310°F / 154°C: Maillard Reactions Become Noticeable
You can thank Maillard reactions for the nice golden-brown color and rich aromas of a Thanksgiving turkey, Fourth of July hamburger, and Sunday brunch toast. Coffee, cocoa, and roasted nuts all rely heavily on Maillard reaction byproducts for their flavors. If you’re still not able to conjure up the tastes brought about by Maillard reactions, take two slices of bread and toast them—one until just before it begins to turn brown, the second until it has a golden-brown color—and taste the difference.
The nutty, toasted, complex flavors generated by the Maillard reaction are created when amino acids in proteins and certain forms of sugars (called reducing sugars) combine and then break down. Named after the French chemist Louis Camille Maillard, who first described it in the 1910s, the Maillard reaction wasn’t well understood until the 1950s. During the reaction, compounds with free amino groups undergo a condensing reaction with reducing sugars. For example, meat has a reducing sugar, glucose (which is the primary sugar in muscle tissue), and also contains amino acids like lysine; with heat, these two compounds easily react with each other to form two new molecules.
The Maillard reaction is much more complicated than the other reactions we’ve talked about so far. One of the two new molecules generated at the start of the reaction is good ol’ H2O, but the other is a complicated, unstable molecule that quickly cascades through more reactions. That cascade of reactions eventually settles down into a few hundred compounds, and it’s these compounds that create the colors and flavors we want.
To complicate matters, the molecule generated by the first condensing reaction depends on which compounds start it all off. Any compound with a free amino group—amino acids, peptides, or proteins—can combine with any carbonyl compound (usually reducing sugars), so the initial starting molecule can take many, many forms. This is why the flavor byproducts from the Maillard reaction will be slightly different if you’re grilling a hamburger or baking bread; the ratios and types of the amino acids and reducing sugars (e.g., glucose, fructose, and lactose) present in the two foods are different. In yet another wrinkle, the byproducts break down into different compounds depending on the pH of the solution they’re in, shifting the flavors as well—it’s complex!
Now that I’ve made it sound really complicated (which it is), how can you control the Maillard reaction and its aromas and colors? Fortunately, understanding the Maillard reaction from a cook’s perspective is far easier than from that of a chemist. There are four ways you can control it, and understanding them requires a simple explanation of the chemistry rules around rates of reactions.
Obviously, a lack of either amino acids or reducing sugars prevents the reaction—both have to be present. There’s a standard chemistry rule: increasing the concentration of reactants increases the rate of reaction. This is why some breads call for milk as an ingredient, and why brushing an egg wash on top of pastry crusts adds color. Both proteins and lactose in milk and the amines in eggs will increase the quantity of reactants and thus generate more Maillard reaction–based flavors and colors. Without them, no dice. If you want more Maillard reactions to occur, the first way is to up the concentration of ingredients that lead to it.
Temperature is the basis of another chemistry rule related to reaction rates. The activation energy—the amount of energy needed for a chemical reaction to occur—is based on the kinetic energy of a molecule. With higher temperatures, there’s a much better chance that a molecule will bounce over the energy barrier necessary for the reaction to happen—but it’s still a probability. At lower temperatures, the reaction can still happen, but much more slowly. (Depending on the type of reaction, there can be a minimum threshold.) Assuming you have plenty of reactants around, upping the temperature of the environment cooking the food is the easiest way to speed up the reactions.
The pH of the environment, which affects so many things in food, also changes how the Maillard reaction happens. The initial step of the reaction depends on free amino groups, but those get tied up under more acidic conditions. This is why adding baking soda to onions speeds up their browning and why dipping pretzel dough in a lye solution makes them browner. There are very few alkalizing ingredients in the kitchen—egg whites, baking soda—but fortunately they’re mostly tasteless in small quantities. If you want to speed up browning on baked goods, try brushing an egg white wash on the surface of the dough; for foods like caramelized onions (a partial misnomer), add a pinch of baking soda to speed up the reaction.
The rate of Maillard reactions also depends on water: not too much or too little. The first step in the Maillard reaction generates a compound that’s easily reversible—it can go back and forth between two states (one of those ⇌ symbols in chemistry diagrams)—and in this case it’s the water molecule from that very first step that can be reabsorbed. When the water molecule is attached to the compound, it prevents the second step in the reaction from happening. If the environment is too wet, the probability of the water molecule staying attached to the compound increases, blocking the reaction from continuing. But if the environment is too dry, then the reaction doesn’t begin either—the amino acid and sugar have to be mobile enough to connect. (The peak for reaction rate, with respect to water, is around 0.6 to 0.7 aw, if you’re familiar with water activity; more practically, about 5% water.) It’s unlikely that changing the amount of water is the fix for any Maillard reaction rate problems you see, but it does explain the difference you can see in a baking test of wetted flour and dry flour cooked on the same sheet.
Maillard reactions won’t readily happen on wet foods. If you’re about to sear meat, give it a quick pat with a paper towel to blot away surface moisture. Adding salt to a lean cut of meat right before cooking it pulls moisture toward the surface, which then takes longer to evaporate away when cooked. Either salt meats well in advance and then pat them dry before cooking, or add salt after cooking.
Accounting for all these variables, in most culinary applications the delicious flavors and pleasing colors from the Maillard reaction still require moderately hot temperatures. The 310°F / 154°C temperature given here serves as a good guideline for when Maillard reactions will begin to occur at a noticeable rate, whether you’re looking through your oven door or sautéing on the stovetop. For most cooking, 350°F / 180°C is a reasonable temperature, either in the skillet or the oven, to develop these flavors. Recipes that call for extended cooking times, like a roast kept in an oven for many hours, can be cooked at 325°F / 160°C. It’s rare to see recipes call for lower oven temperatures than this because of how slowly the Maillard reaction will happen. Avoiding the Maillard reaction—it’s not a flavor you always want, such as in meringue cookies like macaroons (see page 294)—is simple enough: make sure one of water, pH, or temperature is out of the necessary range. Most often this means setting the oven to a very low temperature (say, 250°F / 120°C), which is exactly what’s done for meringue cookies.
We’ll cover the other major browning reaction, caramelization, in the next section, but it’s worth pointing out here that caramelization can deprive the Maillard reaction of the reducing sugars it needs. Searing meats in a too-hot pan will caramelize the glucose in the meat before it has a chance to react with amino acids, so when cooking meats, use a medium-high heat, but not too hot.
Maillard reactions do happen below my 310°F / 154°C guideline—just not very rapidly. Stocks simmering at 212°F / 100°C for many, many hours and with sufficient concentration of the reactants will begin to slowly turn brown and develop the Maillard reaction flavors. (Some chefs swear by pressure cookers for making stocks; higher temperature means faster Maillard reactions!) It’s even possible for Maillard reactions to happen at room temperature, given sufficient time and reagents: some aged cheeses like Manchego and Gouda have minor amounts of some of the Maillard byproducts. It happens elsewhere, too: self-tanning products happen to work via the same mechanism!
356°F / 180°C: Sugar Quickly Caramelizes

Temperatures related to sucrose and baking.
Caramel sauce: delicious, calorie-laden, and made by the simple act of heating sugar. Unlike the Maillard reaction, which is named for the chemist who first described the reaction, caramelization is named for the end result. The word caramelization comes from the 17th-century French for “burnt sugar,” originally Late Latin for “cane” (canna or calamus) and “honey” (mel)—a good visual description of melted, browned sugar!
There are a couple of different ways to burn sugar (besides getting distracted while cooking). The simplest is with dry heat: sugar in a dry pan will thermally decompose—literally, breaking down under heat. In the case of sucrose, the molecular structure will break apart and go through a series of reactions that create over 4,000 different compounds. Some of those compounds are brown (the best-looking tasteless polymerization reactions you’ve ever seen!), while others smell wonderful (you can thank fragmentation reactions for these, as well as blaming them for some of the bitter tastes).
Heating sugar with water, as is done in wet-method caramel recipes, changes things slightly. When wet, sucrose will hydrolyze—a reaction that involves taking in water (hence “hydro”). In the case of sucrose, it hydrolyzes into glucose and fructose, called sucrose inversion. With heat, those molecules then rearrange their structure into another form that kicks off a water molecule and begins a chemical reaction journey. The hydrolysis of sucrose is a simple reaction. Even if you’re not up on your high school chemistry, you can see that the count of atoms on one side of the equation lines up with the count on the other side:
C12H22O11 |
+ |
H2O |
= |
C6H12O6 |
+ |
C6H12O6 |
Sucrose |
+ |
Water |
= |
Glucose |
+ |
Fructose |
This is how pastry chefs make invert sugar syrup! The concentration of sugar, temperature, and pH all speed up the reaction, so if you’ve ever seen a caramel recipe call for cream of tartar, that speeds up the conversion to glucose and fructose. And, because fructose has a lower caramelization temperature (more on that in a minute), a wet caramel sauce should, in theory, caramelize at lower temperatures and have a different chemical makeup than a dry one. The full chemistry of caramelization is still poorly understood—while researchers have been able to describe some of the reactions, the full pathway taken on the chemical reaction journey still has its mysteries.
Describing the temperatures for caramelization is also tricky because of the closeness between melting points and decomposition temperature ranges. Melting, a physical change, is not the same thing as decomposition, a chemical change. By definition, sucrose is a pure substance: it has a specific molecular structure. Pure sucrose melts at 367°F / 186°C, a state change where it goes from solid to liquid. Glucose, likewise, has a melting point of 294°F / 146°C; fructose comes in at a relatively cool 217°F / 103°C.
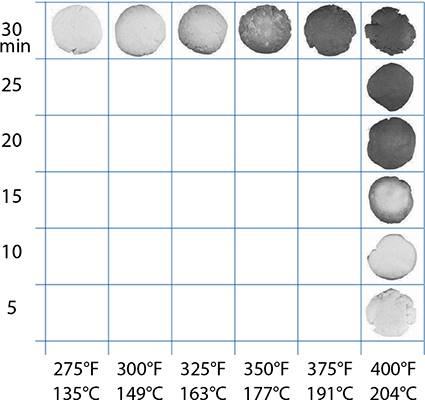
But these sugars begin to thermally decompose at temperatures lower than their melting points. The decomposition happens at a very, very slow rate at modest temperatures and begins to pick up to a noticeable rate as temperatures increase. For sucrose, that inflection point is somewhere around 338°F / 170°C—a good 30°F / 16°C below its melting point. If enough thermal decomposition happens before sucrose is heated to its melting point, granules of sugar will “apparently melt,” to use the phrase coined by the researchers. Heating a granule of table sugar—a bunch of sucrose molecules (with some impurities!) packed into a crystalline structure—to just below its melting point will cause some of the sucrose molecules to convert to other compounds via thermal decomposition. The granule of sugar is no longer a pure substance! This is why a granule of sugar “apparently” melts below its true melting point when heated slowly. Sugar, like everything else that makes up our food, is fascinating and complicated stuff.
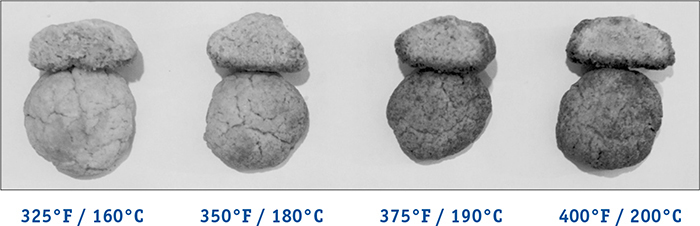
As for flavor, caramelization is like the Maillard reaction in that it generates thousands of compounds, and these new compounds result in both browning and enjoyable aromas. For some foods, these aromas, as wonderful as they might be, can overpower or interfere with the flavors brought by the ingredients. For this reason, some baked goods are cooked at 350°F / 177°C or even 325°F / 163°C so that they don’t see much caramelization, while other foods are cooked at 375°F / 191°C or higher to facilitate it. When cooking, ask yourself if what you are cooking is something that you want to have caramelized aromas, and if so, set your oven to at least 375°F / 191°C or extend the baking times out long enough for the reaction to occur. If you’re finding that your food isn’t coming out browned, it’s possible that your oven is running too cold, so adjust the temperature upward.
Does starch caramelize?
Not directly. Starch is a complex carbohydrate; caramelization is the decomposition of simple carbohydrates, a.k.a. sugars. Given time under heat, starch will break down into dextrin, which is a bunch of glucose molecules linked together. Dextrins are commonly used as adhesives—the stuff you lick on the back of an envelope—and are made by heating starch for many hours. Additional processing converts them to things like maltodextrin (see page 416), but almost all of the browning you see in cooked food comes from sugars (caramelization) and reducing sugars with amino acids (Maillard reactions). Starch can be broken down into glucose, which will caramelize, either via enzymatic reactions or hydrolysis, so there are exceptions. To see the difference, try baking a pinch of dry cornstarch, sugar, and flour alongside slightly wetted versions of each (to see how water changes things) on a lined cookie sheet at 375°F / 190°C for 10 minutes and investigating the results.
Temperatures of common baked goods, divided into those below and above the temperature at which sucrose begins to visibly brown. |
|
Foods baked at 325–350°F / 163–177°C |
Foods baked at 375°F / 191°C and higher |
Brownies |
Breads |
Chocolate chip cookies (chewy 10–12 minute cookies; see page 283) |
Peanut butter cookies |
Sugary breads: banana bread, pumpkin bread, zucchini bread |
Chocolate chip cookies (crispy 12–15 minute cookies; higher temperature means more evaporated water) |
Cakes: carrot cake, chocolate cake |
Flour and corn breads |