Appendix: Traditional Industrial Bioprocesses
Enzymes and microbial cells are used for production of chemicals, pharmaceuticals, foods, flavors, fragrances, and vitamins and for waste treatment. Each of these bioprocesses has unique characteristics in terms of the processing and separation technologies involved. Having covered the basics of bioprocess engineering, this appendix presents some examples of industrial bioprocesses and technologies used for production of various chemicals and pharmaceuticals. Waste-treatment aspects of bioprocess engineering are covered in Chapter 16, “Bioprocesses Utilizing Mixed Cultures.”
A.1. Anaerobic Bioprocesses
Many bulk chemicals have been made industrially by anaerobic processes for a hundred years or more and represented the bulk of bioprocesses until the advert of antibiotics at the end of World War II. Although chemical processes have displaced anaerobic bioprocesses for many products, anaerobic fermentations remain important for several products. Here we review some of the most important of these processes.
A.1.1. Ethanol Production
Ethanol has many applications as a raw material, solvent, and fuel and is utilized in large quantities in the chemical, pharmaceutical, and food industries. Worldwide, 4 million tons of industrial ethanol are produced annually, 80% by fermentation. It is expected that the demand for ethanol as a fuel oxygenate will increase. An annual growth of U.S. ethanol consumption of 3.2% over the next 20 years is predicted by the Energy Information Administration.
Yeasts are the preferred organisms for industrial-scale ethanol production. Different species can be utilized, depending on the composition of the raw material utilized. Saccharomyces cerevisiae, particularly suitable for fermentation of hexoses, has been the major organism used so far. Kluyveromyces fragilis or Candida species can be utilized when lactose or pentoses, respectively, are the available substrates. Other alternative organisms capable of producing ethanol, such as Zymomonas mobilis, Pachysolen species, are not used in industrial production. However, Zymomonas species have significant advantages over yeast and may be used at industrial scale in the future. Other pentose and hexose fermenting organisms, such as Clostridium hermosaccharolyticum and Thermoanaerobacter ethanolicus, are thermophilic and provide significant advantages for ethanol fermentations and separation. However, they produce some undesirable end products and yield dilute ethanol solutions. Genetic engineering has transformed Escherichia coli into a very efficient ethanol producer, reaching ethanol concentrations up to 43% (vol/vol).
Raw materials can constitute up to 70% of the cost of ethanol production. Therefore, the selection of inexpensive materials has an important impact on process economics. The material selected should be readily and economically available in the fermentation plant. In Brazil, cane sugar is extensively used, whereas in the United States, sugar prices make sugar a nonviable raw material. Instead, corn is the most utilized material in the United States for the production of industrial ethanol. Most microorganisms require readily available sugar compounds for fermentation. Such sugar compounds are present in inexpensive raw materials, such as sugar cane, sweet sorghum, sugar beet juices, and molasses. Whey is also utilized in commercial fermentation. Starch- and cellulose-containing materials, such as grains, fruits, vegetables, wood, biomass, waste paper, and agricultural wastes, can also be used, but they need to be hydrolyzed before fermentation. Starch is enzymatically saccharified before fermentation. Acid hydrolysis of starch is not recommended, as it can result in nonfermentable or inhibitory by-products. Hydrolysis of cellulosic materials can be difficult, expensive, and inefficient. However, interest exists in increasing their utilization in an effort to recycle wastes.
Yeast converts hexoses to ethanol and carbon dioxide by glycolysis, as shown by the following reaction:
The theoretical ethanol yield over glucose is 0.51 g/g, and the growth yield over glucose is 0.12 g/g. Usually, by-products such as glycerol, succinic acid, and acetic acid are produced, and the actual yield is about 90% to 95% of the theoretical yield. Optimal temperature and pH values for yeast are 30° to 35°C and 4 to 6, respectively. For thermophilic organisms, optimal temperature may range from 50° to 60°C. Ethanol production is triggered by anaerobic conditions. Trace amounts of oxygen (0.05 to 0.1 mm Hg) are required by yeast for lipid biosynthesis and maintenance of cellular processes.
The feed solution should be balanced in terms of nitrogen, phosphorus, minerals, and some trace elements. Glucose medium is usually supplemented with NH4CL, KH2PO4, MgSO4, CaCl2, and yeast extract. In industry, only some ammonium and phosphate salts need to be added to diluted molasses. Glucose concentration in feed solution has an important effect on the rate and extent of ethanol production. Glucose concentrations above 100 g/l are inhibitory for yeast.
Ethanol and some of the other by-products are inhibitory to yeast above concentrations of 5% (vol/vol). Therefore, the glucose concentration in feed solution in continuous fermentation should be less than 100 g/l, resulting in ethanol concentration in the effluent below 50 g/l. Ethanol-tolerant yeast strains are being developed to avoid ethanol inhibition. Simultaneous removal of ethanol from fermentation broth is another alternative for alleviation of ethanol inhibition.
Conventional ethanol fermentations operate in batch mode under aseptic conditions. Mechanically agitated stainless-steel reactors are used for this purpose. A reactor is filled with a nutrient medium up to 70% of its volume. After pH adjustment, the reactor content is sterilized and cooled to fermentation temperature. Temperature and pH are controlled during operation, and redox potential is kept below –100mV by using reducing agents such as Na2S. A sterile yeast culture is prepared and used for inoculation of the reactor. A batch fermentation cycle lasts nearly 30 to 40 h. Part of the yeast and aqueous medium can be recycled. Batch operation with cell recycle (the Melle–Boinot process) results in reduced fermentation times (10 h) and improved productivities of 6 g/l-h. At the end of batch operation, the reactor content is emptied and yeasts are separated by filtration or centrifugation. The liquid broth is further processed for separation of ethanol by distillation.
Continuous operation in ethanol fermentations has significant advantages over batch operation. With continuous media sterilization and aseptic operation techniques, contamination problems associated with continuous operation can be eliminated. About 95% of sugar can be converted to ethanol in continuous operation with a residence time of 21 h, as compared to batch operation time of 40 h. Under optimized conditions, the residence time for 95% conversion can be as low as 10 h. Continuous operation with cell recycle may increase fivefold the cell concentration in the reactor, resulting in faster conversion. Sedimentation tanks, centrifuges, or filters can be used for cell separation from the fermenter effluent. With cell recycle, the residence time for 95% conversion may be reduced to 1.6 h with a productivity of 30 g/l-h for a feed glucose of 100 g/l. Multistage continuous operation with cell recycle may further improve the productivity of the process. When six fermenters in series are used without cell recycle, it is possible to obtain 95 g/l in 9 h of total residence time and a productivity of 11 g/l-h.
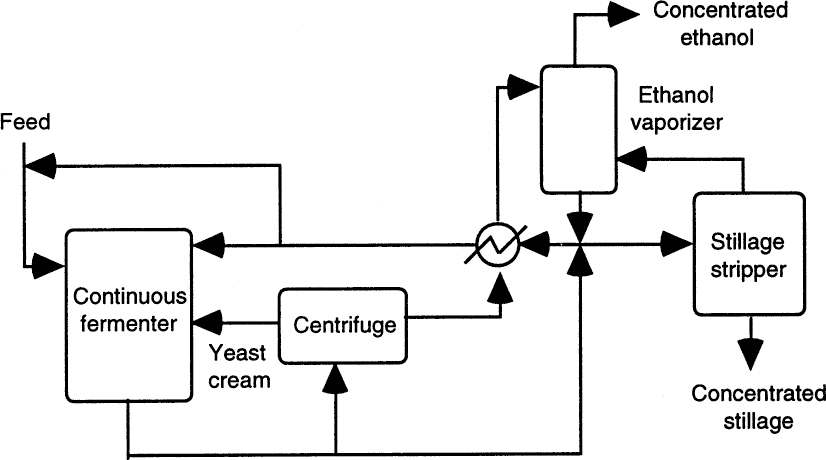
Figure A.1 depicts the biostill process used for ethanol fermentations. This process employs continuous operation with cell recycle and a distillation column for ethanol separation. A mechanically agitated stainless-steel fermenter is used in continuous mode, and the effluent is centrifuged for yeast separation. Part of the separated yeast is recycled back to the fermenter, and the liquid medium is fed to a distillation column for separation of ethanol. Ethanol-free medum is recycled back to the fermenter. Yeast cell recycle provides high conversion rates, and liquid recycle reduces the amount of wastewater generated and dilutes the feed sugar concentration down to noninhibitory levels.
Immobilization of yeast within porous or polymeric matrices results in high cell concentrations in the reactor and, therefore, high ethanol productivities. Immobilized cell reactors may be in the form of packed columns or fluidized beds. Some flocculating yeast strains that settle rapidly may also be used in tower fermenters to obtain high cell concentrations.
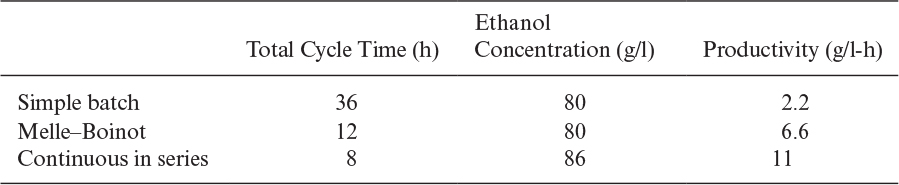
A comparison of industrial processes used for ethanol production is presented in Table A.1.
Ethanol can be separated from the culture vessel during fermentation using low-temperature vacuum distillation, adsorption, or membrane separations. These methods reduce ethanol inhibition but are seldom used in industry because of operating difficulties. Separation of ethanol from fermentation broth is usually accomplished by distillation, which is energy intensive and constitutes more than 50% of the total energy consumption of the plant. The grade of industrial ethanol is usually 95% (190 proof) for chemical and pharmaceutical use, which can be obtained by using distillation columns. However, it is difficult to obtain 100% pure ethanol, since 95% ethanol in water constitutes an azeotropic mixture. A third component is added to alcohol–water mixture to break the azeotrope, but this is expensive and the third component may be toxic.
The cost of ethanol production mainly depends on the raw material and operating costs (chiefly electricity and water cooling). Total operating cost for an ethanol plant producing 190 million liters of ethanol per year is estimated to be 42 cents/l (2001 basis). Raw materials costs constitute 26 cents/l; utilities, 3.4 cents/l; labor, 6 cents/l; and fixed charges (depreciation, taxes, insurance, maintenance), 6.6 cents/l. Plant capital investment for the same size plant is estimated to be $71 million. Produced ethanol can be used as solvent, chemical intermediate (for production of acetaldehyde, acetic acid, ethylene), and fuel. In recent years, there has been an increasing demand for ethanol utilization as fuel in the form of either gasohol (10% alcohol) or pure alcohol. However, the use of ethanol as fuel can be viable only if its cost is comparable to that of oil-derived fuels.
A.1.2. Lactic Acid Production
Lactic acid was first isolated from sour milk (1780) and has two optically active forms called D- and L-lactic acids. The major use of L-lactic acid (more than 50%) is in foods as an acidulant and preservative. Lactic acid is also used as a chemical intermediate to produce other chemicals and in the pharmaceutical industry. Most lactic acid is produced by fermentation.
In industry, usually a mixture of lactic acid bacteria is used to ferment a mixture of carbohydrates. A mixture of strains may result in faster fermentation rates than pure cultures. Selected organisms should grow fast, produce lactic acid with high yields and productivities, and have low nutritional requirements. Lactic acid formation is a mixed growth–associated process that requires high growth rate and cell concentration. Lactic-acid-producing bacteria are classified in two major groups: homolactic (Lactobacillus sp., Streptococcus sp., Pediococcus sp.) and heterolactic bacteria (some Streptococcus sp., Leuconostoc sp.).
Homolactic species of Lactobacillus and Streptococcus are usually used for the industrial production of lactic acid. Homolactic bacteria use the Embden–Meyerhof–Parnas (EMP) pathway to generate two moles of pyruvate from one mole of glucose that are further reduced to lactic acid:
The product yield over glucose is usually above 0.9 g/g. The organisms are facultative anaerobes but generate ATP only by anaerobic fermentation. Industrially important homolactics grow at temperatures above 40°C and pH between 5 and 7. High temperature and low pH (pH <6) reduce the risk of contamination. Homolactic bacteria can produce lactic acid from pentoses as well as hexoses other than glucose. Usually, lactic and acetic acids are produced from fermentation of pentoses. Most of the lactic acid bacteria also require several B vitamins, amino acids, and phosphate. Peptides may increase cell growth rate. Fermentation yield varies, depending on substrate and the organism used.
Heterolactic fermentation is undesirable because of by-product formation. However, depending on microbial flora, energy availability, and fermentation conditions, it may occur. Heterolactic bacteria produce one mole of lactic acid, ethanol, and CO2 from one mole of glucose:
The theoretical lactic acid yield is 0.5 grams per gram of glucose.
Some species of Rhizopus (e.g., R. oryzae) have low nutritional requirements and can be used to produce lactic acid from carbohydrates. They have the advantage of producing stereochemically pure L-(+)-lactic acid. Rhizopus species can also utilize starch for the production of lactic acid.
The ideal raw material must be inexpensive, must result in high rates with high product yields and no by-product formation, should not require significant pretreatment, and should be available year round. Sucrose from sugar cane or sugar beet juice, lactose from cheese whey, and maltose or dextrose from hydrolyzed starch are used as raw materials in industry. Molasses can also be used; however, its complex nature makes separation of lactic acid problematic. Dextrose from corn starch was the most commonly used raw material in the late 1950s. Other processes based on pasteurized milk (L. bulgaricus), dextrose from corn, glucose (Rhizopus), crude sorghum extract, and potato hydrolysate have also been developed. Direct hydrolysis and fermentation of corn starch by certain Lactobacillus species seem to be quite promising from the economical point of view. Nitrogen sources such as malt extract, corn steep liquor, barley, and yeast extract should be added to the fermentation media to improve growth and lactic acid formation.
Industrial processes are operated batchwise. Fermenters are made of stainless steel and are equipped with heat-transfer coils. Vessels are steam-sterilized before being filled with a pasteurized medium. Slow agitation to prevent settling of calcium carbonate is provided with top-mounted mechanical stirrers. Fermentation conditions are different for each industrial producer but are usually in the range of T = 45° to 60°C and pH = 5 to 6.5 for L. delbruckii; T = 43°C, pH = 6 to 7 for L. bulgaricus; and T = 30° to 50°C, pH < 6 for Rhizopus. The fermentation time is 1 to 2 days for a 5% sugar source such as whey and 2 to 6 days for a 15% glucose or sucrose source. Under optimal conditions, the processing time may be reduced to 1 to 2 days. The rate of lactic acid formation depends on temperature, pH, sugar, nitrogen, and lactic acid concentrations. Temperature and pH control at optimum levels improves the rate of lactic acid formation. Produced lactic acid must be neutralized, usually by addition of calcium carbonate or calcium hydroxide. CO2 is continuously released during fermentation, which creates anaerobic conditions in the fermenter. Lactic acid formation productivities are in the range of 1 to 3 kg/m3h. The yield of lactic acid at the end of fermentation is 90% to 95% of initial sugar concentration. Cell mass yield is usually less than 15% of initial sugar concentration; however, the yield may be as high as 30%, depending on the organism and culture conditions.
Continuous, high cell density and immobilized cell reactors have been used for laboratory-scale lactic acid fermentations. Productivity and yield (100 kg/m3) of lactic acid formation are higher in such reactors than in batch cultures. Continuous removal of lactic acid from cultures by dialysis membranes increases production rates.
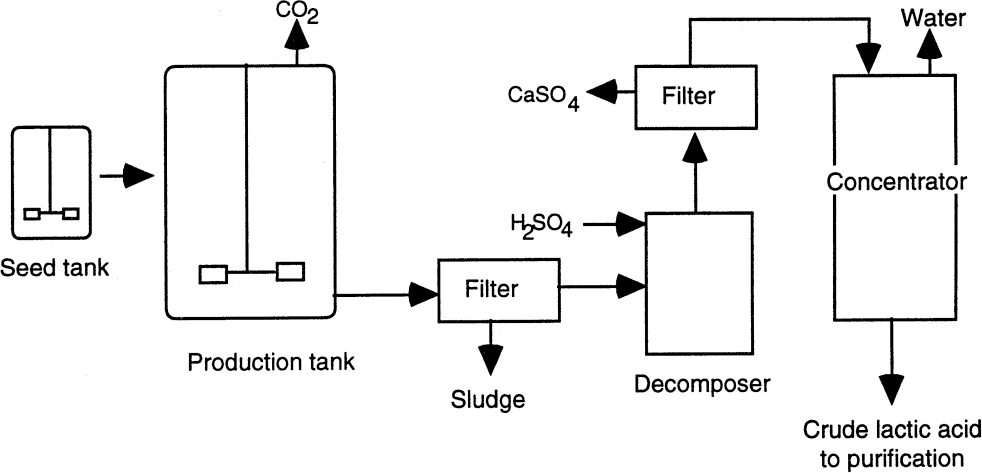
Recovery of lactic acid from the fermentation broth constitutes a significant part of production cost. The use of pure sugar solutions with minimal amounts of nitrogen simplifies product recovery. A process diagram is shown in Figure A.2. The first step is to increase the temperature of the fermenter to 80° to 100°C and the pH to 10 to 11. This procedure kills the organisms, coagulates the proteins, solubilizes calcium lactate, and degrades some of the residual sugars. The liquid is then filtered to remove biomass, and sulfuric acid is added to obtain lactic acid. Calcium sulfate is removed by filtration, and lactic acid is then concentrated. Alternatively, purification of lactic acid may be accomplished by calcium lactate precipitation. The fermenter broth is filtered and evaporated to 25% lactic acid. The calcium lactate is then crystallized and separated from the mother liquor. Several purification procedures can be performed to obtain lactic acid of different grades. These include bleaching with activated carbon, ion exchange, electrodialysis, solvent extraction, and esterification. Edible lactic acid is colorless and contains 50% to 65% lactic acid. Pharmaceutical applications require over 90% pure lactic acid. Traditionally, polymer-grade lactic acid had to be as pure as possible. However, recently polylactate has been obtained from industrial-grade lactic acid.
World demand for lactic acid was about $1.3 billion dollars (800 tons) in 2014. This is divided between fermentation and chemical synthesis where fermentation yields the highest purity lactic acid. About 30% of the market is food and beverage applications, and 10% is pharmaceuticals. The use of lactic acid to make polylactic acid is growing very quickly, as it is a biodegradable, environmentally friendly compound often used in packaging. The annual growth rate for lactic acid is projected to be greater than 15% until 2022.
Major producers of lactic acid are United States, Japan, Belgium, the Netherlands, Spain, and Brazil. China and India are also producers. The price of bulk, food-grade lactic acid is about $1.30/kg (2016). Derivatives of lactic acid such as calcium lactate sell at a slightly higher price (by about 50%). Assuming 20% profit margin, one can estimate the manufacturing price of lactic acid as approximately $1.8/kg.
A.1.3. Acetone–Butanol Production
Acetone is used mainly as a solvent for fats, oils, waxes, resins, lacquers, and rubber plastics. Butanol is used in the production of lacquers, rayon, detergents, and brake fluids and as a solvent for fats, waxes, and resins. Butanol is a better fuel additive than ethanol because of its low vapor pressure, low solubility with water, and complete solubility with diesel fuel. Acetone and butanol (A/B) are produced from petrochemical industry intermediates. However, it is expected that the demand for cleaner processes and shortage of oil-derived products will require production by fermentation.
Clostridium species are used for A/B production. C. acetobutylicum is a strict anaerobe with a versatile metabolic capacity. It has amylolytic and saccharolytic enzymes to hydrolyze gelatinized starch to glucose and maltose. C. acetobutylicum can ferment a large number of carbohydrates such as glucose, lactose, fructose, galactose, xylose, sucrose, maltose, and starch. The fermentation products include acetone, butanol, and ethanol. Acetic and butyric acids, CO2, and hydrogen are also produced in small amounts.
Starch, molasses, cheese whey, Jerusalem artichoke, and lignocellulosic hydrolyzates can be used as raw material for A/B fermentations. More than one of these carbon sources may be utilized simultaneously. The composition of culture medium determines the butanol-to-acetone ratio obtained and can be manipulated to obtain the desired ratio. Molasses can be used as carbon source (6% sucrose) with the addition of nitrogen and phosphate. Higher concentrations of sucrose in molasses increase the ratio of butanol to acetone and ethanol. Average solvent yields of 30% were reported when molasses was used. Utilizing a 5% to 6% starch solution derived from corn, approximately 38% solvent yield can be obtained. A butanol-to-acetone ratio of 2.75 was obtained using Jerusalem artichoke rich in inulin.
Cheese whey with nearly 6% lactose and 1% protein content can be used as carbon and nitrogen source for A/B fermentation. Somewhat different product distribution is obtained when cheese whey is used as carbon source. When corn mash was also added, the butanol-to-acetone ratio was 2. With pure glucose or pure lactose, this ratio is 2.7 and 2.9, respectively. When ultrafiltrate of cheese whey was used, butanol-to-acetone ratio of 10 was obtained without any intermediate buildup of acetic and butyric acids.
Lignocellulosics hydrolyzates (wood, paper, crop residues) contain glucose, galactose, mannose, and pentose sugars, most of which are fermentable by C. acetobutylicum to acetone and butanol. However, since hydrolysis of lignocellulosics is difficult, this raw material is not commonly used for A/B fermentations. Alternatively, cocultures with cellulolytic clostridia can be performed.
A schematic of the process is depicted in Figure A.3. The Weizmann process utilizes starch as raw material for A/B fermentation. C. acetobutylicum hydrolyzes gelatinized corn starch to glucose and maltose with amylolytic enzymes. The grain mesh is first gelatinized at 65°C for 20 min and then sterilized at 105°C for 60 min. The cooked mash is cooled down to 35°C using heat exchangers and is pumped to presterilized fermenters of 250 to 2000 m3. The fermenter is inoculated with a 5% inoculum from a 24 h culture. The final butanol-to-acetone ratio increases as the inoculum age increases. In some cases, 30% to 40% of the total volume of the mash is provided by stillage after solvents from the previous fermentation are stripped. Batch fermentation period is usually 2 to 2.5 days. First, rapid growth and production of acetic/butyric acids and carbon dioxide and hydrogen occur. The initial pH of the medium drops from 6.5 to nearly 4.5 during this phase. In a second phase, growth ceases, and the organisms convert acetic and butyric acids to neutral acetone and butanol. The acidity of the medium decreases, and gas production increases. At the end of the fermentation, the pH is approximately 5.
The South African process utilizes cane molasses as raw material and C. acetobutylicum. Stainless-steel fermenters of 90 m3 are used in batch operation. The effluent contains 2% A/B, and the solvents are recovered by distillation. A/B and ethanol/isopropyl alcohol are obtained as separate fractions. The biomass, rich in riboflavin and B vitamins, is concentrated, dried, and used as animal feed supplement. Due to the high price of molasses, this process is operated intermittently. A process based on utilization of cheese whey for production of A/B has been developed. If the plant is placed near cheese manufacturers, this process can be more attractive than that based on molasses.
Different approaches have been developed to improve solvent yield in A/B fermentations. Phosphate limitation and addition of acetic and butyric acids improve butanol productivity. Calcium present in cheese whey complexes phosphate and also improves butanol yield. Hydrogen saturation in the medium at low agitation rates (25 rpm) and high head-space pressure improves butanol yield. Overall, butanol productivity may be improved by moderate to high agitation (300 rpm) during the acid phase followed by low agitation (25 rpm) during the solvent phase. Simultaneous removal of inhibitory products (butanol, acetone) by adsorption (activated carbon) or extraction (corn oil, paraffin oil) improves fermentation productivities. Organisms can tolerate up to 1.2% (vol/vol) of butanol.
Continuous or fed-batch operations improve solvent productivities. The possibility of controlling the growth rate and environmental and nutritional conditions improves butanol productivities (2.5 g/l-h), as compared to batch cultures (0.8 g/l-h). Immobilized cultures of C. acetobutylicum are also used for A/B fermentations with high productivities (1.2 g/l-h). Biochemical and genetic manipulations of cells may further improve the solvent yield.
To recover A/B, at the end of the solvent phase the broth is transferred to a beer still that concentrates solvents (as shown in Figure A.3). Solvents are then separated by fractionation, and the stillage is dried. An economic evaluation was presented for A/B fermentation from cheese whey in 1982. For a plant producing 45,000 tons of solvents per year, the total capital investment including the waste-water treatment was estimated to be $28 million and the total production cost was nearly $37 million, with an annual income of nearly $53 million and an annual profit of $16 million. Nowadays, production by fermentation is not economically attractive due to low levels of product concentrations (0.7 to 1.5%) and high cost of product recovery. However, fermentation may be the preferred method for production of A/B if a shortage of oil products exists or as demands for environmentally friendly processes increase.
A.2. Aerobic Processes
Aerobic bioprocesses are widely used for the production of organic acids (citric, acetic, gluconic), vitamins, antibiotics, enzymes, flavors and fragrances, and amino acids. Owing to space limitations, only a few examples of aerobic bioprocesses are presented here.
A.2.1. Citric Acid Production
Citric acid present in citrus fruits was first crystalized from lemon juice in the form of calcium citrate. Later on, citric acid was synthesized from glycerol. Production of citric acid from sugar solutions by aerobic bioprocesses was first realized by using Penicillium. Due to low yields obtained from Penicillium, Aspergillus niger was utilized in subsequently developed processes.
Citric acid is used as an acidulant in food, confectionery, and beverages (75%). Pharmaceutical (10%) and industrial (15%) applications are also significant. Citric acid complexes with heavy metals such as iron and copper and can be used as a stabilizer of oil and fats. In the pharmaceutical industry, citric acid can be used in antacids, soluble aspirin preparations, and as a stabilizer of ascorbic acid. Metal salts of citric acid, such as trisodium citrate, are used to prevent blood clotting by complexing calcium. Trisodium citrate can be used in detergents and cleaners as a cleaning agent instead of phosphates.
A. niger is the most widely used organism for citric acid production from molasses or sugar solutions. Candida yeast can also be used for producing citric acid from carbohydrates or n-alkanes, with yields as high as 225 g/l. Beet or cane molasses can be used as source of carbohydrates for citric acid production. The concentration of heavy metals such as iron and manganese must be reduced. Typical trace-element concentrations are 0.3 ppm zinc, 1.3 ppm iron, and Mn < 0.1 ppm. High concentrations of metals can be reduced with the addition of Na- or K-ferrocyanide. Additions of nitrogen, phosphate, and other inorganic salts may not be required. Utilization of pure glucose or sucrose solutions is expensive and requires additions of nitrogen (NH4+), phosphate, and inorganic salts. Because of its low price and nutrient-rich nature, molasses is usually preferred to pure sugar solutions.
Citric acid production is mixed-growth associated, mainly taking place under nitrogen and phosphate limitations after growth has ceased. Since citric acid is a product of primary metabolism, it is produced in high concentrations only under very specific conditions. These include restricted growth, medium deficient in one or more essential elements, high sugar concentration, high dissolved-oxygen concentration, pH below 2, and absence of trace metals. Such conditions provoke an overflow in metabolism that results in an overproduction of citric acid. Oxalic and gluconic acids are also produced if the pH is above 2. Potassium ferrocyanide is added to reduce the concentration of metals and is a growth inhibitor that promotes the production of citric acid. The metabolic imbalance that results in citric acid production also alters the morphology of fungi. The hyphae become short, stubby, and forked in small pellets (0.2 to 0.5 mm in diameter). Limitation of certain nutrients, such as nitrogen and phosphate, stimulates citric acid formation. High dissolved-oxygen concentrations must be maintained throughout the culture. Even short interruptions in oxygen provision can result in irreversible decreases in acid production rate.
Citric acid was historically produced by surface fermentation of beet molasses, and this process is still employed by some manufacturers. It is labor intensive, but power requirements are lower than for submerged fermentation. The surface process is realized on surface-aerated trays with liquid depth of 5 to 20 cm. Sterilized diluted beet molasses, with a sugar concentration of 150 g/l and a pH of 6, is placed on trays in a temperature-controlled (30°C) and aerated clean room. Sterilized additional nutrients and alkali ferrocyanide are added into the medium. Spores of a selected strain of A. niger are spread over the liquid on trays. The clean chamber is aerated with filter-sterilized air to provide oxygen to the organisms and to remove fermentation heat. Mycelium forms a layer on the surface of the medium. After 7 to 10 days of incubation, the trays are emptied, the mycelium is removed, and the medium is transferred to the recovery section. The production of undesirable products such as gluconic and oxalic acids can be avoided by strain selection.
After World War II, submerged fermentation processes utilizing molasses or pure sugar solutions were developed. The submerged process is realized in deep stainless-steel vessels of 100 m3 or larger by batch or fed-batch operation. The fermenters may be mechanically agitated or aerated towers with an internal recycle draft tube. Aeration is provided to the fermenter by air sparging (0.1 to 0.4 vvm), and temperature is controlled with cooling coils. Agitation is usually gentle (50 to 100 rpm) to avoid shear damage on molds. Diluted molasses supplemented with other nutrients is on-line sterilized and added to the fermenter. Sterilization in the fermenter is also possible. The initial pH is adjusted at 2.5 to 3. Spores of A. niger are allowed to germinate in an inoculum medium before being transferred to the main fermenter. In some cases, spores are directly introduced into the fermentation media (5 to 25 × 106 spores/l). Since dissolved-oxygen concentration is critical for citric acid production, oxygen-enriched air may be used in some cases. About 80% of the supplied carbon is converted to citric acid in a typical fermentation. Batch operation usually results in productivities of 0.5 to 1 kg/m3 h. Fed-batch operation can be performed to avoid substrate inhibition and to prolong the production phase 1 or 2 days after growth cessation. Typical volumetric yields of fed-batch processes are around 130 kg/m3. When citric acid production stops, usually after 4 to 5 days, the fermenters are emptied and the biomass is separated from the broth by filtration. The liquid is transferred to the recovery section.
Precipitation is usually accomplished by addition of calcium hydroxide (lime) to the heated fermentation broth to obtain calcium citrate tetrahydrate. The precipitate is then washed and treated with dilute sulfuric acid, yielding an aqueous solution of citric acid and CaSO4 (gypsum) precipitate. After bleaching and crystallization, either anhydrous or monohydrate citric acid is obtained. Solvent extraction is another option for recovery of citric acid, although it is not used commercially. Extraction avoids the use of lime and H2SO4 and gypsum formation; however, it may result in extraction of some impurities present in molasses. Ketones, hydrocarbons, ethers, and esters can be used as extractants. Extraction of citric acid is realized at low temperature, and the solvent is then stripped with hot water.
Almost no information is available on process economics for citric acid production because of the proprietary nature of production schemes. The global citric acid market stood at $2.6 billion in 2014 and is expected to grow to $3.6 billion by 2020.
A.2.2. Production of Baker’s Yeast
Baker’s yeast is essential to the modern baked goods with which we are familiar. The earliest production of baker’s yeast was accomplished by a Dutch process (1781), utilizing grain mash as raw material. Later on, sugar solutions and aeration were used in Germany. The process was further improved to avoid ethanol formation and to improve biomass yield. Today, baker’s yeast worldwide production is at the level of a million tons per year. The process is based on aerobic cultivation of S. cerevisiae on carbohydrates.
Today, the most widely used organism for baking is S. cerevisiae. In the past (prior to the 19th century), other strains of Saccharomyces, such as S. uvarum and S. carlsbergensis (brewer’s yeast), had been used. However, S. cerevisiae is superior to other species of yeast for baking purposes, primarly because of its ability to generate gas (e.g., CO2) in the dough. The most suitable yeast strains are selected in the laboratory and are stored by freeze drying.
Molasses is the most used carbon and energy source for production of baker’s yeast. It is inexpensive, rich in nutrients (nitrogen, phosphorus, and minerals), and available year round. Other carbohydrates, such as glucose, sucrose, fructose, or hydrolyzed starch, can be used. Aqueous ammonia (NH4OH), ammonium salts, or urea may be used as nitrogen sources. Phosphoric acid may also be added as phosphate source. Addition of some magnesium (Mg) and ferrous (Fe) salts may be needed. Some yeast strains require B vitamins as well as Na, K, Mg, Ca, and sulfate ions for effective growth.
A material balance for baker’s yeast formation can be written as follows:
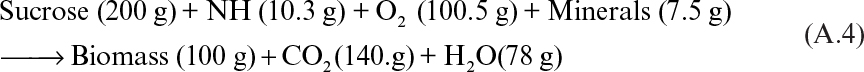
Typical yields are about 0.5 g of cells per gram of substrate and 1 g of cells per gram of oxygen. The maximum specific growth rate of yeast is 0.6 h–1 (doubling time of 1.2 h). The temperature and pH of the fermentation medium are controlled at 30°C and 6 to 7, respectively. High dissolved-oxygen concentrations (above 2 mg/l) are required to promote biomass production. High carbohydrate concentrations may provoke ethanol production even in aerobic conditions (the Crabtree effect). Ethanol is inhibitory to cell growth and reduces biomass yield.
Growth of yeast results in a highly viscous culture broth. Consequently, mechanically agitated fermenters are preferred over airlifts or aerated columns. Typical reactors have a working capacity of 50 m3 to 350 m3 with a height-to-diameter ratio of 3. Vigorous aeration and agitation are required to provide oxygen for biomass production. Reactors are aerated in the bottom by perforated horizontal pipe spargers. Stirred tanks utilized for yeast production typically have oxygen transfer rates of 1 mole O2/l-h and KLa of 600 h–1. Temperature control is accomplished by cooling coils, and pH is controlled by addition of acid or base. Dissolved-oxygen concentration and foam are also controlled. Some plants are computerized and automatically controlled.
To avoid the Crabtree effect, production of baker’s yeast is performed in fed-batch mode. The inoculum is prepared in smaller tanks and is added to the production fermenters. Multistage fermenters can be used for production. Diluted molasses, ammonium or urea, phosphoric acid, and mineral salts are stored in different tanks. The process starts batchwise after filling and sterilization of the fermenter content. Intermittent feeding of continuously sterilized nutrients starts when the initial charge of nutrients is depleted. The feeding rates of nutrients are adjusted to maximize growth rate. Dissolved-oxygen monitoring can be utilized to evaluate the metabolic activity of yeast and is the basis for feedback control systems for nutrient addition.
After 20 to 30 h of culture, the broth is transferred to centrifuges for separation of yeast. The cells are washed several times to remove inert solids. Centrifugal separation results in a light-colored cream with up to 22% solids from yeast. The cream is stored in agitated tanks at 2° to 4°C, and part is used for seeding additional fermentations. Baker’s yeast can be sold in the form of cream, in compressed form (30% yeast solids), or dried (95% yeast solids). Filter press or rotary vacuum filters are used to concentrate cream. The filtered yeast may be mixed with emulsifiers prior to being extruded into yeast cakes or packaged in large paper bags. Fresh baker’s yeast may be marketed as free-flowing particles by adding ingredients such as modified starch or micronized cellulose.
To produce active dry yeast, the filtered yeast cake is extruded into particles that are dried in a hot air stream. Drying temperature, drying rate, and final moisture content of the dried yeast should be controlled during drying. The drying temperature is usually 45°C. Most of the vegetative yeast cells are killed at temperatures above 50°C. Drying time may vary from 20 min to several hours, depending on the type of drier used. The final moisture content of yeasts after drying is about 5% to 10%. Several types of driers can be used. The Roto–Louvre drier is an empty cylinder rotating at 1 to 4 rpm. Heated air at 50° to 60°C is blown to the cylinder containing yeast particles. The drying temperature is 45°C, and the duration is 10 to 15 h. In fluidized bed driers, hot air (50 to 60°C) is blown from the bottom of the column to keep the cells suspended in air. The drying temperature and time are 45°C and 1 to 2 h, respectively. Spray driers are the most widely used. A suspension with 10% to 20% yeast is atomized into a drying chamber and dried with hot air. The drying temperature and time are 50°C and 10 to 20 min, respectively. Product quality depends mainly on gassing activity of yeast. Presence of certain carbohydrates, such as trehalose or glycogenin, increases the gassing activity of yeast. Highly stable active dry yeast can be obtained from cultures with a high protein content. Fluidized bed or spray driers yield fine yeast particles of 0.2 to 2 mm with high protein content and high gassing activity.
A.2.3. Production of Penicillins
Penicillin was discovered by Alexander Fleming (see Chapter 1, “What Is a Bioprocess Engineer?”). Different penicillins are produced by different strains of Penicillium. Chemical structures of penicillins G and V are given in Figure A.4.
Sodium penicillin G (MW = 356.4 KDa, Activity: 1,670 U/mg) is administered parenterally, as it is degraded in acid conditions; penicillin V (MW = 372.4 KDa, Activity: 1,595 U/mg) is acid stable and is orally administered. Both forms are active against gram-positive bacteria by inhibition of cell wall synthesis. Different species of the genus Penicillium produce different forms of penicillin. The strain used by Fleming was P. notatum. Later on, different strains were used, such as P. chrysogenum, which is the most widely used strain in industry. Before utilization at industrial scale, considerable efforts must be spent in strain selection and development to improve the yield and activity of penicillin formation. Selected strains are stored in the form of lyophilized spores. Vegetative cells may be frozen at –70°C with glycerol as a suspending medium.
P. chrysogenum can use a variety of carbohydrates and oils as carbon and energy sources. Among those are glucose, sucrose, hydrolyzed starch, lactose, and molasses. Corn oil supplemented with lactose results in fast production of highly concentrated penicillin. Medium formulation has changed significantly with new developments. The original medium (1945) contained the following compounds: lactose, 3% to 4%; corn steep liquor, 4%; CaCO3, 1%; KH2PO4, 0.4%; and antifoam, 0.25%. Improved media resulting in higher penicillin yields have been developed. A typical composition of such media is glucose or molasses, 10%; corn steep liquor solids, 4% to 5%; phenylacetic acid (continuous feed), 0.5% to 0.8% total; and vegetable-oil antifoam, 0.5% total.
Corn steep liquor (CSL) is used as a nitrogen source, since it results in higher penicillin yields as compared to the other nitrogen sources. Some compounds in CSL are converted to phenylacetic acid or other side-chain precursors. Cottonseed flour or soybean meal may also be used as nitrogen sources; however, they are more expensive than CSL. Continuous addition of ammonium sulfate to keep the ammonium concentration around 250 to 300 mg/l is required for continued synthesis of penicillin and to avoid lysis of the mycelium.
Certain precursors of the penicillin side chain need to be added into the fermentation medium. This constitutes a major cost item. Penicillin G requires 0.47 g sodium phenylacetate and penicillin V requires 0.5 g sodium phenoxyacetate per gram of penicillin produced. Those precursors are fed continuously to avoid possible toxic effects. More than 90% of the precursors are incorporated into the structure of penicillin. Phosphorus supplied by CSL is usually sufficient, since phosphate concentration should be limiting (250 to 300 mg/l) in the media for penicillin production.
Penicillin is a secondary metabolite with a nongrowth-associated production. The process to produce penicillin involves an initial batch phase in which cell growth occurs. In the first 40 h of fermentation, rapid cell growth is achieved with a doubling time of nearly 6 h. After a high cell density has been obtained, nutrients (glucose and CSL) and precursors are added slowly or intermittently to reduce cell growth to 0.02 h–1 and maximize penicillin production. Oxygen, carbon, nitrogen, and phosphate concentrations should be low. A simplified diagram of the process scheme is presented in Chapter 1, Figure 1.5. Culture preparation starts with lyophilized spores and agar slant cultures. Vegetative cells are cultivated in shake flasks and then are transferred to seed fermenters (10 to 100 l). Production fermenters are agitated tanks 200 to 500 m3 in volume made of stainless steel. Mechanical agitation is provided at a rate of 100 to 300 rpm. Temperature is controlled around 25° to 28°C (26°C optimum) by using cooling coils. Antifoam is added to reduce foam formation. Dissolved oxygen is controlled at >2 mg/l and pH at 6.5. Vigorous aeration is supplied from the bottom of fermenters by ring or tube spargers. Due to the high viscosity of the broth, oxygen transfer is a major problem in penicillin fermentations. In some cases, strains from pellets are preferred because the medium is less viscous and oxygen supply is improved. The fermentation is stopped when the oxygen uptake rate of the culture exceeds the oxygen transfer rate of the reactor, or when 80% of the fermenter is full.
The original process for the recovery of penicillin from fermentation broth was based on adsorption on activated carbon. After washing with water, the activated carbon was eluted with 80% acetone. The penicillin was concentrated by evaporation under vacuum at 20° to 30°C. The remaining aqueous solution was cooled to 2°C, acidified to pH of 2 to 3, and the penicillin extracted with amyl acetate. Penicillin was crystallized from amyl acetate with excess mineral salts at pH of 7 under vacuum. This process is uneconomical because of the high cost of activated carbon.
The current recovery process includes filtration, extraction, adsorption, crystallization, and drying. Filtration is usually achieved by using high-capacity, rotary vacuum drum filters for separation of the mycelia. The mycelia are washed on the filter and disposed. The penicillin-rich filtrate is cooled to 2° to 4°C to avoid chemical or enzymatic degradation of the penicillin. In some early processes, the filtrate was further clarified by a second filtration with the addition of alum. In recent years, macroporous filters have been used in some plants for separation of the mycelia.
Solvent extraction is accomplished at low pH such as 2.5 to 3, using amyl acetate or butyl acetate as solvent. Continuous, countercurrent, multistage centrifugal extractors (Podbielniak D-36 or Alfa–Laval ABE 216) are used for this purpose. The distribution coefficient of penicillin G or V between organic and aqueous phase depends strongly on the pH of the medium. At a pH of 3, the distribution coefficient is about 20. Penicillins G and V degrade under acidic conditions with first-order kinetics. To avoid degradation of penicillin during solvent extraction at low pH, temperature is kept around 2° to 4°C and filtration time is kept very short (1 to 2 min). Two extractors used in series result in nearly 99% penicillin recovery. Whole broth extraction (without filtration) is possible by using Podbielniak extractors in series. Due to operational difficulties, however, this approach is not used in practice. Carbon adsorption is used to remove impurities and pigments from penicillin-rich solvent after extraction. Several activated carbon columns in series can be used for this purpose.
Penicillin may be back extracted into water by addition of alkali (KOH or NaOH) or buffer at pH of 5 to 7.5. The water-to-solvent ratio in this extraction is usually between 0.1 and 0.2. A continuous, countercurrent, multistage centrifugal extractor may be used for this purpose. This step is usually omitted in the new separation schemes.
Crystallization may be performed from the solvent or aqueous phase. Na, K, and penicillin concentrations, pH, and temperature need to be adjusted for crystallization. Excess amounts of Na or K are added to the penicillin-rich solvent before crystallization in an agitated vessel. The crystals are separated by a rotary vacuum filter. The crystals may be washed and predried with anhydrous butyl alcohol to remove some impurities. Large horizontal belt filters are used for collection and drying of the crystals. Usually, warm air or radiant heat is used for drying.
Crystalline penicillins G and V are sold as an intermediate or converted to 6-APA (6-aminopenicillanic acid), which is used for production of semisynthetic penicillins. The enzyme penicillin acylase is used for cleavage of penicillin G or V to produce 6-APA. Some bacteria, E. coli, Bacillus megaterum, and P. melanogenum, as well as some molds, produce the enzyme.
Costs for penicillin production utilizing glucose as a substrate were calculated in 1982 as $19/kg. Prices of penicillin decreased to $10/kg in 2015 as a result of new producers in China and India and improvements to technology. This has put pressure on penicillin manufacturers to reduce production costs. The most important costs in penicillin production are raw materials (35%) and utilities (14%). By using cheaper raw materials, such as molasses or starch, and genetically improved strains producing higher penicillin yields, the production costs may be reduced significantly. Worldwide demand for penicillin and related beta-lactams both for medical applications and in animal feed was over $15 billion per year in 2015.
A.2.4. Production of High-Fructose Corn Syrup
High-fructose corn syrup (HFCS) is a low-calorie sweetener commonly used in beverages, desserts, and other sweet foods. Until 1935, the only syrup available was 42 DE (dextrose equivalent) acid-converted corn syrup. In 1940, enzymes were commercially available, and corn starch was hydrolyzed enzymatically to produce corn syrups. In the early 1960s, the first crystalline dextrose derived from corn was marketed. The commercial production of the enzyme glucose isomerase, which converts glucose to its sweeter (approximately 1.7 times) isomer fructose, was a major milestone. The first HFCS was produced in 1967 and contained 15% fructose. Further process improvements yielded 42% and 55% fructose-containing HFCS. The original conversion process was batch; however, immobilized enzyme technology was later used for the production of HFCS by continuous operation. U.S. production of HFCS peaked in 2000 at 9.5 million tons/yr and was about 8.5 million tons/yr in 2015.
Three major HFCS products differ by their fructose content: 42%, 55%, and 90%. HFCS containing 42% fructose is mainly used in most of the food products utilizing liquid sweeteners. HFCS with higher levels of fructose (55%) are mainly used in soft drinks as a replacement for sucrose and in jams and jellies (90%) as a low-calorie sweetener.
Production of HFCS from corn starch is an enzymatic process. The process scheme may be divided into 18 steps and five major operations (Figure A.5). Those operations are dextrose production by enzymatic hydrolysis of corn starch, primary physical and chemical treatment of dextrose syrup, isomerization of dextrose to 42% fructose, secondary refining of fructose corn syrup, and conversion of 42% fructose-to 55% fructose-containing HFCS.
First, corn starch is gelatinized by cooking at high temperatures such as 65°C and is converted to dextrose (liquefaction) by thermostable-amylase in a two-stage continuous reactor. The product is a dextrose-rich syrup containing 10 to 15 DE. The conditions in liquefaction reactors are 105°C and a 5 to 10 min holding time for the first reactor; and 95°C and a 90 to 120 min holding time for the second reactor. The feed starch slurry contains 30% to 35% solids with 0.1% to 10% enzyme at a pH of 6.5. The total and soluble protein contents of the starch slurry should be lower than 0.3% and 0.03%, respectively, to avoid color formation as a result of the Maillard reaction between amino acids and sugars at high temperatures. Saccharification of liquefied starch slurry is achieved by using the enzyme glucoamylase to produce more dextrose from branched chains of the starch. In continuous saccharification reactors, glucoamylase is added to the 10 to 15 DE liquefied starch after temperature and pH adjustment and is fed through a number of reactors in series. The conditions for this step are 60°C, pH of 4.3, and holding time of 65 to 75 h. The feed contains 30% to 35% dry substance and 1 l of glucoamylase solution per ton of dry weight of starch. The product of this step contains 94% to 96% dextrose, 2% to 3% maltose, 1% to 2% higher saccharides, and 30% to 35% dry substance. Environmental conditions must be strictly controlled during liquefaction and saccharification to obtain the 94% to 96% dextrose that is required to obtain 42% fructose HFCS.
The dextrose syrup produced by the liquefaction and saccharification step is refined to remove ash, metal ions, and proteins that may interfere with isomerization. The dextrose syrup is filtered on rotary precoat vacuum filters to remove solids, proteins, and oil. The filtered liquor is then passed through several check and polish filters to remove traces of particles. The color in the filtrate is removed by granular activated carbon in columns. Carbon-treated liquor is filtered again and passed through ion-exchange columns to remove metal ions and ash. These columns are dual-pass cation–anion ion-exchange systems that also remove color. Deionized and decolorized dextrose syrup is evaporated to concentrate dextrose, and Mg ions are added to activate the isomerase.
Conversion of glucose (dextrose) to fructose by the enzyme glucose isomerize is accomplished in a packed column of immobilized enzyme. The reactor conditions are 55° to 65°C, pH of 7.5 to 8, and residence time of 0.5 to 4 h. The optimum temperature is 60°C. Temperatures higher than 60°C cause higher conversion rates and faster inactivation of the enzyme. The feed temperature may be as low as 55°C, resulting in slower conversion and enzyme inactivation rates. Microbial contamination may be a problem at temperatures below 55°C. The optimum pH for maximum enzyme activity is 8 and for stability is between 7 and 7.5. Therefore, the operating pH is adjusted for maximum stability and activity of the enzyme. The feed syrup contains 40% to 45% dry substance, 94% to 96% dextrose, 4% to 6% higher saccharides, and 4 mM of Mg ions as activator. The enzymatic isomerization of glucose to fructose is reversible. With 96% dextrose in the feed, the equilibrium fructose concentration in the effluent is expected to be 48%. However, the exact equilibrium is not reached with 4 h of residence time, and the effluent contains 42% fructose.
The activity of the immobilized enzyme in the column drops exponentially with time. The half-life of the enzyme is 70 to 120 days. Therefore, the residence time for maximum conversion is low for new columns with unused enzymes, and the feed flow rate should be lowered at later stages of operation to obtain 42% fructose. Usually, series and parallel configurations of immobilized enzyme columns are used to compensate for activity loss of the enzyme. Parallel operation of six columns offers a good flexibility and results in good product quality. A number of process variables, such as temperature, feed pH, and flow rate, may be varied to obtain uniform product quality. Constant fructose levels are usually achieved by automatic back blending controlled by a polarimeter. Columns must be replaced two or three times a year. The cost of the enzyme is a major part of the operating cost. By improving the stability and activity of the enzyme, the cost of isomerization may be reduced significantly.
HFCS produced by isomerization of dextrose syrup can be further refined to remove color and ions by carbon treatment and ion exchange, respectively. The refined 42% HFCS is evaporated for shipment to yield 71% solids.
The HFCS from the isomerization step contains 42% fructose, 52% dextrose, and about 6% oligosaccharides. To obtain 55%- and 90%-fructose syrups, the fructose in 42% syrup needs to be concentrated. Fructose preferentially forms a complex with some cations such as calcium. This is used for concentrating fructose in HFCS. There are two commercial processes for enrichment of fructose from 42% syrup. One process utilizes an inorganic resin for selective molecular adsorption of fructose. Another process employs chromatographic fractionation using organic resins. Fructose is selectively held in fractionating columns, but dextrose is not. Deionized and deoxygenated water is used for the elution of fructose from the column. Usually, a column packed with low cross-linked fine-mesh polystyrene sulfonate-Ca cation exchange resin is used for enrichment purpose. The enriched syrup contains nearly 90% fructose and is called very enriched fructose corn syrup (VEFCS). The VEFCS is blended with 42%-fructose syrup to obtain the desired fructose content, such as 55%. The effluent from the isomerization step may be recycled back to the feed solution to obtain 42%-fructose syrup in the effluent of the isomerization column. The raffinate stream rich in oligosaccharides is recycled back to the saccharification step. The water used as an eluent in enrichment columns should be minimized to maximize the solids content of the syrup.
Since 1972, HFCS has replaced sucrose as a low-calorie sweetener to a large extent due to lower cost. This price difference has increased with developments in HFCS production technology. Consequently, HFCS has replaced sucrose and glucose as a low-calorie sweetener used in soft drinks, canned fruits, ice cream, and certain bakery products over the last 20 years. Low-calorie sweetener consumption is expected to level off at 130 lb per capita per year.
A.3. Bioprocess Technologies: Biofuel and Bioenergy Production from Biomass
Renewable energy sources containing cellulose and starch constitute a vast raw material source for production of fuels, chemicals, and energy by using bioprocess technologies. Waste biomass (grains, solid–liquid wastes of food industry) provides inexpensive raw material for production of fuels and energy using bioprocess technologies. Carbohydrate-rich waste materials can be used for liquid and gas fuel production.
A general process scheme for production of biofuels from biomass is depicted in Figure A.6.
A.3.1. Production of Liquid Fuels
Ethanol and methanol are the two major fuels with production potential from waste biomass. In ethanol production, the first step is hydrolysis of cellulose or starch present in biomass by acid or enzymatic hydrolysis. Acid hydrolysis is relatively fast but requires high temperatures, high acid concentrations, and subsequent neutralization. Acid recovery is required for economic reasons. Enzymatic hydrolysis is realized by the enzymes (cellulase, amylases) produced by microorganisms under mild conditions (temperature = 30°C, pH = 7) but is relatively slow and expensive. Sugar solution obtained from hydrolysis of biomass is fermented by either yeast (S. cerevisiae) or bacteria (Zymomonas mobilis) for production of ethanol.
Anaerobic fermentation of glucose solution to ethanol takes place at 30°C and pH of 5 within 48 h. Fermentation effluent contains 5% to 10% ethanol in aqueous solution. Ethanol separation from water can be achieved by distillation, membrane separation, or selective adsorption.
Simultaneous fermentation and separation using vacuum distillation and pervaporation systems make the process economically more feasible. Figure A.7 depicts a typical bioprocess scheme for ethanol production from waste biomass.
Methanol is not an end product of any metabolic pathway. Therefore, production of methanol by fermentation alone is not possible. However, the most suitable method for methanol production is conversion of methane to methanol by enzymatic processes. Methane can be produced by anaerobic digestion of solid wastes or sludges, as described in Chapter 16, Section 16.5. Mono-oxygenase enzymes produced by some bacteria can be used to oxidize methane to methanol. Heat-treated methanotrophic bacteria (Methlococcus sp., Methylomonas sp.) with active mono-oxygenase enzyme systems can also be used for this purpose. Immobilized enzyme or cell systems are more suitable for this conversion.
A.3.2. Production of Gaseous Fuels from Biomass
Methane is the most widely known gaseous fuel produced from waste materials by anaerobic digestion. Steps involved in methane production by anaerobic digestion of waste materials are explained in Chapter 16, Section 16.5. A process scheme for methane production from waste biomass is presented in Figure A.8. The resultant biogas contains nearly 70% methane, 25% CO2, and 5% other gases.
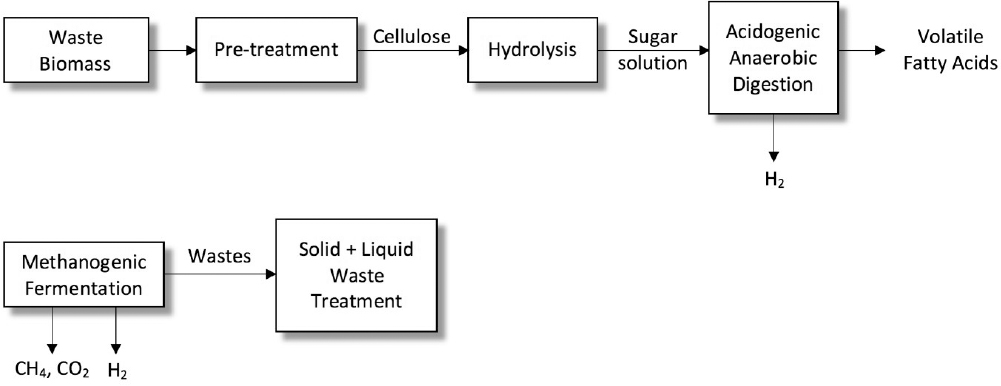
Hydrogen gas is a clean energy source and energy carrier with a high energy content of 122 kJ g–1. Hydrogen can also be used in fuel cells for electricity generation. The major obstacle in utilization of hydrogen is its unavailability in nature. Presently, hydrogen gas is produced by steam reforming of natural gas and other hydrocarbons requiring high energy inputs. Fermentation of carbohydrate-rich raw materials for biohydrogen production offer significant advantages over costly chemical processes due to operation under mild conditions (30 to 35°C, 1 atm). Waste biomass, such as agricultural and domestic wastes, can be used as inexpensive raw materials for biohydrogen production at large scale. Biohydrogen production from carbohydrate-rich wastes requires dark and light anaerobic fermentations. The first step in fermentative hydrogen production from biomass is the acid or enzymatic hydrolysis of biomass to highly concentrated sugar solution, which is followed by dark fermentation by the acetogenic-anaerobic bacteria for production of volatile fatty acids (VFA), hydrogen, and CO2. Volatile fatty acids produced by the dark fermentation are further fermented by the photo-heterotrophic bacteria (light fermentation) such as Rhodobacter species to produce CO2 and H2.
A process scheme for biohydrogen production from waste biomass and carbohydrate-rich wastewater is depicted in Figure A.9. Starch- or cellulose-containing waste is subjected to pretreatment after grinding to make the biomass more suitable for hydrolysis. Glucose syrup obtained after hydrolysis and neutralization is fed to dark fermentation for hydrogen and VFA production. Photo-fermentation of dark fermentation effluent is used for converting VFAs to H2 and CO2.
A.3.3. Bioelectricity Generation from Wastes Using Microbial Fuel Cells
Electrical power generation with simultaneous wastewater treatment can be achieved by using microbial fuel cells (MFCs). A typical MFC consists of anode and cathode chambers separated by a proton exchange membrane (PEM). In an anodic chamber, anaerobic organisms degrade organic compounds present in wastewater or sludge, generating electrons and protons. Generated electrons are transferred through an external circuit, and protons diffuse through the membrane to the cathodic chamber. Electrons and protons transferred to the cathodic chamber form water in the presence of dissolved oxygen. Electron transfer through an external wire provides electrical current generated by the MFC. Anode can be graphite, carbon cloth, or copper sheet, and cathode may be platinum (Pt)-patched graphite, carbon cloth, or gold-covered copper sheet. Originally developed in two-compartment configuration, MFCs are now used in a single-chamber air-cathode configuration. Figure A.10 depicts two-compartment and single-compartment MFC configurations. The voltage generated between the two electrodes can be as high as 1 V, and power densities of 40 W/m2 anode were obtained by using single-chamber air cathode MFCs with considerable chemical oxygen demand (COD) removal from wastewater.
Microbial electrolysis cells (MECs) are used for hydrogen gas formation instead of electricity generation in MFCs. MECs are somewhat similar to MFCs with some differences. Cathode is not in contact with air and is completely immersed in wastewater. A low voltage (0.2 to 0.5 V) is applied to the cathode along with the generated voltage in the system (0.7 to 1 V) for hydrogen formation on cathode. Protons and electrons generated by the microorganisms growing on anode surfaces by anaerobic metabolism of organics combine with the extra electrons supplied by the external voltage source to form hydrogen gas on cathode. MFCs generate electricity; MECs produce hydrogen gas with extra energy input.
Suggestions for Further Reading
ATKINSON, B., AND MAVITUNA, F., Biochemical Engineering and Biotechnology Handbook, 2d ed., Stockton Press, New York, 1992.
BAILEY, J. E., AND OLLIS, D. F., Biochemical Engineering Fundamentals, 2d ed., McGraw-Hill, New York, 1986.
DEMAIN, A. L., “Small Bugs, Big Business: The Economical Power of the Microbe,” Biotechnol. Adv. 18: 499–514, 2000.
FLICKINGER, M. C., AND DREW, S. W., Eds., Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis and Bioseparation, Wiley, New York, 1999.
LOGAN, B. E., Microbial Fuel Cells, Wiley, New York, 2008.
YANG, S. T., EL-ENSASHY, H., AND THONGCHUL, N., Eds., Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals and Polymers, Wiley, New York, 2013.
MOO-YOUNG, M., Ed., Comprehensive Biotechnology, Vol. 3, Pergamon Press, Oxford, UK, 1985.