6
Playing with Chemicals
Chapter Contents
E Numbers: The Dewey Decimal System of Food Additives
Recipes
Bread-and-Butter Quick Pickles
Infused Oils and Herbed Butters
Interviews
Carolyn Jung’s Preserved Lemons
Hervé This on Molecular Gastronomy
HUMANS HAVE BEEN ADDING CHEMICALS TO FOOD FOR MILLENNIA. Salt preserves meats and fish, vinegar turns vegetables into pickles, and egg yolks form emulsions to create sauces like mayonnaise and hollandaise. The last few centuries have brought modern compounds, from alginates to vanillin, that are useful for both commercial and creative applications.
Food itself is made up of chemicals. Corn, chicken, and ice cream cones are just big piles of well-structured chemicals. A cook learns how to manipulate these piles of chemicals using all the techniques we’ve covered so far. But a talented cook has to also know how to manipulate the chemistry of food. Looking at food chemistry—the chemical makeup of ingredients and the changes that occur when they’re combined or processed—is a fun way to explore many cooking techniques. Every type of cook, from the most traditional home cook to the savviest industrial research chef, benefits from an understanding of the chemistry of ingredients.
How are foods structured? What’s happening to them when they’re combined or heated? How can you use a knowledge of chemistry to cook better food? And what creative new ideas can you come up with by understanding chemistry? Let’s look at some historical and modern techniques for chemically manipulating food.

Food Additives
Have you ever wondered how pickles, mayonnaise, or gummy candies are made? They’re not simple foods, at least in the sense of Mother Nature. The next time you’re standing in your kitchen, look at the various jars and packages of foods that you have. What gives those foods their textures and flavors? Invariably, the answer will involve ingredient chemistry. Vinegar pickles rely on acetic acid, mayonnaise wouldn’t exist without lecithin from egg yolks, and gummy candies use gelling agents like gelatin. How do these compounds work? And how can you change textures and flavors using similar compounds? This is where food additives come in.
To start, we should define what food additives are. The US FDA defines them as nonessential items that end up in food or somehow change it. (The full definition they give is a mouthful: they exclude items in use before 1958 or that industry determines are “generally recognized as safe.”) I’m going to use a more colloquial definition of food additive: any chemical with a definable molecular structure that’s used in food. Salt and sugar, by this loose definition, count. They’re chemical compounds—sodium chloride and sucrose, respectively—that can be used to change functional properties in foods. My definition also includes modern compounds like methylcellulose and transglutaminase, which we’ll cover later in the chapter. Food chemistry is much, much broader than food additives, of course. Still, looking at the different uses for food additives is a useful lens for understanding food chemistry.
Before looking at how food additives are used, I want to touch on politics. The use of chemicals in food is often misunderstood. I’m surprised how often the issue of safety is conflated with issues of how our food is produced. Our global food supply is driven by economics, ethics, and politics. These are not science topics, and I want to separate the issues out before diving into the science. (If the other topics interest you, see Marion Nestle’s excellent books What to Eat [North Point Press] and Food Politics [University of California Press].)
A brief digression on “generally recognized as safe,” normally abbreviated to “GRAS”: the US Food Additives Amendment of 1958 defines GRAS additives as compounds considered safe for the intended use based on review by a panel of qualified experts. The panel reviewing the additive is selected by industry, not government, and doesn’t have to disclose their research. In my opinion, the food industry will do better in the long run by communicating more openly about how our food is made and by disclosing everything. The distrust of industry has prompted a consumer aversion to chemicals and a preference for natural ingredients, but that has unintended consequences. “Natural” has no technical definition and isn’t the same thing as healthier! (The number of American consumers who push back on unfamiliar chemical ingredients while happily eating high-sugar, high-sodium foods shows the disconnect.) Anyway, enough with the tangent—let’s have some fun looking at the science of food chemistry!
One way to understand food additives is to look at the reasons they’re used commercially: to extend shelf life, to preserve nutritional value, to address dietary needs, and to aid in manufacturing at scale. Consider the current ingredient list for an Oreo cookie, a delicious marvel of the modern food world. Skipping sugar, cocoa, and salt, which are there for taste, everything else matches at least one of the four reasons:
Baking soda (a.k.a. sodium bicarbonate) and/or calcium phosphate
Aids in manufacturing by speeding up the baking process. Baking soda may seem traditional, but we’ve only used it in cooking since the late 1840s. (Any time you see “and/or” in an ingredient list, this is a tip-off that the manufacturer is choosing between the ingredients based on season, price fluctuations, or baking facility.)
Cornstarch (sometimes called “cornflour”)
Extends shelf life by stabilizing the food and acting as a humectant (something that retains moisture).
Enriched flour (wheat flour, niacin [B3], reduced iron, thiamin mononitrate [B1], riboflavin [B2], folic acid [B9])
Addresses dietary needs by adding in micronutrients that are removed during processing. Fortification is mandatory in over 50 countries; the US FDA requires that white flour be supplemented with B vitamins (to prevent various deficiencies) and iron (to prevent anemia, a low red blood cell count).
High oleic canola and/or palm and/or canola oil
Extends shelf life by providing fats that won’t go rancid as quickly as fats from butter and egg yolks. (“High oleic” refers to fatty acids; for more, see page 152).
Soy lecithin
Aids in manufacturing. A traditional recipe relies on egg yolks for lecithin, which acts as an emulsifier (see page 429), but because Oreos skip the eggs, lecithin needs to be added.
Vanillin (artificial flavor)
Aids in manufacturing at scale—the worldwide demand for vanilla flavoring far, far exceeds the available supply. (We’ll cover vanilla extract later in this chapter; see page 400.)
The Oreo has been around for over a century, but the recipe has shifted as newer additives replace older ones, most recently in 2006 when Nabisco switched from trans-based fatty acids to high oleic ones. Try making your own version: make butter cookies with cocoa powder (see page 224) and add a filling of 1 cup (120g) powdered sugar, 2–3 tablespoons (30–45g) butter, and ¼ teaspoon (1g) vanilla extract.
As you can see, some of the ingredients are compounds that home bakers wouldn’t normally add to their grocery lists: Soy lecithin? Vanillin? High oleic oil? But you’re probably already using some of these compounds, just not by these names. A quick look at a categorization system for food additives will help before we dive into their chemistry.
E Numbers: The Dewey Decimal System of Food Additives
It’s easy enough to find a recipe for cream-filled chocolate cookies, but how do you go about tweaking a recipe to solve certain challenges or create new foods? Heck, figuring out which food additives even exist can be a challenge. Looking at the back of a package of Oreos doesn’t begin to explain the range of possibilities.
The most commonly used index is compiled by the Codex Alimentarius Commission, a commission established by the United Nations and the World Health Organization that has created a taxonomy of food additives called E numbers. Like the Dewey Decimal classification system for books, E numbers define a hierarchical tree. A unique E number (totally unrelated to the number e, ~2.7182) is assigned for each chemical compound that’s approved for food usage in the European Union. E numbers are grouped by functional categories, with the numbering of chemicals determined by each chemical’s primary usage:
E100–E199: |
Coloring agents |
E200–E299: |
Preservatives |
E300–E399: |
Antioxidants, acidity regulators |
E400–E499: |
Emulsifiers, stabilizers, and thickeners |
E500–E599: |
Acidity regulators, anticaking agents |
E600–E699: |
Flavor enhancers |
E700–E799: |
Antibiotics |
E900–E999: |
Sweeteners |
E1000–E1999: |
Additional chemicals |
Many historical additives make an appearance in the list. Good ol’ vitamin C shows up (E300: ascorbic acid), as do vinegar’s acetic acid (E260) and cream of tartar (E334). Some synthetic compounds are listed too, such as propylene glycol (E1520); it’s the liquid in the nonalcoholic vanilla extract sold at your grocery store.
Your local grocery store will stock many of the additives covered in this chapter—pectin, gelatin, agar—but not everything. You can order others online. See http://cookingforgeeks.com/book/additives/ for a list of online suppliers.
Some compounds function in more than one way. Ascorbic acid, listed at E300, is also a preservative (200s) and a color fixer (100s). Lecithin (E322) is almost always culinarily used as an emulsifier (400s), but it’s also an antioxidant. Don’t think of additives as directly mapping to their categories; rather, the categories are a good framework to see the technical purposes for which the food additives are used.
Which additive to use for a particular purpose depends on the properties of the food and your specific goals. You can see some overlap between these categories and the types of colloids mentioned earlier. Some additives work in a broad pH range but are limited to certain temperatures, while others might handle narrower pH ranges but be fine with more heat. For example, agar is a strong gelling agent that can create gels for sweets, but with some ingredients it also exhibits syneresis—liquid weeping out of a gel. Carrageenan does not undergo syneresis but cannot handle an environment as acidic as agar can.
This chapter is loosely structured on these groupings, covering food additives that are common for the home cook, along with a few more playful items. There are a lot more out there, though, if you want to explore! For a full list of E-numbered additives, see http://cookingforgeeks.com/book/enumbers/.
Mixtures and Colloids
There’s one more concept we need to look at before investigating how chemicals interact with food. One of the biggest aha! moments for me in learning to cook was realizing that ingredients are not uniform, consistent things. I’m still learning examples of this—a slice of cucumber from the part nearest the blossom can contain a particular enzyme that turns pickles soft?!—but most ingredients don’t require knowing such trivia. The concept of mixtures and colloids explains why many foods react in more complicated ways than the simple rules of time and temperature would predict.
Very few foods are simple substances, chemically speaking. Water doesn’t even seem so simple to me anymore (even without trace minerals, H2O is complicated—see page 243). Vanilla extract and infused oils carry flavors in ethanol and fats. Jams balance sugars with acids to form gels. Mayonnaise is an emulsion of fats and water that aren’t truly mixed. Chocolate chip cookies are really complicated: pockets of syrupy, sugary liquid surrounded by a breadlike matrix that also has chocolate chips—cocoa solids blended into both liquid and solid cocoa fats. Ice cream gets exceedingly complicated.
To a food scientist, these are examples of mixtures and colloids. A mixture is two or more substances combined together, where the substances remain in their original chemical form. Sugar syrup is a mixture—the sucrose is dissolved in the water, but retains its sweet-tasting chemical structure. The combination of flour and baking soda is also a mixture. A colloid is a type of mixture; specifically, a combination of two substances—gas, liquid, or solid—where one substance is uniformly dispersed in the other, but the two aren’t dissolved together. In other words, the two compounds don’t associate with each other, even if the overall structure appears uniform to the naked eye. Sugar syrup is not a colloid (it’s a different type of mixture—a solution), but milk is, having solid fat particles that are dispersed throughout a water-based solution but that aren’t actually dissolved into the liquid.

Take a look at the table of colloid types. It shows the different combinations of particles and media, along with examples of foods for each colloid type. The medium of a colloid is called the continuous phase (e.g., the watery liquid in milk) and the particles are known as the dispersed phase (for milk, the fat droplets). Foods can be more complicated than this, though. Ice cream is a complex colloid—multiple types of colloids at once—being a water-based liquid containing pockets of air (foam), chunks of ice crystals (suspensions), and fats (emulsion) all at the same time. One surprise in this table is the relatively broad swath of techniques needed to create all these foods. Willy Wonka’s Invention Room would surely have a chart of it on one wall!
These techniques capture much more than traditional culinary techniques. The table is fertile ground for candy makers and experimental chefs alike, and it reveals the basis of many molecular gastronomy–inspired concepts. Whisking up fruit juice with the emulsifier lecithin creates foams that can be used to create a fun topping for an entrée or dessert. Flavorful liquids can be converted into forms that you can nibble, like gummy candies (often shaped like teddy bears or worms). An imaginative use of solid aerosols like smoke can convey intense aromas. Fear not: even if pushing the bounds of culinary possibilities isn’t your thing, most of the items in the table are still of great interest.
DISPERSED PHASE |
||||
Gas particles |
Liquid particles |
Solid particles |
||
CONTINUOUS PHASE |
Gas |
(N/A: gas molecules don’t have a collective structure, so gas/gas combinations either mix to create a solution or separate out due to gravity) |
Liquid aerosols • Mist sprays |
Solid aerosols • Smoke (e.g., when smoking foods) • Aerosolized chocolate |
Liquid |
Foams • Whipped cream • Whisked egg whites • Flavored foams • Ice cream (air bubbles) |
Emulsions • Milk • Mayonnaise • Ice cream (fat in water emulsion) |
Sols and suspensions • Commercial salad dressings • Ice cream (ice crystals and solid fats) |
|
Solid |
Solid foams • Bread • Marshmallows • Soufflés |
Gels • Butter • Cheese • Jelly/gummy candies • Jell-O |
Solid sols • Chocolate |
|
Preservatives
Ahh, salt: responsible for the salvation of many a food (or is that salivation?). The oldest chemical in use, salt was used in prehistoric times and there are records of its use for dry-curing hams in the third century BC by the Roman Cato the Elder. The use of sugar as a preservative wasn’t far behind; the Romans used honey to preserve foods as well. And another historical preservative is vinegar, used as an acidity regulator (sounds delicious when I put it that way, no?).
Chemical preservation has the fundamental purpose of preventing microbial growth. While there are plenty of other ways to preserve food, like smoking or drying, using chemicals doesn’t necessarily change flavors as much. Sausages, vinegar pickles, and fruit preserves all rely on chemicals to keep them safe for eating. Chemicals prevent microbial growth by either disrupting cells’ abilities to function, as nitrite does to sausage, or by changing any of the FAT TOM variables (see page 175) to be inhospitable, such as increasing acidity with vinegar or reducing moisture with sugar in fruit preserves.
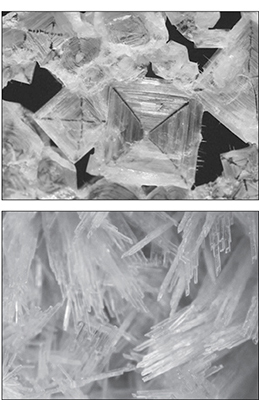
IMAGE COURTESY OF NASA
IMAGE COURTESY OF JUSTIN MEYERS
Different types of salt will form crystals in different shapes, based on the salt’s atomic crystalline structure. Sodium chloride’s crystalline structure is cubic, while potassium nitrate’s crystalline structure has a steep slant to it, creating needlelike crystals.
Salt’s ability to kill pathogens and preserve things isn’t limited to foods. For an adult human, the lethal dose of table salt is about 80 grams—about the amount in the saltshaker on your typical restaurant table. Overdosing on salt is reportedly a really painful way to go, as your brain swells up and ruptures. Plus, it’s unlikely the emergency room physicians will correctly diagnose the cause before it’s too late.
While the chemistry of preservatives may not seem important to everyday cooking, it’s revealing to understand how these ingredients work, and the basics of preservation apply to how most other food additives work. First, a quick refresher on a few definitions that’ll pop up throughout this chapter:
Atom
Basic building block of matter. By definition, atoms have the same number of electrons and protons. Some atoms are stable in this arrangement (e.g., helium), making them less likely to form bonds with other compounds (which is why you don’t see any compounds made of helium). Other atoms (e.g., sodium) are extremely unstable and readily react. A sodium atom (Na) will react violently with water (don’t try licking a sample of pure sodium—it’d ignite due to the water on your tongue), but when an electron is removed it turns into a delicious salty sodium ion (Na+).
Molecule
Two or more atoms bonded together. H = hydrogen atom; H2 = two hydrogen atoms, making it a molecule. When it’s two or more different atoms, it becomes a compound (e.g., H2O). Sucrose (a.k.a. sugar) is a compound with the composition C12H22O11—12 carbon, 22 hydrogen, and 11 oxygen atoms per molecule. Note that the composition doesn’t tell you what the arrangement of the atoms is, but that arrangement is part of what defines a molecule.
Ion
Any atom or molecule that’s charged—that is, where the numbers of electrons and protons aren’t equal. Because of the imbalance, ions can bond with other ions by transferring electrons to (or from) each other.
Cation
An atom or molecule that’s positively charged. Pronounced “cat-ion”—meow!—a cation is any atom or molecule that has more protons than electrons; it’s paw-sitively charged. For example, Na+ is a cation—an atom of sodium that has lost an electron, giving it more protons than electrons and thus a net positive charge. Ca2+ is a cation—a cation of calcium—that has lost two electrons.
Anion
An atom or molecule that’s negatively charged (i.e., one that has more electrons than protons). Cl– is an atomic anion—in this case an atom of chlorine that has gained an extra electron, giving it a net negative charge.
From these definitions, you’ll hopefully deduce that a lot of chemistry is about ions interacting with each other based on differences in electrical charges. Sodium chloride, common table salt, is a classic example: it’s an ionic compound composed of a cation and an anion. In solid form, though—the stuff in your salt shaker—salt is more complicated than one anion plus one cation. It takes the solid form of a crystal of atoms arranged in an alternating pattern (like a 3D checkerboard) based on charge: cation, anion, cation, anion. In water, the salt crystals dissolve and the individual ions are freed (disassociated). The anions and the cations separate out into individual ions, which can then react and form bonds with other atoms and molecules. That’s why salt is so amazing! Sucrose doesn’t do this.
Sodium chloride is one particular type of salt, made up of sodium (a metal, and one that in its pure form happens to react violently when dropped in water) and chloride (chlorine with an extra electron, making it an anion). There are many other types of salts, created with different metals and anions, and they don’t always taste salty. Monosodium glutamate, for example, is a salt that tastes savory and boosts the sensation of other flavors. Epsom salt—magnesium sulfate—tastes bitter.
Multiple types of salts are used to preserve foods. Salmon gravlax is cured with a large amount of sodium chloride, which preserves the fish by increasing osmotic pressure, dehydrating and starving living microbial cells of critical water as well as creating an electrolytic imbalance that poisons them. Many sausages, hams, prosciutto, and corned beef are cured using small quantities of sodium nitrite, which also gives these foods a distinctive flavor and pinkish color. Unlike gravlax, in which the sodium does the preserving, sodium nitrite works because of nitrite; the sodium is merely an escort for the nitrite molecule. Nitrites inhibit bacterial growth by preventing cells from being able to transport an amino acid, meaning they can’t reproduce. (Incidentally, nitrites are also toxic to us at high levels, for presumably the same reason; but without the nitrites, microbial growth would be toxic to us too—dosage matters!)
Sugar can also be used as a preservative. It works like sodium chloride, by changing the osmotic pressure of the environment (see page 386 for more on osmosis in food). With less available water, sugary foods such as candies and jams don’t require refrigeration to prevent bacterial spoilage. Think back to the M in the FAT TOM rule: bacteria need moisture for growth, and adding sugar reduces their ability to drink.
Sugar’s osmotic properties can be used for more than just preserving food. Researchers in the UK have found that sugar can be used as a dressing for wounds, essentially as a cheap bactericidal. The researchers used sugar (sterilized, please), polyethylene glycol, and hydrogen peroxide (0.15% final concentration) to make a paste with high osmotic pressure and low water activity, creating something that dries out the wound while preventing bacteria from being able to grow. Whoever thought of rubbing salt in a wound should’ve tried sugar!
Besides salts and sugar starving microbes of vital water, enzymatic inhibitors and acids are used to prevent their growth. Benzoate is one of the most commonly used modern preservatives, often used in breads to prevent mold growth. (Fans of The Simpsons may recall potassium benzoate as part of the curse of frogurt—see http://cookingforgeeks.com/book/frogurt/.) Like nitrite, benzoate interferes with a cell’s ability to function (in the case of bread, by decreasing fungi’s ability to convert glucose to adenosine triphosphate, thus cutting off the energy supply).
Compounds that lower a food’s pH also preserve the food, and are so critical that acidity regulators get an entire section in the E numbers list. Many of these compounds don’t have uses interesting to the home cook, who already has citric acid (thanks, lemon juice!) and acetic acid (from vinegar) on hand. For industry, the other acidity regulators give a wider range of flavoring options and functional properties, but for home use, there isn’t much repurposing to be explored beyond a few baking tricks like using a pinch of vitamin C (ascorbic acid) to give yeast a boost during fermentation.
Flavorings
The flavor of food is incredibly important—arguably the single most important variable in your enjoyment of a meal. Flavors change our behavior: the smell of freshly baked bread draws us into the bakery, the aroma of fresh herbs and toasted spices in a meal makes us salivate, and the memory of what something tasted like brings us back for another purchase. The loss of smell—anosmia—is considered one of the most severe sensory losses. Think about the last time you had a cold with a plugged-up nose—food becomes so much less appealing without flavor!
Being able to add flavors to foods opens up new possibilities. Industry relies on flavorings as part of mass manufacturing. An acquaintance of mine who used to work at Campbell Soup Company pointed out that meats lose most of their flavor when steam-cooked (which is how chicken for chicken noodle soup is cooked at scale), so flavorings have to be added back in. Coloring extracts are added too, even if their source is traditional ingredients such as turmeric (yellow), paprika (red), or caramel (brown). Flavor is absolutely critical to industry, which knows very, very well that a quickly tapering-off flavor causes you to reach for a second bite, and an appealing flavor triggers a repurchase the next time you’re at the store.
Creating good aromas and flavors is so critical that there are several categories of E numbers just for compounds that change taste. One category, flavor enhancers (E600s), alters the way foods taste. (“Flavor enhancers” is something of a misnomer; “taste enhancers” would be better.) Most of the compounds in this range are glutamic acid salts like MSG (E621), but there are also those that make foods taste sweeter, like the amino acid glycine (E640). Speaking of sweet: artificial sweeteners (E900s) get their own E number category that includes compounds such as sucralose (E955) and stevia’s active chemical (E960). Unless you have some rather impressive lab equipment in your kitchen, making E-numbered compounds isn’t exactly a home project. (Time to whip up some fresh guanylic acid!?) Keep in mind that there are plenty of traditional ways to boost tastes, such as adding ingredients high in glutamic acids (see page 76), or simply a pinch of salt.
But what about actual flavorings? As I mentioned earlier, the E number list isn’t an exhaustive source of food additives. Vanillin doesn’t show up, even though it’s a single molecule with a well-defined structure that’s often added to food. Home cooks use vanilla extract, though, not vanillin powder, and that’s where we can get into some fun, creative experiments: flavor extracts.
Flavoring extracts are used to add new aromas to food or amplify existing ones. Their functional purpose is to carry volatile compounds—ones that easily evaporate—to tickle the sensory apparatus of the nose. Luckily, many volatile compounds in food are also easily dissolved by solvents. Solvents, as we’ll see, are the key to creating extracts that can carry flavors.
In cooking, we use three primary solvents: water, lipids, and alcohol. Each works on different types of compounds, so matching the chemistry of the solvent to the chemistry of the volatile compound is the key to making good extracts. The same chemical principle that allows water to dissolve compounds also applies to lipids and ethanol, so which solvent to use depends on the structure of the compounds being dissolved.
But how does a solvent work? What happens when one molecule bumps into another molecule? Will they form a bond (called an intermolecular bond—one that happens between different molecules) or repel each other? It depends on a number of forces that stem from differences in the electrical charges and charge distributions of the two molecules. Of the four types of bonds defined in chemistry, two are important in flavoring extracts: polar and nonpolar.
A molecule that has an uneven electrical field around it or that has an uneven arrangement of electrons is polar. The simplest arrangement, where two sides of a molecule have opposite electrical charges, is called a dipole. Water is polar because the two hydrogen atoms attach themselves to the oxygen atom such that the molecule as a whole has a negatively charged side—it’s a dipole.
When two polar molecules bump into each other, a strong bond forms between a positive region on the first molecule and a negative region on the second molecule, just like when two magnets are lined up. On the atomic level, the area of the first molecule that has a positive charge is balancing out the area of the second molecule that has a negative charge.
A water molecule is polar because of an asymmetric distributions of charges. This happens because oxygen is more electronegative than hydrogen and the bent shape of the water molecule. This shape gives it a positive charge on one side and a negative charge on the opposite side, making it polar.
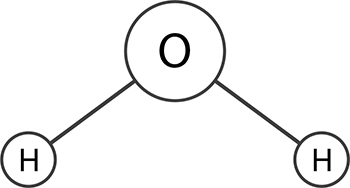
A molecule that has a symmetric shape or atoms with only a small difference in electronegativity has a symmetric charge distribution on all sides—this is called nonpolar. Oil is nonpolar because it is made of mostly carbon and hydrogen, two molecules which have small differences in electronegativity.
In most cases, when a polar molecule bumps into a nonpolar molecule, the polar molecule is unlikely to find an electron to balance out its electrical field. It’s like trying to stick a magnet to a piece of wood: the magnet and wood aren’t actively repelled by each other, but they’re also not actually attracted. A polar and nonpolar molecule won’t form a bond and end up drifting off elsewhere, continuing to bounce around into other molecules.
This is why oil and water don’t normally mix, but sugar and water easily do. The water molecules are polar and form strong intermolecular bonds with other polar molecules—they’re able to balance out each other’s electrical charges. At an atomic level, the oil doesn’t provide a sufficiently strong bonding opportunity for the negatively charged side of the water molecule. Water and sugar (sucrose), however, get along fine. Sucrose is also polar, so the electrical fields of the two molecules are able to line up to some degree.
The strength of the intermolecular bond depends on how well the solvent and solute compounds line up, which is why some things dissolve together well while others only dissolve together to a certain point. A number of organic compounds that provide aromas in food are readily dissolved in ethanol but not in water or fats.
You will invariably encounter dishes where alcohol is used for its chemical properties, either as a medium to carry flavors or as a tool for making flavors in the food available in sufficient quantity for your olfactory system to notice. Alcohol is often added to sauces or stews to aid in releasing aromatic compounds “locked up” in the ingredients. Try adding red wine to a tomato sauce!
Toasting spices in oil—called blooming—causes the oil to capture flavor volatiles from the spices that evaporate as the seeds are heated.
Liquid Smoke, a.k.a. Water-Distilled Smoke Vapor
Smoking foods as a method of preservation was presumably discovered eons ago by cave-dwelling fire builders, but for us today, smoking is carried out for a second reason: smoked foods are delicious. By burning wood or other combustibles and directing the resulting smoke vapors toward fishes or meats, we deposit antimicrobial compounds onto the foods, preventing their spoilage. It’s only a quirk that the preservation process also deposits a whole bunch of smoky aromas that we happen to enjoy. But how can modern apartment dwellers get that delicious flavor without creating a bonfire in the center of the kitchen?

Kent Kirshenbaum demonstrates making liquid smoke using a blowtorch and a round-bottom flask at a New York University talk.
There’s a neat trick to capture smoke flavors into something called liquid smoke. Because those delicious smoky flavors are water-soluble byproducts of the chemical reactions that occur as wood burns, they can be dissolved into water and later applied to food. The commercial food industry does this to infuse smoke flavor into foods that are traditionally smoked but aren’t economical to smoke in large scale, such as bacon, and to add smoky flavors into foods whose flavor is enhanced by smoke essence but that could never actually be smoked, such as “smoked” tofu.
At home, the simplest way of creating a smoked flavor in your cooking—besides actually smoking it—is to include ingredients that are already smoked. You can infuse smoke flavors into your dish by adding spices such as chipotle peppers or smoked paprika, or by using dry rubs with smoked salts or smoked teas such as Lapsang Souchong. However, including smoked ingredients will also bring along the other flavors of the substance being used. Smoked salts, for example, can add too much salt to a dish. This is where liquid smoke comes in.
Liquid smoke isn’t complicated. There shouldn’t be a long list of ingredients on any bottle you buy; it should just read “water, smoke.” In and of itself, liquid smoke is not processed, in that there aren’t any chemical modifications or refining steps that alter or change the compounds that would have been present in traditional smoking.
Making liquid smoke involves heating wood chips to a temperature high enough for the lignins in wood to burn (around 752°F / 400°C) and piping the resulting smoke through water. The water-soluble components of smoke remain dissolved in the water, while the non-water-soluble components either precipitate out and sink or form an oil layer that floats and is then discarded. The resulting product is an amber-tinted liquid that you can brush onto meats or mix in with your ingredients.
Incidentally, the wood chips turn into charcoal in the process; they’re carbonized, but without oxygen present, they can’t combust. You can create your own charcoal by sealing up wood chips inside a container that will vent out smoke but not circulate air back in. You can also make charcoal from materials besides wood. I know one chef who uses leftover corn cobs and lobster shells to create “corn cob charcoal” and “lobster charcoal,” and because some of the flavor molecules from those items are extremely heat-stable, using the charcoal for cooking imparts a whiff of those flavors as well.
In theory, some of the mutagenic, cancer-causing compounds normally present in traditionally smoked foods are present in much smaller quantities in liquid smoke—they end up in the oil phase or precipitate—so using liquid smoke may be somewhat safer for you than traditionally smoking foods. However, liquid smoke still has some amount of mutagenic compounds present. As a substitute for smoked foods, it should be as safe, possibly safer, as traditional smoking, but you probably shouldn’t douse a teaspoon of it on your eggs every morning.
When grilling, make sure your fire is hot enough so that the lignins, not just the celluloses, break down.
Liquid smoke is fascinating stuff, as you can see. Try snagging a bottle of it and revisiting the Salmon Gravlax recipe from page 385, adding 10–15 drops to the salt mixture to give it a smoked flavor. Some of the more unusual recipes use liquid smoke to “smoke” foods that can’t normally be tossed onto a wood-burning grill, such as ice cream. With all the processing that happens to our foods, it’s nice to see that one of the more exotic-sounding ones turns out to be one of the most primitive ones.

Wood chips before heating...

...and after heating.
Charcoal is traditionally made by heating combustible materials like wood in the absence of oxygen (called pyrolysis), evaporating water and breaking down volatile compounds. The result is a solid lump of mostly carbon that combusts more quickly than the original material would have.
Thickeners
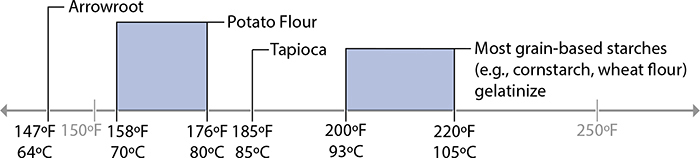
Gelation temperatures of common starches.
Starches are used to thicken many foods—gravies, pies, sauces—because they’re easy to use and easy to find. It’s no surprise that starches are used as thickeners in almost all of the world’s cuisines! Wheat flour is common in Western cooking for its starch, cornstarch is common in Chinese dishes, and tapioca starch and potato starch (sometimes called “potato flour” but not actually a flour) are also common. Many modern thickeners like maltodextrin are also derived from starch, and have been developed for use in everything from baking jam-filled pastries to creating novel textures in high-end cuisine.
Starches are made up of two compounds, amylose and amylopectin. The ratio of these two compounds and how a plant stores them differs between types of plants, which is why starches from wheat flour need near-boiling water to thicken while potato starch will set at lower temperatures. (This is also why different plants need different cooking temperatures—see page 205 for more.)
A starch’s thickening power is determined by both its amylopectin and amylose content. With heat and moisture, starches swell up and gelatinize, absorbing water into the molecular structure and changing its texture. (This is what happens to pasta when you cook it!) Amylopectin absorbs water, and starches with higher levels of it will absorb more moisture (cornstarch and wheat starch are roughly 75% amylopectin, while tapioca and potato are 80–85%). After gelatinizing, another process unfolds—gelation—which is amylose leaving the starch granule and entering the surrounding water. It’s this change that gives starches with higher amylose content their ability to thicken and gel foods.
The starch structure varies based on the plant, which is why different types of starches have different thickening capabilities. When heated in water to near-boiling, 3 teaspoons (8g) of flour is approximately the same as:
1½ |
teaspoons (4g) cornstarch |
1½ |
teaspoons (4g) arrowroot |
1 |
teaspoon (3g) tapioca starch |
2/3 |
teaspoon (6g) potato starch |
Flour isn’t as good a thickening agent as pure starches are, because it contains other stuff in addition to starch: proteins, fat, fiber, and minerals.
How quickly and how much a starch can thicken a liquid depends on the size of the starch granules. (Starch is stored in granules; it will take longer for the amylose to leach out of larger granules.) Likewise, the length and ratios of the molecular structures and variations in the crystalline structure will impact the speed and degree to which a starch will thicken; these structures are determined by the growing conditions of the plant. Moisture, too, is critically important for thickening—the starch has to absorb water in order to swell! This is why adding too much sugar can slow down cooking times for sweet liquids: sugar, being hygroscopic, competes for the water, so be sure to cook sugary liquids longer.
Thickened foods can exhibit a phenomenon known as shear thinning, which is when a substance changes its viscosity under certain conditions. Many sauces, like ketchup, mustard, and mayonnaise, hold their shape—a blob of ketchup doesn’t settle down—but will flow and change shape when pressure is applied (this property is called thixotropy). Squeeze the bottle or tube, and the sauce flows easily, but let go, and it holds its shape. With enough of a thickening agent, a food will firm up to a true solid: one with a defined shape that can be sliced, poked, and prodded.
Arrowroot and Cornstarch
Two of the most common thickeners are arrowroot and cornstarch. The former is derived from a root and the latter from a grain, a fact that is responsible for a number of differences between the two. It’s because of these differences that one or the other will work better, depending upon the chemistry of the dish it’s being used in.
Like all starch-based thickeners, arrowroot and cornstarch thicken mixtures when amylopectin swells to absorb water and amylose leaches out of the starch granules. Depending upon the recipe, they can be used as binders in place of eggs (mix 2 tablespoons / 15g starch with 3 tablespoons / 45 mL of water) or to make fried foods crispier (mix a few spoonfuls of starch and seasoning spices together and toss ingredients like tofu or chicken in the mix before pan-frying them).
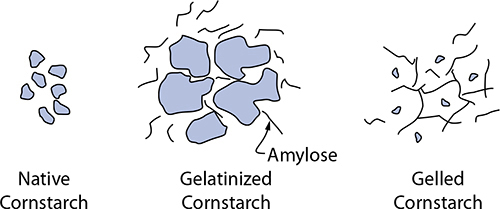
Amylose leaks out of cornstarch granules heated with water; upon cooling, the amylose molecules form a gel.
Arrowroot starch |
Cornstarch |
|
Instructions for kitchen use |
To thicken liquids, use toward the end of cooking: create a slurry in cold liquid like water, mix in, and briefly cook until set. Avoid boiling. |
To thicken liquids, use at the beginning of cooking, create a roux (starch cooked in fat, usually flour in butter), and then whisk other liquids in. |
Temperature |
Root starches require less heat to gelatinize. |
Grain starches require more heat to gelatinize. (Temperatures above ground in direct sunlight can be hot, so the starch granule structure compensates to avoid cooking!) |
Things to avoid |
Avoid dairy. When combined, arrowroot and dairy form a slimy, gooey mixture. Use cornstarch for dairy-based dishes. |
Don’t freeze. Cornstarch-thickened items will “weep,” or expel liquid (called syneresis), when frozen and thawed. |
Industry uses |
Used to form clearer gels (arrowroot forms a clearer gel than cornstarch). |
Cornstarch is gluten-free, so in addition to traditional uses, it’s also used as a thickening substitute for gluten-free items. |
Origin and chemistry |
Derived from the rhizome of the tropical Central and South American plant Maranta arundinacea, arrowroot was first used by Europeans as a supposed cure for poison arrows (hence the name) and other medicinal woes in the 17th century. The 1830s and 1840s saw its introduction as a health food, and it’s been used in culinary applications ever since. |
Derived from corn (shocker, I know), cornstarch was first made in 1842 and commercialized in 1844. Production significantly ramped up by the late 1850s, presumably as a cheaper, less objectionable alternative to arrowroot (which had to be grown in the subtropics and shipped, and whose health-conscious users objected to the slave labor involved at the time). |
Methylcellulose
Methylcellulose isn’t a typical starch-derived thickener, and it’s not used for traditional thickening purposes. It has the unusual property of getting thicker when heated—thermo-gelling, in chem-speak. Take jam: when heated, it loses its gel structure (the pectin melts), causing it to flow out of things like jam-filled pastries. Adding methylcellulose prevents this by absorbing the water in the jam when heated. And since methylcellulose is thermoreversible—it can go back and forth between states based on temperature; in this case between gelled and ungelled—upon cooling after baking, the jam returns to its normal consistency. Magic!
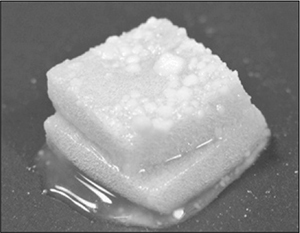
Hollywood movies use methylcellulose to make slime. Add a bit of yellow and green food dye, and you’ve got yourself Ghostbusters-style slime. To get good consistency, whisk it vigorously to trap air bubbles into the mixture.
Methylcellulose has been used in some novel culinary dishes for its thermo-gelling effects. One famous example is hot ice cream in which the ice cream is actually hot cream that’s been set with methylcellulose. As it cools to room temperature, it melts.
Instructions for kitchen use |
Dissolve methylcellulose into hot water (around 122°F / 50°C) and then whisk it while it’s cooling down. Mixing it directly in cold water can be difficult because the powder will clump up as it comes into contact with water. In hot water, though, the powder doesn’t absorb any liquid, allowing it to be uniformly mixed. It’s easiest to stir methylcellulose into your liquid, using between 1.0% and 2.0% of the weight of the total recipe, and let it rest overnight in the fridge to dissolve fully. You can then experiment with setting the liquid. Try baking a small dollop of it, or dropping it by the ice cream scoopful into a pan of simmering water. |
Industry uses |
Methylcellulose is used to prevent “bake-out” of fillings in baked goods. Methylcellulose also has high surface activity, meaning that it can act like an emulsifier by keeping oil and water from separating, so it is also used in low-oil and no-oil dressings and to lower oil absorption in fried foods. |
Origin and chemistry |
Methylcellulose is made by chemically modifying cellulose (if you’re a chemistry geek, via etherification of the hydroxyl groups). There can be great variation between types and derivatives of methylcellulose, in terms of thickness (viscosity), gelling temperature (122–194°F / 50–90°C), and strength of gel (ranging from firm to soft). If your methylcellulose isn’t setting correctly, check the specifications of the type you have; see http://cookingforgeeks.com/book/methylcellulose/ for more. |
Methylcellulose increases surface tension—well, actually, “interfacial tension” because “surface” refers to a two-dimensional shape—which is why it works as an emulsifier.

When cold (on left), water molecules are able to form water clusters around the methylcellulose molecule. With sufficient heat—around 122°F / 50°C—the water clusters are destroyed and the methylcellulose is able to form crosslinks, resulting in a stable gel at higher temperatures.
Maltodextrin
Maltodextrin is a starch that easily dissolves in water with a mildly sweet taste. In manufacturing, it’s spray-dried and agglomerated, which creates a powder that’s very porous on the microscopic level. Because of this structure, maltodextrin is able to soak up fatty substances, making it useful for working with fats when designing food. It also absorbs water, so it’s used as an emulsifier and thickener, as well as a fat substitute: once hydrated, it literally sticks around, mimicking the viscosity and texture of fats.
Since maltodextrin is a powder and absorbs fats, using a lot of it creates a fun, unusual result: it’ll turn liquids and solids that are high in fat into powders. Mix enough of it into olive oil or peanut butter, and they become a colloid of sorts, although in powdered form. And because maltodextrin dissolves in water, the resulting powder dissolves in your mouth, effectively “melting” back into the original ingredient and releasing its flavor. Since maltodextrin itself is generally flavorless (only slightly sweet), it doesn’t substantially alter the flavor of the product that is being “powderized.”
In addition to the novelty and surprise of, say, fish topped with a powder dusting that melts into olive oil in your mouth, powders can carry flavors over into applications that require the ingredients to be solid. Think of chocolate truffles rolled in chopped nuts: in addition to providing flavor and texture contrast, the chopped nuts provide a convenient “wrapper” that allows you to pick up the truffle without the chocolate ganache melting in your fingers. Powdered products can be used to coat the outsides of foods in much the same way.
Instructions for kitchen use |
Add maltodextrin powder slowly to your melted fat or oil for a ratio of about 60% fat, 40% maltodextrin by weight. You can pass the results through a sieve to change the texture from breadcrumb-like to a finer powder. |
Industry uses |
Maltodextrin is used as a filler to thicken liquids (e.g., the liquid in canned fruits) and as a way to carry flavors in prepackaged foods such as chips and crackers. Since it traps fats, any fat-soluble substances can be “wicked up” with maltodextrin and then more easily incorporated into a product. |
Origin and chemistry |
Maltodextrin is derived from starches such as corn, wheat, or tapioca. It’s made by cooking starches at moderate heat for many hours (usually with an acidic catalyst) and running the resulting hydrolyzed starches through a spray-dryer. Chemically, maltodextrin is a sweet polysaccharide typically composed of between 3 and 20 glucose units linked together. |
Gelling Agents
The next time you slather jelly onto a slice of bread, you can thank pectin for its ability to form gels—solid colloidal mixtures with a defined shape that trap liquids. Without gelling agents like pectin we’d have a much less interesting world, at least in the sweets aisle. Gelling agents thicken liquids, and at higher concentrations they create gels. In low concentrations they can also act as emulsifiers (thicker liquids don’t separate as easily) and can prevent sugar crystals from ruining candies or stop water crystals from turning ice cream gritty.
There are two key concepts for gels: strength and concentration. Gram for gram, some gelling agents are able to form stronger gels than others. And, of course, there has to be enough of a gelling agent present for a gel to form.
Weak gelling agents and low concentrations of stronger gelling agents act as thickeners. Without enough of the gelling agent present to create a proper structure, viscosity increases but liquids remain flowable or at least pliable. Jams are a great example of weak gels: they’re flowable and don’t have a proper shape. Some gelling agents are almost always used for weak gels. Carrageenan, for example, is commonly used for thickening. The hazelnut milk I drank this morning had a thick, tongue-coating feel; a quick peek at the ingredient label listed carrageenan. (Presumably the manufacturer thinks consumers want something with the same mouthfeel as regular milk.)
With enough of a strong gelling agent, liquids become true gels—solid colloids. Foods like jelly, Jell-O, and cooked egg whites are gels due to pectin, gelatin, or ovomucin proteins. Gels are formed by tightly interconnected lattices that prevent the food from flowing at all, so they form a solid that you can slice into shapes or unmold and use as a component in a dish. Gels have a memory of their cast shape, meaning that they will revert to that shape when not being pushed or poked at.
In industrial applications, gelling agents are typically used for their functional properties to thicken liquids or modify texture (“improve mouthfeel,” as they say—like my hazelnut milk). Carrageenan is extremely common; half the cream cheese and yogurt in my local store uses it. Agar is used in many sweet Asian desserts, and tapioca is used for balls in bubble tea.
In modern cuisine, gelling agents are used to create dishes in which foods that are normally liquid are turned into something that can be smeared or even made completely solid. Gels can also be formed on surfaces (well, technically, interfaces—where two substances meet) in a technique sometimes called spherification. Let’s see how a handful of different gelling agents work to create everything from everyday jams to novel spherification.
American-style jelly is made with the juice of fruit—no fruit pulp—and turned into a gel using sugar and pectin so that it holds its shape, making it spreadable. Jam includes mashed fruit, making it thick, and uses less pectin, thickening it but not setting it into a true gel.
Pectin
Pectin is amazing stuff: in nature, it acts as glue, holding cells together in plant tissue. The pectin used in cooking acts as a thickener and comes from a family of polysaccharides that, depending on processing, are divided into two broad types: high- and low-ester pectins, sometimes called high-methoxyl (HM) and low-methoxyl (LM). The difference between the high and low types has to do with the esterification of the molecular structure—this is just a detail of the pectin molecules that can vary. The number of esters present in the pectin molecules is naturally high, but with processing it can be reduced, which changes the way pectin forms a gel. High-ester pectin requires sugar and acid in order to link together; low-ester pectin can also create a gel using cations like potassium or calcium.
To complicate matters, the labels of high- and low-ester are based on an arbitrary cutoff point for the degree of esterification. All the requirements for gelling can be satisfied, but the time for the gel to actually set can vary from 20 seconds to 250 seconds. If you’re making jam and pull a sample to test gel level, you may have to wait 4 minutes or so to actually determine if you’ve created the right conditions, depending on the specifics of the high-ester pectin you’re using.
Making jam? Throw some spoons in the freezer before you start. When making the jam, drip the hot jam onto the cold spoon to let it cool for a few minutes to check if it forms a good gel.
Commercially, pectin is extracted from cooked citrus rinds or apple pomace (what’s left after the juice is pressed out of the fruit) and cores. You can use the same method to make your own pectin; you’ll end up with high-ester pectin in liquid. (The process to convert it to low-ester pectin isn’t a home project.) The natural presence of pectin in some fruits is also why a jam recipe may not even call for pectin—it’s already in the ingredients!
High-ester pectin won’t form gels when there’s too much water around. Adding sugar reduces the amount of available water, plus sugar is needed for high-ester pectin to set. High-ester pectin also forms gels only in a pH range of around 2.5 to ~3.5, which is why some recipes add an acid like lemon juice to drop the pH range. Fruits that have more natural sugars will require less added sugar, and likewise more acidic fruits won’t require the addition of something like lemon juice.
Low-ester pectin is made by processing high-ester pectin with an acidified alcohol. This creates a pectin that sets in the much wider pH range of 2.5–6.0 and tolerates more water, although low-ester pectin does still gel better at the lower pH range (below 3.6). Low-ester pectin is more forgiving than high-ester pectin: it can handle more free water and less acidic environments but still does better when treated like high-ester pectin. Low-ester pectin has the benefit of being able to create low-sugar or low-acid foods, allowing for less sugary jams.
In general, if you can get low-ester pectin, use it—it’s just easier to work with, as you can see from the chemistry. Otherwise, be patient with high-ester pectin: use about 1% (by weight), plenty of sugar (60–75%), and enough acid (like lemon juice) to drop the pH.
Carrageenan
Carrageenan is another common gelling agent, used in food as far back as the 15th century. Commercial mass production of carrageenan gums became feasible after World War II, and now it shows up in everything from cream cheese to dog food, acting as a flavorless thickener.
Instructions for kitchen use |
Mix 0.5% to 1.5% carrageenan into room-temperature liquid. Gently stir the liquid to avoid trapping air bubbles in the gel; lumps are okay at this stage. (They’re hard to get out unless you have a vacuum system.) Allow the mixture to rest for an hour or so; carrageenan takes a while to rehydrate. To set the carrageenan, bring it to a simmer either on a stovetop or in an oven. If you are working with a liquid that can’t be heated, create a thicker concentration using just water, heat that, and then mix it into your dish. |
Industry uses |
Carrageenan is used to thicken foods and to control crystal growth (e.g., in ice cream, keeping ice crystals small prevents a gritty texture). Carrageenan is commonly used in dairy (check the ingredients on your container of heavy whipping cream!) and water-based products, such as fast-food shakes (it keeps ingredients in suspension and enhances mouthfeel) and ice creams (it prevents aggregation of ice crystals and syneresis, the expulsion of liquid from a gel). |
Origin and chemistry |
Derived from seaweed (such as Chondrus crispus, a.k.a. Irish moss), carrageenan refers to a family of molecules that all share a common shape (a linear polymer that alternates between two types of sugars). The seaweed is sun-dried, treated with lye, washed, and refined into a powder. Variations in the molecular structure of carrageenan cause different levels of gelification, so you can achieve different effects by using different types of carrageenan (which, helpfully, grow in different varieties of red seaweed). Kappa carrageenan (k-carrageenan) forms a stronger, brittle gel, and iota carrageenan (i-carrageenan) forms a softer brittle gel. |
Technical notes |
||
i-carrageenan |
k-carrageenan |
|
Gelling temperature |
95–149°F / 35–65°C |
95–149°F / 35–65°C |
Melting temperature |
131–185°F / 55–85°C |
131–185°F / 55–85°C |
Gel type |
Soft gel: gels in the presence of calcium ions |
Firm gel: gels in the presence of potassium ions |
Syneresis |
No |
Yes |
Working concentrations |
0.3–2% |
0.3–2% |
Notes |
• Poor solubility in sugary solutions • Interacts well with starches |
• Insoluble in salty solutions • Interacts well with nongelling polysaccharides (e.g., gums like locust bean gum) |
Thermoreversible |
Yes |
Yes |

On the molecular level, carrageenan, when heated, untangles and loses its helical structure (left); when cooled, it reforms helices that wrap around each other and form small clusters (right). The small clusters can then form a giant three-dimensional net that traps other molecules.

Iota carrageenan (left, 2% concentration set in water) creates a soft, elastic gel that sags, while kappa carrageenan (right, 2% concentration set in water) creates a firm, nonelastic gel that’s much more brittle. Both of these are true colloidal gels: solid structures with liquids trapped inside.
Agar
Like carrageenan, agar—sometimes called agar-agar—has a long history in food, but it has only recently become known in Western cuisine because of its use as a vegetarian substitute for gelatin. First used by the Japanese in the firm, jelly-type desserts that they’re known for, such as mizuyokan, agar’s use stretches back many centuries.
When it comes to food additives, agar is one of the simplest to work with. You can add it to just about any liquid to create a firm gel—a 2% concentration in, say, a cup of Earl Grey tea will make it firmer than Jell-O—and it sets quickly at room temperature. It comes in two general varieties: flakes or powder. The powdered form is easier to work with (just add it to liquid and heat). When working with the flake variety, presoak it for at least five minutes and make sure to cook it long enough so that it breaks down fully.
Instructions for kitchen use |
Dissolve 0.5% to 2% agar by weight in cold liquid and whisk to combine. Bring liquid to a boil. As with carrageenan, you can create a thicker concentrate and add that to a target liquid if the target liquid can’t be boiled. Compared to carrageenan, agar has a broader range of substances in which it will work, but it requires a higher temperature to set. |
Industry uses |
Agar is used in lieu of gelatin in products such as jellies, candies, cheeses, and glazes. Since agar is vegetarian, it’s a good substitute in dishes that traditionally call for gelatin, which is derived from animal skins and bones. Agar has a slight taste, though, so it works best with strongly flavored dishes. |
Origin and chemistry |
Like carrageenan, agar is a seaweed-derived polysaccharide used to thicken foods and create gels. When heated above 185°F / 85°C, the galactose in agar melts, and upon cooling below 90–104°F / 32–40°C it forms a double-helix structure. (The exact gelling temperature depends on the concentration of agar.) |
During gelling, the endpoints of the double helices are able to bond to each other. Agar has a large hysteresis; that is, the temperature at which it converts back to a gel is much lower than the temperature at which that gel melts back to a liquid, which means that you can warm the set gel up to a moderately warm temperature and have it remain solid. For more information on the chemistry of agar, see http://cookingforgeeks.com/book/agar/.
Technical notes |
|
Gelling temperature |
90–104°F / 32–40°C |
Melting temperature |
185°F / 85°C |
Hysteresis |
140°F / 60°C |
Gel type |
Brittle |
Syneresis |
Yes |
Concentrations |
0.5–2% |
Synergisms |
Works well with sucrose |
Notes |
Tannic acid inhibits gel formation (tannic acid is what causes overbrewed tea to taste bad; berries also contain tannins) |
Thermoreversible |
Yes |

When heated, the agar molecule relaxes into a relatively straight molecule (first image) that upon cooling forms a double helix with another agar molecule (second image). The ends of these double helices can bond with other agar double helices (third image), forming a 3D mesh (fourth image).
Sodium Alginate
The gels covered so far are all homogenous, in the sense that they are incorporated into the entire liquid and then heated. Sodium alginate, however, sets via a chemical reaction with calcium, not heat, which allows for an interesting application: setting just part of the liquid by exposing it to calcium. One example of this technique is fake cherries, invented in 1942 by William Peshardt (for the curious, see US Patent #2,403,547). Chef Ferran Adrià refined the concept in 2003, and it became something of a trend. It’s a clever yet simple idea: by controlling what regions are exposed to the gelling agents, you can gel selective regions.
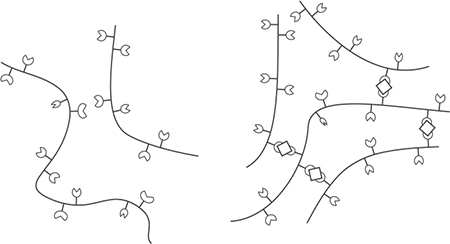
Alginate does not normally bind together (left), but with the assistance of calcium ions is able to form a 3D mesh (right).
You set sodium alginate by adding it to one liquid, adding calcium to a second liquid, and then exposing the two liquids to each other. The sodium alginate dissolves in water, freeing up the alginate, which sets in the presence of calcium ions where the two liquids touch. Imagine a large drop of sodium alginate–filled liquid: the outside of the drop sets once it has a chance to gel with the assistance of the calcium ions, while the center of the drop remains liquid. It’s from this application that the spherification technique mentioned earlier is derived.
Instructions for kitchen use |
Add 1.0–1.5% sodium alginate into your liquid (use water for your first attempt). Let the liquid rest for 2 hours or so to hydrate fully. It will be lumpy at first; don’t stir or agitate the liquid, as doing so will trap air bubbles in the mixture. |
Industry uses |
Sodium alginate is used as a thickener and emulsifier. Since it readily absorbs water, it easily thickens fillings and drinks and is used to stabilize ice creams. It’s also used in manufacturing assembled foods; for example, some pimento-stuffed olives are actually stuffed with a pimento paste that contains sodium alginate. The olives are pitted, injected with the paste, and then placed in a bath with calcium ions to gel the paste. |
Origin and chemistry |
Sodium alginate is derived from the cell walls of brown algae, which are made of cellulose and algin. Alginates are block copolymers—repeating units of the same compounds, in this case mannopyranosyluronic and gulopyranosyluronic acids. Based on the sequence of the two acids, different regions of an alginate molecule can take on one of three shapes: ribbon-line, buckled shape, and irregular coils. Of the three shapes, the buckled shape regions can bind together via any divalent cation. (Recall that a cation—meow!—is just an ion that’s positively charged—that is, missing electrons. Divalent simply means having a valence of two, so a divalent cation is any ion or molecule that is missing two electrons.) |
Emulsifiers
Emulsifiers prevent two liquids from separating, creating liquid–liquid colloids. In cooking, emulsions are almost always water–fat combinations, sometimes fat in water (like salad dressing) or water in fat (like mayonnaise). You might wonder why liquids like oil and water are able to “mix” in the presence of an emulsifying agent, after the earlier discussion about polar (e.g., water) versus nonpolar (e.g., oil) molecules not being able to mix. An emulsifier has a hydrophilic/lipophilic structure: part of the molecule is polar and thus “likes” water, and part of the molecule is nonpolar and orients itself toward oil.
Adding an emulsifier keeps foods from separating by providing a barrier between droplets of oil. Think of it like a skin around the oil droplets that prevents different droplets from touching and coalescing. Emulsifiers reduce the chance that oil droplets will aggregate by increasing what chemists call interfacial tension. The oil and water don’t actually mix; they’re just held apart at the microscopic level.
Emulsifiers stabilize foams by increasing their kinetic stability; that is, the amount of energy needed to get the foam to transition from one state to another is higher. Take bubble bath as an example: the soap acts as an emulsifier, creating a foam of air and water. Water doesn’t normally hold on to air bubbles, but with the soap (the emulsifier), the interfacial tension between the air and water goes way, way up, so it takes more energy to disrupt the system. The more energy it takes, the more kinetically stable the foam is, and the longer it’ll last.

Emulsifiers like lecithin are molecules with both polar and nonpolar regions. Those regions can orient themselves at the interface between two different liquids.
Lecithin
In cooking, lecithin is almost always the emulsifier of choice because it’s easy to use and works in a broad range of foods. Without lecithin, we wouldn’t be able to make mayonnaise; it’s the lecithin in the egg yolks that provides most of the emulsification. Mustard seeds and finely ground spices like paprika can also emulsify foods, at least for a short while—they’ll slow down how quickly the liquids coalesce. This is why mayonnaise recipes often call for mustard. If you ever see a vinaigrette recipe call for mustard, don’t skip it!
Lecithin can create foams for the same reason that it emulsifies. If you’ve ever been served a dish that has a “foam” liquid component—I’ve had both cod topped with a carrot foam and uni (sea urchin) topped with a green apple foam—the chef probably created it by adding lecithin to the liquid and then whipping or puréeing it. It’s a fun way to introduce a flavor to a dish without adding much body.

A photo of a half-water, half-oil solution under a light microscope. (The slide is pressing the oil droplets flat.)
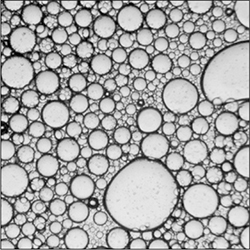
The same mixture with 1% lecithin added. The droplets are stable and do not coalesce into larger drops because of the increased kinetic stability.
Instructions for kitchen use |
For emulsions, add about 0.5–1% lecithin powder (by weight) and whisk. To create foams, add about 1–2% lecithin powder to your liquid (by weight) and use an immersion blender or whisk to froth the liquid. If you have a cream whipper (see page 313), lecithin can be used to make stable foams from liquids that wouldn’t normally hold bubbles after being sprayed. |
Industry uses |
Lecithin is used to create stable emulsions. It’s also used as an antispattering agent in margarines, in chocolate to reduce the viscosity of the melted chocolate during manufacturing, and as an active ingredient in nonstick food sprays. |
Origin and chemistry |
Lecithin is typically derived from soya beans as a byproduct of creating soy-based vegetable oil. Manufacturers extract lecithin from hulled, cooked soya beans by crushing the beans and then mechanically separating out (via extraction, filtration, and washing) crude lecithin. The crude lecithin is then either enzymatically modified or extracted with solvents (e.g., de-oiling with acetone or fractionating via alcohol—I know, sounds yummy). Lecithin can also be derived from animal sources, such as eggs and animal proteins, but animal-derived lecithin is much more expensive than plant-derived lecithin, so it is less common. |
Enzymes
Ever pondered where the word enzyme comes from? It’s based on two words, en and zyme. En is easy (“in”), but zyme? You’d have to either be a language buff or a Greek speaker to recognize zyme as “yeast.” Even though enzyme’s origins are Greek, it was a German doctor who proposed the term in the 1870s while isolating the protein trypsin. He chose it to describe compounds that assisted in fermentation, using the Greek for “in yeast,” not knowing that enzymes appear in almost all living things.
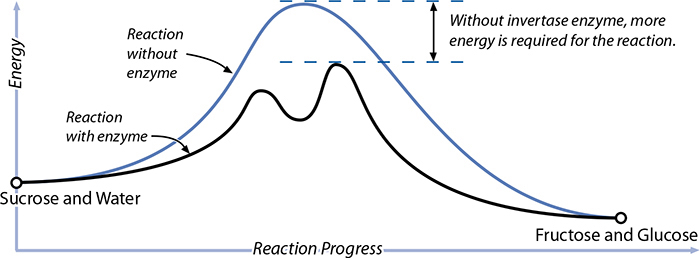
Enzymes are used all over the place in biological systems, working as catalysts that change other compounds. From a chemistry perspective, enzymes can do one of two things. They either provide an alternative reaction pathway—taking a different, easier route to get to the same outcome—or they trigger an entirely different reaction. Enzymes are amazingly selective. They’ll fit very, very few molecular structures, allowing for a biological precision envied by drug makers. (Some drug compounds, like protease inhibitors, are based on inhibiting enzymes!)
While many enzymes are naturally present in food, we sometimes add foreign enzymes to change flavors and textures when cooking. Cheese was traditionally made by exposing milk to parts of a ruminant animal’s stomach, which has a group of enzymes, called rennet, that normally aid in digestion but also cause coagulation (leading to cheese formation). A simpler example is the breakdown of sucrose. Imagine a sucrose molecule, which is one glucose molecule and one fructose molecule, bound together by a common oxygen atom. When heated in the presence of water, the molecule vibrates with more and more kinetic energy, and eventually a water molecule manages to slip in where that oxygen atom is linking them, breaking the sucrose molecule into one glucose and one fructose molecule. (The water takes the place of the oxygen atom in one of them, making this a hydrolysis reaction.)
There’s an enzyme, invertase (enzyme names often end in “ase”), that provides an alternative pathway for this reaction. Invertase wraps around part of the sucrose molecule, gripping it in such a way that a water molecule can more easily slip into where the oxygen atom is holding the two simple sugars together. Once that water molecule slips in, the sucrose breaks down; the invertase enzyme can no longer hold on to the two parts and drifts away. Less energy is required for the reaction, and it’s very selective—other compounds in the system won’t have to be exposed to as much heat to cause the reaction. Enzymes are powerful!
Transglutaminase
One of the more unusual food additives is transglutaminase, an enzyme originally found in blood. It can bond glutamine with other amino acids—it’s protein glue. It can fuse together two or three pieces of meat to create one larger piece, and it can thicken milk and yogurts by lengthening their proteins. It’s also used to firm up pastas and make gluten-free breads more elastic (able to stretch without tearing). Pretty much anywhere proteins are involved, transglutaminase can bind them together.
What could you do in the kitchen with transglutaminase? There’s of course the obvious opportunity to use it to make Frankenstein meats (e.g., chicken stuck to steak), but while these sound fun, they aren’t delicious; plus, the different temperature ranges needed for cooking makes them infeasible. The bacon-wrapped scallop recipe will give you a starting idea, but really the concept of binding proteins can apply to any protein-rich items that you want to manipulate.
Imagine simplifying chicken Kiev—flattened chicken breast that’s traditionally rolled and tied around a center of herbed butter—by sealing the edges of the flattened chicken breast together with transglutaminase. More creative dishes like turducken—an unusual holiday dish of a chicken cooked inside a duck cooked inside a turkey—can be bonded together for stability. Heat-stable aspics and terrines can be made using transglutaminase where heat-sensitive gelatins would fail. You get the idea—experiment!

An example of chicken being bonded to steak. This isn’t delicious, but it shows the concept well. Notice that the cooked steak itself is weaker than the interface where it’s joined to the chicken!
Instructions for kitchen use |
Create a slurry of 2 parts water to 1 part transglutaminase and brush it onto the surfaces of the pieces of meat that you want to join. Press them together and wrap the meat with plastic wrap. (If you have a vacuum sealer, use it to improve the fit between the two pieces of meat.) Store the meat in the fridge for at least two hours. |
Industry uses |
Transglutaminase is used to combine scrap meats into large pieces, such as in imitation crab meat and gluten-free hot dogs. Some cold cuts and lunch meats use transglutaminase too. (That gorgeous ham at the deli counter is not from the rare boneless pig!) |
Origin and chemistry |
Transglutaminase is manufactured using the bacteria Streptomyces mobaraensis. Anywhere that glutamine and a suitable amine are present, transglutaminase can be used to cross-link the two, causing the atoms composing the two groups to line up so that they form covalent bonds, in which two atoms share electrons. |

Before interaction (left), strands of proteins with glutamine and lysine groups are unattached; after interaction (right), the glutamine and amine groups are bonded wherever transglutaminase has a chance to catalyze.