cases, the catalysis for the analyte elimination or generation.
• Semiconductor: A material whose electrical resistivity lies between those of conductors and insulators, and can be influenced externally.
• Field effect: The phenomenon during which a potential acts upon a semiconductor which is doped positively (p) or negatively (n), increasing or decreasing its conductivity.
• Transistor: The three electrode gated device which amplifies a signal, usually a potential, using the field effect phenomenon.
• Transduction: The transcription or conversion of a chemical, biological, molecular change into a readable signal such as electrical or optical.
7.1.2 History
The ability to ‘sense’ chemical species was introduced early in the twentieth century. Based on the observations of Cremer (1906) with glass membranes,1 Fritz Haber and Zygmunt Klemensiewicz published in 1909, a ground-breaking work which paved the ground for chemical sensing technology.2 This work introduced for the first time the use of a thin glass membrane (silicon dioxide) for the measurement of the acidity of a solution, and thus the pH electrode was born. The strong multidisciplinary scientific background of these scientists (biology, chemistry and physics) allowed them to discover that the electrical potential they measured across the thin film of a specific glass was different for different biological solutions. This potential was later shown to be directly related to the amount of hydrogen ions (a very important biochemical parameter). Figure 7.1 shows how this potential is actually generated, based on the difference in hydrogenion activity on the two sides of the glass membrane. It is worth stressing that there are always two potentials that exist on the glass membrane: the inner (or reference) and the outer (or sample) potential. While the inner potential is also very important, it is usually constant (reference). The measured potential difference is thus dependent only on the outer or sample-generated potential.
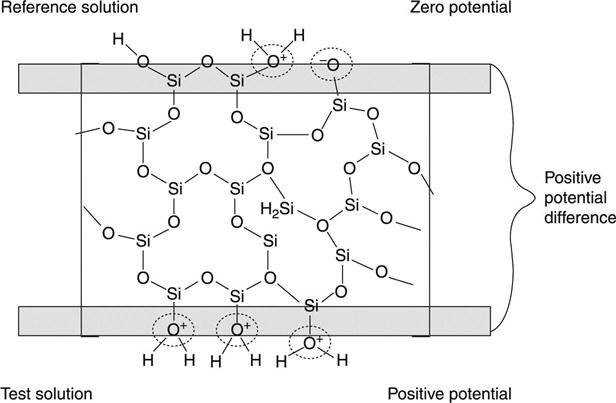
This measured potential difference of the glass membrane when in aqueous solutions was later on described as pH (-log[H+]), a term introduced by S. P. L. Sorensen.3 The discovery of potential generation at a membrane interface apposed or appended the ground for the development of numerous technologies, including chemical sensors, biosensors and Bio-chem-FETs, which we will treat in this chapter.
The ability of a material (in this case the silicon dioxide surface) to selectively recognize a chemical substance (hydrogen ion here) was described early in the twentieth century. It took almost 30 years for this technology to become applicable to real sample measurements. The high resistance of the glass membrane used to make the sensor did not allow easy measurement of the potential developed at the glass solution interface. This problem was solved with the design of a high-impedance electrometer that was introduced in 1934 by Arnold Beckman. Beckman was a chemist who founded Beckman Instruments in order to manufacture the pH meter, the only device in existence at the time with the capability (very high input impedance) to be able to measure acidity (pH). It is of interest to note that later on (1955) Beckman also founded the first transistor company that was based on silicon as a semiconductor.
The sensor technology remained limited to the glass pH electrode up until the early 1960s. At this time there were some new developments that revolutionized the science of sensors. In a publication by Martin S. Frant and James W. Ross Jr. in 1966,4 it was shown that one can use a LaF3 crystal to measure selectively and with high accuracy the activity of the fluoride ion. Again, the fundamental science behind this discovery was the potential development at the LaF3 membrane upon changes of the fluoride activity in the test solution compared with the internal reference (fixed) solution. In the same year, Štefanac and Simon published a paper describing the very successful polymer-based potassium sensor using the highly selective potassium carrier valinomycin.5 Pungor also published within a year a method in which a polymeric membrane can be selective to halide ions, if certain metal salts are incorporated in it.6
Concurrently, a new significant discovery was achieved. In 1962, Clark and Lyons were was able to combine the chemical recognition with biological elements for the development of a new technology, the ‘biosensor’ (Fig. 7.2).7
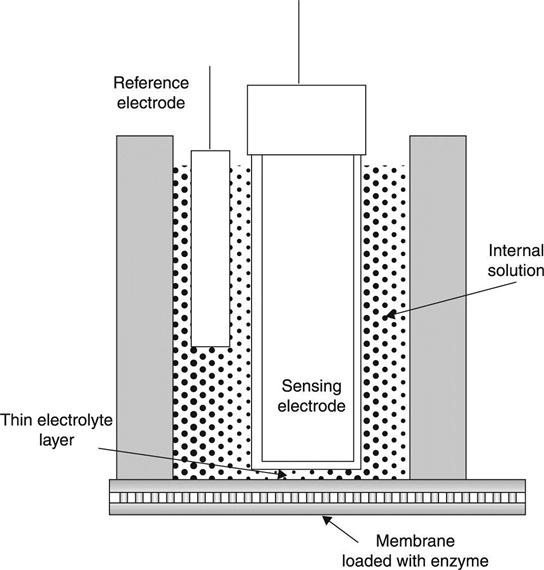
To develop this new device he used the highly selective silicon oxide membrane of the pH sensor discovered almost 60 years earlier, together with the catalytic activity of enzymes. Enzymes, such as glucose oxidase, can catalyze the breakdown of an analyte, such as glucose, and release by-products. It is usually the case that such enzymes either consume a chemical compound, for example oxygen, or generate an analyte that can be measured. Oxygen thus decreases as the activity of the enzyme increases. At the same time the enzyme can produce a product that can also be measured. Classic examples are carbon dioxide, or an acid. The sensing technology provides the means to measure such species rapidly and with high accuracy. Combining the enzyme activity with a transduction (measuring) technology, such as a pH sensor, allows the development of a biosensor. A biosensor is thus a hybrid system in which the recognition is achieved using a catalytic enzyme, while the transduction is performed using a chemical sensing element. Since the species consumed or generated depend on the activity of the enzyme, this field has a wide range of applications.
It is evident from these early works on the development of biochemical detection systems that sensor technology development follows the technological advancements of materials, transducers and biochemistry. Biosensor technology is indeed a multidisciplinary scientific field. Since the areas involved are very different in nature, their fusion for a novel new science has always been very challenging, albeit a highly interesting endeavor.
One of the most interesting such approaches in the history of sensor development has been the combination of a transistor as a transducer with chemical or biological recognition for the development of Chem-FETs and Bio-chem-FETs, respectively. Although the transistor was introduced in the early 1950s, it was not until after the introduction of the ionophores that this was achieved. Bergveld, with a strong background in electronics, combined these two technologies successfully in 1970, in an effort to develop miniaturized sensor systems for in-vivo applications.8 Numerous applications, including biomedicine, biotechnology, the food and drug industry, environmental monitoring, process technology, defense and security, are still being developed using this technology.
7.2 The field effect transistor (FET)
7.2.1 The main components of an FET
Modern electronics are based almost exclusively on transistors. Transistors are semiconductor devices which come in two general types: the Bipolar Junction Transistor (BJT) and the Field Effect Transistor (FET). In this chapter we will deal exclusively with biosensors and chemical sensors that are based on FETs.
We usually know of transistors as devices that are able to amplify a small electrical signal by hundreds or thousands of times its original value. In other words, the transistor is a very sensitive device to input signals.
One of the very important sections of an FET is the gate which is based on a conductor layer connected to the external control circuit. Under operational conditions there is a potential applied between the source and the drain, and this potential is termed source drain voltage, i.e. Vds. This can vary between 2 and 30 V and can be precisely controlled and monitored by external circuits. Another parameter that controls the operation of the FET is the current between the gate and the source. There are thus three parameters that influence the operation of an FET: Vds, Ids, and Vgs. By changing one of these parameters, the other two will adjust their values accordingly. It is usually the case that the Igs is the input signal, and the Ids is the output signal.
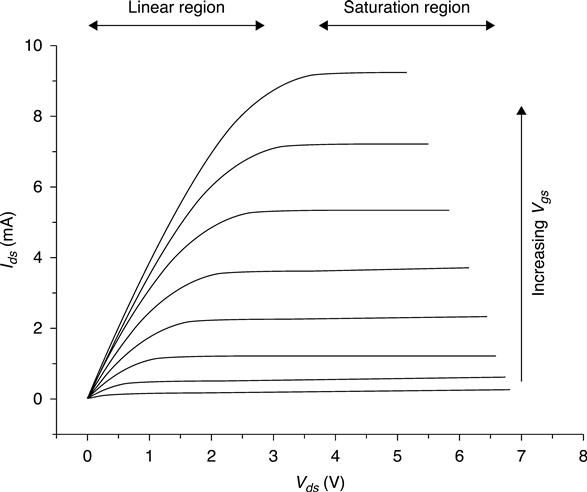
At each Vds there are two distinct regions of operation of the FET: linear and saturation. The saturation region indicates that any further increase in the Vds will not affect the value of the Ids. On the other hand, if within this region the Vgs increases even by a small amount, the Ids will increase significantly. Based on these operational conditions, Vgs is the input signal that gets amplified by the FET, and it is observed as the Vgs. An antenna that is directly connected to the gate, for example, receives a radio signal, and the very weak signal received by the antenna passes through the FET and it is greatly amplified and played by the speakers.
Transistors are now packaged together in arrays of thousands, but they can still be found in individual packages. This gives us the opportunity to experiment with the individual components (especially the gate and the channel), and alter the behavior of the FET.
The gate of the FET is made of a semiconductor, which, when in its off state, is depleted of carriers, and as a result the conductivity of the channel is low. There is no current flowing through from the source to the drain electrode. When, on the other hand, the gate potential increases, there is an accumulation of carriers within the channel region. This results in a drastic decrease of the resistance, with concurrent increase in the current that crosses the channel, forced by the potential difference between the source and the drain electrodes. To demonstrate this effect, we have drawn the channel as an adjustable resistor in Fig. 7.3. Any physical activity or chemical species that can alter this resistance can be directly detected using this FET device.

The control of the conductivity can be achieved very precisely by controlling the potential of the gate. This device operates in two main ways. In the linear mode (or Resistive operation) where Vgs > VT (VT threshold voltage) and a small voltage Vds (Vds < Vgs−VT) is applied between drain and source in this mode FET operates …. In this mode the FET operates like a variable resistor, as it switches between conductive and non-conductive states. In a different mode, where the value of drain to source voltage is further increased (Vds > Vgs−VT), the drain current Ids is weakly dependent upon drain voltage and controlled primarily by the gate is source voltage. This is called saturation mode. In this case the FET is a constant-current source and can be used as a very efficient voltage amplifier. Very small potential changes imposed on the gate result in large changes of the source-to-draincurrent values. This is the mode preferred for weak, high-impedance signals, such as those developed by biosensors and chemical sensors. For this reason all Bio-chem-FETs and Chem-FETs operate in saturation mode9 (Fig. 7.4).
The theory that describes the operation of an FET is summarized in the following equation:
[7.1]
There is a direct relation between the gate to source voltage (Vgs) and the current measured (Ids). By measuring the current one can thus obtain the gate to source voltage (Vgs VT) threshold potential.
7.2.2 The role of semiconductors in the design of Bio-chem-FETs
Semiconductor technology owes its widespread application to the invention of the transistor in 1948.10 The majority of materials used for these devices are based on two semiconductors, silicon and germanium. It has been known since early on that the chemical characteristics of the active area of the semiconductor play a major role in determining the behavior and the performance of the transistor. The precise control of the surface chemistry is very important, and the main parameter to be considered in the application to Bio-c hem-FETs, as well as all other electrochemically based sensors. This is because the surface chemistry controls the surface potential of the semiconductor. Semiconductor surface potential plays an important role in the performance and characteristics of all devices involving surface chemistry.
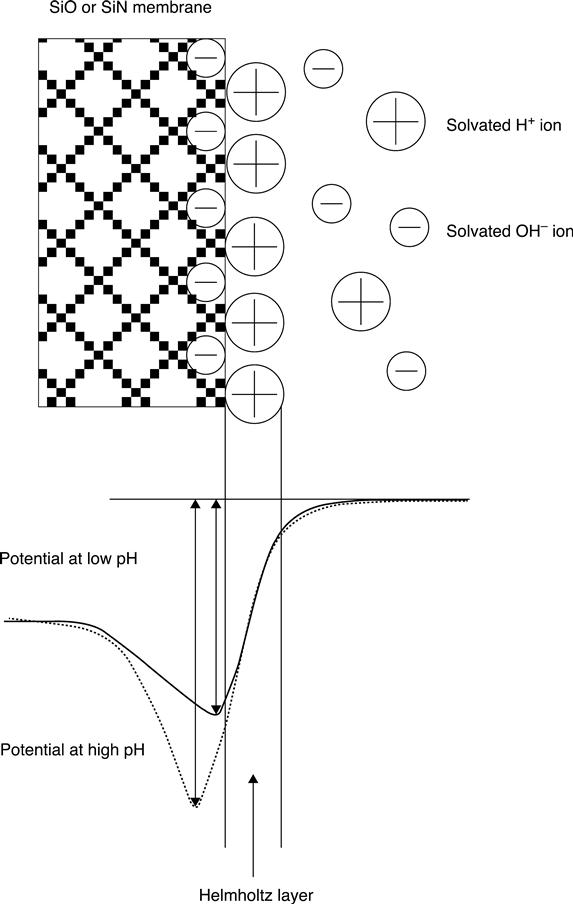
The native and the imposed potential of the gate material are very important in the induced depletion or inversion layer, and the Fermi energy shift or pinning. These parameters are directly related to the chemical composition of the bulk material and to the chemical equilibrium that exists between the surface of the semiconductor and the analyte sensed. The surface potential, and therefore the nature of the space charge double layer associated with the surface, depends onthe chemistry of the adsorbed layers on the electrode surface, as has been known since the early 1930s11–13 (Fig. 7.5). In 1954 Brattain and Garrett actually measured for the first time the effect that different electrolytes, such as HCl, KCl or KOH, had on the half-cell potential of the germanium semiconductor.14 In the samejournal issue, Bardeen and Morrison15 presented the effect that different electrolytes and gases had on the properties of the semiconductor as manifested by the change in the surface space charge barrier. In addition, the effect of both ions and pH on the surface of semiconductors was reviewed a little later by Boddy,16 showing the dependence of surface potential in both germanium17 and silicon semiconductors. It was shown in these early works that the surface chemistry of the material is determined by the active chemical functionalities found at the surface, and to a lesser degree by the crystal orientation. At the same time, the type and amount of the surface chemical functionalities depend on the chemical composition of the material itself, as well as any chemical post-treatment of the surface.
The surface chemical functionalities can influence the physicochemical properties of the semiconductor, as presented early on by Bardeen and Morrison.15 Some of these parameters pertinent to the development of Bio-chem-FETs are the work function or contact potential,18–23 redox reactions,24,25 adsorption,26,27 photoconductivity,28,29 surface conductance–channel effect12 and field–field effect.30–32 All these properties have been used as the basis for the development of Bio-chem-FETs, as the external chemical stimuli can drastically alter these fundamental and easily measurable surface semiconductor properties.
7.2.3 Converting an FET into a Bio-chem-FET
It was established early on that all metal oxide semiconductor capacitors, gate-controlled diodes and Metal Oxide Semiconductor Field Effect Transistors (MOSFETs) are sensitive to an external field that can be transmitted through an insulating gate. In the case of MOSFETs the field is created by applying voltage on the conducting metal gate. When the metal is removed (OSFET), the field can be created by any of the several processes of ion exchange,33 including ionization of neutral groups such as SiOH,34,35 electron exchange, adsorption of charged species or alignment of dipoles at the external gate surface.36 These processes can be enhanced, controlled or made to occur by exposing the OSFET to reactive gases, solvents and electrolytes, and by coating the OSFET with reactive materials such as ion exchangers and redox-sensitive layers.
In the mid-1960s the ground was ready for the introduction of a new sensor technology, based on the combination of a mature transistor technology as the transducer and the well-established chemical-sensing capabilities of inorganic (such as SiO2) and organic (such as nonactin) species. Bergveld, a scientist with a strong background in physics and semiconductors, showed that an open gate Si FET has exceptional sensitivity to pH changes. The silicon oxide that was on the surface of the FET had the same chemical characteristics as the glass pH electrode that had appeared almost 60 years before,1 and thus the Ion Selective Field Effect Transistors (ISFETS) were born. Soon after, the ISFET concept expanded to include sensors for uncharged chemical species. These were described asChemically Modified Field Effect Transistors (CHEMFETs), shown schematically in Fig. 7.6, which also describes the basic principle for the pH Chem-FET. These technologies were based on the fact that surface electron (or hole) exchange, or adsorption,37–39 could take place within a broader category of chemically sensitive semiconductor devices (CSSDs).40

Comparing this with the FET shown in Fig. 7.3, it is clear that the only fundamental difference is the absence of the metal contact to the gate, and in its place there is a chemically sensitive layer in conduct with a solution. The device and the electronic circuit are thus identical, except for the fact that now there is a chemical means, that is a solution, with which we can alter the potential of the SiO2 gate. In a pH-sensitive device, this potential is proportional to the pH of the solution according to the Nernst equation and increases by 59.2 mV for every unit of decrease in pH. If the FET operates in a region where this relatively smallpotential change can influence the source-to-drain current, then the device becomes a pH-sensitive FET, or a pH chem-FET. Soon this idea became a useful miniature pH sensor, which finds more and more applications every day.
The pH-FET was the fundamental building block upon which a wide range of biochemically sensitive FETs have been developed. With the pH-FET work it was shown that the required potential to drastically influence the field effect can be very small, and thus it can be generated using many different biochemical processes. Not only semiconductor membranes such as silicon oxide, but also organic, polymeric and biological membranes can be readily employed. A thin membrane or layer of a material that can generate a potential at its interface can be used for this purpose. The same holds true for other unique materials and systems, such as proteins, enzymes, even whole animal parts. The only requirement is that the surface in contact with the gate material can be charged selectively and reversibly upon a biochemical recognition step.
We have now seen how this ‘simple’ device is capable of ‘transducing’ or converting the response generated by any type of a layer or membrane into a quantitative electrical signal. We can imagine how the electrical field created by a living cell can affect the electron population and thus the conductivity of the gate semiconductor, giving us a valuable signal. Knowing that numerous biological systems show strong charge generation upon interaction with drugs, hormones, electrolytes, endobiotics and xenobiotics opens new horizons and a wide range of possibilities for sensing and monitoring in clinical and medical science. This is as simple as positioning individual cells or any other biochemically active substance in close proximity to the gate of the FET.
7.3 Chemical compounds and biological units as sensing elements in Bio-chem-FETs
As explained in detail above, the discovery of Bio-chem-FETs originated from an increasing need for miniature, implantable sensors for physiological measurements. The success of these devices was based on the fact that ISFETs were very sensitive to electrical interaction at or near the gate insulator/electrolyte interface. Any biochemical reaction/process leading to chemical or electrical changes at this interface can therefore be measured by the Bio-chem-FET. The biological recognition material, immobilized onto the FET gate, is used to recognize and interact specifically with the analyte in test solution (Fig. 7.7). The immobilization of the biological component is a critical step for the construction of biosensors, including Bio-chem-FETs.

A number of immobilization methods, such as physical or chemical adsorption, cross-linking, covalent attachment or entrapment, have been proposed; however, in practical application, several immobilization methods of biological components are sometimes combined to obtain satisfactory immobilization. The biorecognition material could be an enzyme, an antibody, a cell, a tissue slice, a receptor, anucleic acid or an organ. The basic mechanism for the response of all these systems remains the same. There is either an induced potential change caused by a catalytic generation or elimination of H+ or other ion, semiconductor surface polarization or changes in the dipole moment due to the adsorption of charged macromolecules (e.g. polyelectrolytes, proteins, DNA) or affinity binding of molecules (e.g. antigen–antibody affinity reaction, or DNA hybridization), or, finally, potential changes generated by active biological systems (e.g. action potential of nerve cells, metabolic processes of bacteria or cells, ligand–receptor interactions).41 Based on the nature of the biological component employed and the resulting biorecognition process, the Bio-chem-FETs can be distinguished into three main categories: enzyme or catalytic Bio-chem-FETs (utilizing enzymes or tissues as immobilized biocomponents), affinity Bio-chem-FETs (based on antibodies, protein receptors or DNA), and cell-based Bio-chem-FETs, which will be discussed in a separate section.
7.3.1 Catalytic Bio-chem-FETs
Catalytic Bio-chem-FETs are based on enzymes immobilized on the gate insulator of an ISFET. Their working principle is based on enzyme recognition, specific binding and chemical conversion of a substance to a product, any of which may be of analytical interest. The concentration changes of products generated, or reactants consumed, during the enzymatic reaction are converted to signal by the underlying ISFET.
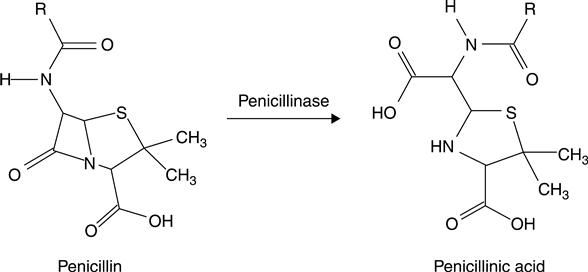
The idea of ISFET-based enzyme sensors was first suggested by Janata and Moss in 197637; however, the first practical application of enzyme ISFETs was reported by Caras and Janata in 1980 for the direct determination of penicillin.42 In this device two pH-sensitive ISFETs were used, one of which possessed a membrane containing cross-linked albumin–penicillinase, and the other which had only a cross-linked albumin membrane. Penicillinase in the membrane catalyzed penicillin hydrolysis, resulting in the production of penicillinic acid, as shown in Fig. 7.8, and therefore in a local pH decrease in the gate area, registered by the pH-sensitive ISFET. The basic advantage of this device was the rapid detection of a small amount of analyte, together with the ability to be used repeatedly. Based on these, the device was suggested for the analysis of complex samples.
Since then, a large number of enzyme-based Bio-chem-FETs differing in their sensing membrane composition and enzyme immobilization method have been reported for numerous target analytes. Some of the most important ones are glucose, urea, penicillin, pesticides, phenolic compounds, steroidal glycoalkaloids and creatinine.43–48 Many of these sensors usually employ a differential pair of ISFETs, one covered with an enzyme-containing membrane and the other with a blank, enzyme-free membrane acting as a reference system.47 In this case, common interferences, such as bulk pH and temperature changes, light sensitivity, sensor drift, etc., can be significantly reduced. The majority of catalytic Bio-chem-FETs are built up of pH-ISFETs that measure the pH changes caused by enzyme-catalyzed reactions at the gate surface. The response of these pH-ISFET-based Bio-chem-FETs is therefore strongly dependent on the buffer capacity of the sample, leading to a narrow dynamic range and low sensitivity of the resulting sensors. The fact that the induced pH change decreases the enzymatic activity also contributes to the non-linear response of the sensor. In order to overcome this problem, several approaches have been proposed, such as additional chargedpolymeric membranes,49 buffer solutions with low capacities or incorporation of other ISFETs instead of pH ISFETs. In the later case, the Bio-chem-FETs detect the concentration changes of another ion produced by the enzymatic reaction and the response is not considered to be affected by the buffer capacity. A large number of such Bio-chem-FETs have been reported, most of them using an NH4+-ISFET, since ammonium ion is known to be produced in many enzyme–substrate reactions, including urease–urea, creatininase–creatinine and amino acid oxidase–amino acid,50,51 An alternative approach for developing enzyme Bio-chem-FETs is based on the measurement of the changes in the redox potential derived from the enzyme-catalyzed reaction.52 The sensitivity of the ISFET-based enzyme sensors is strongly affected by buffer conditions; however, the sensor was not influenced by pH change or buffer capacity.
Despite the remarkable advantages of catalytic Bio-chem-FETs, their inherent drawbacks, such as limited stability issues and limited reproducibility, have hampered their widespread commercial application. Many scientists have been working for many years now towards improving the analytical characteristics of enzyme-based Bio-chem-FETs, and to expand their possible applications. These efforts include improvement of the reference electrode used, since the conventional reference electrode is inconvenient because of its large volume, particularly for miniature detection systems. To do this, different approaches have been proposed. The design and development of reliable miniature reference electrodes,53 the use of a pseudo-reference electrode, and their employment in a differential measuring system54 are some of the approaches utilized. Another way to improve the enzyme loading and stability of the Bio-chem-FETs is the optimization of enzyme immobilization on the ISFET gate. Enzyme-loaded membrane adhesion to the gate and the fabrication of multienzyme membranes which enable the development of multisensors, etc., are some interesting approaches.
Catalytic Bio-chem-FETs have also been shown lately that can be used, albeit in an indirect way, to measure the elusive pyrophosphate ions (PPi).55 Utilizing DNA polymerase reactions designed to take place right on the gate of an FET, the authors showed that PPi can be detected. Such devices can thus be utilized in DNA sequencing. These label-free electrical detection of enzymatic DNA base incorporation reactions can indeed prove to be superior to existing fluorescence and chemiluminescence methods.
7.3.2 Affinity Bio-chem-FETs
Field effect devices are highly sensitive surface-charge (potential) measuring devices. They are able to measure small charge changes occurring on the surface of their gate. Since most biological systems, such as antibodies and antigens, carry highly charged areas on their surface, it is expected that the formation of an antibody–antigen-type complex will drastically influence this charge distribution. Such charge distribution changes can be easily detected by the gate surface of anISFET and can lead to a detectable signal. Affinity Bio-chem-FETs are thus based on the interaction of receptor molecules such as antibodies (immuno Bio-chem-FETs) and DNA (DNA Bio-chem-FETs) with a ligand. In addition, some biological organs that contain receptor molecules (i.e. insect antenna56; Fig. 7.9) which bind molecules irreversibly and non-catalytically can also be used for this purpose.

In general, ImmunoFETs can be label-Sree or labeled according to whether there are labels employed for the functionalization of the antibody or the antigen. The working principle of the label-free ImmunoFET is based on the direct antibody–antigen interaction, which results in changes of the charge distribution that in turn can modulate the current of the ISFET (Fig. 7.10).57,58

Since antibodies and antigens are mostly electrically charged molecules, it is expected that the formation of an antibody–antigen complex on the gate surface of an ISFET would lead to detectable changes in the charge distribution, and thus modulate the drain current of the ISFET. In practice, however, the direct detection of immunological reactions by means of an ImmunoFET was unsatisfactory due to the difficulty in the transduction process upon antibody-antigen recognition action into a measurable signal.57
In the case of DNA-based ISFETs, the signal is generated when single DNA strands, which are immobilized onto the gate surface of ISFETs and which carry a specific charge distribution, coordinate with their complementary ss-DNA. The coordination induces changes in surface potential, thereby allowing excellent performance in DNA sensing. A label-free detection of DNA using an FET device with a real-time electrical readout system is very fast, low-cost and relatively straightforward.
7.3.3 Cell-based Bio-chem-FETs
Cell-based Bio-chem-FETs are unique devices in the sense that they can monitor the activity of a living cell in two ways: by monitoring the metabolic activity of the cell, and by monitoring the charge accumulation on the surface of the cell. A cell–transistor is developed by placing a cell onto the gate of a Bio-chem-FET. This is indeed a hybrid device, since it combines a single cell with the transducer of a microelectronic device, as shown in Fig. 7.11.59 The detection of the cell activity can be achieved in two ways, which sometimes are not easily distinguishable. The reason for this is that the surface of the FET is sensitive to pH changes as well as any potential developed in close proximity.

The main reason for developing a cell-based Bio-chem-FET is the fact that the eternal stimulus to the cell can be directly monitored. At the same time, we can envisage controlling the activity of the cell using a feedback system through the microelectronic FET device.60,61 Bio-chem-FETs can also be used to monitor chemical analytes involved in the cell’s activity. Species such as pH, potassium, calcium, carbon dioxide, chloride ion, etc. could possibly be monitored, either selectively or in conjunction with the charge density of the cell wall.
Cell-based Bio-chem-FETs can provide us with a wealth of information and can thus be utilized in a variety of environments, covering a wide range of sensing applications. Clinical diagnostics, drug efficacy and toxicology,62 food quality and environmental monitoring are some of the important applications. On the other hand, there are many difficulties still to be overcome for these devices to find widespread application. Whole cells immobilized onto an inorganic substrate have limited lifetimes, while the immobilization and adhesion chemistry involved is sometimes harsh and non-reproducible.
More recently, cell-based Bio-chem-FETs with Si3N4 or Al2O3 as pH-sensitive gate insulators have been realized for extracellular acidification,63,64 as well as for respiration (oxygen consumption)65 measurements.
7.4 Nanomaterials and nanoengineering in the design of Bio-chem-FETs
Nanotechnology is a relatively new area of science with broad implications in many disciplines. Its significant advantages over regular macro systems have already made a strong impact in the design of Bio-chem-FETs also. Working at the nano-level, the detection mechanism, analytical performance and overall behavior of a sensor change dramatically.
It is usually common sense that the electrochemical properties of a metallic conductor or electrode are not dependent on size. However, as soon as the active size of this electrode reaches micro-size and below, its properties and behavior startto change significantly. The situation becomes even more dramatic as the size approaches the atomic or nano-scale. At this point all common knowledge about bulk material properties becomes invalid. Even the familiar Ohm’s Law breaks down. Nanosized electrodes can be thought of not as 2D but rather as 1D devices. Since the distance an electron travels between two scattering events is typically much larger than the atomic size, the resistance, for example, becomes independent of the electrode length. In fact, the character of the resistance changes in such a way that it becomes necessary to utilize the wave nature of the electrons within the conductor for a proper description. The energy levels involved are such that quantum effects are observed even at room temperature. The exact chemical composition and nature of the transducer electrode now play a decisive and novel role. These 1D metal wires act as ‘flexible’ electron channels. Intramolecular or intermolecular atomic stresses will result in changes in electrochemical behavior orders of magnitude larger than for bulk materials. The experimental investigation of these phenomena became possible with the introduction of nanoscience, especially the scanning probe microscopy technologies developed by Gerd Binnig and Heinrich Rohrer, for which they were awarded the Nobel Prize in 1986.66 It is evident that nanotechnology by its very nature has eliminated to a large extent the boundaries of chemistry, biology, material sciences and physics. Nanotechnology allows us now to manipulate and monitor the function and properties of the individual biomolecular building blocks67,68 with the use of an incredibly simple scanning tunneling potentiometry metal probe, or, for us, an electrochemical nanotransducer.
One of the most promising nanomaterials for the design of Bio-chem-FETs is graphene. This material is composed of a single layer of carbon atoms, hybridized in sp2 form, prior to any oxidation, except at the edges. For this reason it is very conductive, and, upon slight oxidation, it is prone to functionalization. This material has already been used for sensing applications in FETs (Fig. 7.12).69 It has been shown, for example, that graphene is a channel material superior to other nanomaterials, such as carbon nanotubes (CNTs) or nanofibers (CNFs), in graphene FETs in comparison with carbon nanotube FETs. While CNTs are very useful for label-free biosensing due to their high aspect ratio and exceptional electrical characteristics, their precise quality control remains a major challenge for scientists. On the other hand, graphene is very stable, and can be controlled much more easily due to its 1D structure. Graphene-based Bio-chem-FETs (G-FETs) have already shown their usefulness by extremely low detection limits compared with other nanostructures. Such devices can be used as transducers for the direct detection of proteins and DNA upon either adsorption or hybridization, respectively. The detection of positively charged proteins, for example, after adsorption induces a drastic decrease in the Ids.

As nanotechnology progresses, new methods are being developed for the design of nanostructures that are more and more suited for Bio-chem-FET design. Improvements in the detection of DNA and nucleic acids in general can be achieved using silicon nanowire-based Bio-Chem-FETs (Fig. 7.13).70 Suchsemiconducting nanowires can be fabricated by a top-down method, giving triangular SiNWs with smooth surfaces, and well-ordered crystal structure that allows surface functionalization. Such silicone nanowire Bio-chem-FETs allow the development of sensors that show ultra-high sensitivity, are relativelyinexpensive to make, provide direct electrical readout and have the capability for multiplexed detection.

7.5 Future trends
Bio-chem-FETs have a unique role to fulfill in the area of direct, on-site and implantable biosensing for drugs, DNA, proteins, enzymes, viruses and whole cells. To achieve these goals, and to design a robust, stable and selective Bio-chem-FET, intense and interdisciplinary scientific effort involving chemistry, biology and micro-nanoelectronics is required. Such efforts are very important, since the future of Bio-chem-FETs in biological sensing is still widely open for novel applications, especially for medical and health-related areas. In the long term, there is plenty of room for scientific and technological advancements, considering the fact that nanoscience will play a decisive role in this technology. Nanotechnology, especially nanowires, nanodots and quantum dots, will allow the facile compilation of multielement and multifunctional detection devices. The implementation of these devices into implantable nanoscale electronic devices is thus envisaged to become reality in the future, providing the much-anticipated continuous personalized medicine. The fact that Bio-Chem-FETs are devices that can recognize a biochemical stimulus, transduce it into an electrical signal and transform it into a physical action renders them ideal devices for the direct integration of nanoelectronics and biological systems, which will be the building block for future bionic systems.
