Multiple Reactions 8
Sometimes you just have to jump and build your wings on the way down.
—Carol Craig, entrepreneur
8.1 Definitions
8.1.1 Types of Reactions
There are four basic types of multiple reactions: parallel, series, independent, and complex. These types of multiple reactions can occur by themselves, in pairs, or all together. When there is a combination of parallel and series reactions, they are often referred to as complex reactions.
Parallel reactions (also called competing reactions) are reactions where the reactant is consumed by two different reaction pathways to form different products:
Parallel reactions

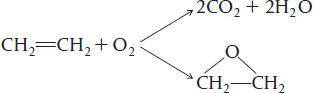
An example of an industrially significant parallel reaction is the oxidation of ethylene to ethylene oxide while avoiding complete combustion to carbon dioxide and water:
Serious chemistry
Series reactions (also called consecutive reactions) are reactions where the reactant forms an intermediate product, which reacts further to form another product:
Series reactions
An example of a series reaction is the reaction of ethylene oxide (EO) with ammonia to form mono-, di-, and triethanolamine:
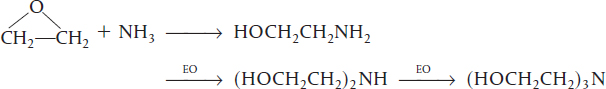
In recent years, the shift has been toward the production of diethanolamine as the desired product rather than triethanolamine.
Independent reactions are reactions that occur at the same time but neither the products nor the reactants react with themselves or one another.
Independent reactions
An example is the cracking of crude oil to form gasoline, where two of the many reactions occurring are
C15H32 → C12C26 + C3H6
C8H18 → C6H14 + C2H4
Complex reactions are multiple reactions that involve combinations of series and independent parallel reactions, such as
A + B → C + D
A + C → E
E → G
An example of a combination of parallel and series reactions is the formation of butadiene from ethanol:
C2H5OH → C2 H4 + H2O
C2H5OH → CH3CHO+H2
C2H4 + CH3CHO → C4H6+H2O
8.1.2 Selectivity
Desired and Undesired Reactions. Of particular interest are reactants that are consumed in the formation of a desired product, D, and the formation of an undesired product, U, in a competing or side reaction. In the parallel reaction sequence
or in the series reaction sequence
The economic incentive
we want to minimize the formation of U and maximize the formation of D because the greater the amount of undesired product formed, U, the greater the cost of separating the undesired product U from the desired product D (see Figure 8-1).
Selectivity tells us how one product is favored over another when we have multiple reactions. We can quantify the formation of D with respect to U by defining the selectivity and the yield of the system. The instantaneous selectivity of D with respect to U is the ratio of the rate of formation of D to the rate of formation of U.
Instantaneous selectivity
In Section 8.3, we will see how evaluating SD/u will guide us in the design and selection of our reaction system to maximize the selectivity.
Another definition of selectivity used in the current literature, , is given in terms of the flow rates leaving the reactor. is the overall selectivity.
Overall selectivity
Using a CSTR mole balance on species D and on species U, it is easily shown that for a CSTR the instantaneous and overall selectivities are identical, i.e., SD/U = See the Chapter 8 Expanded Material on the CRE Web site http://www.umich.edu/~elements/5e/08chap/expanded.html.
For a batch reactor, the overall selectivity is given in terms of the number of moles of D and U at the end of the reaction time.
Two definitions for selectivity and yield are found in the literature.
8.1.3 Yield
Reaction yield, like selectivity, has two definitions: one based on the ratio of reaction rates and one based on the ratio of molar flow rates. In the first case, the yield at a point can be defined as the ratio of the reaction rate of a given product to the reaction rate of the key reactant A, usually the basis of calculation. This yield is referred to as the instantaneous yield YD.
Instantaneous yield based on reaction rates
The overall yield, , is based on molar flow rates and defined as the ratio of moles of product formed at the end of the reaction to the number of moles of the key reactant, A, that have been consumed.
For a batch system
Overall yield based on moles
For a flow system
Overall yield based on molar flow rates
As with selectivity, the instantaneous yield and the overall yield are identical for a CSTR (i.e., = YD). From an economic standpoint, the overall selectivities, , and yields, , are important in determining profits, while the instantaneous selectivities give insights in choosing reactors, operating conditions, and reaction schemes that will help maximize the profit. There often is a conflict between selectivity and conversion because you want to make as much as possible of your desired product (D) and at the same time minimize the undesired product (U). However, in many instances, the greater the conversion you achieve, not only do you make more D, but you also form more U.
8.1.4 Conversion
While we will not code or solve multiple reaction problems using conversion, it sometimes gives insight to calculate it from the molar flow rates or the number of moles. However, to do this we need to give conversion X a subscript to refer to one of the reactants fed.
For Species A
and for Species B
For a semibatch reactor where B is fed to A
The conversion for the different species fed is easily included in the numerical software solutions.
8.2 Algorithm for Multiple Reactions
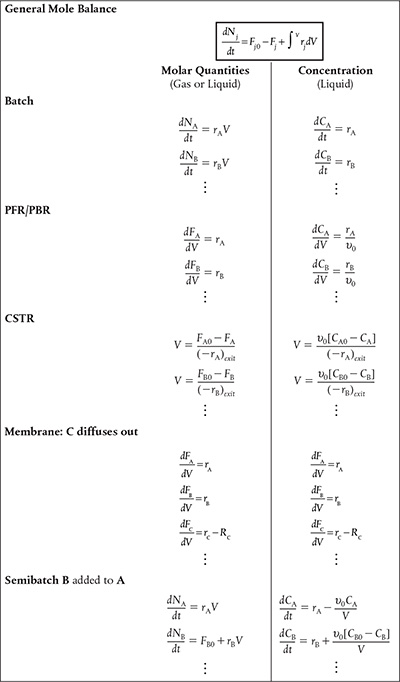
The multiple-reaction algorithm can be applied to parallel reactions, series reactions, independent reactions, and complex reactions. The availability of software packages (ODE solvers) makes it much easier to solve problems using moles Nj or molar flow rates F;· rather than conversion. For liquid systems, concentration is usually the preferred variable used in the mole balance equations.
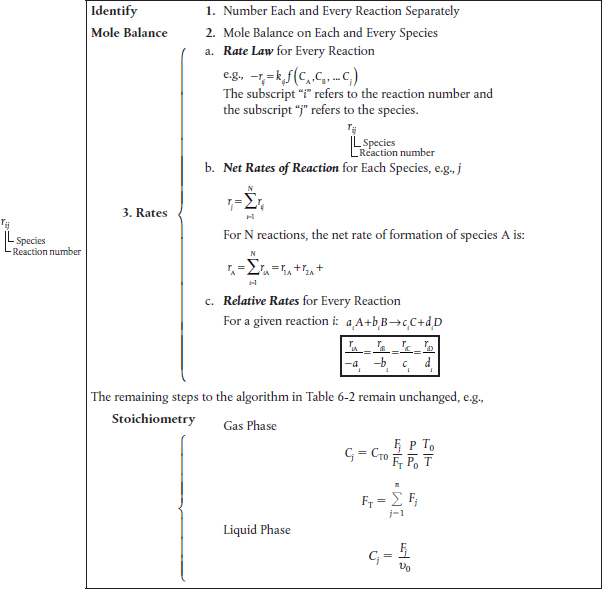
After numbering each and every reaction involved we carry out a mole balance on each and every species. The mole balances for the various types of reactors we have been studying are shown in Table 8-1. The rates shown Table 8-1, e.g., rA, are the net rates of formation and are discussed in detail in Table 8-2. The resulting coupled differential mole balance equations can be easily solved using an ODE solver. In fact, this section has been developed to take advantage of the vast number of computational techniques now available on laptop computers (e.g., Polymath).
Just a very few changes to our CRE algorithm for multiple reactions
8.2.1 Modifications to the Chapter 6 CRE Algorithm for Multiple Reactions
There are a few small changes to the CRE algorithm presented in Table 6-2, and changes to our CRE we wj|| describe these changes in detail when we discuss complex reactions in pie reactions Section 8.5. However, before discussing parallel and series reactions, it is necessary to point out some of the modifications to our algorithm. These changes are highlighted by brackets in Table 8-2. When analyzing multiple reactions, we first number every reaction. Next, we must perform a mole balance on each and every species, just as we did in Chapter 6 to analyze reactions in terms of the mole balances for different reactor types. The rates of formation shown in the mole balances in Table 6-2 (e.g., rA, rB, rj) are the net rates of formation. The main change in the CRE algorithm in Table 6-2 is that the Rate Law step in our algorithm has now been replaced by the step Rates, which includes three substeps:
• Rate Laws
• Net Rates
• Relative Rates
The concentrations identified in the lower bracket in Table 8-2 are the same concentrations that were discussed in Chapter 6.
For the competing reactions such as
the rate laws are
Rate laws for formation of desired and undesired products
The net rate of disappearance of A for this reaction sequence is the sum of the rates of formation of U and D:
where α1 and α2 are positive reaction orders. We want the rate of formation of D, rD, to be high with respect to the rate of formation of U, rO. Taking the ratio of these rates [i.e., Equation (8-6) to Equation (8-7)], we obtain the instantaneous selectivity, SD/U, which is to be maximized:
Instantaneous selectivity
8.3.2 Maximizing the Desired Product for One Reactant
In this section, we examine ways to maximize the instantaneous selectivity, , for different reaction orders of the desired and undesired products.
Case 1: αλ > α2. The reaction order of the desired product, α1; is greater than the reaction order of the undesired product, α2. Let a be a positive number that is the difference between these reaction orders (a > 0):
α1 – α2 = a
For α1 > α2 , make CA as large as possible by using a PFR or batch reactor.
Then, upon substitution into Equation (8-10), we obtain
To make this ratio as large as possible, we want to carry out the reaction in a manner that will keep the concentration of reactant A as high as possible during the reaction. If the reaction is carried out in the gas phase, we should run it without inerts and at high pressures to keep CA high. If the reaction is in the liquid phase, the use of diluents should be kept to a minimum.1
1 For a number of liquid-phase reactions, the proper choice of a solvent can enhance selectivity. See, for example, Ind. Eng. Chem., 62(9), 16. In gas-phase heterogeneous catalytic reactions, selectivity is an important parameter of any particular catalyst.
A batch or plug-flow reactor should be used in this case because, in these two reactors, the concentration of A starts at a high value and drops progressively during the course of the reaction. In a perfectly mixed CSTR, the concentration of reactant within the reactor is always at its lowest value (i.e., that of the outlet concentration) and therefore the CSTR should not be chosen under these circumstances.
Case 2: α2 > α1. The reaction order of the undesired product is greater than that of the desired product. Let b = α2 – α1, where b is a positive number; then
For the ratio rD/rU to be high, the concentration of A should be as low as possible.
For α2 > α1 use a CSTR and dilute the feed stream.
This low concentration may be accomplished by diluting the feed with inerts and running the reactor at low concentrations of species A. A CSTR should be used because the concentrations of reactants are maintained at a low level. A recycle reactor in which the product stream acts as a diluent could be used to maintain the entering concentrations of A at a low value.
Because the activation energies of the two reactions in cases 1 and 2 are not given, it cannot be determined whether the reaction should be run at high or low temperatures. The sensitivity of the rate selectivity parameter to temperature can be determined from the ratio of the specific reaction rates
Effect of temperature on selectivity
where A is the frequency factor and E the activation energy, and the subscripts D and U refer to desired and undesired product, respectively.
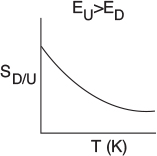
Case 3: ED > Ευ. In this case, the specific reaction rate of the desired reaction feD (and therefore the overall rate rD ) increases more rapidly with increasing temperature, T, than does the specific rate of the undesired reaction kv. Consequently, the reaction system should be operated at the highest possible temperature to maximize SD/U.

Case 4: Ευ > ED. In this case, the reaction should be carried out at a low temperature to maximize S^, but not so low that the desired reaction does not proceed to any significant extent.
Example 8-1 Maximizing the Selectivity for the Famous Trambouze Reactions
Reactant A decomposes by three simultaneous reactions to form three products, one that is desired, B, and two that are undesired, X and Y. These liquid-phase reactions, along with the appropriate rate laws, are called the Trambouze reactions (AIChE J., 5, 384).
1)
2)
3)
The specific reaction rates are given at 300 K and the activation energies for reactions (1), (2), and (3) are El = 10,000 cal/mole, E2 = 15,000 cal/mole, and E3 = 20,000 cal/mole.
(a) How, and under what conditions (e.g., reactor type(s), temperature, concentrations), should the reaction be carried out to maximize the selectivity of species B for an entering concentration of species A of 0.4 M and a volumetric flow rate of 2.0 dm3/s?
(b) How could the conversion of B be increased and still keep selectivity relatively high?
Solution
Part (a)
The instantaneous selectivity of species B with respect to species X and Y is
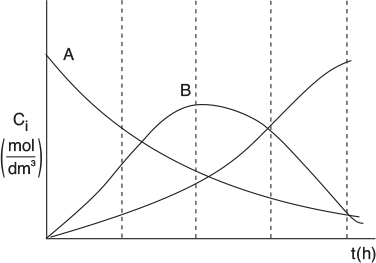
When we plot SB/XY vs. CA, we see that there is a maximum, as shown in Figure E8-1.1.
As we can see, the selectivity SB/XY reaches a maximum at a concentration . Because the concentration changes down the length of a PFR, we cannot operate the PFR at this maximum. Consequently, we must use a CSTR and design it to operate at this maximum of SB/XY. To find the maximum, , we differentiate SB/XY with respect to CA, set the derivative to zero, and solve for . That is
Solving for
We see from Figure E8-1.1 that the selectivity is indeed a maximum at = 0.112 mol/dm3.
Operate at this CSTR reactant concentration: = 0.112 mol/dm3.
Therefore, to maximize the selectivity SB/XY, we want to carry out our reaction in such a manner that the CSTR concentration of A is always at . The corresponding selectivity at is
We now calculate the CSTR volume when the exit concentration is . The net rate of formation of A is a sum of the reaction rates from Equations (1), (2), and (3)
Using Equation (E8-1.5) in the mole balance on a CSTR for this liquid-phase reaction (v = v0) to combine it with the net rate we obtain
CSTR volume to maximize selectivity
For an entering volumetric flow rate of 2 dm3/s, we must have a CSTR volume of 1564 dm3 to maximize the selectivity, SB/XY.
Maximize the selectivity with respect to temperature.
We now substitute in Equation (E8-1.1) and then substitute for in terms of k1and k3, cf. Equation (E8-1.3), to get SB/XY. in terms of k1, k2, and k3.
At what temperature should we operate the CSTR?
For the activation energies given in this example
So the selectivity for this combination of activation energies is independent of temperature!
What is the conversion of A in the CSTR?
If a greater than 72% conversion of A is required, say 90%, then the CSTR operated with a reactor concentration of 0.112 mol/dm3 should be followed by a PFR because the conversion will increase continuously as we move down the PFR (see Figure E8-1.2(b)). However, as can be seen in Figure E8-1.2, the concentration will decrease continuously from , as will the selectivity SB/XY as we move down the PFR to an exit concentration CAr. Consequently the system
How can we increase the conversion and still have a high selectivity SB/XY?
would give a higher conversion than the single CSTR. However, the selectivity, while still high, would be less than that of a single CSTR. This CSTR and PFR arrangement would give the smallest total reactor volume when forming more of the desired product B, beyond what was formed at CA in a single CSTR.
Figure E8-1.2 illustrates how as the conversion is increased above X* by adding the PFR reactor volume; however, the selectivity decreases.
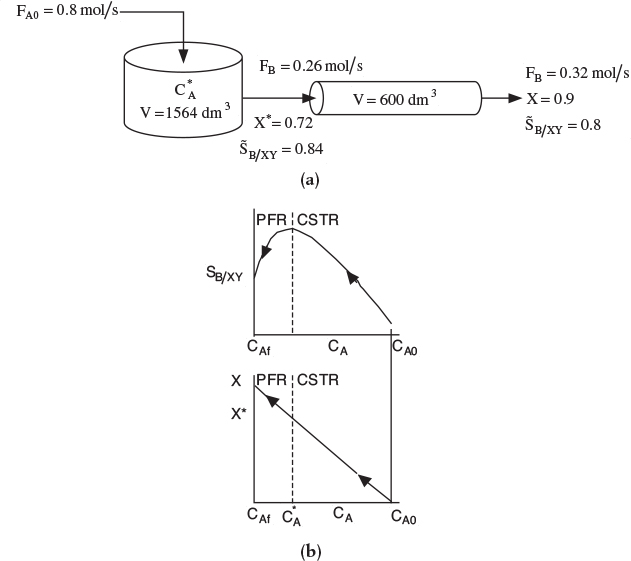
Figure E8-1.2 Effect of adding a PFR to increase conversion, (a) Reactor arrangement; (b) Selectivity and conversion trajectories.
This calculation for the PFR is carried out in the Chapter 8 Expanded Material on the CRE Web site (http://www.umich.edu/~elements/5e/08chap/expanded.html). The results of this calculation show that at the exit of the PFR, the molar flow rates are Fx = 0.22 mol/s, FB = 0.32 mol/s, and FY = 0.18 mol/s corresponding to a conversion of X = 0.9. The corresponding selectivity at a conversion of 90% is
Do you really want to add the PFR?
Analysis: One now has to make a decision as to whether adding the PFR to increase the conversion of A from 0.72 to 0.9 and the molar flow rate of the desired product B from 0.26 to 0.32 mol/s is worth not only the added cost of the PFR, but also the decrease in selectivity from 0.84 to 0.8. In this example, we used the Tram-bouze reactions to show how to optimize the selectivity to species B in a CSTR. Here, we found the optimal exit conditions (CA = 0.112 mol/dm3), conversion (X = 0.72), and selectivity (SB/XY = 0.84). The corresponding CSTR volume was V = 1564 dm3. If we wanted to increase the conversion to 90%, we could use a PFR to follow the CSTR, but we would find that the selectivity decreased.
8.3.3 Reactor Selection and Operating Conditions
Next, consider two simultaneous reactions in which two reactants, A and B, are being consumed to produce a desired product, D, and an unwanted product, U, resulting from a side reaction. The rate laws for the reactions
are
The instantaneous selectivity
Instantaneous selectivity
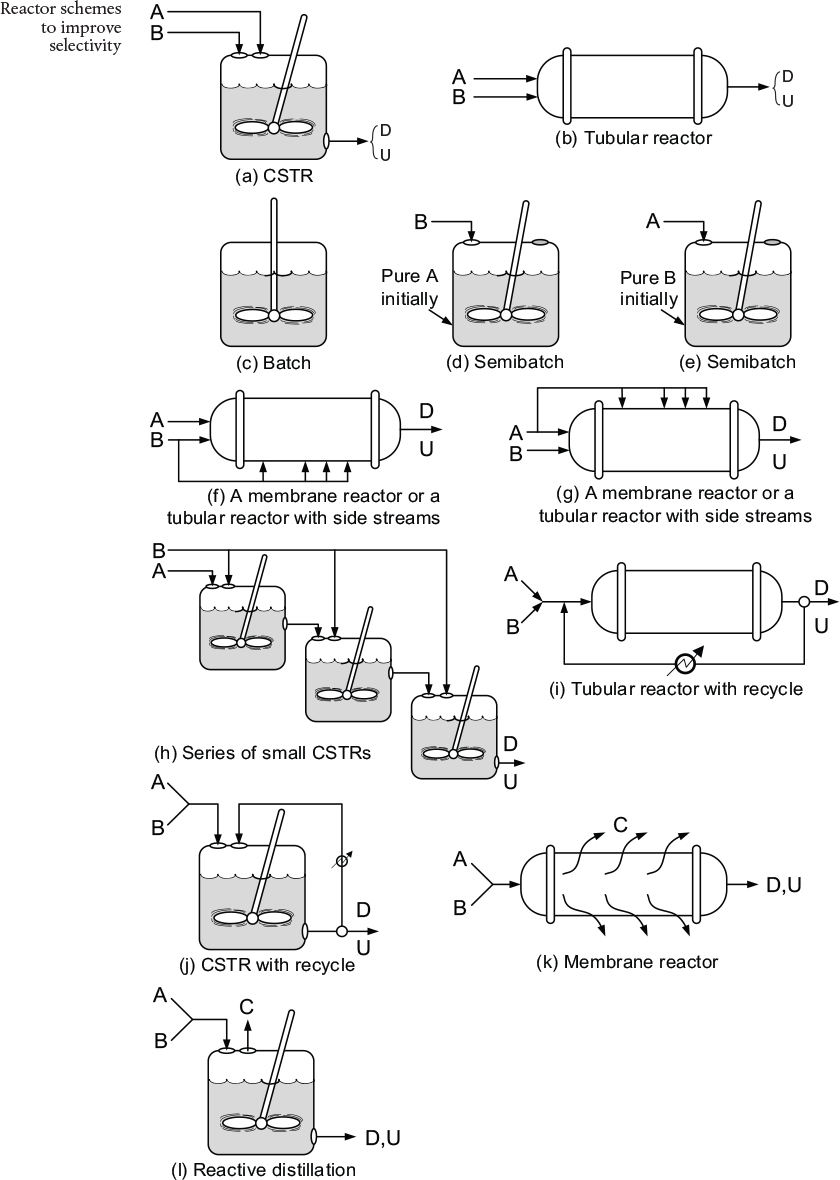
is to be maximized. Shown in Figure 8-2 are various reactor schemes and conditions that might be used to maximize SOjV.
The two reactors with recycle shown in (i) and 0) can also be used for highly exothermic reactions. Here, the recycle stream is cooled and returned to the reactor to dilute and cool the inlet stream, thereby avoiding hot spots and runaway reactions. The PFR with recycle is used for gas-phase reactions, and the CSTR is used for liquid-phase reactions.

The last two reactors in Figure 8-2, (k) and (1), are used for thermodynam-ically limited reactions where the equilibrium lies far to the left (reactant side)
and one of the products must be removed (e.g., C) for the reaction to continue to completion. The membrane reactor (k) is used for thermodynamically limited gas-phase reactions, while reactive distillation (1) is used for liquid-phase reactions when one of the products has a higher volatility (e.g., C) than the other species in the reactor.
In making our selection of a reactor, the criteria are safety, selectivity, yield, temperature control, and cost.
Figure 8-2 Different reactors and schemes for maximizing SD|u in Equation 8-16. Note unreacted A and B also exit the reactor along with D and U.
Reactor Selection
Criteria:
• Safety
• Selectivity
• Yield
• Temperature control
• Cost
Example 8-2 Chowe of Reactor and Conditions to Minimize Unwanted Products
For the parallel reactions
consider all possible combinations of reaction orders and select the reaction scheme that will maximize SD/U .
Solution
Case 1: α1 > α2, β1 > β2 . Let a = α1 — α2 and b = β1 — β2 , where a and b are positive constants. Using these definitions, we can write Equation (8-16) in the form
To maximize the ratio rO/rv, maintain the concentrations of both A and B as high as possible. To do this, use
• A tubular reactor, Figure 8-2 (b).
• A batch reactor, Figure 8-2(c).
• High pressures (if gas phase), and reduce inerts.
Case 2: α1 > α2 , β1 < β2 . Let a = α1 — α2 and b = β2 — β1, where a and b are positive constants. Using these definitions, we can write Equation (8-16) in the form
To make SD/U as large as possible, we want to make the concentration of A high and the concentration of B low. To achieve this result, use
• A semibatch reactor in which B is fed slowly into a large amount of A as in Figure 8-2 (d).
• A membrane reactor or a tubular reactor with side streams of B continually fed to the reactor as in Figure 8-2 (f).
• A series of small CSTRs with A fed only to the first reactor and small amounts of B fed to each reactor. In this way, B is mostly consumed before the CSTR exit stream flows into the next reactor as in Figure 8-2(h).
Case 3: α1 < α2 , β1 < β2 . Let a = α2 — α1 and b = β2 — β1, where a and b are positive constants. Using these definitions, we can write Equation (8-16) in the form
To make SD/U as large as possible, the reaction should be carried out at low concentrations of A and of B. Use
• A CSTR as in Figure 8-2(a).
• A tubular reactor in which there is a large recycle ratio as in Figure 8-2(i).
• A feed diluted with inerts.
• Low pressure (if gas phase).
Case 4: α1< α2 , β1 > β2. Let a = α2 — α1 and b = ß1 — β2, where a and b are positive constants. Using these definitions, we can write Equation (8-16) in the form
To maximize SD/U, run the reaction at high concentrations of B and low concentrations of A. Use
• A semibatch reactor with A slowly fed to a large amount of B as in Figure 8-2(e).
• A membrane reactor or a tubular reactor with side streams of A as in Figure 8-2 (g).
• A series of small CSTRs with fresh A fed to each reactor.
Analysis: In this very important example we showed how to use the instantaneous selectivity, SD/U, to guide the initial selection of the type of reactor and reactor system to maximize the selectivity with respect to the desired species D. The final selection should be made after one calculates the overall selectivity for the reactors and operating conditions chosen.
8.4 Reactions in Series
In Section 8.1, we saw that the undesired product could be minimized by adjusting the reaction conditions (e.g., concentration, temperature) and by choosing the proper reactor. For series (i.e., consecutive) reactions, the most important variable is time: space time for a flow reactor and real time for a batch reactor. To illustrate the importance of the time factor, we consider the sequence

in which species B is the desired product.
If the first reaction is slow and the second reaction is fast, it will be extremely difficult to produce a significant amount of species B. If the first reaction (formation of B) is fast and the reaction to form C is slow, a large yield of B can be achieved. However, if the reaction is allowed to proceed for a long time in a batch reactor, or if the tubular flow reactor is too long, the desired product B will be converted to the undesired product C. In no other type of reaction is exactness in the calculation of the time needed to carry out the reaction more important than in series reactions.
Example 8-3 Series Reactions in a Batch Reactor
The elementary liquid-phase series reaction
is carried out in a batch reactor. The reaction is heated very rapidly to the reaction temperature, where it is held at this temperature until the time it is quenched by rapidly lowering the temperature.
(a) Plot and analyze the concentrations of species A, B, and C as a function of time.
(b) Calculate the time to quench the reaction when the concentration of B will be a maximum.
(c) What are the overall selectivity and yields at this quench time?
CA0 = 2M, k1=0.5h-1, k2 =0.2h-1
Solution
Part (a)
0. Number the Reactions:
The preceding series reaction can be written as two reactions
1. Mole Balances:
1A. Mole Balance on A:
a. Mole balance in terms of concentration for V = V0 becomes
b. Rate law for Reaction 1: Reaction is elementary
c. Combining the mole balance and rate law
Integrating with the initial condition CA = CA0 at t = 0
Solving for CA
IB. Mole Balance on B:
a. Mole balance for a constant-volume batch reactor becomes
b. Rates:
Rate Laws
Elementary reactions
Relative Rates
Rate of formation of B in Reaction 1 equals the rate of disappearance of A in Reaction 1.
The net rate of reaction of B will be the rate of formation of B in reaction (1) plus the rate of formation of B in reaction (2).
c. Combining the mole balance and rate law
Rearranging and substituting for CA
Using the integrating factor gives
There is a tutorial on the integrating factor in Appendix A and on the CRE Web site.
At time t = 0, CB = 0. Solving Equation (E8-3.13) gives
1C. Mole Balance on C:
The mole balance on C is similar to Equation (E8-3.1).
The rate of formation of C is just the rate of disappearance of B in reaction (2), i.e., rc = -r2B = k2CB
Substituting for CB
and integrating with Cc = 0 at t = 0 gives
Note that as t —>∞ then Cc = CA0 as expected. We also note the concentration of C, Cc, could have been obtained more easily from an overall balance.
Calculating the concentration of C the easy way
The concentrations of A, B, and C are shown as a function of time in Figure E8-3.1.
Part (b)
1. Concentration and Time at the Maximum
We note from Figure E8-3.1 that the concentration of B goes through a maximum. Consequently, to find the maximum we need to differentiate Equation E8-3.14 and set it to zero.
Solving for tmax gives
Substituting Equation (E8-3.20) into Equation (E8-3.5), we find the concentration of A at the maximum for CR is
Series Reaction
Similarly, the concentration of B at the maximum is
2. Evaluate: Substituting for CA0 = 2 mol/dm3, k1 = 0.5h-1, and k2 = 0.2h-1 in Equations (E8-3.5), (E8-3.14), and (E8-3.18), the concentrations as a function of time are
Substituting in Equation (E8-3.20)
The time to quench the reaction is at 3.05 h.
At tmax = 3.05 h, the concentrations of A, B, and C are
The concentration of C at the time we quench the reaction is
Cc = CA0 - CA - CB = 2 - 0.44 - 1.07 = 0.48 mol/dm3
Part (c) Calculate the overall selectivity and yield at the reaction quench time. The selectivity is
The yield is
Analysis: In this example, we applied our CRE algorithm for multiple reactions to the series reaction A —> B —> C. Here, we obtained an analytical solution to find the time at which the concentration of the desired product B was a maximum and, consequently, the time to quench the reaction. We also calculated the concentrations of A, B, and C at this time, along with the selectivity and yield.
We will now carry out this same series reaction in a CSTR.
Example 8-4 Series Reaction in a CSTR
The reactions discussed in Example 8-3 are now to be carried out in a CSTR.
(a) Determine the exit concentrations from the CSTR.
(b) Find the value of the space time τ that will maximize the concentration of B.
Part (a)
1. a. Mole Balance on A:
IN |
- |
OUT |
+ |
GENERATION |
= |
0 |
FA0 |
- |
FA |
+ |
rAV |
= |
0 |
u0 CA0 |
- |
v0 CA |
+ |
rA V |
= |
0 |
Dividing by υ0, rearranging and recalling that τ=ν/υ0, we obtain
b. Rates
The laws and net rates are the same as in Example 8-3.
C. Combining the mole balance of A with the rate of disappearance of A
Solving for CA
We now use the same algorithm for species B we did for species A to solve for the concentration of B.
2. a. Mole Balance on B:
IN |
- |
OUT |
+ |
GENERATION |
= |
0 |
0 |
- |
Fb |
+ |
rBV |
= |
0 |
- |
v0 CB |
+ |
rBV |
= |
0 |
Dividing by v0 and rearranging
b. Rates
The laws and net rates are the same as in Example 8-3.
Net Rates
c. Combine
Substituting for CA
Rates
Part (b) Optimum Concentration of B
To find the maximum concentration of B, we set the differential of Equation (E8-4.8) with respect to τ equal to zero
Solving for τ at which the concentration of B is a maximum at
The exiting concentration of B at the optimum value of τ is
Substituting Equation (E8-4.11) for τmax in Equation (E8-4.12)
Rearranging, we find the concentration of B at the optimum space time is
Evaluation
At τmax, the concentrations of A, B, and C are
The conversion is
The selectivity is
The yield is
Real data compared with real theory.
SUMMARY TABLE
Time |
|||
Batch |
tmax = 3.05 h |
2.3 |
0.69 |
CSTR |
τmax = 3.16 h |
1.6 |
0.61 |
Analysts: The CRE algorithm for multiple reactions was applied to the series reaction A —» B —» C in a CSTR to find the CSTR space time necessary to maximize the concentration of B, i.e., τ = 3.16 h. The conversion at this space time is 61%, the selectivity, SB/C , is 1.60, and the yield, Y"B , is 0.63. The conversion and selectivity are less for the CSTR than those for the batch reactor at the time of quenching.
PFR
If the series reaction were carried out in a PFR, the results would essentially be those of a batch reactor where we replaced the time variable “t” with the space time, “τ". Data for the series reaction
is compared for different values of the specific reaction rates, k1 and k2, in Figure 8-3.
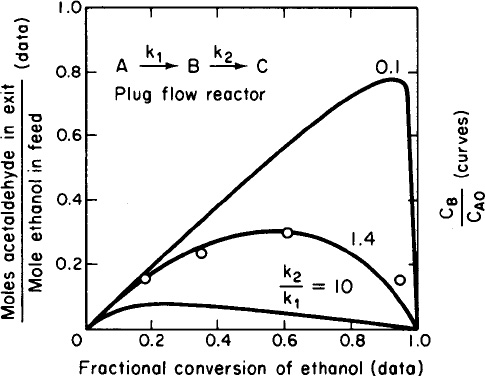
Figure 8-3 Yield of acetaldehyde as a function of ethanol conversion. Data were obtained at 518 K. Data points (in order of increasing ethanol conversion) were obtained at space velocities of 26,000, 52,000,104,000, and 208,000 h-1. The curves were calculated for a first-order series reaction in a plug-flow reactor and show yield of the intermediate species B as a function of the conversion of reactant for various ratios of rate constants k2 and fea. (McCabe, Robert, W., and Patricia J. Mitchell. Oxidation of ethanol and acetaldehyde over alumina-supported catalysts. Industrial & Engineering Chemistry Product Research and Development 1983 22(2), 212-217. Copyright © 1963, American Chemical Society. Reprinted by permission.)
A complete analysis of this reaction carried out in a PFR is given on the CRE Web site.
Living Example Problem
8.5 Complex Reactions
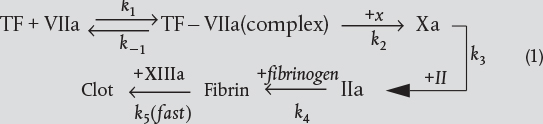
A complex reaction system consists of a combination of interacting series and parallel reactions. Overall, this algorithm is very similar to the one given in Chapter 6 for writing the mole balances in terms of molar flow rates and concentrations (i.e., Figure 6-1). After numbering each reaction, we write a mole balance on each species, similar to those in Figure 6-1. The major difference between the two algorithms is in the rate-law step. As shown in Table 8-2, we have three steps (3, 4, and 5) to find the net rate of reaction for each species in terms of the concentration of the reacting species. As an example, we shall study the following complex reactions
In business, it is usually important to keep your reactions proprietary.
This industrially important complex reaction, which has been coded as A, B, C, and D for propriety and security reasons, embodies virtually all the nuances needed to gain a thorough understanding of multiple reactions occurring in common industrial reactors. The following three examples model this reaction in a PBR, a CSTR, and a semibatch reactor, respectively.
8.5.1 Complex Gas-Phase Reactions in a PBR
We now apply the algorithms in Tables 8-1 and 8-2 to a very important complex reaction carried out in a PBR. To protect the confidential nature of this reaction the chemicals have been given the names A, B, C, and D.
Example 8-5 Multiple Gas-Phase Reactions in a PBR
The following complex gas-phase reactions follow elementary rate laws
and take place isothermally in a PBR. The feed is equimolar in A and B with FA0 = 10 mol/min and the volumetric flow rate is 100 dm3/min. The catalyst weight is 1,000 kg, the pressure drop is α = 0.0019 kg-1, and the total entering concentration is CT0 = 0.2 mol/dm3.
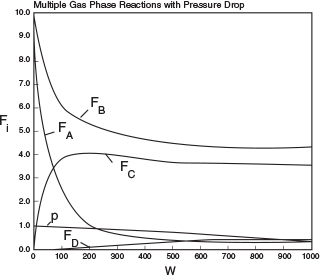
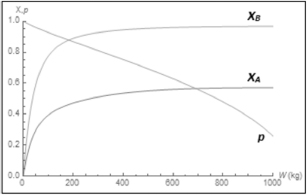
Plot and analyze FA, FB, Fc, FD, y, and SC/D as a function of catalyst weight, W.
Solution
Following the algorithm in Table 8-2 and having numbered our reactions, we now proceed to carry out a mole balance on each and every species in the reactor.

Gas-Phase PBR
1. Mole Balances
(1)
(2)
(4)

Following the Algorithm
2. Rates
Net Rates
(5)
(6)
(7)
(8)
Rate Laws
(9)
(10)
Relative Rates
(11)
(12)
(13)
(14)
The net rates of reaction for species A, B, C, and D are
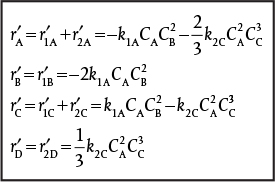
Selectivity
Tricks of the Trade
One observes that at W = 0, FD = 0 causing SC/D to go to infinity, Therefore, we set SC/D = 0 between W = 0 and a very small number, W = 0.0001 kg, to prevent Polymath, as well as other ODE solvers such as MATLAB and Excel, from crashing. In Polymath, this condition is written
3. Stoichiometry Isothermal T = T0
4. Parameters
(24) CT0=0.2mol/dm3
(25) α = 0.0019 kg-1
(26) u0=100dm3/min
(27) k1A = 100 (dm3/mol)2/min/kg-cat
(28) k2C = 1,500 (dm15/mol4)/min/kg-cat
(29) FT0 =20mol min
(30) FA0 = 10
(31) FBO = 10
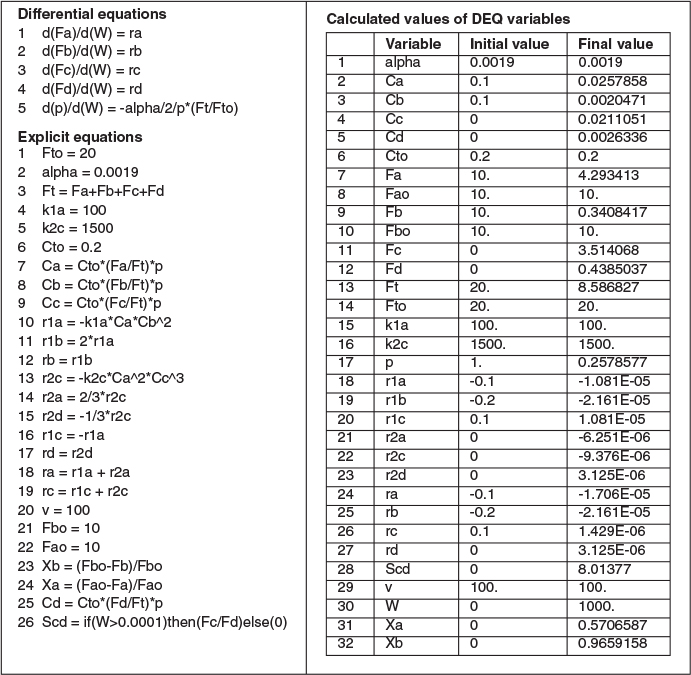
Entering the above equations into Polymath’s ODE solver, we obtain the following results in Table E8-5.1 and Figures E8-5.1 and E8-5.2.
Analysis: This example is a very important one as it shows, step by step, how to handle isothermal complex reactions. For complex reactions occurring in any of the reactors shown in Figure 8-2, a numerical solution is virtually always required. The Polymath program and sample results are shown below. This problem is a Living Example Problem, LEV, so the reader can download the Polymath code and vary the reaction parameters (e.g., k1A, α, k2c, νo) to learn how Figures E8-5.1 and E8-5.2 change (http://vmriv.umich.edu/~elements/5e/08chap/live.html).
In looking at the solution we note from Figure E8-5.2 that the selectivity reaches a maximum very close to the entrance (W ≈ 60 kg) and then drops rapidly. However, 90% of A is not consumed until 200 kg, the catalyst weight at which the desired product C reaches its maximum flow rate. If the activation energy for reaction (1) is greater than that for reaction (2), try increasing the temperature to increase the molar flow rate of C and selectivity. However, if that does not help, then one has to decide which is more important, selectivity or the molar flow rate of the desired product. In the former case, the PBR catalyst weight will be 60 kg. In the latter case, the PBR catalyst weight will be 200 kg.
The molar flow rate profiles are shown in Figure E8-5.1, while the selectivity and conversion profiles are shown in Figures E8-5.2 and E8-5.4, respectively.
8.5.2 Complex Liquid-Phase Reactions in a CSTR
For a CSTR, a coupled set of algebraic equations analogous to the PFR differential equations must be solved. These equations are arrived at from a mole balance on CSTR for every species coupled with the rates step and stoichiometry. For q liquid-phase reactions occurring where N different species are present, we have the following set of algebraic equations:
We can use a nonlinear algebraic equation solver (NLE) in Polymath or a similar program to solve Equations (8-17) through (8-19).