5
Fun with Hardware
Chapter Contents
Sous Vide Cooking: Low-Temperature Poaching
Sous Vide Cooking and Food Safety
Cooking Times for Fish, Poultry, Beef, and Fruits and Veggies
How to Make a 500-Pound Doughnut
WHAT WOULD IT LOOK LIKE IF YOU HAD SUPERPOWERS IN THE KITCHEN? You know, if you could slow time down? Or had heat vision? Or could suck all the air out of the room? Okay, maybe that last one isn’t so awesome sounding—VacMan?!—but interesting stuff happens when you have superpower control over the basic variables of cooking. We usually work with the variables of time, temperature, air, and water (discussed in the previous two chapters) at moderate values: making a soft-cooked egg in 6 minutes, baking pizza at 450°F / 230°C, or churning ice cream at –20°F / –29°C for half an hour. What happens when we stray outside these usual ranges?
Increasing air pressure changes the boiling point of water, speeding up how quickly foods cook. Separation techniques and tools from dehydrators to centrifuges change textures and flavors in multiple ways. Or consider sous vide cooking: essentially ultra-low-temperature poaching. When upping the variable of time, we have to drop temperature to keep time-and-temperature reactions in line. But something fascinating happens: as we dial down the temperature, it eventually has to equal the target temperature of the cooked food. It becomes impossible to accidentally overcook foods. This is amazing!
What if you take temperature beyond the limits of your kitchen thermometer? Ice cream made with liquid nitrogen at –320°F / –196°C sets in 30 seconds, and it’s great—the water crystals don’t form large aggregates, so this method creates the smoothest ice cream you’ll ever have. And at 900°F / 480°C, thin-crust pizza bakes in under a minute, and it’s delicious! Let’s see what techniques and fun culinary creations come out of using hardware that lets you play with these variables.

High-Pressure Situations
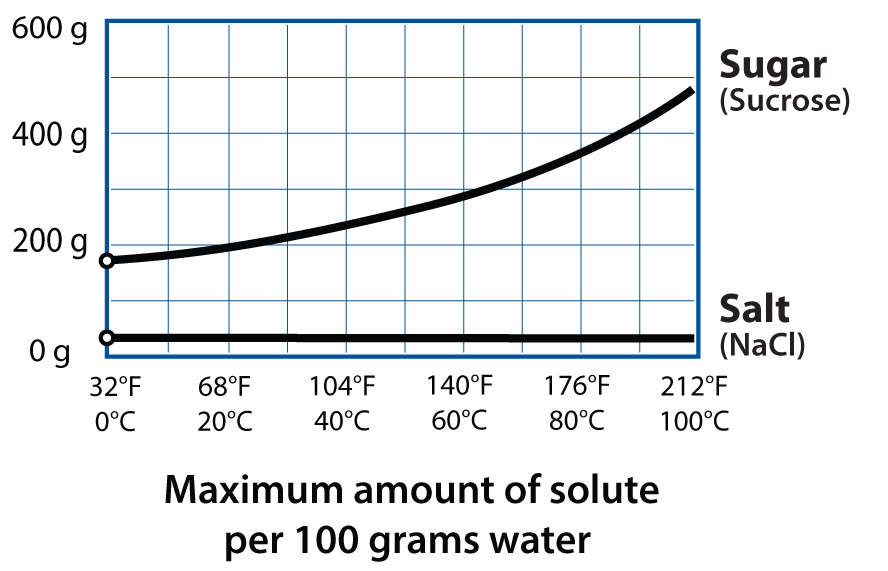
As I’ve learned more about food science, I’ve come to realize just how critical water is! It impacts cooking in so many different ways: transmitting heat via steam, dissolving trace minerals to change how gluten forms and yeast multiplies in breads, and altering textures in cookies (chewy or crispy) and dried foods. Water is everywhere.
One water variable that doesn’t change much in the kitchen is its boiling point. Adding salt can increase its boiling point by a few degrees, but what could we do if we raised it even more? If most heat-related reaction rates in cooking roughly double with every 18°F / 10°C increase, then increasing water’s boiling point from 212°F / 100°C to 230°F / 110°C should, in theory, cut braising times in half and cook rice twice as quickly. Raising it again, to 248°F / 120°C, would slash cooking times by up to a whopping 75%. And this is exactly what happens under pressure.
How much pressure, you ask? There’s a nifty type of science chart called a phase diagram that shows a substance’s phase—solid, liquid, or gas—at various pressures and temperatures. Here’s one for water at the various temperatures and pressures normally found in the kitchen.
A quick primer on how to read this: consider the line at 14.7 psi (1,013 hPa—that’s hectopascals) equal to one atmospheric pressure or what you’d experience at sea level on an average day. The freezing point at one atmosphere is 32°F / 0°C; the boiling point is 212°F / 100°C. Move that line down a little bit to 12.1 psi (834 hPa), equivalent to an altitude of 5,280 feet (1,609 meters) above sea level, and you’ll see why water boils at 203°F / 95°C in Denver, Colorado. Go up to 30 psi (2,070 hPa), and voilà! Water boils at roughly 248°F / 120°C. This is the science behind what makes pressure cookers amazing. I know, I know, getting excited by the idea of keeping water liquid at a higher temperature may seem strange, but trust me, you’ll love what it can do.

Phase Diagram of Water for the Kitchen
What else happens when we increase pressure? Water is more complicated than this simple phase diagram suggests, because nothing in the kitchen is a pure substance. Your salt has trace minerals in it—probably silica too. Table sugar isn’t actually 100% sucrose; a spoonful of it includes ash, proteins, and inorganic impurities. And water, even purified distilled water, isn’t actually 100% H2O: there’s gas dissolved in there. With pressure, we can dissolve more gas into liquids like water for both fun and useful purposes.
Food is always mixtures of solids, gases, and liquids. (Actually, food is almost always mixtures of mixtures, and figuring out how to separate them has its own challenges, as we’ll see later in this chapter.) We talked about humidity—dissolved water vapor in air—in the previous chapter, but what do we call dissolved air in water? It’s what fish breathe, but we don’t even have a word for it!
Gases dissolve into liquids all the time—think carbonated drinks, or the small bubbles you see when heating water to boil—and changes in pressure change how much gas can be dissolved. This is known as Henry’s Law: essentially, the higher the pressure of a gas above a liquid, the more soluble that gas becomes. (Huh. There’s no Potter’s Law yet. Probably too late; all these laws seem to have been named about two centuries ago. The English chemist William Henry came up with this one in 1803.) You can dissolve gases into foods to make foams like whipped cream (and Aero chocolate!), and you can use a pressurized container to make wild things like carbonated fruit.
In the following sections, we’ll take a look at how to cook with pressure cookers and cream whippers, covering what they are and how to use them.
Dropping the temperature of a liquid increases the amount of gas that will dissolve into it. If you’re trying to saturate gas into a liquid, cool the liquid down first.
Pressure Cookers
Pressure cookers are like an old-fashioned version of the microwave: a convenient appliance that speeds up cooking. Our grandparents used a manual version, essentially fancy pots with locking lids that went on the stovetop. Manual versions are still available today—now enhanced with safety locks and over-pressure release valves to prevent accidents—and are a worthwhile investment for the serious pressure cooker enthusiast. Manufacturers also make electric units, which can safely be left unattended and are what I suggest for first-time buyers. If you have a tiny kitchen, get an electric unit that has modes for slow cooking and cooking rice.
It’s not the pressure itself that changes how foods cook, but the impact of pressure on physical and chemical processes. Increasing pressure always increases the boiling point of water. In wet cooking methods, the temperature differential between the food and the liquid heating it is what determines how quickly the food heats up. (See page 139 for more.) Increasing the pressure increases the boiling point of water, but it’s not boiling water per se that does the cooking—it’s the larger temperature difference between the higher-temperature liquid and colder food that results in a faster rate of heat transfer.
How much faster heat will transfer into food depends on the maximum temperature at which the liquid can boil. Depending on the make and model, a pressure cooker can increase the pressure by 11–15 psi (758–1,034 hPa). There’s no formal standard for how much pressure a cooker should operate at, but most recipes are written assuming a high pressure of 15 psi (1,034 hPa) and low pressure of 8 psi (550 hPa). (Underwriters Laboratories won’t certify units above 15 psi, which is why you don’t see higher pressures than that.) You will need to adjust cooking times based on the pressure at which your model operates!

Pressure vs Boiling Point of Water
Figure out your starting air pressure, add the operating pressure of your cooker, and check the boiling point of water for that absolute pressure.
Pressure increases are relative to your current atmospheric pressure, so the maximum boiling point of water is based on your current air pressure plus the additional pressure your unit adds to it. If you live at sea level and have a higher-pressure unit, you’ll be able to get water up to 29.7 psi (2,048 hPa) for a boiling point of 250°F / 121°C, but a mile up and with a unit that only adds 11 psi (758 hPa), you’ll only raise the boiling point to 236°F / 113°C. Take a look at this zoomed-in part of the phase diagram of water from the previous section, cropped to the starting and maximum pressures possible in pressure cooking. Add the operating pressure of your pressure cooker to the air pressure at your elevation to look up how hot (and thus how quickly!) your foods will cook.
One fun science comment: Maillard reactions don’t readily occur in most wet cooking methods. It’s not that water inhibits the reaction itself; there actually needs to be some water present in the food (see page 213). The limiting factor is that water, when used as a source of heat, prevents the necessary temperatures from being reached, at least at atmospheric pressure. Some combinations of amino acids and reducing sugars will begin to undergo Maillard reactions just above water’s normal boiling point. Lysine/glucose, for example, will combine at around 212–230°F / 100–110°C in a solution with a pH between 4 and 8, reacting much faster at the hotter and more basic ranges. A few novel recipes use this quirk to create Maillard reactions in soups using baking soda, but it’s not even a minor part of most pressure cooking. Fortunately, Maillard reactions don’t happen too much at the pressures used in pressure cooking (you’d need to go to ~70 psi / ~4,800 hPa to really see them). If they did happen, foods would have Maillard reactions center-to-edge and taste disgusting—too much of a good thing, in this case, gives horrible flavor.
Pros
• Speed! Raising the boiling point of water from 212°F / 100°C to 248°F / 120°F roughly quadruples how quickly culinary reactions can occur, cutting cooking times by ~60–70% (it takes some time to heat up the food; otherwise, it’d be closer to 75%). Pressure cookers are fantastic for cooking slow-cooking grains and legumes (rice and lentils in 5–8 minutes instead of 30), beans (30 minutes for dry, unsoaked beans to be ready to serve), and higher-collagen meats (ribs, pot roast, and pulled pork can all be cooked in under an hour are deliciously easy).
The French scientist Denis Papin served the very first pressure cooker meal way back in 1679 to a group of scientists in London, calling it a bone digester and serving bones rendered to something like jelly (along with cooked meats). Oh, 17th-century British cooking...
• Electric units are extremely energy efficient, meaning they’re great for summer cooking where you want something that’s normally slow-braised for hours—say, pulled pork—but don’t want to adds lots of heat to your kitchen.
Cons
• You won’t be able to poke at food while it cooks to adjust seasoning or check if it’s done. What goes in the pot at the start is what you’re going to get at the end, as when baking a cake. If you’re an intuitive cook, winging it as you go, treat pressure cooking as a way of cooking one ingredient that you’ll then use as a component in your meal.
• With the faster rate of reactions, overcooking will happen much more quickly. It’s better to slightly undercook something and continue cooking it “off pressure.” Take notes on cooking times on your recipes. (Use the low-pressure setting on vegetables to avoid overcooking.) Different pressure cookers will run at slightly different pressures, so treat times for recipes as starting points and take notes.
• Pressure cookers rely on boiling water or steam for transmitting heat—it’s a wet cooking method—and trap and condense most of the moisture, making it hard to reduce sauces. You may need to reduce the liquids after cooking. On the flip side, don’t skimp on the liquid: make sure that you have at least a cup or two of water in the unit; otherwise, there won’t be anything to turn into steam and you’ll end up burning the bottom of whatever you’re cooking.
Pressure frying uses oil instead of water to cook at even higher temperatures, creating crispy, browned outsides and moist interiors on foods like breaded chicken drumsticks. Pressure frying is how Harland Sanders made his original “Kentucky Fried Chicken,” and why it was so successful! Unfortunately, pressure fryers are industrial appliances; there’s no safe consumer version for the home. This is one you don’t want to try hacking: using oil in a standard pressure cooker can melt the sealing gaskets and lead to explosive decompression with hot oil spraying everywhere.
Tips and tricks
• If you want to adapt other recipes to a pressure cooker, think about things that normally cook via steaming, braising, or any wet cooking method, and try cooking for a third of the suggested time. Make sure not to fill the cooker more than two-thirds full; some ingredients will expand as they cook, and blocking the release valve is bad. If using ingredients that foam while cooking—applesauce, barley, oatmeal, pasta—don’t fill the cooker more than one-third full. Be aware that dairy curdles under pressure, so add any dairy ingredients after pressure cooking.
• If you have a stovetop unit, you can rinse it under cool tap water to cool it down quickly after cooking; this is useful for quick-cooking foods like vegetables or polenta where the residual heat would continue cooking them.
• Many electric pressure cookers operate at 12 psi (830 hPa) instead of 15 psi (1,034 hPa), meaning cooking times for recipes based on the slightly higher pressure may need to be extended by 15–20%. Check the manual for the operating pressure, not the rated air pressure—manufacturers tend to list the maximum pressures their units reach and bury the true pressures at which they cook.
• Steam vegetables and artichokes using a metal steaming tray to raise them above the water level. You can also cook small quantities of food in a small glass or metal bowl this way—just remember to pour a cup or two of water into the pressure cooker! Don’t use plastic containers in a pressure cooker; they’ll melt.
• Pressure cookers are great for making stock. Save bones from meals in a container in the freezer. Once the container is full, transfer the contents to the pressure cooker, cover them with water, and cook for 30 minutes. Cool and strain the liquid.
• Try using your pressure cooker to render tallow or lard: toss chopped-up fatty meats into a jar, cover them with water, add a cup of water to the pressure cooker, and render for about 2 hours. Let the fat cool to a safe-to-handle temperature and pour through a strainer.
Cream Whippers
Most of us are familiar with whipped cream in a pressurized can. A cream whipper is a reusable version of the can that you fill with cream or whatever else you like. It is a simple yet clever design: pour your contents into the container, screw on the lid, and pressurize using a small disposable gas cartridge that provides either nitrous oxide or carbon dioxide to the can through a one-way valve. Presto! You now have the ability to increase pressure and dissolve more gas into a liquid, opening up some fun culinary techniques.
Cream whippers take their name from their primary purpose: making whipped cream. With a whipper, you can control the quality of the ingredients and the amount of sugar used. Once filled up, they’re no different than the more familiar whipped cream cans. The obvious extension is to create flavored whipped cream. Toss some orange zest and maybe a bit of vanilla sugar into a pint of organic cream, screw the lid on and pressurize with a gas cartridge, and spray away. Try tea-infused cream: steep some Earl Grey in cream and transfer it to the whipper, or go smoky and use Lapsang Souchong tea. (Strain any tea leaves out before filling the canister of the whipper!) You can also spike the cream—make amaretto cream to go on your coffee with 4 parts heavy cream, 2 parts amaretto liqueur, and 1 part powdered sugar.
But the real fun with cream whippers is passing other liquids through them. You can whip any liquid or mixture that has the ability to hold air. Chocolate mousse can be made instantly with a cream whipper. Adding a small amount of gelatin or lecithin (see page 430) to liquids will give them the capability of foaming, producing a light, bubble-bath-like foam that’s edible and flavored. Foamed carrot juice sounds strange, but as part of an avant-garde meal can be amazing. You can even put pancake batter in a cream whipper (and yes, some entrepreneur has already tried commercializing “pancakes in a can”). Because the contents are ejected under pressure, any small, pressurized bubbles come along for the ride and expand instantly, leading to mechanical injection of air into the liquid. This is why cream turns into whipped cream, although the foam that’s generated isn’t as stable as manually whisked whipped cream.
One downside of cream whippers is the expense of the disposable gas cartridges. They add up, but if you’re a regular user of whipped cream, the long-term savings alone will make buying a whipper worthwhile, not to mention the quality gains. If you want to play around with textures and flavors in the kitchen, it’s a downright cheap option.
Whippers come in an insulated variety, made of metal with an insulated center, that’s useful for keeping contents cold. These are handy if you’re using the whipper only for whipped cream. These thermal versions can’t be heated in a warm water bath, though, making it harder to do hot foams or to partially poach the contents à la sous vide for egg-based custards, so snag a noninsulated one for those purposes.
You can also use a whipper as a source of pressure. One technique uses an adapter you can find in the plumbing section of your local hardware store to connect the threaded spray nozzle of the whipper to a length of plastic tubing. Fill the tubing with a hot liquid and some agar or another gelling agent (which we’ll talk about later—see page 418), let it set, and use the whipper as a source of compressed air to force-eject the “noodle.”
Don’t overlook the fact that cream whippers are also pressurized containers themselves, if you ignore the spray valve. Volatile compounds—most odors are volatile; otherwise, how could we smell them?—will dissolve into a liquid more readily under higher pressure. Dropping flavored items into the container (spices, fruit, peppers), covering them with a liquid (water, alcohol, oil), and pressurizing the container will rapidly infuse the liquid with the flavors. Then you just vent the pressure with the container in the upright position, taking care not to spray, unscrew the top, and pour your infused liquid through a strainer.
Another thing to try is using a CO2 cartridge to create “whipper fizzy fruit”—fruit that has been carbonated, giving it a fizzy texture. Try popping grapes, strawberries, or sliced fruit such as apples and pears into the canister and pressurizing it. Let it rest for an hour, depressurize the can, and remove the fruit. Not exactly haute cuisine, but fun to do as a party trick. Fizzy raspberries make a great basis for a mixed drink.
A few things to keep in mind when you’re working with a whipper:
• Make sure to get a whipper that allows for liquids other than cream—some manufacturers make “mini whippers” only usable with cream.
• Check that the gasket is properly seated and the threads on the lid are clean when screwing on the lid, unless you want chocolate cake batter, cream, or pancake mix sprayed 10 feet in a random direction.
• Always run your liquid through a strainer (~500 micron is fine—see page 348) to remove any particles that might clog the nozzle. You can skip straining things like plain cream, of course.
• When working with heavier batters, you can double-pressurize the canister. After pressurizing with one cartridge, remove it and pressurize with a second one. You’ll find that the pressure decreases as you run through the contents, because the airspace in the whipper increases as the contents are ejected.
• If your liquids fail to foam correctly, make sure they are cold! Cream will not foam when it is even slightly warm. Also try adding some gelatin, which provides structure. If you don’t mind taking a shortcut, try using flavored Jell-O.
• Don’t use gas cartridges made for nonfood applications, like BB guns. They aren’t food grade, and contaminants like manufacturing oils and solvents can come along for the ride.

A Few Low-Pressure Tricks
If high pressure raises the boiling point and increases the solubility of gases into liquids, then it follows that dropping air pressure decreases the boiling point and can remove dissolved gases from liquids. But you can do other fun tricks using a vacuum system that creates low pressures. Craving pickles right now? Air bubbles in your batter ruining cakes or clouding up your soups? Wondering how certain restaurants create “watermelon steaks” or the food industry makes chocolate candies like Aero or freeze-dried ice cream? The answer to all these questions lies within vacuum systems that create low-pressure situations.
• Instant pickles: Some foods—typically ones that pickle well—will spring back to their former shape after being exposed to a vacuum and having the pressure restored. You can take advantage of this property to cleverly suck a liquid into plant tissue. Squeeze a damp sponge and air will leave; let go while holding it under water and what was once air will become water. In cooking, this technique is called flash pickling. Microscopic air pockets in foods like cucumbers and onions will be pulled out and replaced with brine or other liquids, from flavor-infused oils to alcohol, and in a process that takes only minutes instead of days. Instant pickles!
• Removing air bubbles: As pressure drops, the volume a gas occupies increases and the density of the volume decreases. In a viscous liquid (e.g., soup, batter), the decrease in air density means any air bubbles present are going to become more buoyant. Just as the less-dense hot air inside a hot air balloon causes it to rise due to the relative difference of density between it and its environment, less-dense air bubbles in liquids become more buoyant and more likely to rise to the surface. This follows from Stokes’ Law—essentially, viscous liquids exert a drag force around really small spherical objects—and increasing the difference in densities can overcome that drag force. (Shaking or knocking a cake pan against a counter won’t remove smaller bubbles for this reason.) Soups and liquids can become cloudy during cooking due to microscopic air bubbles being blended in; drawing a vacuum on them will clarify them by buoying up the small bubbles. This isn’t beneficial just for visual reasons; removing those air bubbles can change the way a liquid tastes and the way custards bake.
• Translucent fruits: Under vacuum, air bubbles in fruits like pineapple and watermelon will expand with catastrophic results. Cells rupture and tissue walls collapse; upon return to atmospheric pressure, such foods can go through a serious case of the bends and become smaller, denser, and possibly more translucent due to the new absence of light-obscuring air pockets.
• Foamed foods: You can inject air bubbles into liquids and then expand them by dropping the pressure of the air around them. Whipped cream in a can, of course, is the familiar version of this: increase the pressure, dissolve the gas into the liquid, and then rapidly drop the pressure. The dissolved air comes out of solution, and our friend Stokes’ Law keeps it in place (at least for a while, in the case of whipped cream). It works with solids, too: creating chocolate foam, first attempted in the 1930s, involves adding bubbles into melted chocolate, changing the size of those bubbles with pressure changes, and then letting the chocolate set. In industry, manufacturers do this by increasing the pressure around liquid chocolate to drive the gas into suspension (as with whipped cream), then rapidly decompressing it to foam up the chocolate and allowing it to set. Seeing as even professional chefs don’t have rapid decompression chambers, the culinary way to do this is to use a cream whipper to force nitrous oxide into melted chocolate, spray it, evacuate a chamber with the chocolate inside it to cause the bubbles to expand, and then let it set.
• Freeze-dried foods: In our continuing series of “water is wonderfully weird,” the freezing and boiling points of water converge under a strong enough vacuum—called the sublimation point. The phase diagram on page 306 shows a point where solid, liquid, and gas all converge; at pressures below ~0.08 psi (6 hPa), ice converts directly to water vapor, skipping the liquid phase. Freeze-drying can create amazing results and also preserves a large amount of the nutritional value and flavor of the food. The few commercially available freeze-dried goods you might have experienced (such as freeze-dried instant coffee) may not taste as good as traditionally prepared foods, but this is more about economics and the ingredients being used than the freeze-drying process.
So now that I’ve hopefully got you excited about using vacuums, how can you actually do this at home? No, your vacuum cleaner won’t draw a strong enough vacuum. (Plus: eww, gross.) Luckily, there’s already a kitchen device that’s mostly up to the task: the vacuum sealer. Vacuum sealers are traditionally used by home cooks to seal and store foods. Instead of storing food in containers or plastic-wrapped bowls, food is placed into a food-safe plastic bag, air is sucked out of it by the unit’s air pump, and the bag is sealed by a fusing bar that melts and seals the opening of the bag. (Some units use one-way valve systems instead of fusing bars, but they don’t seal as well; avoid those.) The benefit? Removing air from the bag reduces oxidation of fats and minimizes odors that lead to freezer burn, plus the sealed food can be easily defrosted in water before being opened. Sous vide cooking, which we’ll cover next, also relies on vacuum sealers to package the foods being cooked.
PHOTOS USED BY PERMISSION OF CARL HILL-POPPER
To use techniques like flash pickling, look for a vacuum sealer that supports canning attachments. These are devices that fit over the lid of a wide-mouth glass jar and pull air out of the jar—which, being rigid, doesn’t collapse and squish the food, as happens with traditional vacuum sealing bags. Normally these tools are used to extend food’s storage life by reducing oxygen exposure in the jar, but for our purposes they allow for many of the tricks just listed. (True freeze-drying, I’m afraid, requires too strong a vacuum for extended times—many hours—and lower temperatures than are easily manageable at home.)
Vacuum sealing doesn’t pasteurize or sterilize foods.
Removing air does decrease the impact spoilage bacteria can have, but increases some pathological bacteria’s ability to grow. Unless you’re following specific instructions for canning or sterilizing foods, treat vacuum-sealed foods like any other perishable items: store them in the fridge and use them within a few days, or freeze them.
One more note: if you happen to have a vacuum chamber sealer, consider yourself lucky. Vacuum chamber sealers are countertop units with an interior chamber that can easily drop the pressure of that chamber to ~10% of atmospheric pressure. Like consumer vacuum sealers, they’re normally used for rapidly sealing food storage bags, but they operate more quickly and have the benefit of not drawing out liquid or squishing the food during sealing (just upon repressurization). Most of the tricks listed here are super simple with a good vacuum chamber.
Sous Vide Cooking: Low-Temperature Poaching
With a name like sous vide, this cooking technique sounds French, and for good reason: it was French chef Georges Pralus who introduced it to the culinary world in the 1970s. While the name may be unusual-sounding to non-Francophiles, sous vide cooking is not complicated and is one of the most useful techniques to appear in the professional cooking world in the past few decades.
In sous vide cooking, food is immersed in a temperature-controlled liquid bath, usually water, where the temperature of the bath is the same as the target temperature of the food being cooked. Given enough time, an egg will set to soft–poached stage at around 144°F / 62°C. To cook a soft-poached egg sous vide–style, you would drop an egg (still in its shell) into a liquid bath at 144°F / 62°C and leave it there until enough of the proteins denature (about an hour). Perfectly cooked poached eggs! As we’ll see, the same concept applies for many other foods.
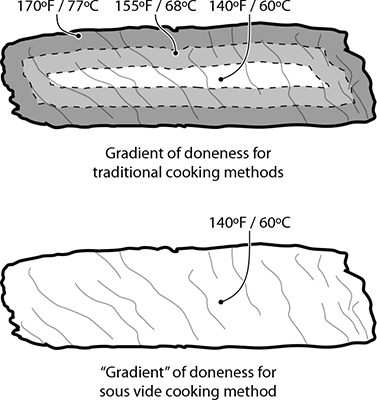
Without a temperature gradient, sous vide cooked foods have a uniform level of doneness.
Another benefit of sous vide cooking is that the entire portion of food cooks to the same uniform temperature. There’s no overcooked outer portion with items like meats; the entire piece of food has a uniform temperature and uniform doneness. With traditional cooking techniques, cooking something like a pork chop is a race to tie at the finish line: you want the internal temperature to reach one temperature at the same time that the external surface temperature reaches a different one. Steering two different temperatures at the same time isn’t difficult per se, but it does require skill. Sous vide cooking separates the task of reaching those two temperatures into two different stages: first, bring the entire piece of food up to the desired internal temperature (say, pork chops to 140°F / 60°C); once that’s done, bring the surface temperature way up by dropping the item into a hot pan or tossing it under a broiler for a minute to brown the outside via the Maillard reaction.
Sous vide is a funny name; it should have been called “water bath cooking” because the actual heat source is the water.
The name sous vide translates from the French “under vacuum.” Traditionally, you start the sous vide process by placing foods into a heat-safe vacuum-sealed plastic bag. Using vacuum sealers removes all the air in the bag, which allows the heated liquid of the bath to transfer heat into the food while preventing the liquid from coming into direct contact with the food. This means the heating liquid (e.g., water) does not chemically interact with the food: the flavors of the food remain stronger, because the liquid can’t dissolve and carry away any compounds from the food.

The steak tip on the left was cooked sous vide at 140°F / 60°C; the one on the right was pan-seared. Note that the sous vide steak has no “ring of doneness”: it’s medium-rare, center to edge.
Sous vide cooking doesn’t have to be done with a vacuum-sealed bag. Eggs, for example, are already sealed (ignoring the microscopic pores). If using plastic is a concern for you, you can use small glass jars with oils or marinades inside with the food submerged, making sure that there’s no air getting in. If you use this method, make sure the glass container is small enough that the contents come up to temperature quickly for food safety reasons.

Cooking eggs in a sous vide bath at 144°F / 62°C.
Picking the temperature of the liquid bath is, in theory, simple: understand the chemistry of the food being cooked and pick a temperature range that’s warm enough to trigger desirable reactions and cool enough to avoid triggering other reactions. For the variable of time, the food needs to cook long enough for sufficient quantities of the desired reaction to occur, exactly as we talked about when discussing the rate of reactions (see page 136). Normally these time and temperature ranges are based on certain families of proteins (e.g., collagen, myosin, actin) or polysaccharides like pectin and hemicellulose. We’ll cover temperature ranges for sous vide cooking, along with tips for various items, in the coming pages.
After cooking an egg sous vide, crack it open and drop the egg (without the shell!) into a pot of just-boiled water. Then fetch the egg out immediately. The hot water will rapid-set the outside of the egg for better appearance and easier handling.
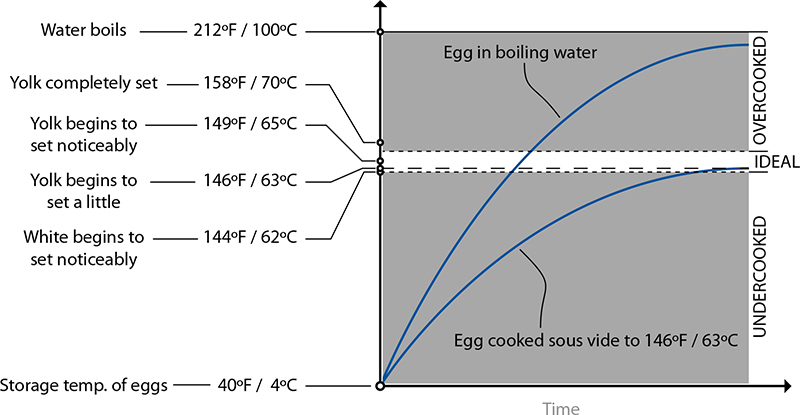
A “perfect” soft-cooked egg should have a slightly runny, custard-like yolk and a mostly set white. Eggs cooked in near-boiling water will overcook if the temperature of the egg ramps up above the “ideal” range before the egg is pulled out. In sous vide, the temperature of the water is equal to the ideal temperature of the cooked egg, so it cannot overcook.
Other points about sous vide cooking:
• Other fluids can be used instead of water, such as oil, or even melted butter. Meats don’t absorb fats the same way that they can water, so using one of these as the liquid medium can allow you to skip sealing, or to seal with fats inside the bag. This can be extremely useful for those foods that might be difficult to vacuum seal. Chef Thomas Keller, for example, has a recipe for poaching lobster tails in a bath of butter and water (beurre monté—melted butter with water whisked in—which will remain emulsified at higher temperatures than butter alone). Even if you’re not using a liquid bath of fats, adding a small amount of liquid inside your bag will help prevent the food from being “squeezed” flat by the vacuum step.
• Temperature-controlled air wouldn’t work, as the rate of heat transfer is too slow compared to water—roughly 23 times slower. Given the low temperatures involved, something like chicken in a 140°F / 60°C “air bath” (like a low-temperature oven) would take so long to come up to temperature that the chicken would spoil. (Thinly sliced meats, though, do heat up quickly enough—this is how beef jerky is made!) Using a liquid such as water ensures that heat can penetrate the food via conduction—liquid touching container touching food—rather quickly.
• Sous vide cooking doesn’t work on all meats and fish: the textures of some foods will break down when held at temperature for any extended period of time. Some species of fish break down due to enzymatic reactions that normally occur at such a slow rate that they are not noticeable in traditional cooking methods.
Sous Vide Hardware
Sous vide cooking requires very little in the way of hardware: a source of heat to keep a liquid bath at temperature, something to hold the liquid, and a way to package the food being cooked. Commercial chefs tend to use expensive professional immersion recirculators dropped in large pans and chamber vacuum sealers; fortunately, there are now several products available for the home cook who wants to try sous vide.
There are two general styles of sous vide units: “clip-on” ones that attach to the side of a pot, and standalone units that have a reservoir that’s better insulated, and thus more energy efficient. Which to use is a matter of counter space and preference. If you’re unsure, go for a clip-on one; they’re cheaper to buy and use less counter space. Search online, or see http://cookingforgeeks.com/book/sousvidegear/ for suggestions on current products.
Besides commercial consumer products that supply the heat, a number of emerging products provide the sous vide logic for setups based on “BYOHS” (bring your own heat source). These work by controlling the heat for stovetop burners or appliances like slow cookers: stick a probe thermometer in a pot of liquid or the bowl of the slow cooker, and the device will adjust the burner knob or cycle the appliance power on and off to moderate the temperature. While not as accurate as heater-based sous vide gear, which typically has a water agitator to circulate water to prevent cold spots, the BYOHS method is appealing for its simplicity and cheapness, and many sous vide dishes cooked this way will turn out fine. Hopefully we’ll see more kitchen appliances from mainline manufacturers incorporate digital probes as part of the hardware—why not have a USB port on your stovetop for a probe?—but in the meantime, there’s plenty of fun to be had with wire cutters and rigging up your own sous vide setup.
The other hardware needed is something to package the food being cooked. Traditionally vacuum sealers are used, hence the cooking method’s name. Vacuum-packing food removes air from the package so that air bubbles don’t insulate the food or cause the bag to float, which would prevent the face-up side of the bag from heating up. If you get a sous vide cooker, you really should get at least a cheap consumer vacuum sealer (make sure to get vacuum sealing bags that are heat-safe!).
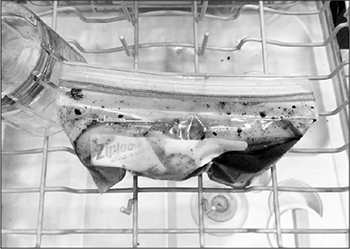
In a pinch, you can use certain brands of resealable sandwich and storage bags (freezer bags work best). To use such a bag, place the food in the bag and add a small amount of marinade, water, or oil (this will help remove air pockets). Then submerge most of the bag in a container of lukewarm water, leaving just the sealing strip at the top above water. Massage the bag to work out any air bubbles, and then seal it. If you’re concerned about plastic, use small canning jars filled with food and marinade (no air!), making sure they are small enough to heat up quickly in the water bath.
Make sure that any plastic you’re using is heat-stable (formulated with plasticizers that are okay with heat) and free of BPA. SC Johnson, the manufacturer of Ziploc bags, does not use BPA and claims its bags are heat-safe up to 170°F / 76°C.
Sous Vide Cooking and Food Safety
Sous vide cooking creates amazingly tender chicken, perfect soft-cooked eggs, and succulent steaks. It can also create the perfect breeding ground for not-so-succulent bacteria if done improperly. Here are a few things to be aware of when cooking sous vide:
• The heat involved in sous vide cooking is very low, so it’s possible to violate the “40–140°F / 4–60°C danger zone” rule (see page 170) and its derivative rule, “Thou shalt pasteurize all potentially contaminated foods.” Meats can be cooked to a point where they are texturally done—medium rare, proteins denatured—but have not been held for enough time at temperature to be pasteurized (i.e., had enough bacteria and parasites rendered nonviable). With sous vide cooking, pay attention to hold times to correctly pasteurize foods. A perfect medium-rare hamburger can be cooked and pasteurized with correct hold times!
• Pasteurization is not always an instantaneous process. When cooked to lower temperatures, food must be held at temperature for a sufficient period of time for the appropriate reduction of bacteria to occur. Guidelines that say to cook chicken breasts to 165°F / 74°C are easy to understand because they don’t rely on hold time, plus have a wide margin of error for inaccurate thermometer reading. But you can pasteurize foods at lower temperatures, given longer hold times and accurate thermometers. The hold time for chicken breasts at 140°F / 60°C is around half an hour, meaning the meat needs to reach 140°F / 60°C and then sit at that temperature for at least that long.
• In the US FDA’s Bad Bug Book, the highest survival temperature listed for a foodborne pathogen is 131°F / 55°C, for the relatively uncommon Bacillus cereus; the next highest survival temperature listed is 122°F / 50°C. While these temperatures are below those used in cooking meats sous vide, there’s still a safety issue: given enough time during the heating-up phase, some pathogens can produce harmful toxins. To be safe, make sure that the core temperature of your food product reaches the target temperature within two hours.
• For individuals concerned about safety, sous vide can be a great cooking method: you have the tools to properly pasteurize pathogens. As a guideline, get food above 136°F / 58°C—the lowest temperature given in the US FSIS food guidelines—within a two-hour window and hold it for long enough to pasteurize it. Pay attention to hold times!
You can hold food above 140°F / 60°C for as long as you want; it’s actually safer than storing food in the fridge. The downside is that some reactions, such as enzymatic activity, will continue to occur, leading to potential textural issues when the food is held too long.
• Sous vide cooking methods are either cook-hold or cook-chill. In cook-hold, the food is heated up and held at that temperature until it is served. In cook-chill, the food is heated up, cooked, then rapidly chilled in the fridge or freezer for later use. (Use an ice-water bath to quickly drop the temperature.) With the cook-chill approach, a greater amount of cumulative time is spent in the danger zone: first while the food is being heated, then while it’s being chilled, and then while it’s being heated again. Give preference to the cook-hold method.
Cooking Times for Fish, Poultry, Beef, and Fruits and Veggies
While the principle of sous vide cooking is the same regardless of the food in question, the temperatures required to correctly cook and pasteurize any given food depend upon its specifics. Different meats have different levels of collagen and fats, and denaturation temperatures for proteins such as myosin also differ depending upon the environment that the animal came from. Fish myosin, for example, begins to denature at temperatures as low as 104°F / 40°C, while mammalian myosin needs to get up to 122°F / 50°C. (Good thing, too; otherwise, hot tubs would be torture for us.) Slight changes in cooking temperature can yield improvements in quality—experiment!
Data for the graphs in this section are from Douglas Baldwin’s “A Practical Guide to Sous Vide Cooking”; see the interview with him on page 327 to learn more. If you are going to be using sous vide cooking in a professional setting, I highly recommend consulting Chef Joan Roca’s book Sous Vide Cuisine (Montagud Editores, 2005).
Fish and other seafood
Fish cooked sous vide is amazingly tender, moist, and succulent. Unlike fish that has been sautéed or grilled—cooking methods that can lead to a dry and rough texture—sous vide fish can have an almost buttery, melt-in-your-mouth quality. Other seafood, such as squid, also responds well to sous vide cooking, although the temperatures do vary.
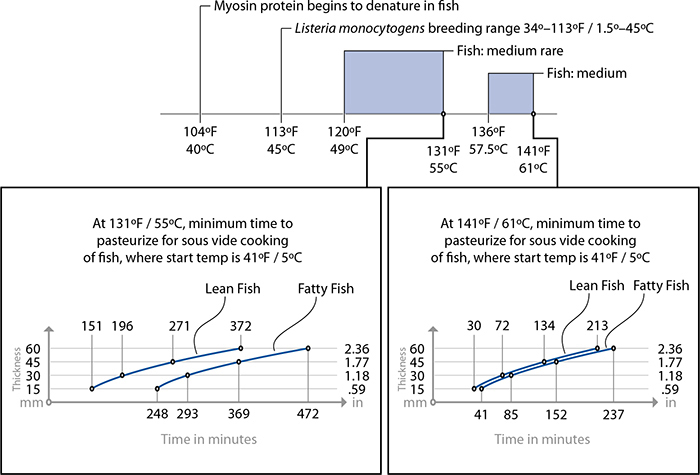
Cooking fish sous vide is so straightforward that you don’t need a recipe to understand the concept. The following tips should help in your experimentation with sous vide fish:
• Fish cooked to a doneness level of medium rare (131°F / 55°C) or more undergoes pasteurization by being held at temperature for a sufficient length of time (see the time-for-thickness graphs provided for lean and fatty fish).
• Lean fish, such as sole, halibut, tilapia, striped bass, and most freshwater fish, requires less time to cook and pasteurize than fattier fish, such as arctic char, tuna, and salmon.
• For fish cooked to a doneness level of only rare (i.e., cooked in a water bath set to 117°F / 47°C), pasteurization is not possible. Thus, if you are poaching salmon at 117°F / 47°C, be mindful that it will not actually get hot enough to kill all types of bacteria commonly implicated in foodborne illnesses. Cooking fish at 117°F / 47°C for less than two hours presents no worse an outcome than eating the fish raw, so the usual precautions for fish intended to be served raw or undercooked apply: buy sashimi-grade, previously frozen fish to eliminate parasites (see page 180), and don’t serve the fish to at-risk individuals.
The US FDA’s 2005 Food Code excludes certain species of tuna and “aquacultured” (read: farm-raised) fish from this requirement, depending upon the farming conditions (see US FDA Food Code 2005 Section 3-402.11b).
• If the type of fish you cook ends up having white beads on the surface after cooking (coagulated albumin proteins), try brining it the next time. Use a 10% salt solution for 15 minutes before cooking. This will “salt out” the albumin via denaturation.
Chicken and other poultry
One of the greatest travesties regularly foisted upon our dinner plates is overcooked chicken. Properly cooked chicken is succulent, moist, and bursting with flavor—never dry or mealy. The challenge in cooking chicken is that, from a food safety perspective, ensuring pasteurization (sufficient reduction of the bacteria that cause, say, salmonellosis) also overcooks it. “Instant” pasteurization, which is what most recipes call for, happens at 165°F / 74°C, but at this temperature most proteins also denature, giving the chicken that unappealing dry, mealy texture. However, pasteurization can be done at lower temperatures, given longer hold times. Sous vide is, of course, extremely well suited for this: so long as you hold the chicken for the minimum pasteurization time required for the temperature you’re cooking it at, you’re golden. Even if you hold it too long, as long as it’s below the temperature at which actin denatures, the chicken will remain moist. Another benefit of sous vide!

Sous vide chicken breast
As with fish, you don’t need a recipe in the traditional sense to try out sous vide cooking with chicken. Here are some general tips:
• Chicken has a mild flavor that is well suited to aromatic herbs. Try adding rosemary, fresh sage leaves, lemon juice, and black pepper, or other standard flavors, in the bag. Avoid garlic, however, because it tends to impart an unpleasant flavor when cooked at low temperatures. When adding spices, remember that the items in the bag are held tightly against the meat, so herbs will impart flavors primarily in the regions that they touch. I find that finely chopping the herbs or puréeing them with a bit of olive oil works well.
• As with other sous vide items, allow space between the individual items in the vacuum bag to ensure more rapid heat transfer, or place individual portions in separate bags.
Beef and other red meats
There are two types of meats, at least when it comes to cooking: tender cuts and tough cuts. Tender cuts are low in collagen, so they cook quickly to an enjoyable texture; tough cuts require long cooking times for the collagen to dissolve. You can use sous vide for both kinds of meat; just be aware of which type of meat you’re working with.
Many chemical reactions in cooking are a function of both time and temperature. While myosin and actin proteins denature essentially instantly at traditional cooking temperatures, other processes, such as the denaturation and hydrolysis of collagen, take noticeable amounts of time at those same temperatures (collagen is a really complicated molecule; see page 162). As with most temperature-dependent reactions, the rate of reaction increases as the temperature increases, so while mammalian collagen will begin to break down at around 155°F / 68°C, poultry legs and stews are often simmered at or above 170°F / 77°C. Even at this temperature, the collagen still takes hours to break down.
The drawback to cooking either type of meat with traditional cooking methods—searing low-collagen meats or stewing high-collagen ones—is that other proteins also denature. Fattier cuts of meat will mask the resulting dryness—hence the premium paid at the butcher counter for meats with good fat marbling. There is another way, though: cooking the meat in a sous vide environment will denature some of the proteins (e.g., myosin) and hydrolyze others (with sufficient time, collagen) while leaving other proteins native, preventing the dryness associated with traditional cooking methods. For low-collagen cuts of meat, the results are stunning: perfectly medium-rare meats in under an hour. For tougher cuts of meats, though, the catch is that the rate of reaction for collagen hydrolysis at these temperatures is so slow that the cooking time stretches into days. This isn’t a problem, technically speaking, as long as you don’t mind the wait.
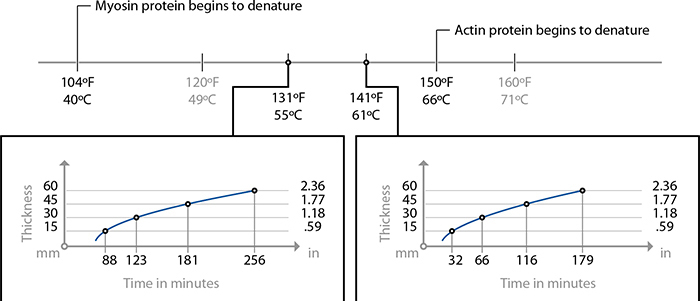
Cooking times for low collagen cuts of meat. For high-collagen cuts, use 24–48 hours at 141 F / 61 C to break down collagen.
Fruits and vegetables
Vegetables, like fish and meats, can also be cooked sous vide. Unlike proteins, the starches in vegetables don’t begin to break down until much warmer temperatures, typically around 180–190°F / 82–88°C. For professional cooks, where consistency and exact reproducible steps are important, cooking vegetables sous vide will produce better results than traditional poaching and blanching.
For the home cook, though, sous vide vegetables raise an “Is it worth it?” question. If you’re sous vide cooking two different parts of a meal at the two different temperatures, then you need to either cook them sequentially, or be willing to pay for two sous vide setups. You can see where this is headed. I’ll confess here that I cook my vegetables the traditional way: blanching in a pot of hot water with a watchful eye, taking care to not overcook them.
Still, you should give sous vide cooking of vegetables a try and compare the outcome with traditional methods. Keeping fruits and vegetables in a sealed bag does keep their juices up against the tissues, leading to stronger flavors. Also consider that the ratios of sugars to starches in your fruits and vegetables will vary, even for the same source, over the course of the year, as weather changes. Expect to adjust cooking times as necessary.
Try this: drop some peeled and halved small carrots or asparagus into a bag, along with olive oil, salt, and pepper; seal the bag; and cook the vegetables at 185°F / 85°C for 10–15 minutes. You may need to rig up some insulation around your sous vide setup if it struggles to maintain temperature; covering with plastic wrap will also help. Asparagus cooked at 185°F / 85°C will remain vivid green; at 203°F / 95°C, it’ll have begun to fade.
Cooking times at 185°F / 85°C
Soft fruits (e.g., peaches, plums): 20–60 minutes
Firm fruits (e.g., apples, pears): 25–75 minutes
Tender vegetables (e.g., asparagus, sliced fennel, peas): 10–60 minutes (longer for thicker slices)
Root vegetables (e.g., potatoes, beets): 2–4 hours
Beyond cooking veggies sous vide, you can do some other useful tricks with them in a water bath. The geeky way to think about cooking is to consider the addition of heat to a system. Adding heat isn’t a spontaneous thing: there will always be a heat gradient, and the difference between the starting and target temperatures of the food will greatly affect both the cooking time and the steepness of the gradient.
This is one reason to let a steak rest at room temperature for 30 minutes before grilling: 30 minutes is short enough that bacterial concerns are not an issue, but long enough to shrink the temperature difference between raw and cooked steak by a third. You can use a water bath to the same effect for vegetables: reduce the heat delta by holding them in a moderate-heat water bath (say, 140°F / 60°C) for 15–20 minutes, and then steam or sauté them.
I often cook steak tips at the same time that I preheat hardy green vegetables like kale or collard greens, using the same water bath for both the steak and the veggies. The veggies don’t actually cook at the temperature that the meat is cooking at! When I’m ready to serve the dinner, I fetch the greens out and give them a quick sauté in a frying pan. Because the greens are already warm, they reach a pleasant cooked texture in just a minute or two. Then I transfer them to a plate and, using the same frying pan, I give the steak tips a quick sear to brown the outsides and finish them off in no time.
There’s another fun trick you can do with vegetables, and this is one where a sous vide setup is definitely needed. Ever wonder why vegetables like carrots in some canned soups are mushy, textureless blobs, but are firm in other brands? It’s not because of differences in the variety of carrots being used. Some vegetables—carrots, beets, but not potatoes—exhibit a rather counterintuitive behavior when precooked at 122°F / 50°C: they become “heat resistant,” so they don’t break down as much when subsequently cooked at higher temperatures. Holding a carrot in a water bath at around 122°F / 50°C for 30 minutes causes enhanced cell-cell adhesion, science lingo for “the cells stick better to each other,” which means that they’re less likely to collapse and get mushy when cooked at higher temperatures or stored for extended periods of time.
During the precooking stage, calcium ions help form additional crosslinks between the walls of adjoining cells, literally adding more structure to the vegetable tissue. Since “mushy” textures occur because of ruptured cells, this additional structure keeps the vegetable tissue firmer by reducing the chance of cellular separation.
The normal solution to mushy vegetables is to refrain from adding them until close to the end of the cooking process. This is why some beef stew recipes call for adding vegetables such as carrots only in the final half-hour of cooking.
For industrial applications (read: canned soups), this isn’t always an option. In home cooking, while you won’t need this trick, it’s a fun experiment to do. Try holding carrots at 120–130°F / ~50–55°C for half an hour and then simmering them in a sauce mixed in with a batch of sliced carrots that hasn’t been heat-treated. (You can cut the heat-treated carrots into slightly different shapes—say, slice the carrot in half and then into half-rounds, versus full-round slices—if you don’t mind your experiment being obvious.)

Try par-cooking vegetables in your sous vide setup at the same time that proteins are cooking. When you’re ready to serve the meal, give the greens a quick sauté to finish them.
Making Molds
We tend to take the shapes of foods for granted, but that doesn’t mean we should. There are lots of amazing things you can do with molds besides making heart-shaped chocolates or ice cream cones. You probably don’t think of cake pans or cookie cutters as molds, but they change the shapes of foods: cake pans confine the 3D volume that batters can fill and cookie cutters define the 2D shape of rolled doughs. What sorts of fun arts-and-crafts things can we do to make our own shapes?
A quick primer on molds: molds can be either rigid or flexible and heat-safe or not. Rigid, heat-safe molds are almost always metal (historically copper) and are used for baked foods such as cakes and madeleines as well as cold-setting foods like gels (mmm, Jell-O), chocolates, and sugar decorations. Flexible culinary molds are made with either plastic or food-grade silicone rubber, the latter also being heat-stable.
Before making a mold, think about what food you want to put in or on it. Do you need a heat-safe mold? Does the mold need to be flexible for you to be able to remove the food? Gels are flexible and don’t need to be heated, so even rigid plastic molds work. Jell-O will work (rather boringly so); or get creative and use a flavored panna cotta recipe (see page 424). Heat-safe molds are needed for sugar work—say, shaped lollipops—as well as batters that need to be baked (Bundt cake, anyone?). For these foods, use either metal or silicone molds, opting for either a stiff or a flexible one based on the food.
Enough talk about how molds are normally used. What I want to discuss is how to make your own—in whatever shape you like!
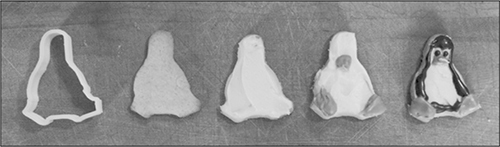
Tux the Penguin cookies, made using a CNC-printed cookie cutter. See http://cookingforgeeks.com/book/cookie-cutter/ for files.
Cookie cutters are easy to create, and making one is a fun holiday project. Plus, you probably already have everything you need on hand. Want an R2-D2–shaped cookie? Grab an empty aluminum soda can, your kitchen shears, and a pair of needle-nose pliers. Snip a round strip from the can, fold in the top and bottom of the strip to create clean edges, and use the pliers to bend and shape the strip. (A cardboard cutout template of the desired shape, R2-D2 or otherwise, will help.) If you happen to have access to a CNC (computer numeric control) printer, you can print a cookie cutter out of ABS plastic molds and wrap it in aluminum foil (ABS plastic isn’t food grade, and some extruder heads contain lead).
Simple crude chocolate and sugar candy molds can be made by pressing an object into a layer of cornstarch. Like with sand casting, pressing an object into the cornstarch and removing it leaves a “footprint” behind. Fill that void with chocolate or hard-crack state sugar (sugar syrup heated to 300°F / 150°C),, let it cool, and voilà! While this method is quick—mmm, chocolate LEGOs—the cornstarch tends to stick to the finished food and the mold doesn’t pick up much in the way of details. It’s a fun experiment that doesn’t require much work, but not likely to be a regular technique.

Some molds are just surfaces on which foods are added, cooled, and then removed, such as for making ice cream cones and chocolate leaves. To make chocolate leaves, coat the back side of lemon or rose leaves with tempered chocolate, rest at room temperature for an hour or two, and peel the leaf off. Try coating the leaf with a thin layer of white chocolate first to highlight the leaf venation.
Silicone rubber molds are great at picking up detail (e.g., chocolate coins with recognizable faces) and are usable between –65°F / –53°C and 450°F / 230°C. The downside is availability and cost—you’ll have to order supplies online, and the cost for larger molds can add up. Still, it’s worth it: silicone rubber molds that you see at the store are one-part molds without much detail; the beauty of the DIY option is in detailed multipart molds that can be baked and flexed to pop off of various shapes. Flat objects (coins, keys) and convex objects (no concave shapes, so oddly, asparagus) are easiest to make molds of: drop the object into a flat tray, coat it to cover, let the mold set, remove and flip the object, and then coat the other side. Vintage plastic toys that come from simpler molds (e.g., cars, toy soldiers, dinosaurs) are easy enough to make molds of too: place the toy into a plastic container, coat it to cover, let the mold cure, then unmold the toy and carefully cut the mold in half. (You may need to cut a sprue in before being able to pour food in.)
Plaster of Paris, a.k.a. calcium sulfate, is used to make plaster bandages, which are heat-safe and nontoxic. Plaster bandages are rolls of cloth coated with calcium sulfate; a strip is cut off, dipped in a bowl of water, and wrapped around the object (historically, a broken arm; these days, mainly objects for arts-and-crafts applications). Coat whatever you’re going to wrap—beach ball, tree branch, large tire inner tube—with a generous layer of shortening first, which will act as a mold release, and then cover the object with three to five layers of plaster bandage. If you need to cut the plaster bandages after they’re dry, use an angle grinder with a grit disc. Food-grade calcium sulfate is hard to find (it’s used in making tofu but not in plaster bandages), so you may want to line the mold with parchment paper, depending upon your use.

You don’t have to make a mold to get creative: use existing molds in different ways. Bake an “Apple” apple pie in a square cake pan. Use a knife to cut the logo, or do what Lenore Edman and Windell Oskay of Evil Mad Scientist did: use a laser cutter. See http://cookingforgeeks.com/book/appleapplepie/ for details.
How to Make a 500-Pound Doughnut
Doughnuts. Is there anything they can’t do?
—Homer Simpson (“Marge vs. the Monorail”)
Making a monster doughnut on network television is one of those crazy life stories that you never expect to happen to you. But after the first edition of this book came out, I got a call from a production company looking for a food science geek to be in a show for a “network” that “deals with food.”
“How big of a doughnut do you think you could make?” they wanted to know. The show idea was simple: two chefs, each assisted by a pastry chef and a food science geek, would compete to make the tastiest, prettiest, and biggest version of an assigned food. The first (and only) episode of Monster Kitchen was broadcast on July 19, 2011, and showed us making a giant doughnut.
After some reading—yes, a baked doughnut still qualifies as a doughnut—I came up with a plan. I’d make a mold that we could bake in a room-sized industrial oven, Pastry Chef Amy Brown would mix up the batter in a cement mixer, and Chef Eric Greenspan would take care of the doughnut filling. The entire plan hinged on the ability to make a monster mold in the shape of a doughnut. A 5-foot-wide (1.5 meter) mold. As big as Chef Greenspan himself. Yikes.

Silicone rubber is often used for food molds, but the curing time would be too long, plus the mold would be too thick for the dough to cook. Metals like copper are also common for food molds, but at this scale a copper mold would be either too flimsy to hold its shape or too thick to manipulate. This left plaster—and plaster bandages, rolls of gauze coated with plaster powder. Bingo! Wet the strips and lay them on the surface of the object you want to create a mold of, and a few hours later they’ll have cured into a hard cast that’s also heat-safe.
For the positive—the item that provides the model for the mold—I needed something torus-shaped, like a white-water-rafting tube. At smaller sizes I could make a positive by crumpling up aluminum foil into the approximate shape and working it, but no way for a 5-foot one. After an admittedly grueling phone and online hunt (thanks, Chris!), we found a tire tube that was just over 5 feet in diameter. I had my materials to make a true torus-shaped mold and something to fabricate the mold around. Add cake batter, bake, and glaze, and you’ve got a monster doughnut.

Mini-monster doughnut mold (for ~1-foot-diameter / 30 cm doughnuts)
1 Make and check a mold positive by crumpling up aluminum foil into a torus shape and then wrapping it in plastic wrap. Check that it’ll fit into your oven (or if you’re daring, your deep-fat fryer) before continuing.
2 Coat the positive with shortening, which will act as a mold release.
3 Create the mold using plaster bandage strips, available online or at craft supply stores. Cut a strip a few feet long, wet it, and wrap it around the positive. Depending upon the size of your mold, either loop the strips around the torus, in which case you’ll have to cut it later, or wrap only the sides and top such that you can remove the aluminum/plastic wrap core later. Repeat until the entire tube is covered, at least 4 or 5 times. (In the show, the mold was wrapped 8–10 times; that was just barely enough for that size.)
4 Allow mold to cure, ideally 24–48 hours.
5 Remove the positive. If you left the bottom uncovered, flip the mold over and pull out the aluminum foil and plastic wrap. If you fully wrapped the mold, cut the top off with an angle grinder (wear a dust mask and eye protection!).
Cake doughnut recipe
The following doughnut recipe, based on Amy Brown’s work, makes fantastic cake-based doughnuts. (Doughnuts are often raised with yeast, but we opted to go with a baking powder and baking soda–based dough for a quicker rise.) Don’t let the “500-Pound Doughnut” title here fool you into thinking you can’t make regular doughnuts with this recipe.
1 In a bowl (for 1 dozen regular doughnuts), Hobart mixer (1-foot-diameter / 30 cm doughnut), or twenty 5-gallon food-grade buckets (5-foot-diameter / 1.5m doughnut), mix:
1 dozen regular doughnuts |
1-foot-diameter doughnut |
5-foot-diameter doughnut |
|
Flour (g) |
516 |
6,192 |
103,200 |
Sugar (g) |
238 |
2,856 |
47,600 |
Baking soda (g) |
3 |
36 |
600 |
Baking powder (g) |
9 |
108 |
1,800 |
Salt (g) |
3 |
36 |
600 |
Nutmeg (g) |
2 |
24 |
400 |
2 In another large bowl (for 1 dozen regular doughnuts or a 1-foot-diameter doughnut), or four 5-gallon food-grade buckets (5-foot-diameter doughnut), mix:
See notes on page 278 for buttermilk substitution
1 dozen regular doughnuts |
1-foot-diameter doughnut |
5-foot-diameter doughnut |
|
Buttermilk (mL) |
192 |
2,304 |
38,400 |
Butter (g) |
64 |
768 |
12,800 |
Vanilla extract (mL) |
4 |
48 |
800 |
Eggs, large (count) |
2 |
24 |
400 |
Egg yolks (count) |
1 |
12 |
200 |
3 Mix dry and wet ingredients, using a spatula for the smaller versions; for a 5-foot-diameter doughnut, mix in four batches in a stand cement mixer. Transfer dough to mold.
For regular doughnuts, roll the dough out to about ½” / 1 cm thickness. Cut it into doughnut shapes using a round punch (a large yogurt container, flipped upside down, will work); punch out the center as well. Fry dough in oil at 375°F / 190°C until golden brown, taking care to keep the heat at the appropriate temperature (use more oil and cook them one or two at a time). Flip doughnuts partway through baking. Once they’re done, transfer to a cooking sheet lined with paper towels to cool.
For 1-foot-diameter doughnuts, bake in an oven set to 350–375°F / 175–190°C until the middle reaches 195°F / 90°C. Remove the doughnut from the oven, cool for at least 30 minutes, and remove the mold. If you choose, optionally fry the doughnut at this point to set the outside to a crispier, mahogany brown color: transfer the doughnut to a sturdy cooling rack and, using a wire to hold that rack, lower the doughnut into a large pot of hot frying oil, fry it, and then lift it out.
For a 5-foot-diameter doughnut, start by baking it in a large oven, somewhere around 350–375°F / 175–190°C, for half a day or so, until the internal temperature reaches 180°F / 80°C. To fry it...well, it’s complicated, involving cranes, sand blasters, welders, dumpsters, and about a million BTU of burners. Thankfully, someone else was footing the bill.
Filling and glazing
As for filling and glazing the doughnut, that’s a personal choice. On the show, we used an egg custard filling and maple glaze, with chocolate-coated bacon strips as sprinkles. Personally, I think powdered sugar is pretty good, and way easier.

Wet Separations
Separating foods is an intriguing chemistry and physics problem that calls for clever solutions (pardon the pun). Many ingredients—olive oil, flour, butter, orange juice—start out as mixtures that involve a separation process to split them, like removing oil from an olive or splitting fat from milk.
How to separate out various parts of an ingredient depends on its properties. There’s the obvious property of size—straining pasta from water is easy—and some liquids, like fresh whole milk, separate on their own when given time. But how would you remove salt from water or separate flavors from liquids? The tools of the industrial world can help us answer these questions, based on differences in aspects like density, boiling points, and even magnetic properties. Here are some of the ways the food industry separates out liquid things:
• Mechanical filtration easily strains solids from liquids, separating pulp from pressed juices and turning cloudy liquids clear. Sometimes a double-whammy approach is taken: adding a temporary ingredient that sticks to cloudy stuff in liquid and then is filtered out to create crystal-clear liquids.
• Centrifuges separate mixtures based on differences in density and are easier to use than the previous option in industrial operations (no filters to clean). Countertop centrifuges can spin a small quantity of liquid, but industry needs something with way more capacity. One option is a decanting centrifuge, which takes a mixture in on one end and then spins the liquid, causing the denser items (pulp in juices, yeast in beverages) to separate out through one pipe while lighter items (fat from milk, vegetable oil from pressed vegetable matter) travel farther and exit through another pipe. Continuous-feed centrifuges are a rather clever way of scaling up mechanical filtration and natural separation.
• Drying, a.k.a. dehydration, works by evaporating water, reducing the moisture content for shelf stability; it changes the texture, color, and flavors of foods.
• Distillation works by evaporating a liquid into a gas and then condensing the gas into another container. The entire liquor industry is based on distillation, and perfumes and many fragrances are also separated out from water this way.
We’ll dive into potential kitchen uses of these separation techniques over the next few pages.
I’d be remiss if I didn’t include two other separation techniques and fun demos of how they work. Neither is useful in the kitchen, but they’re interesting!
• Magnetic separation is used in industrial processing to remove any “ferrous foreign bodies” like bolts or metal shavings that have accidentally entered the food supply. While a consumer countertop magnetic separator isn’t going to do much—toxic metals like mercury won’t respond to a magnetic field—there is a fun demo to show how this works with iron. Toss a handful of iron-fortified breakfast cereal into a blender or plastic bag, pulverize until it’s a powder, and then run a strong magnet through the powder. You should see small black specks coating the surface of the magnet.
• Chromatography separates compounds based on how quickly they travel through another material. I haven’t seen any home applications of chromatography, perhaps because it separates things on such a small scale that it doesn’t lead to culinary bliss. Try marking a paper towel with a row of lines from a few different pens and dipping the edge parallel to the lines into a glass of water. After a few minutes, you should see the various dyes separating out as the water travels through the marks and carries the various pigments different distances.
Mechanical Filtration
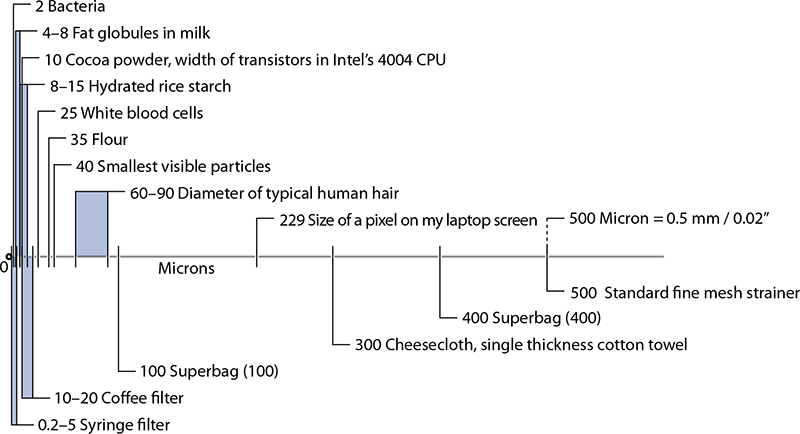
Sizes of common items (top portion) and common filters (bottom portion).
Filtering is a process for separating solids from liquids in a slurry. Separation creates two quantities: the liquid and the solids that are filtered out. Usually it’s the liquid that we want, but the solids can be desirable in some cases. Which filtration method to use depends on the particle size of the solids. You probably don’t think of straining spices and larger particles from something like broth as filtration, but it is (use a simple wire mesh strainer; plastic ones have larger holes, plus they invariably break). In commercial kitchens, a chinois—a conical strainer also used for milling soups and mashed potatoes into a finer texture—is commonly used too.
The interesting stuff with filtration happens at much smaller sizes, and is done in two general ways: the old-fashioned “hey, smaller and smaller holes” way (like running puréed nut milks through a strainer lined with cheesecloth), and a modern method using gels.
You can make your own almond milk by pureéing presoaked almonds and then filtering the purée through a standard kitchen strainer lined with cheesecloth or a clean terrycloth towel, which can trap particulates down in the ~300 micron range (gather up the edges of the towel and squeeze to let the liquid through, if necessary). Or “go industrial” and filter out fine particles with mesh filter sieves—these are heat-safe, reusable, and highly durable filters, typically used by the food industry in pressurized systems but fun to play with at home (search for part 6805K31 on http://www.mcmaster.com).
The more interesting way to filter really small particles is with gels, which can be used to separate out the smallest of solids that produce cloudiness in otherwise clear liquids. Gels work by trapping the particulates, and then the gel structure itself can be removed. The technique isn’t modern: consommé, a clear soup made by clarifying stock or broth, is traditionally made by simmering egg whites in the liquid, which bind to particulates and then coagulate and float, forming a large mass that’s easily removed. And beer and wine makers use isinglass, a collagen derived from fish bladders, to filter the liquids: the isinglass binds with yeast, making denser particles that flocculate and precipitate out. (Sorry, vegetarian beer lovers.)
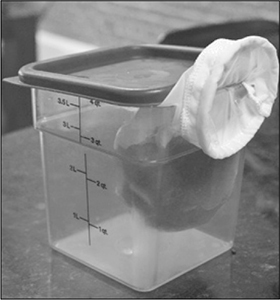
A modern technique for making clarified liquids is to freeze the liquid and drip-thaw it through a mesh filter sieve.
The two common compounds for creating gels for gel filtration are gelatin and agar. If you’re clarifying stock, the gelatin will already be present (from the bones); otherwise, add gelatin or agar (see page 423 for more on agar) to set the gel and then squeeze it through a towel—like the previous description of making almond milk—taking care not to squeeze too hard lest you end up with the gel breaking down and squeezing out of the towel too.
There’s a second, even-more-clever trick that you can do with gel clarifications: drip-thawing. Instead of squeezing the gel through a cheesecloth or towel, freeze and then thaw the gelled liquid in a towel-lined strainer over a bowl (make sure it’s totally frozen before thawing it; and if it’s going to take longer than an hour or two to thaw, thaw it in the fridge for food safety reasons). If the liquid you’re clarifying doesn’t naturally gel, you should add either gelatin (at ~0.5% concentration) or agar (at ~0.25% concentration) to your liquid. The downside of drip-thawing is the time it takes, but you can get clear liquids without needing to hit up the nearest centrifuge.
Centrifuges in the Kitchen
Centrifuges are like your washing machine’s spin cycle gone mad. By spinning objects—clothes, sample vials, tomato juice—at high speeds around a fixed axis, centripetal acceleration causes denser items to separate out faster from lighter things by a process called sedimentation.
Sedimentation normally happens with gravity and time. This causes seasonings to drift to the bottom of salad dressings or packaged items to settle beneath all the foam packing peanuts in a box (although I swear sometimes shippers just stick my order in the box first).
Centrifuges apply way more acceleration than gravity. A thousand g-forces—that’s a thousand times the force Earth’s gravity would exert!—is considered weak by centrifuge standards, although strong enough for many culinary uses. Centrifuges generate a lot of force (well, technically, acceleration), and that force separates compounds via sedimentation very quickly. To put this in perspective, astronauts experience about 2 g-forces during takeoff!
In the culinary world, centrifuges are used by industry. (Low-fat milk does not come from a cow on a diet!) High-end chefs use them, too. Tomato juice, when spun up, separates into three phases, with the middle one looking like clear water (not yellowish at all!) but tasting like tomato. Crushed plant matter—ground nuts, puréed fruits or veggies—can also be spun up to similar effect, making nut oils in minutes (the oil separates to the top, being less dense) and separating out flavorful fats from other plants, which would otherwise be impossible. If you’re lucky enough to have access to a centrifuge, start by spinning tomato juice; the shock of seeing water but tasting and smelling tomato is an unforgettable experience. Check out http://cookingforgeeks.com/book/centrifuge/ to see more.
Drying
You might not think of drying as a separation, but it is: water is separated via evaporation or sublimation when foods are dehydrated. Natural air drying of foods is perhaps the oldest preservation method, and it’s a simple method for transforming foods into shelf-stable versions that won’t spoil or go moldy. Even with modern refrigeration, we still dry foods this way for desirable changes to their texture, creating firm dried fruits, chewy beef jerky, and crispy kale chips.
If you’re lucky enough to live in a warm, arid region, any place where the summertime sunshine pushes the needle above 85°F / 30°C and keeps the humidity well below 60% (ah, California), drying fruit is an easy task. Pick fully ripened fruits, wash and clean them, cut stone fruits in half (removing any pit) and other fruits in slices (peppers and tomatoes, both biologically fruits!), and soak them for 10–15 minutes in lemon juice (or a ~4% solution of vitamin C). Pat the fruits dry, lay them out on a sheet of cheesecloth on top of an oven rack, and dry them during the day for a week or so (bring them indoors at night). If you think your fruit has any insects or insect eggs, freeze the dried fruit (below 0°F / –18°C) for two days or cook it at 160°F / 70°C for half an hour.

Dry low-moisture herbs like oregano, rosemary, sage, and dill by hanging them upside down in a dark, dry place until the leaves are brittle and crumble when pinched, several days to several weeks.
Why bother? Well, besides handling an influx of 20 pounds of apricots in a week (I grew up with an apricot tree in the backyard), creating your own dried foods can give you access to ingredients that far surpass what you can buy or that don’t even exist commercially. Store-bought paprika, even from good spice sellers, simply cannot compete with what you can make at home. Snag some peppers for making paprika, optionally smoke them if you like smoked paprika (it’s great with chicken; see page 28), and dry them. Once they’re dried, toss them into a blender to pulverize them. (If you have a green thumb, look for NuMex R Naky or Paprika Supreme seeds.)
Freeze-drying is also a dehydration process and works via sublimation—ice evaporating straight to vapor. While it has little impact on shape, flavors, or nutritional values, it’s expensive to do, so it’s generally used only in cases where water weight is an issue, like backpacking and space travel.
If you don’t live in an arid climate—or it’s not summertime—look into getting a food dehydrator. These are essentially boxes with a fan and a heater that speed up evaporation by maintaining air temperature and blowing aside water vapor. The heater isn’t for cooking so much as for maintaining temperature. Water, as it evaporates, will drop the food’s surface temperature, leading to a slower rate of evaporation; the heater fixes that issue and gives a slight bump in temperature to speed up evaporation. Toss in a bunch of sliced apricots or tomatoes and wait a few hours, and presto, they’re dried. (Dip those apricots in melted dark chocolate, by the way. You’re welcome.)
Food dehydrators can be put to other uses as well. Beef jerky, made the old-fashioned way, can spoil or have food safety issues when dried too slowly. A dehydrator fixes that. You can make other jerkies, too: salmon, deboned and sliced ¼” / ½ cm thick and dried for 3–6 hours turns out delicious. Or make your own fruit leathers (thin sheets of chewy dried fruit): purée fruit mixed with a teaspoon of lemon juice per cup of fruit and optionally add sugar to taste, smear the purée on a silicone sheet, and dry it. DIY Fruit Roll-Ups!
While water boils at 212°F / 100°C, it evaporates based on vapor pressure at lower temperatures, assuming the relative humidity is below 100%. You don’t have to heat foods to evaporate their water, although increasing the temperature will increase the rate of evaporation due to changes in vapor pressure.
Chilling Out with Liquid Nitrogen and Dry Ice
If there were one food-related science demo to rule them all, ice cream made with liquid nitrogen would surely be the winner. Large billowy clouds, the titillating excitement of danger, evil mad-scientist cackles, and it all ends with something delicious for everyone? Sign me up.
While the gimmick of liquid nitrogen ice cream never seems to grow old (heck, they were making it over a hundred years ago at the Royal Institution in London), a number of more recent culinary applications are moving liquid nitrogen (LN2, for those in the know) from the “gimmick” category into the “occasionally useful” column.

Common and uncommon cold temperatures.
But first, a digression into the dangers of liquid nitrogen. Nitrogen is mostly inert and in and of itself harmless, making up 78% of the air we breathe. The major risks are thermal shock and freeze burns, suffocation, and explosions. Let’s take each of those in turn:
• It’s cold. Liquid nitrogen boils at –320°F / –196°C. To put that in perspective, it’s further away from room temperature than oil in a deep-fat fryer: seriously cold. Thermal shock and breaking things are very real concerns with liquid nitrogen. Think about what can happen when you’re working with hot oil, and show more respect when working with liquid nitrogen. Pouring 400°F / 200°C oil into a room-temperature glass pan is not a good idea (thermal shock), so avoid pouring liquid nitrogen into a glass pan. Splashes are also a potential problem, especially for your eyes. Gloves, eye protection, and closed-toe shoes are all good ideas.
• It’s not oxygen. This means that you can asphyxiate as a result of the oxygen being displaced in a small room. When using liquid nitrogen, make sure you’re in a relatively well-ventilated space. Dorm room with the door closed = bad; big kitchen space with open windows and good air circulation = okay.
• It’s boiling. When things evaporate, they like to expand, and when they can’t, the pressure goes up. When the pressure gets high enough, the container fails and turns into a bomb. Don’t ever store liquid nitrogen in a completely sealed container. The container will rupture at some point. Ice plugs can form in narrow-mouth openings, too, so avoid stuffing things like cotton into the opening.
“Yeah, yeah,” you say, “thanks, but I’ll be fine.”
Probably. But that’s what most people think who end up being given, posthumously (posthumorously?), a Darwin Award (for stupid actions that eliminated them from the gene pool). What could possibly go wrong once you get it home? One German chef blew both hands off while attempting to follow a liquid nitrogen–based recipe. And then there’s what happened when someone at Texas A&M University removed the pressure-release valve on a large dewar—an insulated container designed to handle liquid gases—and welded the opening shut. From the accident report:
The cylinder had been standing at one end of a ~20’ × 40’ laboratory on the second floor of the chemistry building. It was on a tile-covered, 4–6” thick concrete floor, directly over a reinforced concrete beam. The explosion blew all of the tile off of the floor for a 5’ radius around the tank, turning the tile into quarter-sized pieces of shrapnel that embedded themselves in the walls and doors of the lab... The cylinder came to rest on the third floor, leaving a neat 20”-diameter hole in its wake. The entrance door and wall of the lab were blown out into the hallway. All of the remaining walls of the lab were blown 4 to 8” off of their foundations. All of the windows, save one that was open, were blown out into the courtyard.
“Okay, I promise to be safe. Where do I get some?”
Look for a scientific gas distributor in your area. Some welding supply stores also carry liquid nitrogen. You’ll need a dewar. Dewars come in two types: nonpressurized and pressurized. Nonpressurized dewars are essentially large insulated containers and are what you should use. The pressurized variety has a pressure-release valve—allowing the liquid nitrogen to remain liquid at higher temperatures, increasing the hold time—and is generally supplied with larger industrial orders.
Standard lab safety protocols for driving small quantities of liquid nitrogen around usually state that two people should be in the car and that you should drive with the windows down.
Small quantities of liquid nitrogen in nonpressurized dewars don’t require hazmat licenses or vehicle placarding when properly secured and transported in a private car (at least where I live). Some jurisdictions consider it a hazardous material, though—after all, handled improperly, it can and will cause death—so check into transportation of “material of trades” for your locale.
When it comes to working with liquid nitrogen, I find it easiest to work with a small quantity in a metal bowl placed on top of a wooden cutting board. Keep your eyes on the container, and avoid placing yourself in a situation where, if the container were to fail, you would find yourself getting splashed.
Don’t sit at a table while working with it. And placing a noninsulated container, such as a metal bowl, directly on the countertop is not a good idea. I once cracked a very nice countertop with an empty but still cold bowl during a talk (I’m still sheepishly apologizing for it).
One final tip: when serving guests something straightaway after contact with liquid nitrogen, use a digital thermometer to check the temperature to make sure the food is warm enough. As a guideline, standard consumer freezers run around –10°F / –23°C.
Making Dusts
One of the classic “silly things you can do with liquid nitrogen” is to freeze a leaf or a rose and then whack it against something to shatter it. Unlike traditional methods of freezing, liquid nitrogen freezes the water in the plant so quickly that the ice crystals do not have time to aggregate into crystals large enough to pierce the cell walls and destroy the tissue, meaning the leaf or flower won’t wilt when thawed.
In culinary applications, you can use this same property to create “dust” from plant material. Lavender flowers, for example, can be rapidly frozen, crushed with a mortar and pestle (which need to be chilled in a freezer to keep the frozen plant material from thawing), and then allowed to thaw back out. Some chefs have frozen larger items—beets, for example—causing them to shatter in an organic pattern that couldn’t be obtained with a knife.
Making Ice Cream
The standard formula for LN2 ice cream goes something like this:
cream + flavoring + liquid nitrogen + whisking / mixing = 30-second ice cream
Unlike with traditional ice cream bases, you don’t have to worry about the ratio of fats to water to sugar, at least for ice cream that will be consumed immediately. (Like there’s any other type.) Traditional ice cream bases rely on precise ratios of the ingredients to get something that has a freezing point over a broad range, making for some fascinating microscopic-level structures. Liquid nitrogen ice cream is closer to soft-serve ice cream: it’s frozen right at the point of consumption and isn’t set as hard. Freezing a batch of LN2 ice cream will give you something closer to a block of frozen milk if the milk fat ratios aren’t high enough.
The other benefit to LN2 ice cream is the lower temperatures in play, which are cold enough to freeze ethanol. While you can make ice cream with a small quantity of alcohol using traditional methods, in those versions the alcohol brings only a mild flavor. With liquid nitrogen, you can make a scoop of ice cream flavored with enough alcohol to really kick the flavor up, producing results unlike anything you’ve had before.


Be sure to take the necessary safety precautions!
To watch a video of making liquid nitrogen ice cream, see http://cookingforgeeks.com/book/icecream/.
Cooking with a Lot of Heat
20 minutes at 300 degrees is the equivalent of 5 minutes at...let’s see...(mumbles) 1,200 degrees.
—Marge Simpson, baking a cake (“24 Minutes”)
Common and uncommon hot temperatures.
If cooking at 300°F / 150°C produces something yummy, surely cooking at 1,200°F / 650°C must do the same in a quarter the time. Well, okay, not quite—if I said anything otherwise, hopefully your mental model of how heat is transferred through food and the importance of time and temperature would have you slamming this book shut while muttering something unfit to print.
But there are some fun edge cases, just as there are with “cold cooking,” where extremely high heat can be used to achieve interesting results. (And dangerous results—lighting your barbecue in two seconds with liquid oxygen? Yikes.) Let’s take a look at a few dishes that you can make by transferring lots of heat using blowtorches and high-temperature ovens, without melting the appliances.
In cooking, we generally avoid heating the surface temperatures of food above 380°F / 195°C, for good reason: that’s about the upper limit for burnt sugar to not taste like charcoal. Above this temperature, the next set of chemical reactions involves proteins and carbohydrates just tasting nasty. But in small quantities, we enjoy some of these reactions, using words like charred—char having an etymology of “to blacken,” hence charcoal. Charred, grilled, barbecue: all these words describe foods whose surface temperatures get hot enough to just barely burn, and that’s where broilers, searing-hot pans, and grills all come in: they transfer a lot of heat into food. But what about cases where you want to transfer a lot of heat to just one part of a food? Break out a blowtorch.

You can create a quick work surface for blowtorching by flipping a cookie sheet upside down and setting the ramekins on top.

“Upgrade” Bananas Foster—a simple and tasty dessert with butter and sugar and cooked bananas—by sprinkling sugar on the cooked bananas and then using a blowtorch to caramelize the sugar. Need a quick work surface? Flip a cast iron pan upside down and line it with foil.
Blowtorches can be used to provide very localized heat, enabling you to cook just those parts of the food at which you aim the flame. (Flamethrowers can be used too. I’m not going to confess to how much time I spent watching videos I found when searching for “flamethrower cooking.”) Torching tuna sushi keeps the interior raw but adds that charred flavor. Roasting peppers blisters their skin off without overcooking the inside. Browning meats cooked at low heat (sous vide or poached) quickly finishes off the outside. And, of course, creating the sugary crust on crème brûlée is the canonical excuse for a blowtorch in the kitchen. (A broiler will work too, if you run out of fuel.)
When it comes to buying a torch, skip the “gourmet” torches and head to a hardware store to pick up a real blowtorch. The smaller torches sold specifically for the kitchen work okay, but they don’t pack the same thermal punch as the hardware-store variety, which have larger nozzles and thus larger flames. Depending on taste sensitivity, some people may notice an “unburned fuel” taste (sometimes called “torch taste”) created by the fats and compounds in meat breaking down under extremely high temperature (fat is fuel, after all, and undergoes chemical reactions). If you’re noticing this, back off on the heat, and if your torch allows for it, increase the amount of air being mixed in!
Practice using a blowtorch by melting sugar sprinkled on a sheet of aluminum foil on top of a metal cookie sheet or cast iron pan. Don’t get the flame too close; this is the most common mistake people make when cooking with a blowtorch. The blue part of the flame is hottest, but the surrounding air beyond the tip will still be plenty hot. (You’ll know you’re definitely too close when the aluminum foil begins to melt, which happens at around 1,220°F / 660°C.) The trick with blowtorches is to heat up the area in passes, waving the torch back and forth over the total surface, so that you don’t linger on one spot and overheat it.
How to Cook a High-Heat Pizza
A serious examination of pizza is clearly a must-have for a book called Cooking for Geeks. Pizza covers so many variables: flavor combinations, Maillard reactions, gluten, fermentation, moisture levels, and temperature. We’ve covered most of these elsewhere in the book, but we haven’t yet talked about temperature, which is key to a good crust.
Great thick-crust pizzas have a great interior that comes from good dough that’s baked at moderate temperatures. My local delicious thick-crust pizza place runs its oven at 450°F / 230°C in the winter, 350°F / 180°C in the summer. (The oven can’t be run any hotter in summer without the kitchen becoming unbearable; they just bake the pizzas longer.) Easy enough.
But if you want to make a crispy thin-crust pizza, high heat is critical for creating a great crust. The lower temperature bound I’ve found acceptable for great flat-crust pizza was 600°F / 315°C. At 700°F / 370°C, the crust becomes noticeably better. The best thin-crust pizzas I’ve had were cooked in wood-fired brick ovens or on a grill over wood charcoal with temperatures running between 750°F / 400°C and 900°F / 480°C. Sadly, most ovens max out at 550°F / 290°C, making great thin-crust pizza hard to do in the oven. What’s a thin-crust-pizza-loving geek to do? If only there were a flow chart for this...

Wood charcoal grill temperature: 742°F/ 394°C.
Wood grill method
This is by far the easiest method. Grills fueled by charcoal or wood get hot, easily up into the 800°F / 425°C temperature range. (Propane grills tend to run cooler, even though propane itself technically burns hotter.)
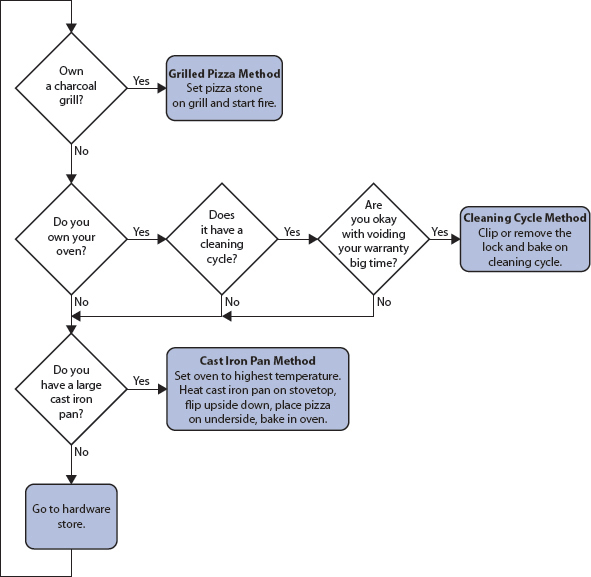
Place a pizza stone on top of the grill and light the fire. Once the grill is hot, transfer the pizza with toppings onto the grill. Depending upon the size of your grill, you may be able to cook the pizza directly on top of the grill, sans stone—give both a try!
Superhot cast iron pan method
Grill-lusting apartment dwellers have to get creative to create high-heat pizza. While most ovens limit the temperature to 550°F / 290°C, both the oven’s broiler and the stovetop reach higher temperatures.
Preheat the oven to 550°F / 290°C, or as hot as it goes.
Heat up an empty cast iron pan on the stovetop at maximum heat for at least 5 minutes.
Switch the oven to broiler mode, transfer the hot cast iron pan to the oven, flipping it upside down and setting under the broiler set to high. Par-bake the pizza dough until it just begins to brown, about 1–2 minutes.
Superheated cast iron under a broiler.
Transfer the dough to a cutting board and add sauce and toppings. Transfer the pizza back to the cast iron pan and bake until toppings are melted and browned as desired.
Cleaning cycle method (a.k.a. “oven overclocking”)
While consumer ovens top out at 550°F / 290°C, that doesn’t mean they can’t get hotter. It’s just dangerous, voids your warranty, and, given that the alternative ways of getting this kind of heat are far easier, is really not worth doing. Still, in the name of science...
To make your base, see the No-Knead Pizza Dough recipe and accompanying pizza-making instructions in “Pizza Dough—No-Knead Method” on page 271.
Ovens get a lot hotter—a lot, lot hotter—when they run in the cleaning cycle. The problem is that ovens mechanically lock the door, preventing you from slipping a pizza in and out, and leaving a pizza in for the entire cleaning cycle will result in less-than-tasty charcoal.
Cut or remove the lock, however, and ta-da! You’ve got access to a superheated oven. After some fiddling, I took my oven to over 1,000°F / 540°C. The first pizza we tried took a blistering 45 seconds to cook, with the bottom of the crust perfectly crisped and the toppings bubbling and melted.
However, the center of the pizza never had a chance to heat up, so the 1,000°F / 540°C pizza wasn’t quite right. Another attempt at around 600°F / 315°C was remarkably good but didn’t capture the magic of the crispy thin crust and toasty-brown toppings. At around 750–800°F / 400–425°C, however, we started getting pizzas that were just right.

Ovens aren’t designed to have their doors opened when running in the cleaning cycle. Honestly, I don’t recommend this approach. I broke the glass in my oven door and had to “upgrade” it, although it is cool to have bragging rights to an oven sporting a piece of PyroCeram, the same stuff the military used for missile nose cones in the 1950s. Given that an upside-down cast iron pan under a broiler or a wood-fired grill turns out delicious flat-crust pizzas, I’d recommend skipping the oven overclocking, as much fun as it is.