7. From Ancient Molds to Modern Miracles: The Discovery of Antibiotics
To the villagers who had lived and worked on its slopes for centuries, the 3,000-foot mountain must have seemed idyllic in its beauty and bounty of food that sprang from its rich, fertile soil. Rising from the Bay of Naples along the southwest coast of Italy, its slopes were covered by vineyards, cereal crops, and fruit orchards. Stands of oaks and beech trees rose to its summit and were populated by deer and wild boar. And grazing land was plentiful for the goats that provided milk and cheese. Given that there had been no sign of trouble for more than 1,000 years, it’s no wonder that inhabitants of the two towns on its western and southeastern sides—Pompeii and Herculaneum—had no idea that all of this bucolic splendor sat atop a smoldering time bomb, a volcano that would one day erupt with a horrible and fatal fury.
It was on the morning of August 24, AD 79, that Mount Vesuvius, after only a few warning tremors earlier in the month, awoke with sudden violence, sending an enormous “black and terrible cloud” of toxic gas, ash, and cinder ten miles into the sky. During the afternoon, the dark cloud moved southeastward toward Pompeii and began raining down volcanic debris. By the end of the day, the town was covered by a three-foot-deep blanket of ash. While many residents fled in fear, others remained behind, hiding under roofs for shelter. They would meet their fate at around 6 the next morning, when subsequent eruptions overwhelmed the town with burning dust, cinder, and gases, killing as many as 2,000 of its 20,000 residents.
But by that time, the smaller town of Herculaneum—less than 10 miles away on the other side of Vesuvius—had already been devastated. Just hours earlier, at around 1 a.m., an enormous explosion had sent a blast of dense volcanic debris rushing down the western slope at more than 150 miles per hour. Within seconds, Herculaneum was smothered under a 100-foot-deep inferno of ash and cinder. Although most of Herculaneum’s 5,000 residents managed to escape in the preceding hours, it was not until 2,000 years later—in 1982—that archeologists excavating a nearby ancient beach discovered the skeletons of 250 individuals who, in their attempt to flee, had not been so lucky. The skeletons were found in various poses on the beach and inside nearby boat sheds and, due to the unusual circumstances of their deaths—instant burial beneath a fine volcanic dust as hot as 1,112 degrees Fahrenheit—were nearly perfectly preserved.
Take two figs and call me in the morning?
Since modern archaeologists began exploring ancient Herculaneum in the 1980s, it’s no surprise that they have uncovered a wealth of insights into the lives of these ancient Romans in the days leading up to their deaths. The findings ranged from well-preserved wooden chests and cupboards, to a variety of food remnants, including olive oil, plum jam, dried almonds and walnuts, goat cheese, hardboiled eggs, wine, bread, dried figs, and pomegranates. Nor is it surprising that with modern scientific tools, researchers have been able to learn some remarkable details about the health and diseases suffered by the people whose skeletons were recovered on that ancient beach, including skull lesions caused by scratching lice, rib damage from continuous inhalation of indoor cooking smoke, and foot injuries from Roman shoes and sandals.
What was surprising, however, is what the scientists didn’t find: Evidence of infections.
As described in a 2007 article in the International Journal of Osteoarchaeology, in a study of 162 of the 250 Herculaneum skeletons, only one showed evidence of general infections. This finding was a “true enigma” because such infections are usually more common in ancient populations due to the poor sanitary conditions of the time.
Why were infections so rare among the ancient inhabitants of Herculaneum? A closer look at the villagers’ diet uncovered a key clue: Microscopic examination of two particular foods—dried pomegranates and figs—revealed that the fruit was contaminated by Streptomyces bacteria. Streptomyces are a large and widespread group of generally harmless bacteria that are fascinating for several reasons. For one thing, they are abundant in the soil, where they release a variety of substances that play a critical role in the environment by decomposing plant and animal matter into soil. But equally significant, Streptomyces are known today for their ability to produce an astonishing variety of drugs, including up to two-thirds of the antibiotics now used in human and veterinary medicine. And one of these antibiotics, tetracycline, is commonly used today to treat a variety of general infections, including pneumonia, acne, urinary tract infections, and the infection that causes stomach ulcers (Helicobacter pylori).
Sure enough, when scientists tested the bones of the Herculaneum residents, they found clear evidence that they’d been exposed to the antibiotic tetracycline. Could the villagers have ingested the tetracycline by eating Streptomyces-contaminated fruit? In fact, the researchers found that their pomegranates and figs were “invariably contaminated” by the bacteria, probably due to the Roman method of preservation, in which fruit was dried by burying it in beds of straw. That solved one mystery. By eating Streptomyces-contaminated pomegranates and figs, the ancient villagers unknowingly dosed themselves with tetracycline antibiotics and thereby protected themselves from general infections. Yet that immediately raises another question: Just how “accidental” was this treatment?
According to historical records, at the same time in history in other parts of the Roman Empire, ancient physicians prescribed a variety of foods to treat infections—including figs and pomegranates. For example, in the first century AD, physician Aulus Cornelius Celsus used pomegranates to treat tonsillitis, mouth ulcers, and other infections, and other Roman physicians used figs to treat pneumonia, gingivitis, tonsillitis, and skin infections. While there’s no hard evidence that the physicians of ancient Herculaneum intentionally “prescribed” bacteria-laden fruit to treat infections, it does raise the question: Might these findings cast a new light on exactly who discovered the “first” antibiotic?
* * *
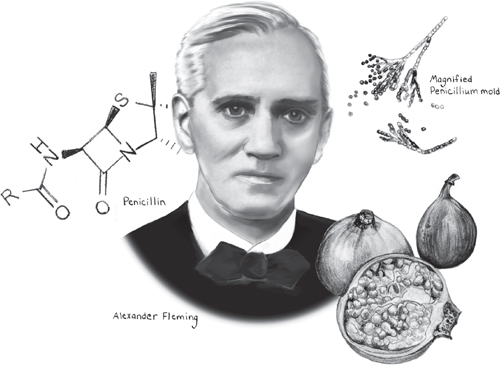
Medical historians need not fear: Neither dried fruits, Ancient Romans, nor Streptomyces are likely to usurp the credit traditionally given to three individuals who, 2,000 years later, were awarded the 1945 Nobel Prize in Physiology or Medicine for discovering the first antibiotic: penicillin. The award is well-deserved because when penicillin was first discovered by Alexander Fleming in 1928, and later refined by Howard Florey and Ernst Chain into a more potent version for widespread use, the impact was profound: transforming commonly fatal infections into easily treated conditions and saving millions of lives. Antibiotics, the general term for drugs that inhibit or kill bacteria while leaving normal cells unharmed, became the classic “miracle drug” of the twentieth century and one of the greatest breakthroughs in the history of medicine.
But the story of antibiotics is not without its share of irony and controversy. In the late-nineteenth century, the discovery that bacteria could cause dangerous diseases prompted scientists to search for antibiotics that could fight those diseases. Today—a victim of our own success—the over-use of antibiotics is forcing scientists to search again for new antibiotics to treat the same diseases.
Setting the stage: from ancient healers to a war among the microbes
To many people, the story of how Alexander Fleming discovered penicillin conjures up a yucky image of mold, the microscopic fungi that make its unwelcome appearance as dark green splotches on a damp shower curtain, old carpet, or loaf of bread. While it’s true that many antibiotics—including penicillin—are produced by molds, Fleming did not discover his unique mold in a breadbox or dank bathroom, but on a glass culture plate in his laboratory. Nevertheless, it’s fitting that the first known antibiotic was produced by a mold, given that the curative powers of these fuzzy fungi have been recognized by healers and physicians throughout history and in all cultures.
The first written reference to the healing power of mold can be found in the oldest known medical document, a papyrus attributed to the Egyptian healer Imhotep and dating back to around the thirtieth century BC. In that ancient writing, healers are advised that when treating open wounds, they should apply a dressing of fresh meat, honey, oil—and “moldy bread.” Later historical accounts report that holy men in Central Asia once applied moldy preparations of “chewed barley and apple” to surface wounds and that in parts of Canada, a spoonful of moldy jam was once commonly ingested to cure respiratory infections. More recently, a physician reported in the 1940s the “well-known fact” that farmers in some parts of Europe once kept loaves of moldy bread handy to treat family members who had been injured by a cut or bruise. The physician wrote that “A thin slice from the outside of the loaf was cut off, mixed into a paste with water, and applied to the wound with a bandage. No infection would then result from such a cut.”
But despite such stories, the therapeutic use of mold in folk medicine had no role in the modern discovery of antibiotics. In fact, it was not until the late 1800s that scientists, intrigued by the discovery of bacteria and “germ theory,” began to ponder whether it might be possible to cure disease by using one type of microbe to fight another.
One of the earliest reports was by Joseph Lister, the physician who first used antiseptics to prevent surgical infections. In 1871, Lister was experimenting with a species of mold called Penicillium glaucum—related to, but not the same as, the mold that led to the discovery of penicillin—when he made an odd discovery: In the presence of this mold, bacteria that normally scurried busily about in every direction across his microscope slide were not only “comparatively languid,” but “multitudes were entirely motionless.” Lister was so intrigued that he hinted to his brother in a letter that he might investigate whether the mold had a similar effect in people. “Should a suitable case present itself,” Lister wrote, “I shall employ Penicillium glaucum and observe if the growth of the organisms be inhibited in human tissues.” But tantalizingly close as he was to being the first person to discover antibiotics, Lister’s investigations did not go far.
A few years later, in 1874, English physician William Roberts made a similar observation in the Royal Society Note, pointing out that he was having difficulty growing bacteria in the presence of the same mold. “It seemed,” Roberts wrote, “as if this fungus... held in check the growth of bacteria.” Two years later, physicist John Tyndall described the antagonistic relationship between Penicillium and bacteria in more colorful terms. “The most extraordinary cases of fighting and conquering between bacteria and Penicillium have been revealed to me,” wrote Tyndall. But Tyndall missed his chance at fame when, instead of investigating whether Penicillium was attacking the bacteria through the release of some substance, he mistakenly believed the mold was simply suffocating the bacteria.
Soon, other scientists were making similar observations: To their surprise, the tiny, silent world of microbes was in reality a tumultuous landscape wracked by warfare, not only between molds and bacteria, but between different species of bacteria. In 1889, French scientist Paul Vuillemin was sufficiently impressed by these battles to coin a new term that would foreshadow the coming breakthrough: antibiosis (“against life”).
Given these early and intriguing clues, why was it not until 1928—another three decades—that Fleming finally discovered the first antibiotic? Historians note that several factors may have distracted scientists from pursuing drugs that could fight infections. For one thing, in the late 1800s and early 1900s, physicians were enamored by other recent medical breakthroughs, including antiseptics (chemicals that can kill bacteria on the surface of the body but can’t be taken internally) and vaccines. What’s more, scientists’ knowledge of fungi in the nineteenth century was not necessarily to be trusted. In fact, in the early studies of bacteria-fighting fungi, the experimenters might have been referring to any species of Penicillium mold—or, for that matter, any green fungus.
And as it turned out, the Penicillium mold that led to the discovery of antibiotics was not any old fungus growing on your bathroom wall. It was a specific and rare strain, and the antibiotic substance it produced, penicillin, was fragile, difficult to isolate, and—to be blunt—it was a miracle that Fleming discovered it at all.
Milestone #1 “That’s funny”: the strange and serendipitous discovery of penicillin
Although most of us prefer to not think about it, just as we are surrounded by countless bacteria, we are similarly exposed to numerous invisible mold spores that waft in daily through our windows and doors, seeking moist surfaces on which to land and germinate. This was pretty much what Alexander Fleming was thinking when, in the summer of 1928, he returned from a long holiday and noticed something growing on a glass culture plate he had left in a corner of his laboratory bench. Fleming was a physician and bacteriologist in the Inoculation Department at St. Mary’s Hospital in London, and he had previously inoculated the culture plate with staphylococcus bacteria as part of a research project. Returning from vacation, Fleming had randomly grabbed the glass plate, removed its lid, and was about to casually show it to a colleague, when he peered inside and said, “That’s funny...”
Fleming wasn’t surprised to see that the surface of the plate was speckled by dozens of colonies of staphylococcus bacteria—that was part of his experiment. Nor was he surprised that one side of the plate was covered by a large splotch of mold. After all, he had been away for two weeks and was planning to discard the culture plate anyway. What caught his eye was something he didn’t see. Although bacterial colonies covered most of the plate, there was one spot where they came to screeching halt, forming a translucent ring around something they clearly did not like: the giant patch of mold. What’s more, the bacteria closest to the mold were clearly disintegrating, as if the mold were releasing something so potent that it was killing them by the millions.
Fortunately, Fleming—who only a few years earlier had discovered lysozyme, a natural bacteria-fighting substance made by a number of tissues in the body—recognized an important discovery when he saw it. As he later wrote, “This was an extraordinary and unexpected appearance, and seemed to demand investigation.” Over the next few months, Fleming did exactly that, growing cultures of the mold and studying how the mysterious yellow substance it released affected other types of bacteria. He soon realized that the mold was a specific type of Penicillium, and that the substance it released was able to inhibit or kill not just staphylococcus, but many other types of bacteria. A few months later, in 1929, he named the substance “penicillin” and published his first paper about its remarkable properties.
What made penicillin so special? First of all, unlike the lysozyme he had discovered a few years earlier, penicillin stopped or killed many types of bacteria known to cause important human diseases, including staphylococcus, streptococcus, pneumococcus, meningococcus, gonococcus, and diphtheria. What’s more, penicillin was incredibly potent. Even in its relatively crude form, it could be diluted to 1 part in 800 before losing its ability to stop staphylococcus bacteria. At the same time, penicillin was remarkably nontoxic to the body’s cells, including infection-fighting white blood cells.
But apart from penicillin’s antibiotic properties, perhaps most amazing was that Fleming discovered it at all. For despite Fleming’s own long-held belief, the mold that produced his penicillin did not come from a random spore that happened to drift in through an open window in his laboratory and land on his culture plate one summer day. As evidence later showed, the arrival of that particular mold spore, the timing of Fleming’s vacation, and even local weather patterns all conspired in series of eerie coincidences.
The curious mysteries of a migrant mold
The mystery first came to light when, decades later, a scientist who worked in Fleming’s department in the late 1920s recalled that the windows of the laboratory in which Fleming worked were usually closed—partly to prevent culture dishes often left on the window sill from falling onto the heads of people walking by on the street below.
If the mold spores didn’t drift in from outside, where did they come from?
As it turns out, the laboratory in which Fleming worked was located one floor above the laboratory of a scientist named C. J. La Touche. La Touche was a mycologist, a fungi specialist, whose “messy” laboratory just happened to include eight strains of Penicillium mold, one of which was later found to be identical to Fleming’s mold. But with the windows closed, how did La Touche’s mold spores find their way upstairs and into Fleming’s culture dish? In another bit of unlikely fortune, Fleming’s and La Touche’s laboratories were connected by a stairwell whose doorways on both levels were almost always open. Thus, the spores from La Touche’s laboratory must have found their way up the open stairwell and onto Fleming’s culture plate. Not only that, but the spores had to have appeared at the exact moment when Fleming had removed the lids of his culture plates, either while he was inoculating them with staphylococcus bacteria or perhaps when he was inspecting them under a microscope.
But the serendipity of Fleming’s discovery does not end there. Initially, other scientists were unable to repeat Fleming’s experiment when their samples of penicillin, strangely, had no effect on staphylococcus bacteria. This mystery was later solved when scientists realized that penicillin can only stop bacteria while they are still growing. In other words, penicillin has no effect on bacteria once they’re fully developed, and the same is true in the body: Whether in the blood or other tissues, penicillin only works against growing bacteria. Which raises another question: How did Fleming’s random mold spores manage to germinate and produce penicillin with the exact timing needed to kill the staphylococcus bacteria while they were still growing?
In 1970, Ronald Hare, a professor of bacteriology at the University of London, proposed an extraordinary yet plausible explanation. Researching the weather and temperature conditions at the time of Fleming’s vacation, Hare found that Fleming’s culture was probably exposed to the mold spores in late July when the temperature was cool enough for the spores to germinate and produce penicillin. Later, weather records showed that the temperature warmed enough for the staphylococcus colonies to grow, just when mold was sufficiently mature to release its penicillin and kill the nearby and still-growing bacteria. If the temperature patterns had been different, the mold might have released its penicillin too late—after the bacteria had stopped growing—and would have been impervious to its antibiotic effects. And Fleming would have seen nothing “funny” about his culture plate when he returned from vacation.
Finally, how likely was it that the spores that happened to land on Fleming’s culture were from a penicillin-producing mold and not some other random fungus? While it might seem fairly likely given that the spores came from the nearby laboratory of a fungi specialist, consider this: In the 1940s, scientists undertook an intensive search to find other molds that were as good as Fleming’s mold at producing penicillin. Of the approximately 1,000 mold samples they tested, only three—Fleming’s and two others—were high-quality producers of penicillin.
* * *
Alexander Fleming’s discovery of penicillin in 1928 is considered the launching point for the revolution in antibiotics—but you’d never know it from the amount of attention it received over the following 10 years. Although some scientists read his 1929 paper and were intrigued, and a few physicians tried it in a handful of patients, penicillin was soon all-but-forgotten. As Fleming later explained, he himself was discouraged by several obstacles. First, penicillin was unstable and could lose its potency within days. Second, Fleming didn’t have the chemical knowledge to refine it into a more potent form. Finally, Fleming’s clinical interest may have been stifled by his physician peers, who were underwhelmed by the idea of treating their patients with a yellow substance made from a moldy broth. And so Fleming soon abandoned penicillin and turned his attention to other work.
Although it would be nearly a decade before penicillin would be “rediscovered,” in the meantime two other milestones occurred. One of these was the first authenticated “cure” with penicillin—a milestone achievement by a physician whose name almost no one knows today.
Milestone #2 Like nobody’s business: the first successful (but forgotten) cures
Dr. Cecil George Paine was a student at St. Mary’s Hospital when he first became intrigued by a lecture Fleming gave and his 1929 paper on penicillin. A few years later, while working as a hospital pathologist, Paine decided to try it out for himself. Around 1931, he wrote to Fleming and asked for cultures of his Penicillium mold and, after Fleming complied, soon produced his own samples of crude penicillin. All he needed now were some patients. As Paine later recounted, “I was friendly with one of the eye men, so I asked if he’d like to try out its effects.”
The “eye man,” Dr. A. B. Nutt, was an assistant ophthalmic surgeon at the Royal Infirmary and apparently the trusting type. He let Paine administer his penicillin treatment in four newborn babies with ophthalmia neonatorum, an eye infection caused by exposure to bacteria in the birth canal. According to the records, one three-week-old boy had a “copious discharge from the eyes” and one six-day-old girl had eyes that were “full of pus.” Paine administered his penicillin and recalled later that, “It worked like a charm!” Three of the babies showed significant improvement within two or three days. What’s more, Paine later administered penicillin to a mine worker whose lacerated eye had become infected, and “It cleared up the infection like nobody’s business.”
But despite these historic first cures, Paine abandoned penicillin when he was transferred to another hospital and began pursuing other career interests. He never published his findings and did not receive credit for his work until much later. When once asked where he placed himself in the history of penicillin, Paine replied regretfully, “Nowhere. A poor fool who didn’t see the obvious when it was stuck in front of him... It might have come on to the world a little earlier if I’d had any luck.”
But if even Paine had published his findings in the early 1930s, was the world even ready for the idea of an “antibiotic” drug? Many historians don’t think so because the concept was too novel. After all, how could a drug kill the bacteria that caused an infection, while not harming the patient’s own cells? The medical world was simply not ready, and might not have been, were it not for another key milestone in 1935.
Milestone #3 Prontosil: a forgotten drug inspires a world-changing breakthrough
With penicillin shelved and forgotten by the early 1930s, scientists were investigating a variety of even stranger candidates they hoped could be used to defeat infections. Indeed, some you would sooner expect to find streaming through the iron pipes of a factory than the blood vessels of a human. But, in fact, the idea of using chemicals to treat disease had proven itself in 1910 when Paul Ehrlich—the scientist whose theory of cell receptors in 1885 helped illuminate the immune system and how vaccines work—applied his knowledge of industrial dyes to develop an arsenic-based drug called Salvarsan. Salvarsan was a big hit: The first effective treatment for syphilis, it soon became the most prescribed drug in the world.
But after Salvarsan, and up until the early 1930s, scientists had little luck with the use of chemicals to treat infections. One good example of a bad idea was the attempt to use mercurochrome to treat streptococcal infections. Today, the red liquid antiseptic is applied externally to disinfect skin and surface wounds, but in the 1920s some thought infections could be cured with intravenous injections of mercurochrome. Fortunately, not everyone bought into this notion: In 1927, a group of researchers argued that any recovery seen in patients injected with mercurochrome was not due to its antibiotic properties, but the patient’s subsequent “formidable constitutional disturbances” and “violent purging and rigors.”
The push in the 1930s to find any antibiotic compound, industrial chemical or otherwise, was understandable. At that time, before the discovery of antibiotics, many infections could quickly turn deadly, including common streptococcal infections, such as strep throat, scarlet fever, tonsillitis, various skin infections, and puerperal (childbirth) fever. The horror of a spreading and unstoppable infection is easily recalled from the story of Mary Wollstonecraft and her agonizing death in 1797 shortly after childbirth (Chapter 3). But though Semmelweis’ work in the 1840s eventually helped reduce the incidence of childbirth fever, streptococcal infections remained common and dangerous, particularly if they spread to the blood.
And so it was in this context that, in 1927, German scientist Gerhard Domagk began working in the laboratory of I. G. Farbenindustrie in search of industrial compounds that could fight streptococcal infections. Finally, on December 20, 1932, after testing many chemicals used in the dye industry, Domagk and his associates came upon a chemical from a group of compounds known as sulphonamides. They tested it in the usual way, injecting a group of mice with fatal doses of streptococci bacteria and then, 90 minutes later, giving half of them the new sulphonamide compound. But what they discovered four days later, on December 24, was most unusual. While all of the untreated mice were dead from the streptococci bacteria, all of the sulphonamide-treated mice were still alive.
The miracle new drug—later named Prontosil—was soon famous throughout the world. As scientists soon discovered, unlike any previous drug they’d tested, Prontosil could be taken internally not only to treat streptococcal infections, but also gonorrhea, meningitis, and some staphylococcal infections. Soon, other sulphonamide drugs—commonly called “sulfa drugs”—were found, and though none were as effective as Prontosil, in 1939 Domagk was awarded the Nobel Prize in Physiology or Medicine for his work.
Looking back now at the Nobel presentation speech in honor of Domagk’s achievement, one can’t help noticing an odd disconnect. True, Domagk was properly credited for the “Thousands upon thousands of human lives... being saved each year by Prontosil and its derivatives.” But some of the presenter’s words seemed to speak to another, even greater, milestone yet to come, particularly when he referred to “a discovery which means nothing less than a revolution in medicine” and “a new era in the treatment of infectious diseases.”
But even though Domagk’s milestone would soon be overshadowed by penicillin, Prontosil is now widely recognized for opening the medical world to a new way of thinking: It was possible to create drugs that stopped bacterial infections without harming the body. And, in fact, Domagk’s discovery later helped prod other scientists to take another look at a drug that had been abandoned a decade earlier. As Alexander Fleming himself once noted, “Without Domagk, no sulphonamides; without sulphonamides, no penicillin; without penicillin, no antibiotics.”
Milestone #4 From bedpans to industrial vats: the revolution finally arrives
In the late 1930s, two researchers at Oxford University in England began studying the properties of Fleming’s antibiotic discovery—not penicillin, but lysozyme, the natural antibiotic Fleming had found in tears and other body fluids several years before the discovery of penicillin. Although the two researchers, German biochemist Ernst Chain and Australian pathologist Howard Florey, were impressed by lysozyme’s ability to dissolve bacterial cell walls, by 1939 they had finished their work and were ready to move on. But before writing up their final paper, Chain figured he should take one final look at the literature, and that was when he came across an obscure paper written by Fleming in 1929. Chain was intrigued by what he read about penicillin—not because he was dreaming of antibiotic miracle drugs, but its unique ability to break down bacterial cell walls.
Chain persuaded Florey that they should take a closer look at penicillin, though that was easier said than done: Could they even find a sample of the mold nearly a decade after Fleming had abandoned his experiments? But though Fleming’s original mold was long gone, Florey and Chain didn’t have to look far for its offspring. By coincidence, another staff member at the school had previously obtained a sample from Fleming and had kept it growing ever since. “I was astounded at my luck,” Chain later recalled upon learning of the mold. “Here, and in the same building, right under our noses.”
Chain began studying the mold, and by early 1940, he applied his background in biochemistry to achieve something Fleming had been unable to do: He produced a small amount of concentrated penicillin. Indeed, compared to the “crude” penicillin that Fleming had given up on—which inhibited bacteria at dilutions of 1 part per 800—Chain’s concentrated extract was 1,000 times more potent and able to stop bacteria in dilutions of 1 part per million. And yet, amazingly, it was still nontoxic to the body.
Well aware of the recent success of Prontosil and the new hope that drugs could be used to treat infections, Chain and Florey quickly changed their research goals. Penicillin was no longer an abstract curiosity in their study of bacterial cell walls, but a potent antibiotic, a therapeutic drug that might be used to cure human diseases. Excitement began to build as Chain and Florey made plans to test their newly potent penicillin in animals. On May 25, 1940, eight mice were given a lethal dose of streptococci, after which four of the mice were given penicillin. The researchers were so excited that they stayed up all night, and by 3:45 a.m., they had their answer: All of the untreated mice were dead, while the mice that received penicillin were still alive.
But once again the researchers were confronted with an obstacle: It had taken Chain considerable time and effort to produce the tiny quantities needed to treat four mice; how could they possibly make enough penicillin for humans? Focusing on the immediate goal of treating just a few people in a clinical trial, research associate Norman Heatley soon found a creative solution. He procured bedpans—hundreds of them—in which the mold could be grown and then used the silk from old parachutes—suspended from a library bookcase—to drain and filter the moldy broth. Chain then chemically extracted penicillin using the methods he’d developed. By early 1941, they had enough penicillin to treat six patients severely sickened by staphylococcus or streptococcus infections. The researchers gave five of the patients intravenous penicillin and one (an infant) oral penicillin. Although one patient eventually died, the other five responded dramatically.
But once again the researchers’ excitement was tempered by a seemingly insurmountable challenge: How could they now produce enough penicillin for a larger trial, much less thousands of patients worldwide? By this time, mid-1941, word about the initial trials of penicillin was spreading quickly. It wasn’t just that a new antibiotic had been discovered, but that penicillin seemed much more promising than Prontosil and other sulphonamides. As The Lancet pointed out in August, 1941, penicillin had a “great advantage” over Prontosil because it not only could fight a greater variety pathogenic bacteria, but it was not affected by pus, blood, or other microbes—exactly what you needed in a drug for treating infected wounds.
Yet, given the production limits of bedpans and old parachutes, Florey and Chain still had to figure out how to make large quantities of penicillin. Unfortunately, British pharmaceutical companies were unable to help, as their resources were “stretched to the limit” by Britain’s involvement in World War II. And so in June, 1941, Florey and Heatley set out for the United States to seek help from American government and business. Within six months, thanks to good connections and good fortune, Heatley found himself in a laboratory in Peoria, Illinois. Not just any laboratory, but a Department of Agriculture fermentation research laboratory, with the capacity to “brew” an estimated 53,000 gallons of mold filtrate. While hardly enough to treat thousands of patients—100 was closer to the mark—it was far better than the three gallons per hour Heatley had been making back in England.
At this time serendipity appeared twice more in the story of penicillin. First, the Peoria researchers found they could increase penicillin production by about ten times if they augmented the fermentation process with corn steep—a byproduct of cornstarch production that, at the time, could only have been found in the Peoria facility. Then, in another stroke of good fortune, a worker found some mold that, by chance, was growing on a rotting cantaloupe and that produced six times more penicillin than Fleming’s mold.
With fate and fortune working their magic on both sides of the Atlantic, pharmaceutical companies in America and Britain were soon producing enough penicillin to treat soldiers wounded in World War II—from simple surface wounds to life-threatening amputations. In fact, the increased production was remarkable. In March, 1942, there was barely enough penicillin to treat a single patient; by the end of 1942, 90 patients had been treated; by August, 1943, 500 patients had been treated; and by 1944, thanks to “submerged fermentation” technology developed by Pfizer, there was enough penicillin to treat all of the soldiers wounded in the Invasion of Normandy, as well as a limited number of U.S. civilians.
The discovery of antibiotics—and the antibiotic revolution—had finally arrived. But who was the first patient in the United States to actually be saved by penicillin?
Milestone #5 “Black magic”: the first patient to be saved by penicillin
In March, 1942, 33-year-old Anne Miller lay dying in Yale-New Haven hospital from a serious streptococcal infection that had spread through her body following a miscarriage. For the past month, doctors had tried and failed to cure her with drugs, surgery, and transfusions. Now, as her condition began to worsen, Miller was slipping in and out of consciousness, and she was not expected to live much longer. That was when her private physician, Dr. John Bumstead, came up with an idea that might just save her life.
Bumstead had been reading about a new drug for treating bacterial infections. He was well aware that only tiny quantities of penicillin had been produced so far, but he also knew one other critical piece of information: Another physician in the hospital, Dr. John Fulton, happened to be an old Oxford friend of one of the few people in the world who might be able to obtain some of the drug: Howard Florey. Oddly enough, Fulton himself lay in a nearby hospital bed, suffering from a severe pulmonary infection. Nevertheless, intent on saving his patient, Bumstead approached the ailing physician and asked if he could somehow persuade Florey to send some of the rare drug. Despite his feeble condition, Fulton agreed and began working the phone from his hospital bed. Persistence and patience paid off, and on Saturday, March 14, a small package arrived in the mail. Inside was a vial containing a pungent, brown-red powder.
As a group of physicians gathered around the small quantity of penicillin, it wasn’t clear exactly what they should do with it. After some discussion, they decided to dissolve it in saline and pass it through a filter to sterilize it. Then, bringing it to the bedside of the dying Anne Miller, they gave her an IV injection of 5,000 units. A medical student then gave her subsequent doses every four hours. Prior to that first treatment on Saturday, Miller’s temperature was spiking close to 106 degrees Fahrenheit. But with penicillin now coursing through her blood, the effect was immediate and dramatic: Overnight, Miller’s temperature dropped sharply. By Monday morning, her fever had plunged to 100 degrees, and she was eating hearty meals. When doctors came to her bed for morning rounds, one senior consultant looked at her temperature chart and was heard to mumble, “Black magic...”
Miller’s treatment continued for several months, until her temperature stabilized and she was released. Rescued from her death bed, Miller lived another 57 years, finally passing away in 1999 at the age of 90. While the physicians who treated her in 1942 couldn’t know that this remarkable new drug would enable her to live so long, Miller’s recovery did have one immediate impact. News of her recovery helped inspire American pharmaceutical companies to dramatically ramp up their production of penicillin, from 400 million units in the first five months of 1943 to 20.5 billion units in the next seven months—an increase of 500 times. By 1945, penicillin was being churned out at a rate of 650 billion units a month.
* * *
While the discovery of penicillin, from moldy culture plates in England to giant fermentation vats in Peoria, had its share of serendipity, calculated hard work also played a role in the discovery of antibiotics. In particular, the focused efforts of two highly diligent organisms, bacteria and humans, led medicine into the next era—an era where antibiotics seemed to literally—in fact, did literally—spring from the ground.
Milestone #6 The battle in the soil: discovery of the second antibiotic (and third and fourth and...)
Dirt: Is there anything simpler, cheaper, or more ubiquitous? We sweep, scrape, and wash it away with a disdain for something whose value is so negligible that it has become the measly standard against which all “dirt cheap” things are compared. Yet to Selman Waksman, dirt held a fascination that dated back to 1915, when he became a research assistant in soil bacteriology at the New Jersey Agricultural Experiment Station. To Waksman, dirt was nothing less than a vast universe populated by a wealth of richly important inhabitants.
Waksman wasn’t just interested in the role that microscopic bacteria and fungi play in breaking down plant and animal matter into the organic humus that enables plants to grow. Rather, it was the battle that microbes in the soil continually wage against each other and the chemical weaponry they produced to wage those wars. Scientists had known about this microbial warfare for years—it was, you’ll recall, why Vuillemin coined the term “antibiosis” in 1889. But what intrigued Waksman was not just that bacteria were continually fighting each other, but that previous findings had shown that something in the soil was able to kill one specific bacteria: tubercle bacillus, the bacteria that causes tuberculosis. By 1932, Waksman had shown that whatever that “something” was, it seemed to be released from other bacteria during their ongoing warfare in the soil.
And so in 1939, as other scientists across the Atlantic were taking a second look at a penicillin-inducing mold, Waksman and his associates at Rutgers University in New Jersey began studying dirt and its microbes, hoping that one of them might produce a substance useful in fighting tuberculosis and other human infections. But unlike Fleming’s work, serendipity would play no role in Waksman’s laboratory. Focusing on a large group of bacteria known as actinomycetes, Waksman’s team began a rigorous and systematic investigation in which they looked at more than 10,000 different soil microbes. Their efforts were soon rewarded with the discovery of two substances that had antibiotic properties—actinomycin in 1940 and streptothricin in 1942. While both turned out to be too toxic for use in humans, in September, 1943, Albert Schatz, a PhD student in Waksman’s lab, hit “pay dirt” when he found two strains of Streptomyces bacteria that produced a substance that could stop other bacteria cold. And not just any bacteria, but tubercle bacillus—the microbe that caused tuberculosis.
The new antibiotic was named streptomycin, and in November, 1943, just weeks after Schatz’s discovery, Corwin Hinshaw, a physician at the Mayo Clinic, requested a sample to test in animals. It took five months to get the sample, and it was barely enough to treat four guinea pigs, but it was well worth the wait: The effect of streptomycin on tuberculosis was “marked and striking.” Now all Hinshaw needed was a guinea pig of a different sort.
In July, 1943, as she was being admitted to the Mineral Springs Sanitarium in Goodhue County, Minnesota, 19-year-old Patricia Thomas made a confession to her physician: She often spent time with a cousin who had tuberculosis. Her physician was hardly surprised. Thomas herself had now been diagnosed with “far-advanced” tuberculosis, and was deteriorating rapidly. Over the next 15 months, a cavity would form in her right lung, an “ominous” new lesion would appear in her left lung, and she would develop a worsening cough, night sweats, chills, and fever. And so, on November 20, 1944, a year after his success with the rodent, Dr. Hinshaw asked Thomas if she was willing to act as a guinea pig of another sort and become the first human with tuberculosis to be treated with streptomycin. Thomas accepted, and it turned out to be a wise decision. Within six months, she had a rapid—some would say miraculous—recovery. Treatment was stopped in April, 1945, and subsequent X-rays showed significant clearing of disease. Rescued from impending death, Thomas eventually married and gave birth to three children.
Although streptomycin proved to be far from perfect, it was a major milestone in the development of antibiotics. For one thing, like penicillin, streptomycin could fight bacteria in the presence of pus or body fluids. But equally important, streptomycin gave physicians a tool they had never had before: the first effective treatment for tuberculosis. As noted when Waksman received the 1952 Nobel Prize for the discovery, streptomycin had a “sensational” effect on two frequently deadly forms of tuberculosis. In the case of tuberculosis meningitis, an “always fatal” form in which TB bacteria infect the membrane that covers the brain and spinal cord, streptomycin treatment “can be dramatic... Patients that are unconscious and have a high fever may improve rapidly.”
Within a few years, streptomycin was known across the world, quickly emerging from a laboratory curiosity to a pharmaceutical best seller being produced at a rate of more than 55,000 pounds a month. Waksman later wrote that the fast rise of streptomycin was partly due to the success of penicillin between 1941 and 1943. Yet streptomycin was a major milestone in its own right, curing thousands of patients with TB who would not have been helped by penicillin. By the end of the 1950s, streptomycin had lowered the death rates of TB in children in some countries by as much as 90%. And streptomycin was only the beginning. Between 1940 and 1952, Waksman and his associates isolated a number of other antibiotics—including actinomycin (1940), clavacin (1942), streptothricin (1942), grisein (1946), neomycin (1948), fradicin (1951), candicidin (1953), and candidin (1953)—though streptomycin and neomycin proved to be the most useful for treating infections.
Waksman also earned one other claim to fame. In the early 1940s, as scientists were publishing more and more papers about “bacteria-fighting” substances, it occurred to Dr. J. E. Flynn, editor of Biological Abstracts, that the world needed a new word to call these substances. Flynn asked several researchers for suggestions and considered such terms as “bacteriostatic” and “antibiotin.” But Flynn finally settled on a word Waksman had proposed in 1941 or 1942. “Dr. Waksman’s reply came back,” Flynn later recalled, “and this was the first time I had ever seen the word used in its present sense... as a noun.” First used in Biological Abstracts in 1943, Waksman’s new word—“antibiotic”—is now one of the most recognized terms in medicine throughout the world.
Antibiotics today: new confidence, new drugs, new concerns
Since their discovery in the 1940s, antibiotics have transformed the world in many ways—good, bad, predictable, and unpredictable. Today, it’s difficult to imagine the fear patients must have felt prior to the 1940s, when even minor injuries and common diseases could erupt into fast-spreading deadly infections. With antibiotics, physicians were suddenly empowered with the most satisfying tools imaginable—pills, ointments, and injections that could dramatically save lives.
Yet some also claim that antibiotics have a brought out a darker side of human nature, the mere knowledge of their availability creating a sense of false confidence and a willingness to indulge in risky behaviors. For example, antibiotics may have helped create a society more focused on the convenience of treatment, than the hard work of prevention. Equally alarming, some claim antibiotics have contributed to an increase in immoral behaviors, as seen in the epidemics of sexually transmitted diseases. Finally, even though antibiotics have saved millions of lives, it’s important to remember that they are not available to everyone and do not always work: Each year, as many as 14 million people around the world still die from infections.
* * *
Although many antibiotics have been discovered since the 1940s—90 antibiotics were marketed between 1982 and 2002 alone—it’s helpful to remember that all antibiotics share one common principle: the ability to stop the infecting microbe without harming the patient’s own cells. They achieve this by capitalizing on a vulnerability found in the microbe but not in human cells. Based on this principle, antibiotics generally fall into one of four categories:
- Folate antagonists—Prontosil and other sulfa drugs in this category prevent bacteria from synthesizing folic acid, a substance they need to grow and replicate.
- Inhibitors of cell wall synthesis—Penicillin and related drugs in this group prevent bacteria from making their cell walls.
- Protein synthesis inhibitors—Streptomycin, neomycin, tetracycline, and many others in this category target ribosomes (tiny structures inside bacteria that make proteins).
- Quinolone antibiotics—Often used to treat urinary tract infections, these agents inhibit an enzyme bacteria needed to replicate their DNA.
How do physicians choose an antibiotic among the many available drugs? One key factor is whether the infecting bacteria have been identified and are known to be sensitive to a specific antibiotic. Other factors include the location of the infection, patient health (a healthy immune system is needed to help defeat the infection), side effects, and cost. But perhaps the first and most important question is whether an antibiotic should be used at all. Even back in 1946, Fleming cautioned that penicillin has no effect on “cancer, rheumatoid arthritis... multiple sclerosis, Parkinson’s disease... psoriasis, and almost all the viral diseases, such as small pox, measles, influenza, and the common cold.” If some of these seem laughably obvious, consider that Fleming added, “These are merely some of the diseases of many suffers who, in the past two years, have as a result of press reports written to me for relief.”
Unfortunately, improper use still hovers like a dark cloud over one of the ten greatest discoveries in medicine. The issue centers around the emergence of resistance—the ability of bacteria to adapt, survive, and thrive despite treatment with an antibiotic—which can occur when antibiotics are improperly used. Bacteria are notoriously clever at developing resistance, for example by acquiring genetic mutations that protect them against the drug or by producing enzymes that inactivate the drug. As bacteria pass on such traits to new generations, they can evolve into “superbugs” that are resistant to multiple antibiotics and that transform once-treatable diseases into potentially deadly infections. While natural processes play a role in resistance, it’s now clear that careless and inappropriate use by humans is a major factor.
Mis-use and neglect: an age-old problem causes a brand-new danger
The warning signs were evident as early as the 1940s, when the presenter of the Nobel Prize to Selman Waksman cautioned that one complication already seen in the treatment of tuberculosis was “the development of strains of bacteria that become more and more resistant to streptomycin...” Other warnings about resistance appeared in the 1950s and early 1960s, when Japanese physicians reported an epidemic of bacterial dysentery that had become resistant to streptomycin, tetracycline, and chloramphenicol. And in 1968, doctors reported the first outbreak of bacterial infections that were resistant to methicillin and other penicillin-type drugs. Since then, those resistant bacteria—called methicillin-resistant Staphylococcus aureus, or MRSA—have become a worldwide concern.
S. aureus is a common bacteria found on the skin and normally quite harmless, even when it enters the skin through a cut or sore and causes a local infection such as a pimple or boil. But in people with weakened immune systems, such infections can turn deadly if they spread to the heart, blood, or bone, and particularly if antibiotics lose their effectiveness against them. Unfortunately, this is exactly what began to happen in the 1970s when MRSA began appearing in hospitals and killing as many as 20 to 25% of the people it infected. Worse, in the past decade MRSA has begun to venture outside hospitals, with so called “community-acquired” MRSA outbreaks now occurring in prisons, nursing homes, and school sports teams. A recent article in the New England Journal of Medicine found that emerging strains of MRSA are now showing resistance to vancomycin, another important antibiotic. The authors wrote that the problem stems not only from improper use of antibiotics, but is “exacerbated by a dry pipeline” for new antibiotics. They concluded that “a concerted effort on the part of academic researchers and their institutions, industry, and government is crucial if humans are to maintain the upper hand in this battle against bacteria—a fight with global consequences.”
Describing the development of new antibiotics as a “dry pipeline” may seem odd given how many have been produced since the 1940s, but as it turns out, most commonly used antibiotics today were discovered in the 1950s and 1960s. Since then, pharmaceutical companies have mostly tweaked them to create new chemical variations. But as one author pointed out in a recent issue of Biochemical Pharmacology, “It is still critically important to find new antibiotic classes [given] the increasing incidence of resistant pathogens. If we do not invest heavily in discovering and developing new antibiotic classes, we might well end up in a situation akin to the pre-antibiotic era...”
While some have hoped that biotechnology would lead to revolutionary new antibiotics, so far such technologies have produced limited advances at best. For this reason, other researchers suggest that we indeed may need to return to the “pre-antibiotic” era by taking a harder look at the natural world, the microbes that have been making antibiotics for far longer—a half-billion years or so—than humans.
Overcoming resistance: a way out through the past?
Given that two-thirds of our current antibiotics already come from Streptomyces bacteria, some might wonder if it really makes sense to continue to investigate “natural resources” for new antibiotics. But in fact, we have barely seen the tip of the iceberg.
How big an iceberg? In a 2001 issue of the Archives of Microbiology, researchers made an eye-opening claim: They found that Streptomyces bacteria, which include 500 or more separate species, may be capable of producing as many as 294,300 different antibiotics. If you’re wondering how a group of one-celled organisms could be so productive, consider the genetic engines packed into these tiny one-celled creatures. In 2002, other researchers announced in Nature that they had decoded the entire genetic sequence of a representative species of Streptomyces, uncovering an estimated 7,825 genes. This was the largest number of genes found in a bacterium, and nothing to sneer at given that it’s about one-third the number found in humans. With that kind of genetic abundance, perhaps it’s not surprising that these microbial super-specialists, rather than putting their genes to work making multicellular arms, legs, and cerebrums, are capable of producing so many different antibiotics.
* * *
In the early 1980s, anthropologists uncovered the skeletons of an ancient group of people who died more than 1,000 years ago and whose remains were remarkably well-preserved. Conducting fluorescent studies, the scientists found evidence of the antibiotic tetracycline in their bones and postulated that it may have been produced by Streptomycetes bacteria present in the food that the people ate at the time. The researchers also speculated that the tetracycline in their food might account for the “extremely low rates of infectious diseases” found in these people.
No—we’re not talking about the villagers of Herculaneum in 79 AD, but a group of Sudanese Nubians who lived on the west bank of the Nile River a few hundred years later, in 350 AD. And the source of their dietary tetracycline was not dried pomegranates or figs, but wheat, barley, and millet grains they had stored in mud bins. The scientists speculated that the mud storage bins provided the ideal environment needed for Streptomycetes—which comprise up to 70% of the bacteria in the desert soils of Sudanese Nubia—to flourish. It was unclear whether the tetracycline found in the ancient Nubians was produced by the same species of Streptomyces that produced the tetracycline found in the people of ancient Herculaneum.
But that’s exactly the point. In an age of emerging resistance and potentially deadly infections, could this remarkable genus of bacteria—provider of antibiotics to ancient peoples, source of nearly a dozen antibiotics discovered in the 1940s and 1950s, producer of two-thirds of the current antibiotics in use today, and yet still barely tapped for their potential of nearly 300,000 antibiotics—could this remarkable genus of bacteria be trying to tell us something?
